Abstract
For children with severe asthma, guideline-based management focuses on the escalation of anti-inflammatory and bronchodilatory medications while addressing comorbid conditions. Bronchoscopy, in this context, has been relegated to ruling out asthma mimickers. More recently, however, there have been questions surrounding the clinical utility of bronchoscopy in severe childhood asthma. In this solicited lecture summary, we discuss the past, present, and potential future applications of bronchoscopy in severe childhood asthma.
Keywords: Airway inflammation, Chlamydophila, Mycoplasma, Pneumocystis
The Truth is Out There: Controversial Topics Breed Opposing Sentiments
“What is the role of bronchoscopy in childhood asthma?” This query, when posed to the contemporary pediatric pulmonologist, typically elicits one of a variety of responses ranging from “it’s an unethical abuse of medical technology” to “it’s an irreplaceable tool for proper diagnosis and management”. The response provided depends largely on one’s preconceived beliefs, personal experience, and quite frankly, “gut feeling”. The paucity of published evidence on the use of bronchoscopy in childhood asthma is neither strong enough to convince the skeptics of its merit nor weak enough to dissuade the believers of its use. The skeptics may point to the available literature, which is almost exclusively case-based, noting the prominent lack of randomized, placebo-controlled trials from which to interpret data. The believers, on the other hand, may give credence to general logic and deduction, as it would seem unlikely that the direct examination of a diseased body compartment would be of no practical value to the treating clinician. As is the case for many controversial topics, the truth likely lies somewhere between the two extremes. In this solicited lecture summary, we discuss the past, present, and potential future of bronchoscopy in childhood asthma using a combination of documented literature, personal experience, logic, deduction, and admittedly, some unsubstantiated opinion.
Old School Ingenuity: The Earliest Applications of Bronchoscopy in Asthma
It might come as a surprise to many contemporary pediatric pulmonologists that the use of bronchoscopy in asthma is not simply a byproduct of recent technological advances. Bronchoscopy has been employed in the direct clinical management of patients with asthma since the early 1900s (Figure 1 provides a timeline of major milestones). In fact, a pre-1970 PubMed search using the keywords “bronchoscopy and asthma” yields a myriad of literature highlighting bronchoscopy’s use in clinical care. The earliest manuscript, published in 1917 by W. S. Syme in the British Medical Journal, is actually entitled “Bronchoscopy in the treatment of asthma”1. Physicians in the early twentieth century routinely turned to bronchoscopy as a means of escalating therapy in patients with difficult to manage asthma, either by clearing secretions, performing bronchoalveolar lavage (BAL), formulating autogenous vaccines, or even delivering topical medications directly into the lung, most notably silver nitrate and “asthma solution”, a mixture of adrenaline, cocaine, and normal saline1–4. While some of the aforementioned techniques might elicit skepticism or even shock from the contemporary clinician, the ambitions of our predecessors should not be overlooked, giving nod to the complexities of asthma as a disease process and the ingenuity that comes with trying to individualize therapy. That being said, the use of bronchoscopy as a treatment modality in asthma largely fell out of favor after 1970 when the successful commercialization of safe, effective, and user-friendly inhaled medications, including selective beta agonists and corticosteroids, resulted in better asthma symptom control and dampened the need for invasive management strategies5.
Figure 1.
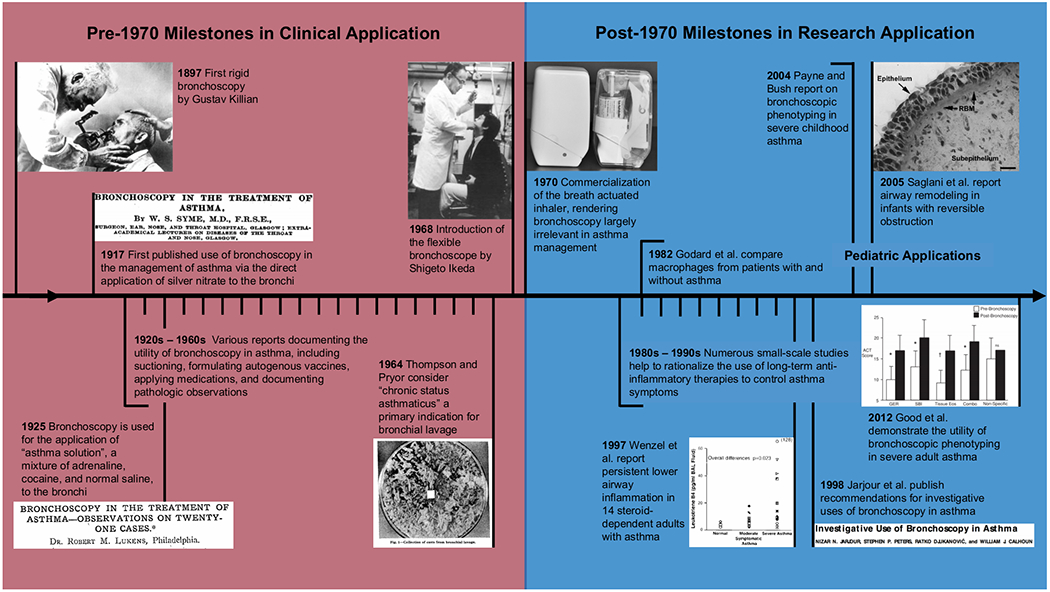
Major milestones in the use of bronchoscopy for asthma
That is not to say that bronchoscopy is never employed by contemporary pulmonologists in the management of patients with asthma. More accurately, bronchoscopy has secured a limited role in the diagnosis and management of “not asthma”. Few would disregard its role in diagnosing airway abnormalities that cause chronic cough, persistent wheeze, or other respiratory symptoms unresponsive to aggressive asthma therapy (Figure 2). Indeed, numerous citations exist demonstrating the utility of bronchoscopy in identifying, and in some cases treating, dynamic and fixed airway abnormalities, airway tumors, vascular compression anomalies, foreign bodies, and even leeches in patients mistakenly diagnosed with asthma, thus popularizing the phrase “All that wheezes is not asthma”6–10. Nevertheless, bronchoscopy for true asthma has never fully regained its pre-1970 status as an important component in asthma diagnosis and management. Bronchoscopy does not have a role in the current consensus statements regarding asthma management11–12, and asthma is not an application put forth by either of the two most recent consensus statements addressing pediatric flexible bronchoscopy13–14.
Figure 2.
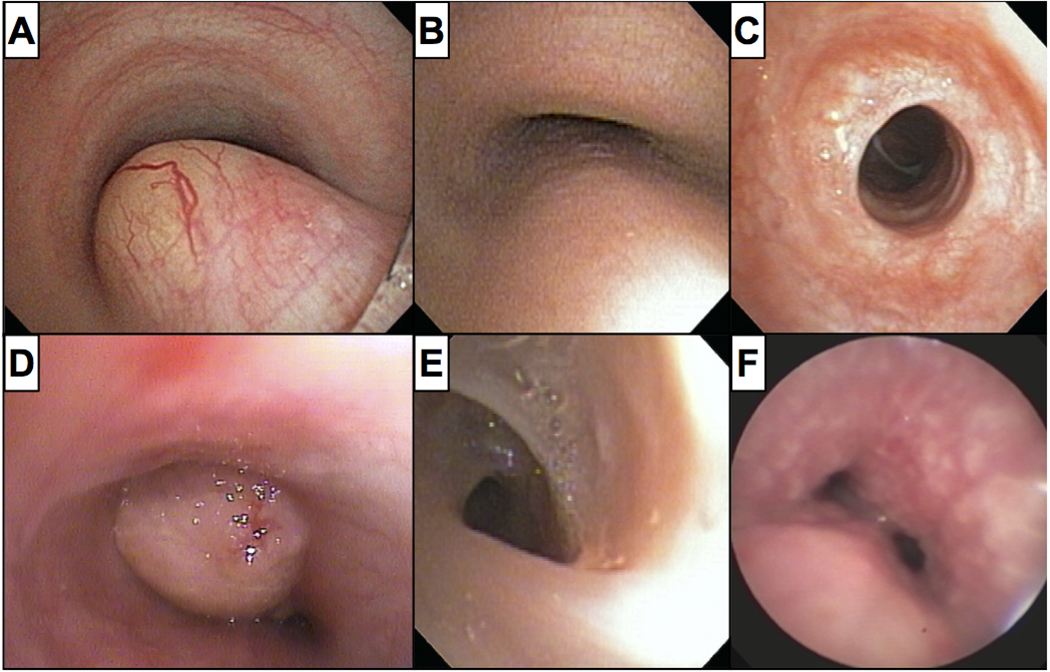
Examples of airway abnormalities in children referred for severe asthma, including (A) a foregut duplication cyst, (B) tracheomalacia in an adolescent, (C) short segment tracheal stenosis, (D) a myofibroblastic tumor, (E) a spiral-shaped web, and (F) granulomatous disease (Vicencio, personal archive)
Keeping Up with the Times: Bronchoscopy as a Research Tool
While many pre-1970 clinicians highlighted the use of bronchoscopy in the direct clinical management of patients with asthma, some also made note of pathologic findings that would ultimately prove critical to the modern understanding of asthma, commenting on airway eosinophilia, mucus composition, and bronchial epithelium changes. While less invasive therapies rendered bronchoscopy largely irrelevant for the clinical care of patients with asthma, post-1970 clinicians adapted the procedure for use in research, aided significantly by the introduction of the flexible bronchoscope in 196815. In 1974, Reynolds and Newball were among the first to publish their use of flexible bronchoscopy in human investigation, reporting the feasibility of analyzing respiratory cells and secretions obtained from human volunteers16. In 1982, Godard et al. used similar techniques to compare macrophages collected from the lower airways of patients with and without asthma17. These early studies paved the way for continued asthma investigation employing flexible bronchoscopy as a primary tool. Subsequent studies were instrumental in demonstrating the importance of inflammation in asthma pathogenesis and helping to rationalize the use of anti-inflammatory medications in the control of symptoms18. Notably, the use of bronchoscopy, an invasive procedure with, at that time, limited therapeutic utility for asthma, garnered scrutiny as a research instrument; the ethics of performing the procedure were questioned, and the lack of a standardized protocol between studies was criticized.
In an effort to address these concerns, Jarjour et al. published in 1998 a manuscript entitled “Investigative use of bronchoscopy in asthma”, which reviewed the utility, technique, and safety of bronchoscopy in asthma based largely on evidence collated from hundreds of preceding independent studies19. Included in this statement are general recommendations regarding patient assessment and preparation for the procedure as well as more granular recommendations regarding specific techniques, including BAL, segmental allergen challenge, and biopsy. The authors concluded by stating, “We are confident that current and future meticulously designed and executed research studies utilizing bronchoscopic techniques will significantly add to our knowledge of disease mechanisms and lead us to new and improved treatments for asthma”19.
One of the first groups to “meticulously design and execute” such studies was National Jewish Hospital in Denver, CO, USA. In 1997, Wenzel et al. noted that “almost no clinical, physiologic or pathologic data exist on severe, steroid-dependent asthmatics”20. To correct this deficiency and possibly formulate better management strategies, they reported bronchoscopic findings in fourteen such patients, concluding that “…inflammation remains in severe symptomatic asthmatics despite treatment with high dose glucocorticoids which may be due to the severity of disease…or other as yet undefined factors”20. Quickly establishing themselves as a national and international referral center that routinely offers bronchoscopy for patients with severe asthma, Good et al. eventually published a manuscript in 2012 entitled “Refractory asthma: importance of bronchoscopy to identify phenotypes and direct therapy”21. To our knowledge, this manuscript remains the only prospective study to objectively document improvement in patients with severe asthma based predominately on information obtained via bronchoscopy. Specifically, they identified “…five mutually exclusive phenotypes [in 58 individuals]…based on bronchoscopic evaluation…so as to better individualize therapeutic options and improve asthma control”21. Indeed, the investigators demonstrated robust improvements in asthma control test (ACT) scores and more modest, though significant, improvements in forced expiratory volume in one second (FEV1) measures. It should be noted, however, that the highlighted phenotypes and their proposed treatments may not be clinically relevant in current times. For example, one phenotype, persistent airway eosinophilic inflammation, was treated with omalizumab, the only biologic therapy available for use in asthma at that time. Today, such treatment is dictated by analysis of peripheral blood, and additional biologic therapies are available that specifically target eosinophils. The methods described by Good et al. were not widely adopted by other clinicians at the time, in part, because some of the phenotypes discussed required tools that were not widely available, such as polymerase chain reaction (PCR) to identify the presence of subacute atypical bacterial infection.
The concept of bronchoscopic phenotyping in severe asthma was not completely foreign to pediatric clinicians. In fact, a very similar manuscript entitled “Phenotype-specific treatment of difficult asthma in children” was published by Payne and Bush (Royal Brompton Hospital, London, England) eight years before the Good et al. report22. In their manuscript, Payne and Bush suggested that cyclosporine, azithromycin, and subcutaneous terbutaline might represent therapeutic options for persistent eosinophilic, neutrophilic, or pauci-granulocytic inflammation, respectively. Unlike Good et al., Payne and Bush did not provide objective measures of improvement using this approach. This deficiency, coupled with heightened controversy regarding the use of an invasive procedure in children, likely contributed to the lack of adoption of such techniques among contemporary clinicians. Nevertheless, Payne and Bush paved the way for important investigations in children over subsequent years.
Between 2000 and the present day, there have been limited high-quality publications that employ bronchoscopy in severe childhood asthma, many of which are either directly or indirectly linked to the Royal Brompton group. In this regard, federal regulations in the United States require that interventions in clinical studies that pose greater than minimal risk to pediatric human subjects also hold the prospect for direct clinical benefit. Although one can reasonably argue that patients with poorly controlled severe childhood asthma may benefit from undergoing bronchoscopy, design of controlled trials comparing bronchoscopy-directed care vs. standard care is typically limited by such considerations. Nevertheless, such investigations, when done, have yielded important information regarding airway remodeling23–27 and inflammatory pattern alterations27–29 in children. Most recently, Robinson et al. analyzed the microbiome from the lower airways of children with severe asthma, suggesting that such analysis is a superior method for subcategorizing disease compared to more traditional clinical parameters30.
Despite their important contributions in general knowledge, these investigations thus far have done little to alter current management. As such, to the contemporary pediatric pulmonologist treating a patient with severe childhood asthma, the role of bronchoscopy remains somewhat of an enigma.
Back to the Future: The Re-emergence of Bronchoscopy in Clinical Management?
In recent years, the clinical utility of bronchoscopy in severe childhood asthma seems to be resurfacing in select circles – this solicited lecture summary being one testament – fueled in part by ongoing research as well as a general frustration regarding the paucity of options for some patients. Currently, however, there are no standard protocols in place for the use of bronchoscopy in childhood asthma. Those groups who have chosen to employ the procedure typically reserve it for the minority of patients who fail standard therapy. For example, Teague et al. recently reported their experience over a 9-year period. Of approximately 2800 children referred to their asthma center for management, 311 underwent bronchoscopy after failing guideline-based treatment; 185 were found to have an alternate diagnosis causing persistent symptoms, while 126 retained the primary diagnosis of asthma31. Although this and other studies have documented the safety of bronchoscopy in childhood asthma, studies demonstrating clinical efficacy are still lacking31–33. In this section, we briefly discuss specific conditions for which we believe bronchoscopy may have immediate, or at the very least expectant, applications.
Subacute Infection:
In 2010, we used PCR to analyze BAL fluid from two separate cohorts of children with chronic unexplained cough and/or refractory asthma for the presence of atypical bacteria, demonstrating the presence of Chlamydophila pneumoniae in 33-50% of samples34. Notably, C. pneumoniae positivity was associated with neutrophilia and increased IL-8 levels, suggesting that the presence of C. pneumoniae alters inflammatory and gene expression profiles. Although this study does not prove causation, subsequent investigations using animal models have demonstrated robust release of histamine from neutrophils in the setting of subacute atypical infection with C. pneumoniae and Mycoplasma pneumoniae specifically, a response not observed with other bacterial species, which may represent a pathophysiologic link between atypical infection and asthma35–36. Importantly, clinical trials using targeted antimicrobial therapy in adults with poorly controlled asthma yielded conflicting results. Specifically, Kraft et al. demonstrated improvement in lung function with macrolide therapy in patients who were PCR-positive for atypical infection in BAL fluid and/or on endobronchial biopsy37, while Sutherland et al., using PCR on biopsy alone, failed to show similar improvements38.
In a similar manner, we and others have long proposed that subacute fungal infection may contribute to severe asthma, although proof of such infection has remained elusive39–45. Recently, using mycobiome techniques, we reported the unexpected abundance of Pneumocystis jirovecii in BAL fluid from children with asthma compared to controls (Figure 3), strengthening prior independent reports that have implicated P. jirovecii in asthma pathogenesis46–51. As our current findings are associative in nature, further study is required to determine causality. However, with modern point-of-care testing, specifically commercially-available PCR, and inexpensive treatment options, the use of bronchoscopy to potentially diagnose subacute atypical bacterial or P. jirovecii infection in children with severe asthma seems quite tantalizing.
Figure 3.

Data from Goldman et al46 demonstrating significant differences in several fungal species, most notably P. jirovecii, in the lower airways of children with severe asthma (SA) compared with non-asthma controls (nonA)
Inflammatory Assessment:
Although control of airway inflammation remains the standard approach for the treatment of severe childhood asthma, few studies have investigated whether inflammatory markers commonly employed in the clinical setting, including analysis of peripheral blood, sputum, and more recently fractional excretion of nitric oxide (FENO), accurately reflect the inflammation seen in the lower airways of children. Currently, standard therapy, including inhaled corticosteroids, is dictated almost exclusively by symptoms rather than assessment of inflammation. While FENO has become an important tool in guiding asthma therapy for many children, it is still unclear whether this modality accurately reflects the inflammatory milieu in children with severe disease52–54. With the recent introduction of biologic therapies directed against specific inflammatory cells, like the eosinophil, objective data are becoming increasingly important for children with severe disease.
Ullman et al. were the first to prospectively and rigorously analyze surrogate markers of airway inflammation in children with severe asthma, concluding that “peripheral blood counts are not reliable in characterizing airway inflammation in severe asthmatic children exposed to high dose steroid therapy, therefore bronchoscopy with BAL should be considered”55. More recently, Ribeiro et al. demonstrated similar discordance between blood and BAL eosinophils, also highlighting the possibility that analysis of BAL eosinophils may help to identify patients who might be candidates for anti-IL-5 therapy despite having blood values outside the recommended parameters for treatment56. Similarly, Teague et al. concluded that “in 32% of children evaluated, BAL revealed corticosteroid-refractory eosinophilic infiltration amenable to anti-TH2 biological therapies”31. While data from these studies are insufficient to routinely inform treatment changes at present, ongoing investigations may identify a larger role for the assessment of lower airway inflammation in clinical care. Indeed, recent reports suggest that analysis of inflammatory patterns in bronchial biopsies may be useful in predicting future lung function57–58.
Other Conditions:
During the course of performing bronchoscopy in children with severe asthma, we have periodically stumbled upon unexpected pathology. For example, Spencer et al. previously reported a small case series of three patients whose bronchoscopies demonstrated extensive nodularity throughout the tracheobronchial tree (Figure 4)59. Cryobiopsy of the lesions revealed either dense islands of lymphoplasmacytic cells or non-caseating granulomas. In the two years since that report, we have identified several other patients with identical findings (Spencer and Vicencio, unpublished). The vast majority of these patients demonstrate positive serologic markers of autoimmune disease, including antinuclear antibodies (≥1:160), antineutrophilic cytoplasmic antibodies, and/or rheumatoid factor, prompting the label “airway autoimmune inflammatory response (AAIR) syndrome”. Interestingly, although none of these patients fit clinical criteria for a specific rheumatologic disorder, targeted treatment for some, specifically twice daily trimethoprim-sulfamethoxazole or daily azathioprine, resulted in dramatic improvement in not only symptom control but also mucosal nodularity. A similar condition, termed “asthmatic granulomatosis”, was previously described in adult patients and successfully managed with either azathioprine, mycophenolic acid, methotrexate, or infliximab60.
Figure 4.

Two patients diagnosed with AAIR syndrome adapted from Spencer et al59. Pathology demonstrates dense lymphoplasmacytic islands (top panel) and well-formed non-caseating granulomas (bottom panel)
Ongoing Studies:
In late 2015, we initiated an IRB-approved protocol to bank matched biologic specimens, specifically blood, BAL fluid, and nasal and bronchial brush biopsies, from children with severe asthma undergoing bronchoscopy. At the time of this editorial’s submission, 52 patients have been enrolled into this study, 22 of whom have completed analysis for atypical bacterial infection, P. jirovecii infection, discordant BAL/blood eosinophils, and AAIR syndrome. Ten patients (45%) were positive for one or more of these potentially actionable conditions (Goldman, Januska, Bunyavanich, and Vicencio, unpublished). Ongoing studies in this regard may help to facilitate a more personalized approach to the child with severe asthma.
Based on these recent findings as well as our prior experience, we present our general approach to the application of bronchoscopy in childhood asthma (Figure 5), fully acknowledging that its efficacy has not been validated. Indeed, we expect that our protocol will change as additional information becomes available, either through our own future discoveries or those from other groups.
Figure 5.

Example of our approach to the application of bronchoscopy in childhood asthma adapted from the EPR-3: Guidelines for the Diagnosis and Management of Asthma11 with modifications highlighted in black boxes. BAL, bronchoalveolar lavage; EPR-3, expert panel report 3; ICS, inhaled corticosteroids; LABA, long-acting beta agonist; LTRA, leukotriene receptor antagonist; PCR, polymerase chain reaction
The Truth is (Still) Out There: Closing Remarks and Additional Opinions
The discipline of childhood asthma encompasses an army of bright, evidence-driven clinicians and investigators in search of scientific truth. It also includes pockets of passionate individuals with an unwavering desire to change the norm, even if by unconventional means and despite periodic criticism. At various points in our careers, the authors listed on this very summary reflected such diversity. In fact, the corresponding author (Vicencio) previously deflected numerous solicitations by a non-pulmonologist colleague (Goldman) to employ bronchoscopy in the investigation of subacute fungal infection in severe childhood asthma. As demonstrated by this co-authored summary and ongoing collaborations, a skeptic can indeed become a believer.
At present, it is unclear how frequently or infrequently bronchoscopy is employed in the care of children with asthma, and concrete numbers are difficult to ascertain. Appropriately, the reservations of many focus on the risk of performing an invasive procedure on children with uncontrolled disease, including the potential impact of prolonged general anesthesia on brain development in children less than three years of age61. Much less discussion, however, centers around the counterfactual risk of bronchoscopy, the risk of not performing the procedure. In this regard, current therapies for children with severe asthma are fraught with uncertainty, perhaps rendering concerns regarding general anesthesia somewhat less significant considering that the vast majority of these children are typically over the age of three and will undergo very short anesthesia times. Adverse effects of chronic systemic steroids render this option unpalatable, and newer biologic therapies lack long-term safety data and have unfavorable financial implications. As such, we believe that bronchoscopy may indeed become a preferred tool for diagnosis and management in the not-too-distant future. Currently, protocols for its use in childhood asthma and subsequent treatments vary widely among clinicians and are based largely on personal experience, logic, deduction, and availability of resources. As it stands, the “correct” approach is unknown.
In closing, it should be noted that our original question remains, “what is the role of bronchoscopy in childhood asthma?” Clearly, we have not definitively answered the question. However, we hope that this summary can, at a minimum, help bridge the gap between the skeptics and the believers and serve as a starting point for the exchange of ideas, expertise, and resources toward the common goal of finding the truth.
References
- 1.Syme WS. Bronchoscopy in the treatment of asthma. Br Med J. 1917;1(2948):868–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans EH. Bronchoscopy in asthma and other cases. Ind Med Gaz. 1933;68(11):627–628. [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson HT, Pryor WJ. Bronchial lavage in the treatment of obstructive lung disease. Lancet. 1964;2(7349):8–10. [DOI] [PubMed] [Google Scholar]
- 4.Lukens RM. Bronchoscopy in the treatment of asthma – observations on twenty-one cases. The Laryngoscope. 1925;35(3):227–234. [Google Scholar]
- 5.Stein SW, Thiel CG. The history of therapeutic aerosols: a chronological review: J Aerosol Med Pulm Drug Deliv. 2017;30(1):20–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maguire A, Gopalakaje S, Eastham K. All that wheezes is not asthma: a 6-year-old with foreign body aspiration and no suggestive history. BMJ Case Rep. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinberger M, Abu-Hasan M. Pseudo-asthma: when cough, wheezing, and dyspnea are not asthma. Pediatrics. 2007;120(4):855–864. [DOI] [PubMed] [Google Scholar]
- 8.Koul PA, Khan UH, Shah TH, Dar AM. All that wheezes is not asthma. BMJ Case Rep. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keng LT, Chang CJ. All that wheezes is not asthma: adult tracheomalacia resulting from innominate artery compression. Postgrad Med J. 2017;93(1095):54–55. [DOI] [PubMed] [Google Scholar]
- 10.Lee P, Tan ZY, Pham T. All that wheezes is not asthma. Thorax. 2018;73(8):792. [DOI] [PubMed] [Google Scholar]
- 11.National Asthma Education and Prevention Program, third expert panel on the diagnosis and management of asthma. Expert panel report 3: guidelines for the diagnosis and management of asthma. Bethesda (MD): National Heart, Lung, and Blood Institute (US), 2007. [Google Scholar]
- 12.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2019. Available from: www.ginasthma.org.
- 13.Faro A, Wood RE, Schechter MS, Leong AB, Wittkugel E, Abode K, Chmiel JF, Daines C, Davis S, Eber E, et al. Official American Thoracic Society technical standards: flexible airway endoscopy in children. Am J Respir Crit Care Med. 2015;191(9):1066–1080. [DOI] [PubMed] [Google Scholar]
- 14.Eber E, Anton-Pacheco JL, de Blic J, Doull I, Faro A, Nenna R, Nicolai T, Pohunek P, Priftis KN, Serio P, et al. European Respiratory Society statement: interventional bronchoscopy in children. Eur Respir J. 2017;50(6). [DOI] [PubMed] [Google Scholar]
- 15.Ikeda S, Yanai N, Ishikawa S. Flexible bronchofiberscope. Keio J Med. 1968;17(1):1–16. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds HY, Newball HH. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J Lab Clin Med. 1974;84(4):559–573. [PubMed] [Google Scholar]
- 17.Godard P, Chaintreuil J, Damon M, Coupe M, Flandre O, Crastes de Paulet A, Michel FB. Functional assessment of alveolar macrophages: comparison of cells from asthmatics and normal subjects. J Allergy Clin Immunol. 1982;70(2):88–93. [DOI] [PubMed] [Google Scholar]
- 18.Djukanovic R, Roche WR, Wilson JW, Beasley CR, Twentyman OP, Howarth RH, Holgate ST. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990;142(2):434–457. [DOI] [PubMed] [Google Scholar]
- 19.Jarjour NN, Peters SP, Djukanovic R, Calhoun WJ. Investigative use of bronchoscopy in asthma. Am J Respir Crit Care Med. 1998;157(3):692–697. [DOI] [PubMed] [Google Scholar]
- 20.Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, Martin RJ. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med. 1997;156(3):737–743. [DOI] [PubMed] [Google Scholar]
- 21.Good JT, Kolakowski CA, Groshong SD, Murphy JR, Martin RJ. Refractory asthma: importance of bronchoscopy to identify phenotypes and direct therapy. Chest. 2012;141(3):599–606. [DOI] [PubMed] [Google Scholar]
- 22.Payne D, Bush A. Phenotype-specific treatment of difficult asthma in children. Paediatr Respir Rev. 2004;5(2):116–123. [DOI] [PubMed] [Google Scholar]
- 23.Cokugras H, Akcakaya N, Seckin I, Camcioglu Y, Sarimurat N, Aksoy F. Ultrastructural examination of bronchial biopsy specimens from children with moderate asthma. Thorax. 2001;56(1):25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Payne DN, Rogers AV, Adelroth E, Bandi V, Guntupalli KK, Bush A, Jeffery PK. Early thickening of the reticular basement membrane in children with difficult asthma. Am J Respir Crit Care Med. 2003;167(1):78–82. [DOI] [PubMed] [Google Scholar]
- 25.Barbato A, Turato G, Baraldo S, Bazzan E, Calabrese F, Tura M, Zuin R, Beghe B, Maestrelli P, Fabbri LM, Saetta M. Airway inflammation in childhood asthma. Am J Respir Crit Care Med. 2003;168(7) 798–803. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins HA, Cool C, Szefler SJ, Covar R, Brugman S, Gelfand EW, Spahn JD. Histopathology of severe childhood asthma: a case series. Chest. 2003;124(1):32–41. [DOI] [PubMed] [Google Scholar]
- 27.Saglani S, Malmstrom K, Pelkonen AS, Malmberg LP, Lindahl H, Kajosaari M, Turpeinen M, Rogers AV, Payne DN, Bush A, et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med. 2005;171(7):722–727. [DOI] [PubMed] [Google Scholar]
- 28.Andersson CK, Adams A, Nagakumar P, Bossley C, Gupta A, De Vries D, Adnan A, Bush A, Saglani S, Lloyd CM. Intraepithelial neutrophils in pediatric severe asthma are associated with better lung function. J Allergy Clin Immunol. 2017;139(6):1819–1829.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grunwell JR, Stephenson ST, Tirouvanziam R, Brown LAS, Brown MR, Fitzpatrick AM. Children with neutrophil-predominant severe asthma have proinflammatory neutrophils with enhanced survival and impaired clearance. J Allergy Clin Immunol Pract. 2019;7(2):516–525.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson PFM, Pattaroni C, Cook J, Gregory L, Alonso AM, Fleming LJ, Lloyd CM, Bush A, Marsland BJ, Saglani S. Lower airway microbiota associates with inflammatory phenotype in severe preschool wheeze. J Allergy Clin Immunol. 2019;143(4):1607–1610.e3. [DOI] [PubMed] [Google Scholar]
- 31.Teague WG, Lawrence MG, Shirley DT, Garrod AS, Early SV, Payne JB, Wisniewski JA, Heymann PW, Daniero JJ, Steinke JW, et al. Lung lavage granulocyte patterns and clinical phenotypes in children with severe, therapy-resistant asthma. J Allergy Clin Immunol Pract. 2019;7(6):1803–1812.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Payne D, McKenzie SA, Stacey S, Misra D, Haxby E, Bush A. Safety and ethics of bronchoscopy and endobronchial biopsy in difficult asthma. Arch Dis Child. 2001;85(5):423–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webley WC, Salva PS, Andrzejewski C, Cirino F, West CA, Tilahun Y, Stuart ES. The bronchial lavage of pediatric patients with asthma contains infectious Chlamydia. Am J Respir Crit Care Med. 2005;171(10):1083–1088. [DOI] [PubMed] [Google Scholar]
- 34.Patel KK, Vicencio AG, Du Z, Tsirilakis K, Salva PS, Webley WC. Infectious Chlamydia pneumoniae is associated with elevated interleukin-8 and airway neutrophilia in children with refractory asthma. Pediatr Infect Dis J. 2010;29(12):1093–1098. [DOI] [PubMed] [Google Scholar]
- 35.Patel KK, Webley WC. Respiratory Chlamydia infection induce release of hepoxilin A3 and histamine production by airway neutrophils. Front Immunol. 2018;9:2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X, Zhang D, Zhang H, Wolters PJ, Killeen NP, Sullivan BM, Locksley RM, Lowell CA, Caughey GH. Neutrophil histamine contributes to inflammation in mycoplasma pneumonia. J Exp Med. 2006;203(13):2907–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraft M, Cassell GH, Pak J, Martin RJ. Mycoplasma pneumoniae and chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121(6):1782–1788. [DOI] [PubMed] [Google Scholar]
- 38.Sutherland ER, King TS, Icitovic N, Ameredes BT, Bleecker E, Boushey HA, Calhoun WJ, Castro M, Cherniack RM, Chinchilli VM, et al. A trial of clarithromycin for the treatment of suboptimally controlled asthma. J Allergy Clin Immunol. 2010;126(4):747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wise F, Sulzberger MB. Urticaria and hay fever due to Trichophyton (Epidermophyton interdigitale). JAMA. 1930;95:1504. [Google Scholar]
- 40.Denning DW, O’Driscoll BR, Powell G, Chew F, Atherton GT, Vyas A, Miles J, Morris J, Niven RM. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: The Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med. 2009;179(1):11–18. [DOI] [PubMed] [Google Scholar]
- 41.Vicencio AG, Muzumdar H, Tsirilakis K, Kessel A, Nandalike K, Goldman DL. Severe asthma with fungal sensitization in a child: response to itraconazole therapy. Pediatrics. 2010;125(5):e1255–e1258. [DOI] [PubMed] [Google Scholar]
- 42.Vicencio AG, Chupp GL, Tsirilakis K, He X, Kessel A, Nandalike K, Veler H, Kipperman S, Young MC, Goldman DL. CHIT1 mutations: genetic risk factor for severe asthma with fungal sensitization? Pediatrics. 2010;126(4):e982–e985. [DOI] [PubMed] [Google Scholar]
- 43.Goldman DL, Vicencio AG. The chitin connection. MBio. 2012;3(2):e00056–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moss RB. Treatment options in severe fungal asthma and allergic bronchopulmonary aspergillosis. Eur Respir J. 2014;43(5):1487–1500. [DOI] [PubMed] [Google Scholar]
- 45.Vicencio AG, Santiago MT, Tsirilakis K, Stone A, Worgall S, Foley EA, Bush D, Goldman DL. Fungal sensitization in childhood persistent asthma is associated with disease severity. Pediatr Pulmonol. 2014:49(1):8–14. [DOI] [PubMed] [Google Scholar]
- 46.Goldman DL, Chen Z, Shankar V, Tyberg M, Vicencio A, Burk R. Lower airway microbiota and mycobiota in children with severe asthma. J Allergy Clin Immunol. 2018;141(2):808–811.e7. [DOI] [PubMed] [Google Scholar]
- 47.Swain SD, Meissner N, Han S, Harmsen A. Pneumocystis infection in an immunocompetent host can promote collateral sensitization to respiratory antigens. Infect Immun. 2011;79(5):1905–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swain SD, Meissner N, Siemsen DW, McInnerney K, Harmsen AG. Pneumocystis elicits a STAT6-dependent, strain-specific innate immune response and airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2012;46(3):290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schnipper S, Small CB, Lehach J, Kaufman A, Grizzanti JN, Rothstein M, Minkowitz S, Rosenstreich DL. Pneumocystis carinii pneumonia presenting as asthma: increased bronchial hyperresponsiveness in Pneumocystis carinii pneumonia. Ann Allergy. 1993;70(2):141–146. [PubMed] [Google Scholar]
- 50.Gingo MR, Wenzel SE, Steele C, Kessinger CJ, Lucht L, Lawther T, Busch M, Hillenbrand ME, Weinman R, Silvka WA, et al. Asthma diagnosis and airway bronchodilator response in HIV-infected patients. J Allergy Clin Immunol. 2012;129(3):708–714.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eddens T, Campfield BT, Serody K, Manni ML, Horne W, Elsegeiny W, McHugh KJ, Pociask D, Chen K, Zheng M, et al. A novel CD4(+) T cell-dependent murine model of Pneumocystis-driven asthma-like pathology. Am J Respir Crit Care Med. 2016;194(7):807–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Syk J, Malinovschi A, Johansson G, Unden AL, Andreasson A, Lekander M, Alving K. Anti-inflammatory treatment of atopic asthma guided by exhaled nitric oxide: a randomized, controlled trial. J Allergy Clin Immunol Pract. 2013;1(6):639–648. [DOI] [PubMed] [Google Scholar]
- 53.Petsky HL, Kew KM, Chang AB. Exhaled nitric oxide levels to guide treatment for children with asthma. Cochrane Database Syst Rev. 2016;11:CD011439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bjerregaard A, Laing IA, Backer V, Sverrild A, Khoo SK, Chidlow G, Sikazwe C, Smith DW, Le Souef P, Porsbjerg C. High fractional exhaled nitric oxide and sputum eosinophils are associated with an increased risk of future virus-induced exacerbations: a prospective cohort study. Clin Exp Allergy. 2017;47(8):1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ullmann N, Bossley CJ, Fleming L, Silvestri M, Bush A, Saglani S. Blood eosinophil counts rarely reflect airway eosinophilia in children with severe asthma. Allergy. 2013;68(3):402–406. [DOI] [PubMed] [Google Scholar]
- 56.Ribeiro V, Andrade J, Rose S, Spencer C, Vicencio A, Bunyavanich S. Children with severe persistent asthma have disparate peripheral blood and lower airway eosinophil levels. J Allergy Clin Immunol Pract. 2019. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson WC 3rd, Apter AJ, Dutmer CM, Searing DA, Szefler SJ. Advances in asthma in 2016: designing individualized approaches to management. J Allergy Clin Immunol. 2017;140(3):671–680. [DOI] [PubMed] [Google Scholar]
- 58.Lezmi G, Deschildre A, Abou Taam R, Fayon M, Blanchon S, Troussier F, Mallinger P, Mahut B, Gosset P, de Blic J. Remodelling and inflammation in preschoolers with severe recurrent wheeze and asthma outcome at school age. Clin Exp Allergy. 2018;48(7):806–813. [DOI] [PubMed] [Google Scholar]
- 59.Spencer CY, Millman J, Veiga K, Vicencio AG. Airway autoimmune inflammatory response (AAIR) syndrome: an asthma-autoimmune overlap disorder? Pediatrics. 2018;141(3). [DOI] [PubMed] [Google Scholar]
- 60.Wenzel SE, Vitari CA, Shende M, Strollo DC, Larkin A, Yousem SA. Asthmatic granulomatosis: a novel disease with asthmatic and granulomatous features. Am J Respir Crit Care Med. 2012;186(6):501–507. [DOI] [PubMed] [Google Scholar]
- 61.U.S. Food and Drug Administration. FDA drug safety communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm532356.htm.


