Abstract
Objective:
We described progression-free survival and overall survival in patients with primary mucinous ovarian cancer receiving adjuvant gynecologic versus gastrointestinal chemotherapy regimens.
Methods:
We identified all primary mucinous ovarian cancer patients receiving adjuvant gynecologic or gastrointestinal chemotherapy regimens at a single institution from 1994–2016. Gynecologic pathologists using strict pathologic/clinical criteria determined diagnosis. Adjuvant therapy was coded as gynecologic or gastrointestinal based upon standard agents and schedules. Clinical/pathologic/treatment characteristics were recorded. Wilcoxon Rank-Sum Test was used for continuous variables, Fisher’s exact test for categorical variables. Progression-free and overall survival were calculated using Kaplan-Meier method, applying landmark analysis.
Results:
Of 62 patients identified, 21 received adjuvant chemotherapy: 12, gynecologic; 9, gastrointestinal. Median age at diagnosis: 58 (range 25–68) gynecologic cohort, 38 (range 32–68) gastrointestinal cohort (P=0.13). Median BMI at first postoperative visit: 25 kg/m2 (range 18–31) gynecologic cohort, 23 kg/m2 (range 18–31) gastrointestinal cohort (P=0.23). History of smoking: 6/12 (50%) gynecologic cohort, 3/9 (33%) gastrointestinal cohort (P=0.66). Stage distribution in gynecologic and gastrointestinal cohorts, respectively: Stage I, 9/12 (75%) and 3/9 (33%); Stage II, 2/12 (17%) and 1/9 (11%); Stage III, 1/12 (8%) and 5/9 (56%) (p=0.06). Grade distribution in gynecologic and gastrointestinal cohorts, respectively: 8/12 (67%) and 1/9 (13%), Grade 1; 4/12 (33%) and 7/9 (88%), Grade 2/3 (P=0.03). Three-year progression-free survival: 90.9% (95%CI 50.8–98.7%) gynecologic, 53.3% (95%CI 17.7–79.6%) gastrointestinal. Three-year overall survival: 90.9% (95%CI 50.8–98.7%) gynecologic, 76.2% (95%CI 33.2–93.5%) gastrointestinal.
Conclusion:
Ongoing international collaborative research may further define associations between chemotherapy regimens and survival.
Keywords: Mucinous ovarian cancer, Chemotherapy, Gastrointestinal, Oncologic outcomes
INTRODUCTION
Primary mucinous ovarian cancer is a rare tumor, clinically and pathologically unique among epithelial ovarian cancers. It was previously thought that these tumors comprised 11–15% of all primary ovarian carcinomas. However, it then became clear that many lesions previously diagnosed as primary mucinous ovarian cancers actually represented metastases from gastrointestinal or pancreatobiliary primary sites. It is now believed that only 3–4% of all primary ovarian carcinomas are primary ovarian cancers [1–3].
The overall prognosis of primary mucinous ovarian cancer is favorable because a majority of patients are diagnosed with early-stage disease. However, patients with advanced disease have worse overall survival than those with high-grade serous ovarian cancer, primarily because these tumors respond poorly to standard adjuvant therapy regimens [4]. In GOG-182/ICON5, an analysis of patients by histologic subtype demonstrated that those with primary mucinous ovarian cancer had worse progression-free and overall survival, in all study arms, compared to patients with other histologic types [5]. In a retrospective review of several Hellenic Cooperative Oncology Group trials, Pectasides et al compared outcomes of patients with Stage III and IV primary mucinous ovarian cancer to patients with Stage III and IV high-grade serous ovarian cancer, and found an overall response rate to platinum-based chemotherapy of 38.5% and 70%, respectively [6]. These data, along with the pathologic and molecular similarities of primary mucinous ovarian cancer to gastrointestinal cancers, led to consideration of chemotherapy regimens used to treat colorectal cancer. In an in vitro model of human ovarian mucinous adenocarcinoma cell lines, Sato et al demonstrated activity to oxaliplatin, 5-fluorouracil, and etoposide, and resistance to cisplatin and paclitaxel. They also demonstrated synergistic effects and improved survival using combination oxaliplatin and 5-fluorouracil in a xenograft mouse model [7]. In vivo, Jain et al reported a case of a woman with recurrent metastatic mucinous ovarian cancer who had a partial response to combination therapy with oxaliplatin, 5-fluorouracil, and bevacizumab [8]. Investigators embarked on an international randomized trial to compare the efficacy of a gynecologic chemotherapy regimen versus a gastrointestinal chemotherapy regimen in women with primary mucinous ovarian cancer; however, given the rarity of this disease, the trial closed early due to poor accrual.
Based on prior studies suggesting the efficacy of gastrointestinal regimens in this setting [7, 8], we sought to describe the oncologic outcomes in patients treated with standard gastrointestinal regimens and outcomes in patients treated with standard epithelial ovarian cancer regimens. We evaluated our clinical experience with respect to progression-free and overall survival in patients receiving each regimen.
METHODS
This study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center. Patients diagnosed with primary mucinous ovarian cancer, treated at our institution between 1994–2016, were identified using institutional databases. Data was not available prior to 1994, and 2016 was chosen as the last year to collect data, allowing enough follow-up time for completion of the prescribed chemotherapy regimens and adequate follow-up to identify recurrence or progression. Patients were included if the following criteria were met: diagnosed with primary mucinous ovarian cancer of any stage and any grade; had primary surgical cytoreduction (with or without fertility preservation); received adjuvant chemotherapy with either a standard gynecologic or standard gastrointestinal regimen; received chemotherapy at Memorial Sloan Kettering Cancer Center. Patients were excluded due to any of the following: did not have primary surgical treatment; primary mucinous ovarian cancer was not confirmed on pathology re-review and normal upper/lower endoscopy; received adjuvant chemotherapy at an outside institution; presented to our institution with recurrent disease.
Baseline clinical characteristics and treatment information were extracted from the medical record, including age, stage (per International Federation of Gynecology and Obstetrics 2014 staging), body mass index (BMI; kg/m2), tumor grade as defined by the Shimizu and Silverberg system [9], tumor size, surgical treatment, adjuvant chemotherapy regimen and number of cycles completed, date of diagnosis, date of last follow-up, disease status at last follow-up. Given the data suggesting that mucinous tumors of the ovary, gastrointestinal and pancreatobiliary tracts are associated with a history of smoking, we also collected smoking history. Patients were considered to have a history of smoking if they reported regular cigarette use currently or in the near or distant past.
Gynecologic pathologists used strict pathologic and clinical criteria to diagnose invasive primary mucinous ovarian cancer. Pathologic criteria included the presence of confluent cells with intracytoplasmic mucin measuring >10mm2 and at least 5mm in one linear dimension, and unilateral tumor size ≥10cm [2, 10, 11]. In advanced-stage cases, normal upper and lower endoscopy and prolonged clinical follow-up, without evidence of a separate gastrointestinal or pancreatobiliary primary cancer, were required.
Adjuvant chemotherapy was coded as a conventional gynecologic (gynecologic cohort) or gastrointestinal (gastrointestinal cohort) regimen, based upon standard agents and schedules in epithelial ovarian and colorectal/appendiceal/gastric cancer regimens, respectively. Examples of gynecologic regimens included intravenous carboplatin and paclitaxel, and intravenous and intraperitoneal cisplatin and paclitaxel. Examples of gastrointestinal regimens included intravenous 5-fluorouracil (5-FU) and oxaliplatin (FOLFOX), and oral capecitabine and IV oxaliplatin (CapeOx).
Descriptive statistics were provided. Comparison between baseline characteristics of the two adjuvant treatment groups was performed using Wilcoxon Rank-Sum Test for continuous variables and Fisher’s exact test for categorical variables. Median survival times and survival rates, including progression-free and overall survival, were calculated using the Kaplan-Meier method. Because of the time-dependent nature of adjuvant therapy, landmark analysis with landmark time of 6 weeks after diagnosis was applied.
RESULTS
Of the 62 patients treated for primary mucinous ovarian cancer at Memorial Sloan Kettering Cancer Center between 1994–2016, 21 received adjuvant chemotherapy: 12 in the gynecologic cohort, 9 in the gastrointestinal cohort. Though not statistically different, patients in the gastrointestinal cohort trended towards younger age at diagnosis (median 38 yrs. vs. 58 yrs., p=0.13). Patients in the gastrointestinal cohort had higher-grade tumors (12.5% Grade 1 vs. 66.7% Grade 1, 87.5% Grade 2/3 vs. 33.3% Grade 2/3; p=0.03). In the gynecologic cohort 9/12 (75.0%) had Stage I disease, 2/12 (16.7%) Stage II, 1/12 (8.3%) Stage III. In the gastrointestinal cohort 3/9 (33.3%) had Stage I disease, 1/9 (11.1%) Stage II, 5/9 (55.6%) Stage III (p=0.06). There were no cases of Stage IV disease in either cohort (Table 1).
Table 1.
Patient characteristics by adjuvant chemotherapy group
| Characteristic | Adjuvant Chemotherapy Regimen | P value | |
|---|---|---|---|
| GYN (n=12) | GI (n=9) | ||
| Age (years), median (range) | 58 (25–68) | 38 (32–68) | 0.13 |
| BMI (kg/m2), median (range) | 25 (20–40) | 23 (18–31) | 0.23 |
| 0.66 | |||
| No | 6 (50%) | 6 (67%) | |
| 0.06 | |||
| IV | - | - | |
| 0.08 | |||
| Unknown | - | 1 (11%) | |
| Tumor size (cm), median (range) | 15 (11–30) | 16 (4–30) | 1.00 |
| 0.37 | |||
| No | 6 (50%) | 7 (78%) | |
Median year of diagnosis in the gynecologic cohort was 2007 (range, 2000–2014). Median year of diagnosis in the gastrointestinal cohort was 2011 (range, 2004–2015). Primary mucinous ovarian cancer was an incidental finding in 3 (25%) patients in the gynecologic cohort and 2 (22%) in the gastrointestinal cohort. All other patients presented with symptoms, primarily abdominal distention and pain. Preoperative CA-125 was elevated (defined as value >35 U/mL) in 6 patients in the gynecologic cohort (50%) and 3 in the gastrointestinal cohort (33%). Preoperative CEA was elevated (defined as >5 ng/mL) in 2 of the 3 patients with available data in the gynecologic cohort and 1 of the 5 patients with available data in the gastrointestinal cohort. All patients had unilateral tumors, except for 1 in the gynecologic cohort and 1 in the gastrointestinal cohort; both these patients had at least one tumor ≥10cm (13cm and 14cm, respectively). Available immunohistochemistry and pathologic characteristics are reported in Table 2. All patients had primary surgical management. None had neoadjuvant chemotherapy. Details of surgical procedures are shown in Table 3. All patients had optimal initial resection: 11 in the gynecologic cohort and 8 in the gastrointestinal cohort had no residual disease; 1 in each cohort had residual disease of <1 cm.
Table 2:
Available immunohistochemical and pathologic characteristics by chemotherapy group
| Characteristic | Adjuvant Chemotherapy Regimen | |
|---|---|---|
| GYN (n=12) | GI (n=9) | |
| Unknown | 7 (58%) | - |
| Unknown | 11 (92%) | 5 (56%) |
| Unknown | 4 (33%) | 5 (56%) |
| Unknown | 4 (33%) | 5 (56%) |
Table 3:
Surgical procedures performed in each cohort
| Surgical Procedure, n (%) | Adjuvant Chemotherapy Regimen | |
|---|---|---|
| GYN (n=12) | GI (n=9) | |
| Hysterectomy | 10 (83%) | 6 (67%) |
| USO | 1 (8%) | 3 (33%) |
| BSO | 11 (92%) | 6 (67%) |
| Appendectomy | 10* (83%) | 8* (89%) |
| Omentectomy | 12 (100%) | 9 (100%) |
| Peritoneal biopsies | 10 (83%) | 8 (89%) |
| Pelvic lymphadenectomy | 10 (83%) | 7 (78%) |
| Paraaortic lymphadenectomy | 10 (83%) | 6 (67%) |
| Bowel resection | 1 (8%) | 1 (11%) |
One patient in each cohort had her appendix removed prior to her diagnosis
Adjuvant regimens in the gynecologic cohort were intravenous carboplatin/intravenous paclitaxel (10/12, 83%), intraperitoneal cisplatin/intravenous paclitaxel (1/12, 8%), intravenous carboplatin (1/12, 8%). Adjuvant regimens in the gastrointestinal cohort were FOLFOX (1/9, 11%), intravenous cisplatin/intravenous irinotecan (2/9, 22%), CapeOX (6/9, 67%) (Figure 1). The median number of completed chemotherapy cycles was higher in the gastrointestinal cohort (12 [range 2–12] vs. 6 [range 3–6], p=0.02) due to standard regimens that routinely utilized more cycles. Eleven patients (92%) in the gynecologic cohort and 6 (67%) in the gastrointestinal cohort completed the prescribed regimens (p=0.27).
Figure 1:
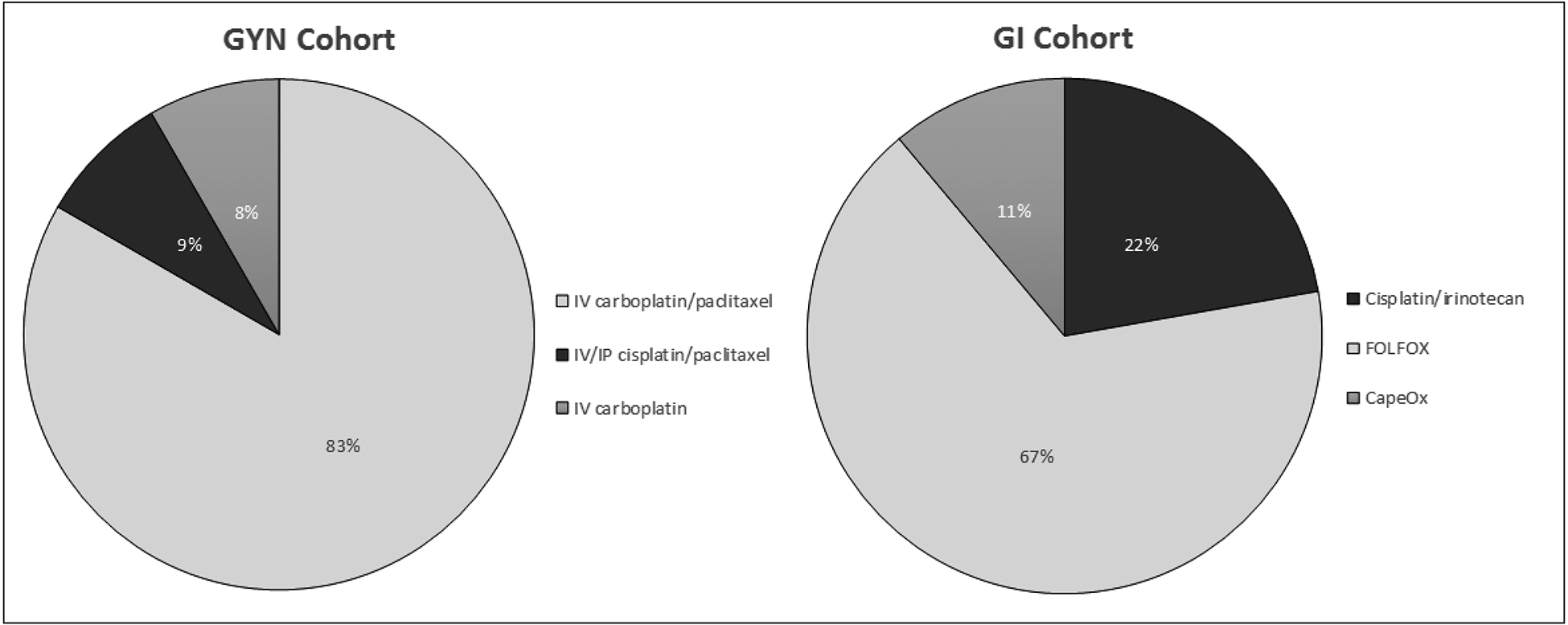
Adjuvant chemotherapy regimens by cohort
Median follow-up for the entire cohort was 47.4 months (range, 7.6–194.3 mos). Three-year progression-free survival was 90.9% (95% CI: 50.8–98.7%) in the gynecologic cohort, 53.3% (95% CI: 17.7–79.6%) in the gastrointestinal cohort. Three-year overall survival was 90.9% (95% CI: 50.8–98.7%) in the gynecologic cohort, 76.2% (95% CI: 33.2–93.5%) in the gastrointestinal cohort (Figure 2). One patient in the gynecologic cohort recurred. This patient had Stage IIIC disease, was treated with intraperitoneal cisplatin and intravenous paclitaxel and recurred after 66.9 months; she then received liposomal doxorubicin but had progression of disease. Four patients in the gastrointestinal cohort recurred. One patient with Stage IIIB disease received FOLFOX and recurred 7.8 months after diagnosis; she then received 5-FU/irinotecan (FOLFIRI) with bevacizumab. Another patient with Stage IIIC disease received CapeOX and recurred at 19.3 months; she subsequently received FOLFIRI with bevacizumab. One patient with Stage IIIA2 disease received FOLFOX and recurred 19.9 months after diagnosis; she then received carboplatin and weekly paclitaxel. Another patient with Stage IIIC disease progressed while on cisplatin/irinotecan 2.7 months after diagnosis, and received no additional chemotherapy. In the gynecologic cohort, 11/12 patients did not recur, including 1 with Stage IA disease, 3 with Stage IC1, 5 with Stage IC3, 2 with Stage IIB disease. In the gastrointestinal cohort, 5/9 patients did not recur, including 3 with Stage IA, 1 with Stage IIB, 1 with Stage IIIB disease.
Figure 2:
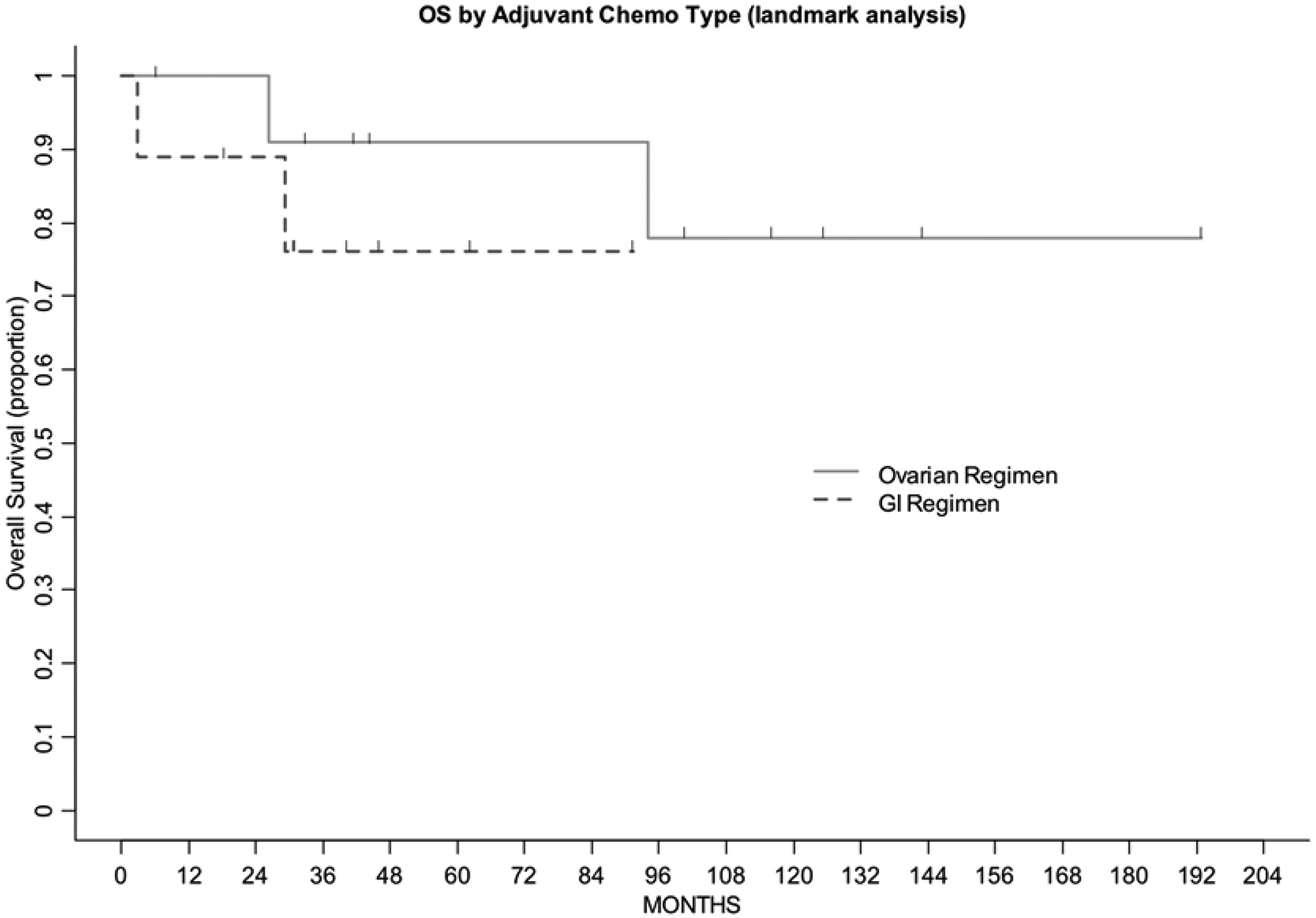
Overall survival by adjuvant chemotherapy cohort
DISCUSSION
Primary mucinous ovarian cancer is a rare tumor sharing some pathologic features with gastrointestinal cancers. This, along with the generally poor response of these tumors to chemotherapeutic regimens used for high-grade serous ovarian cancer, has rationalized the use of gastrointestinal regimens. Herein we describe outcomes of primary mucinous ovarian cancer patients, of all stages, who received either a standard gynecological or gastrointestinal chemotherapy regimen following initial surgical cytoreduction. Three-year progression-free survival was 90.9% for those receiving a gynecologic versus 53.3% for those receiving a gastrointestinal regimen. Three-year overall survival was 90.9% for patients receiving a gynecologic versus 76.2% for those receiving a gastrointestinal regimen. These data should be viewed cautiously, however, because patients who received a gastrointestinal regimen had higher-grade tumors and trended toward higher-stage disease.
It is unclear if the response to these treatment regimens is any better than the response to standard ovarian cancer regimens. GOG-241, an international randomized clinical trial comparing an epithelial ovarian cancer regimen to a gastrointestinal regimen in advanced or recurrent primary mucinous ovarian cancer, closed early due to poor accrual. This was a 2×2 factorial study designed to compare the use of carboplatin and paclitaxel +/− bevacizumab, versus oxaliplatin and capecitabine +/− bevacizumab, and to evaluate the efficacy of adding bevacizumab to standard regimens in primary mucinous ovarian cancer. The trial accrued 50 of a targeted 322 patients: 26 in the oxaliplatin/capecitabine +/− bevacizumab arm, and 24 in the carboplatin/paclitaxel +/− bevacizumab arm. Preliminary data (presented at the annual meeting of the American Society of Clinical Oncology in 2015) demonstrated a response rate of 15.4% (4/26) in the oxaliplatin/capecitabine +/− bevacizumab arm, 25% (6/24) in the carboplatin/paclitaxel +/− bevacizumab arm [12]. However, the data was insufficient to draw any conclusions. Median progression-free survival was 17.4 months in patients who received bevacizumab, versus 8.8 months in those who did not receive bevacizumab (p=0.72); but again, it was difficult to draw any conclusions based on such a small number of patients [13]. Kurnit et al presented data on a cohort of 55 patients (at the Society of Gynecologic Oncology Annual Winter Meeting in 2019) demonstrating borderline improvement in progression-free survival with a gastrointestinal regimen compared to a gynecologic regimen (median progression-free survival 26 months for gynecologic vs. not reached for gastrointestinal, HR 0.4 [95% CI 0.2–1.0]). They also showed improved overall survival with an adjuvant gastrointestinal regimen (median overall survival 67 months for gynecologic vs. not reached for gastrointestinal, HR 0.3 [95% CI 0.1–0.9]). However, there was a marked imbalance between the two groups in use of bevacizumab, with only 1 patient (3%) in the gynecologic cohort receiving bevacizumab versus 14 patients (54%) in the gastrointestinal cohort. Therefore, they were unable to reach any conclusions about the potential benefit of bevacizumab [14].
Any investigation into primary mucinous ovarian cancer is plagued by the heterogeneity of the disease. Previously published molecular data suggest frequent TP53, KRAS, ERBB2, and CDKN2A alterations, although there is one distinct molecular signature that has not been identified [15–18]. Our group recently reported the genetic alterations in a cohort of primary mucinous ovarian cancers compared to genetic alterations in high-grade serous ovarian carcinomas, mucinous colorectal carcinomas, mucinous gastric carcinomas, and mucinous pancreatic carcinomas. Comparison of our primary mucinous ovarian cancer cohort with other mucin-producing malignancies identified significant differences in the mutational profiles of all histologies except that of mucinous pancreatic carcinoma. Our previous study also highlighted the clinical value of using molecular profiling to diagnose primary mucinous ovarian cancer and identify potentially targetable mutations and copy number alterations [17]. A recently published paper by Meagher et al advocates for molecular profiling of mucinous ovarian cancer of uncertain primary origin. Their cohort included all mucinous tumors suspected of being primary mucinous ovarian cancers; however, they did not require strict pathologic or molecular criteria to exclude another primary. This created a cohort that potentially bears the greatest similarity to what is seen in clinical practice—because, in practice, it can be difficult to confirm that a mucinous ovarian mass is a true primary mucinous ovarian cancer. By comparing these to mucinous cancers in other sites (colon/rectum, pancreas, appendix) the authors found that the greatest similarity was with pancreatic and appendiceal tumors. They proposed molecular profiling of all mucinous tumors suspected to be primary mucinous ovarian cancers, and the development of basket trials to evaluate targeted therapies based on these profiles [18]. Basket trials are ideal in this setting but are limited to tumors with targetable alterations. However, the majority of primary mucinous ovarian cancers and mucinous ovarian cancers of uncertain primary origin, do not have alterations that are targetable as of yet. Therefore, these tumors are treated with standard chemotherapy, but the exact regimen remains undetermined. Given the pathologic and molecular similarities to mucinous pancreatic carcinomas, reported in our study as well as by Meagher et al [17, 18], the use of pancreatic cancer treatment regimens warrants investigation. The nearly 40% positive staining for PD-1 demonstrated in these tumors indicates that there may be a potential role for immunotherapy. This, too, warrants investigation.
Immunohistochemistry is also helpful in distinguishing between primary mucinous ovarian cancer and gastrointestinal cancer metastatic to the ovary. Primary ovarian cancers tend to be PAX8 positive and have CK7 staining stronger than or equal to CK20. Gastrointestinal tumors tend to be PAX8 negative and have either negative CK7 staining or CK7 staining that is weaker than CK20. We report our available immunohistochemistry results in Table 2. Although the sample size is small, it appears that the tumors treated with a gastrointestinal regimen tended to have immunohistochemistry staining most consistent with gastrointestinal tumors. Though we cannot comment on whether this improved survival outcomes, it is hypothesis-generating and should be evaluated further.
We need more effective treatment for primary mucinous ovarian cancer. Though patients who present with early-stage disease have a favorable prognosis, the prognosis of those with advanced or recurrent disease is very poor. This is largely due to lack of response to standard chemotherapy. Given the molecular heterogeneity of these tumors and the not-infrequent presence of actionable mutations/copy number alterations, the implementation of a small gene panel, with or without immunohistochemistry, to help diagnose primary mucinous ovarian cancer and identify potentially targetable mutations, should be considered in the setting of advanced or recurrent disease [13, 14]. Multi-institutional and international collaboration may identify valuable methods for investigating novel therapeutic strategies.
The greatest strength of our study is its cohort of patients with primary mucinous ovarian cancers, receiving chemotherapy at a single specialty center offering expertise in the diagnostic and therapeutic complexities of this disease. Our ability to perform any meaningful statistical comparison of survival between the two treatment cohorts was limited, due to the small number of patients. Like other retrospective studies, ours has inherent limitations; these include lack of standardized chemotherapy regimens and an imbalance in baseline characteristics between the two cohorts, with apparent stage and grade imbalance favoring the gynecologic cohort.
In conclusion, primary mucinous ovarian cancer is a rare and challenging entity. No universally effective treatment regimen exists for patients with advanced or recurrent disease. In this study, we described the outcomes of patients with all stages of primary mucinous ovarian carcinoma receiving either a standard gynecologic or a standard gastrointestinal regimen. The study was too small, and perhaps imbalanced, to draw any definitive conclusions. Given the finding that neither regimen has clear superiority over the other, the current practice at Memorial Sloan Kettering Cancer Center is to present these cases at our multidisciplinary treatment planning conference and make collaborative decisions regarding adjuvant treatment, based upon patient and tumor characteristics. Future studies should involve multiple institutions and should investigate chemotherapy regimens used in mucinous pancreatic cancer and/or immunotherapy, for the treatment of patients with advanced or recurrent primary mucinous ovarian cancer who are not eligible for targeted therapy.
Acknowledgements:
This study was presented as a poster at the 49th SGO Annual Meeting on Women’s Cancer, March 24-27, 2018, New Orleans, LA, USA.
All contributors meet the criteria for authorship and are listed in the Contributors section, below.
Funding: This study was funded in part through the NIH/NCI Support Grant P30 CA008748.
Disclosures: Dr. Abu-Rustum reports grants from Stryker/Novadaq, grants from Olympus, and grants from GRAIL, outside the submitted work.
Dr. O’Cearbhaill reports personal fees from Clovis and personal fees from Tesaro, outside the submitted work.
Footnotes
Competing Interests: None declared.
REFERENCES
- 1.Frumovitz M, Schmeler KM, Malpica A, et al. Unmasking the complexities of mucinous ovarian carcinoma. Gynecol Oncol 2010; 117:491–496. doi: 10.1016/j.ygyno.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seidman JD, Kurman RJ, Ronnett BM. Primary and Metastatic Mucinous Adenocarcinomas in the Ovaries. Am J Surg Pathol 2003; 27:985–993. [DOI] [PubMed] [Google Scholar]
- 3.Kosary CL. Cancer of the Ovary. In: Reis LAG, Young JL, Keel GE, et al. , eds. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988–2001, Patient and Tumor Characteristics. Publication No. 07–6215. Bethesda, MD: National Cancer Institute, 2007;133–144. [Google Scholar]
- 4.Schiavone MB, Herzog TJ, Lewin SN, et al. Natural history and outcome of mucinous carcinoma of the ovary. Am J Obstet Gynecol 2011; 205:480e1–8. doi: 10.1016/j.ajog.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 5.Zaino RJ, Brady MF, Lele SM, et al. Advanced Stage Mucinous Adenocarcinoma of the Ovary Is Both Rare and Highly Lethal: A Gynecologic Oncology Group Study. Cancer 2011; 117:554–562. doi: 10.1002/cncr.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pectasides D, Fountzilas G, Aravantinos G, et al. Advanced stage mucinous epithelial ovarian cancer: The Hellenic Cooperative Oncology Group experience. Gynecol Oncol 2005; 97:436–441. doi: 10.1016/j.ygyno.2004.12.056. [DOI] [PubMed] [Google Scholar]
- 7.Sato S, Itamochi H, Kigawa J, et al. Combination chemotherapy of oxaliplatin and 5-fluorouracil may be an effective regimen for mucinous adenocarcinoma of the ovary: A potential treatment strategy. Cancer Sci 2009; 100:546–551. doi: 10.1111/j.1349-7006.2008.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain A, Ryan PD, Seiden MV. Metastatic Mucinous Ovarian Cancer and Treatment Decisions Based on Histology and Molecular Markers Rather Than the Primary Location. J Natl Compr Canc Netw 2012; 10:1076–1080. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu Y, Kamoi S, Amada S, et al. Toward the development of a universal grading system for ovarian epithelial carcinoma. I. Prognostic significance of histopathologic features—problems involved in the architectural grading system. Gynecol Oncol 1998; 70:2–12. [DOI] [PubMed] [Google Scholar]
- 10.Riopel MA, Ronnett BM, Kurman RJ. Evaluation of diagnostic criteria and behavior of ovarian intestinal-type mucinous tumors: atypical proliferative (borderline) tumors and intraepithelial, microinvasive, invasive, and metastatic carcinomas. Am J Surg Pathol 1999;23:617–635. [DOI] [PubMed] [Google Scholar]
- 11.Brown J, Frumovitz M. Mucinous Tumors of the Ovary: Current Thoughts on Diagnosis and Management. Curr Oncol Rep 2014; 16:389. doi: 10.1007/s11912-014-0389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perren TJ. Mucinous epithelial ovarian carcinoma. Ann Oncol 2016; 27(Suppl 1):i53–i57. doi: 10.1093/annonc/mdw087. [DOI] [PubMed] [Google Scholar]
- 13.Xu W, Rush J, Rickett K, Coward JI. Mucinous ovarian cancer: A therapeutic review. Crit Rev Oncol Hematol 2016; 102:26–36. doi: 10.1016/j.critrevonc.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Kurnit KC, Sinno A, Fellman BM, et al. Gastrointestinal adjuvant chemotherapy regimens improve survival outcomes in women with mucinous ovarian cancer. In: Proceedings from the 2019 SGO Annual Winter Meeting;. January 17–19, 2019; Lake Tahoe, CA. [Google Scholar]
- 15.Ryland GL, Hunter SM, Doyle MA, et al. ; Australian Ovarian Cancer Study Group, Gorringe KL, Campbell IG. RNF43 is a tumour suppressor gene mutated in mucinous tumours of the ovary. J Pathol 2013; 229:469–476. doi: 10.1002/path.4134. [DOI] [PubMed] [Google Scholar]
- 16.McAlpine JN, Wiegand KC, Vang R, et al. HER2 overexpression and amplification is present in a subset of ovarian mucinous carcinomas and can be targeted with trastuzumab therapy. BMC Cancer 2009; 9:433. doi: 10.1186/1471-2407-9-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller JJ, Schlappe BA, Kumar R, et al. Massively parallel sequencing analysis of mucinous ovarian carcinomas: genomic profiling and differential diagnoses. Gynecol Oncol 2018;150: 127–135. doi: 10.1016/j.ygyno.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meagher NS, Schuster K, Voss A, et al. Does the primary site really matter? Profiling mucinous ovarian cancers of uncertain primary origin (MO-CUP) to personalise treatment and inform the design of clinical trials. Gynecol Oncol 2018; 150:527–533. doi: 10.1016/j.ygyno.2018.07.013. [DOI] [PubMed] [Google Scholar]


