Abstract
Chikungunya virus (CHIKV) is a single-stranded RNA virus belonging to the family Togaviridae and genus Alphavirus, that causes an acute febrile illness, chikungunya fever, transmitted to humans by Aedes species mosquitoes. During acute illness, patients have high fever, polyarthralgias/polyarthritis, maculopapular rash, headache, and myalgia that lasts for days to weeks. Following resolution of acute infection, a significant proportion of patients develop chronic chikungunya arthritis that can resemble rheumatoid arthritis. In this review, we first consider the historical background of infectious causes of inflammatory arthritis, and then the pathogenic and clinical manifestations of chronic chikungunya arthritis as an rheumatoid arthritis mimic. We believe that chronic chikungunya arthritis may be a post-infectious, inflammatory process and that an understanding of the parallels and differences between chronic chikungunya arthritis and rheumatoid arthritis may offer insights into better diagnosis and treatment of both diseases.
Keywords: chikungunya, rheumatoid arthritis, pathogeneses, methotrexate, virus
Introduction
Chikungunya fever is caused by the chikungunya virus (CHIKV), an arbovirus transmitted by Aedes species mosquitoes. First isolated in 1952 in Tanzania, CHIKV has caused intermittent outbreaks in Africa, Asia, the Indian Ocean islands, and in southern Europe. In 2013, CHIKV spread to the Western Hemisphere. 46 countries and territories have documented local transmission with 1.7 million cases reported [1]. At the present time, it is estimated that 39% of the world’s population lives in countries endemic for CHIKV and are at risk for infection. [2]
Acute chikungunya fever is characterized by the abrupt onset of high fever, disabling polyarthritis, and maculopapular rash associated with other symptoms including headache, myalgia, nausea, and vomiting [3]. Chikungunya fever occurs in widespread epidemics and is defined by (1) a history of acute febrile arthralgia (acute attack) with duration ≥ 48 hours and (2) positive anti-CHIKV specific immunoglobulin M; or (3) RNA virus by reverse transcriptase polymerase chain reaction; or (4) post-exposure anti-CHIKV specific immunoglobulin G positive serologic test detected by ELISA [4].
Although chikungunya fever resolves within 5–14 days in the majority of patients, [5] more than 40% develop arthritic manifestations lasting more than 3 months after the acute illness. Rheumatic features include symmetrical polyarthritis often affecting the hands and feet similar to rheumatoid arthritis, non-specific arthralgias consistent with post-viral arthritis, or asymmetric oligo or mono arthritis similar to seronegative spondyloarthritis. Post-viral polyarthralgia, fibromyalgia, chronic joint pain, adhesive capsulitis, and plantar fasciitis can also occur [6,7,8]. When these arthritic manifestations last more than 3 months following the onset of chikungunya fever, the illness can be called chronic chikungunya arthritis [9].
During acute chikungunya fever, patients have viremia and an interferon response consistent with an acute viral infection [4]. Treatment during this phase includes supportive care, short-term opioid analgesics, paracetamol/acetaminophen, and non-steroidal anti-inflammatory drugs (NSAIDs). The mechanism by which the disease transitions in some but not all patients to chronic chikungunya arthritis is uncertain. Since studies suggest similarities with rheumatoid arthritis in regards to clinical features and cytokine expression, patients who develop chronic rheumatic symptoms have been treated with NSAIDs, corticosteroids, chloroquine, hydroxychloroquine, methotrexate, sulfasalazine, and/or biological agents such as etanercept or abatacept with some evidence of efficacy [9,10,12,13,14,15].
In this review, we consider the historical background of infectious causes of inflammatory arthritis, the clinical manifestations of chronic chikungunya arthritis as an rheumatoid arthritis mimic, and compare the pathogenesis of chronic chikungunya arthritis with rheumatoid arthritis. We also compare the burden of chronic chikungunya arthritis with rheumatoid arthritis on patient quality of life. Our goal is to stimulate further studies on pathogenesis and treatment of chronic chikungunya arthritis, to improve outcomes in the same way that rheumatoid arthritis treatment has progressed. Over the past quarter century, chronic chikungunya arthritis has emerged as an important viral infection causing debilitating chronic rheumatic disease throughout the world. There is an urgent need to advance the recognition and management of chronic chikungunya arthritis.
Viral causes of arthritis
Many viruses cause arthritis, including parvovirus B19, rubella virus, alphaviruses including chikungunya, o’nyong-nyong, Mayaro, Sindbis, Okelbo, Barmah Forest, and Ross River virus, hepatitis C virus, human T-cell lymphotropic virus type I, and human immunodeficiency virus [16,17,18].
Several mechanisms by which viruses have been implicated in the etiopathogenesis of rheumatic diseases have been investigated. One involves molecular mimicry between host antigens and viral proteins. This may lead to loss of immune tolerance and cause arthritis as a consequence of cytokine signaling and upregulation of other pro-inflammatory factors [19], such as IL-1, IL-6, and TNF-α. Alternatively, viral infection of genetically-susceptible hosts can cause T and B cell dysfunction, resulting in autoimmunity [16]. For example, in human immunodeficiency virus −1 infection, secretion of T helper type 1 (Th1) cytokines, interleukin 2 (IL-2), interferon γ (IFN-γ), and IL-12, which are required for maintenance of classical T cell mediated immunity, is decreased while production of Th2 cytokines, in particular IL-4, IL-5, IL-6, and IL-10, promoting B cell function, is increased similar to postulated mechanisms of autoimmunity in systemic lupus erythematosus [20,21].
In addition, it is hypothesized that viruses may cause or exacerbate pre-existing rheumatic disease more generally. For example, parvovirus B19 infection can mimic systemic lupus erythematosus clinically and could play a role in disease pathogenesis [22]. Similarly, hepatitis C virus infection may share common clinical and serologic features with systemic lupus erythematosus, polyarteritis nodosa, sarcoidosis, and Sjögren’s syndrome, imitating those diseases. Extrahepatic manifestations of hepatitis C virus such as arthralgia, myalgia, sicca syndrome, and antinuclear antibody (ANA) positivity can mimic systemic lupus erythematosus [23]. Rarely, hepatitis C virus infected patients develop scleroderma and inflammatory myositis [17]. Infection by human T-cell lymphotropic virus type-I has been associated with polymyositis and Sjögren’s syndrome [24]. Many studies have linked Epstein-Barr virus to the development of systemic lupus erythematosus, rheumatoid arthritis, and Sjögren’s syndrome, in part because several immune escape mechanisms and immunomodulatory proteins have been described for Epstein-Barr virus [25].
Viral infections and rheumatoid arthritis pathogenesis
Several viruses and their products, including Epstein-Barr virus, cytomegalovirus, human immunodeficiency virus, human T-cell lymphotropic virus type I, alphaviruses, and flaviviruses, have been implicated in the pathogenesis of rheumatoid arthritis, possibly through molecular mimicry (Table 1) [26,27]. During infection, the formation of immune complexes may trigger the induction of rheumatoid factor, a high-affinity autoantibody against the Fc portion of immunoglobulin, which is implicated in rheumatoid arthritis pathogenesis [28]. In this hypothesis, viral antigens bear structural similarity to self-antigens (i.e., similar epitopes). As a result, an immune response to pathogens could lead to cross reactivity with self-antigens. The similarity of pathogen and self-antigen structures is a widespread phenomenon, as in the case of Coxsackie B4 virus in type I diabetes and the encephalomyocarditis virus in autoimmune myositis [29].
Table 1.
| Epstein-Barr virus |
| Hepatitis C virus |
| Human T cell lymphotropic virus I |
| Coxsackievirus |
| Adenovirus |
| Parvovirus B19 |
| Rubella virus |
| Cytomegalovirus |
| Alphaviruses |
Another mechanism by which viral infections could potentiate rheumatoid arthritis is through epitope spreading, in which the immune cell response extends beyond the original epitope, optimizing the protection against newly encountered pathogens and assisting in clearing inflammatory sites of damaged endogenous proteins of the body’s own tissues [30]. Under abnormal circumstances, such as in rheumatoid arthritis, epitope spreading could lead to the development of an autoimmune response to the epitopes themselves.
One study proposed that cytomegalovirus -specific CD8+ T cells may contribute to tissue-induced rheumatoid arthritis inflammation by local release of proinflammatory cytokines [31,32]. T cells are implicated in the pathogenesis of rheumatoid arthritis with associated T cell clonal expansion and increased CD4+ CD28− T cell populations in rheumatoid arthritis patients [32].
Since almost everyone has repeated viral infections, but most people do not develop autoreactive diseases, other factors, particularly genetic susceptibility, must determine the risk for virus induced rheumatic disease [33]. For example, Saal et al demonstrated that motif-positive HLA-DRB1 QK / RRAA patients who expressed synovial Epstein-Barr virus DNA had a significantly higher risk for rheumatoid arthritis than negative controls, indicating the possible role of Epstein-Barr virus exposure for rheumatoid arthritis in genetically susceptible individuals [34].
Clinical manifestations of chronic chikungunya arthritis as an rheumatoid arthritis mimic
In 55 to 65% of cases, rheumatoid arthritis has an insidious onset over weeks to months [35]. Initial symptoms may either be systemic or articular. In some patients, fatigue, malaise, swollen hands, and diffuse musculoskeletal pain may be the initial symptoms, with the joint involvement occurring later. Morning stiffness is a cardinal symptom of rheumatoid arthritis that can appear even before synovitis and may be related to the accumulation of edema within inflamed tissues during sleep [36].
The most commonly involved joints initially in rheumatoid arthritis are the metacarpophalangeal joints, the proximal interphalangeal joints, the metatarsophalangeal joints, and the wrists [36,37]. Initial laboratory tests can show thrombocytosis, mild normochromic anemia, ESR ≥ 30 mm/ hour, elevated C-reactive protein level, positive RF test (about 70% to 80% of patients) and positive anti-CCP antibodies (50% to 90%) [38].
In many patients, chronic chikungunya arthritis mimics rheumatoid arthritis clinically. Some studies report patients with chronic chikungunya arthritis whose clinical features are so similar to rheumatoid arthritis that they meet the 2010 American College of Rheumatology (ACR) diagnostic criteria for rheumatoid arthritis [7,8,9]. As in rheumatoid arthritis, most patients with chronic chikungunya arthritis are middle-aged and are women [6]. Commonly affected joints include the metacarpophalangeal, proximal interphalangeal, and wrist joints as well as the knees and ankles in a symmetric, polyarticular pattern [6,9]. Joint edema, bursitis, and tendonitis may occur [39]. Fatigue, insomnia, myalgias, morning stiffness, memory or concentration problems, and asthenia or depression may occur as in rheumatoid arthritis patients with co-existing fibromyalgia [9,40,41] In one study, most patients (90%) reported symmetrical joint involvement, 63% had joint swelling, and 39% had chronic myalgias [41]. Inflammatory markers such as ESR and CRP are usually elevated in patients with chronic musculoskeletal symptoms, and some patients develop radiographic evidence of joint damage [8]. MRI findings shared between these diseases include joint effusions, bony erosions, marrow edema, synovial thickening, tendinitis, and tenosynovitis [42]. In addition to these clinical similarities, a minority of chronic chikungunya arthritis patients have positive rheumatoid factor [6] and less commonly positive anti-cyclic citrullinated peptide antibody test results [7,8].
Similarities in the pathogenesis of rheumatoid arthritis and chronic chikungunya arthritis
As in rheumatoid arthritis, the pathogenesis of chronic chikungunya arthritis is not well understood, but both diseases may involve similar mechanisms. For example, in some studies, chronic chikungunya arthritis has been associated with high circulating levels of pro-inflammatory cytokines including IL-6, GM-CSF, IFN-α, and IL-17 [43,44]. IL-6 is well-recognized in rheumatoid arthritis joint inflammation and increases the production of cartilage-destroying enzymes [43]. In chronic chikungunya arthritis, plasma levels of IL-6 and GM-CSF have been found to be significantly higher in patients with persistent arthralgia compared with those who had recovered [45]. In another study, the cytokine profile in chronic chikungunya arthritisincluding IFN-α, IL-5, IL6, IL-10, and particularly IL-7 and IL-15, was similar to rheumatoid arthritis [46].
In chronic chikungunya arthritis, increased IL-1β and IL-6 levels and reduced expression of the chemokine CCL5 (RANTES) correlate with severe disease [46]. Jaller Raad et al observed increased levels of IL-6, IL-8, IL-17, and IFN-γ in 95% of patients with chikungunya arthritis [47]. The profile of elevated levels of cytokines such as IL-1β, TNF, IL-6, and IL-17 during chronic chikungunya arthritis resembles that observed in rheumatoid arthritis [27,33]
An important issue in both rheumatoid arthritis and chronic chikungunya arthritis is whether persistent infection is responsible for chronic rheumatic symptoms. Chang et al did not find CHIKV in synovial fluid in chronic chikungunya arthritis patients. This study also did demonstrate the cytokine profile in chronic infection mimics rheumatoid arthritis, including IFN-α, IL-1β, IL-5, IL-6, IL- 10, IL-7, IL-15, and TNF-α [48].
IL-17 is an important cytokine in both rheumatoid arthritis and chronic chikungunya arthritis. IL-17 may drive matrix and bone destruction through stimulation of IL-6, TNF, IL-1β, matrix metalloproteinases, and the nuclear activator receptor kB-nuclear activator receptor kB ligand (RANKL) system [49]. IL-17R (receptor) activation also induces the production of other inflammatory cytokines and chemokines that initiate the recruitment of neutrophils, macrophages and lymphocytes, and is important in rheumatoid arthritis pathogenesis [49]. In both diseases, IL-6 stimulates RANKL and inhibits osteoprotegerin released by osteoblasts [50].
In genetically susceptible hosts, viral infection may lead to T and B cell dysfunction and autoimmunity [51]. Usually, the immune system develops a potent virus specific immune response that rapidly eliminates the virus with only minimal self tissue injury. Typically, only small amounts of self-antigens, insufficient to induce autoreactive B and T lymphocytes, are released and autoimmune disease does not ensue. But in the event that the host and the virus share antigenic determinants, viral infection may result in autoimmunity as virus-specific cells and antibodies are cross reactive with self-antigens. In this hypothesis, an infecting virus is usually eliminated by the host immune response [16]. If there is an inefficient antiviral response due to disturbed immune cell function (natural killer cell [NK], T cell, B cell, etc.), there may be persistence of the virus and/or chronic arthralgia. To date, studies have failed to demonstrate such persistence of CHIKV in synovial fluid [48,52].
Treatment of rheumatoid arthritis and chronic chikungunya arthritis
Similarities in pathogenesis could explain why chronic chikungunya arthritis seems to respond to treatments effective in rheumatoid arthritis [7,9,53]. In particular, methotrexate (MTX), a mainstay in the treatment of rheumatoid arthritis, has been investigated in chronic chikungunya arthritis [9,10,53,54]. MTX inhibits purine and pyrimidine synthesis, suppresses transmethylation leading to accumulation of polyamines, and reduces antigen dependent T-cell proliferation [55]. In addition, MTX promotes adenosine-mediated suppression of inflammation [56]. In rheumatoid arthritis, MTX induces increased extracellular adenosine, inhibition of purine and pyrimidine synthesis, and downregulation of proinflammatory cytokines [57].
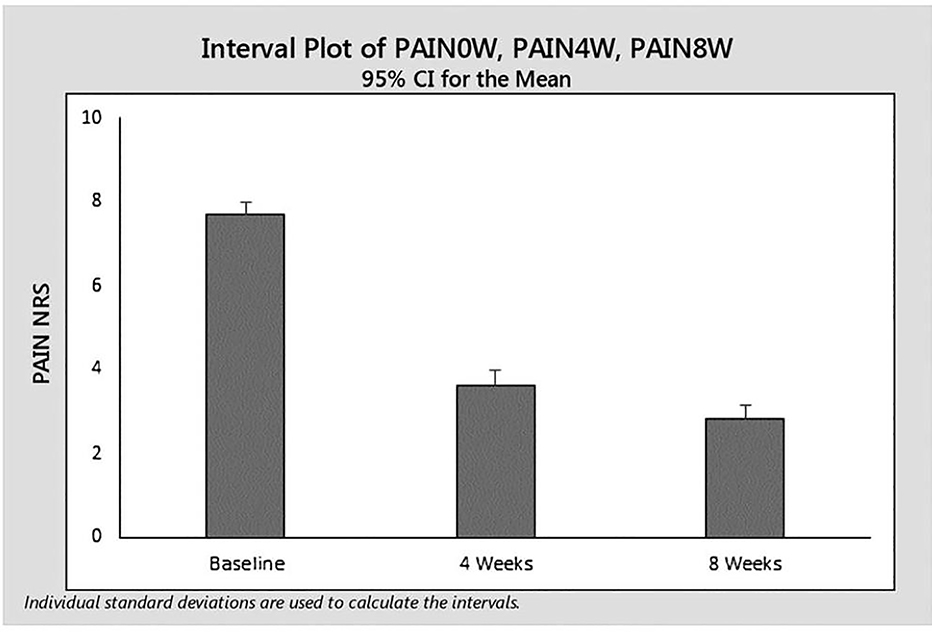
We conducted a systematic review to evaluate the safety and effectiveness of MTX in chronic chikungunya arthritis [53]. Among 131 reports, only six met our evaluation criteria. These were retrospective, uncontrolled, and unblinded studies. Nonetheless, they suggest that MTX may be effective in chronic chikungunya arthritis. In addition, no safety signals implicating reactivation of latent viral infection have been reported to date. We also reported upon our experience with MTX in 48 Brazilian patients with chronic chikungunya arthritis seen over 14.2 ± 4.2 months after disease onset [9]. We assessed pain reduction using a visual analog scale (VAS). Following administration of low dose MTX of 9.2 mg ± 3.2 mg per week, the mean reductions in pain at 4 and 8 weeks, compared to baseline, were 4.3 ± 3.0 (p<0.0001) and 4.5 ± 2.6 (p<0.0001) respectively. MTX treatment was well tolerated [9]. (Figure 1) We believe that the available studies suggest the need for prospective, blinded, randomized evaluation of MTX in chronic chikungunya arthritis treatment.
Figure 1.
Pain scores at W0 and W4 in patients treated with MTX for CCA (as observed data). NRS indicates numerical rating scale.
Conclusion
Over the past two decades, understanding of the immune-mediated pathogenesis of rheumatoid arthritis has greatly improved in parallel with treatment advances that have significantly benefitted rheumatoid arthritis patients. During this same period, CHIKV infection has spread from local African endemic disease to global epidemics, affecting millions of individuals [1,3]. At onset, chikungunya virus is a viral infection. But in many patients, the disease progresses to chronic chikungunya arthritis, causing chronic pain and disability to individual patients [58] and economic harm to vulnerable communities. As described in this review, chronic chikungunya arthritis may be a post-infectious inflammatory process with clinical and pathogenic features that mimic rheumatoid arthritis. Emerging evidence demonstrates that low dose MTX, a widely available and relatively safe disease modifying drug in rheumatoid arthritis, could also be effective in the treatment of chronic chikungunya arthritis [9,53,59]. Given the growing disease burden associated with chronic chikungunya arthritis, it is important that the pathogenesis and treatment of chronic chikungunya arthritis continues to be elucidated. rheumatoid arthritis provides insights into future directions for these investigations.
Table 2.
Similarities and differences between chronic chikungunya arthritis and rheumatoid arthritis.
| Chronic chikungunya arthritis | Rheumatoid arthritis | |
|---|---|---|
| Similarities | Presentation: small joint symmetric polyarthritis (most commonly). | |
| Patients: middle-aged females (most commonly affected demographic). | ||
| Symptoms: fatigue, arthralgias, arthritis, myalgias, and morning stiffness. | ||
| Labs: normochromic anemia; thrombocytosis, and elevated ESR/CRP. | ||
| Radiographic: joint effusions, bone erosions, marrow edema, synovitis, tendinitis, and/or tenosynovitis. | ||
| Serum cytokine profile: ↑ IL-1β, IL-6, IL-17, and TNF (chronic disease) | ||
| Synovial cytokine profile: ↑ IL-1β, IL-6, IL-7, IL-8, IL-10, IL-15, IL-17, GM-CSF, IFN-α, IFN-γ, and TNF (chronic disease) | ||
| Disability: can be moderate-to-severe (chronic disease) | ||
| Differences | Presentation: medium and/or large joint asymmetric mono- or oligoarthritis (less commonly). | |
| Signs and Symptoms: memory and concentration problems and asthenia/depression can be more predominant than in rheumatoid arthritis. | Signs and Symptoms: association with pulmonary (interstitial) disease and/or rheumatoid nodules. | |
| Serologies: anti-CHIKV IgM and/or IgG antibodies. | Serologies: anti-cyclic citrullinated peptide antibodies (anti-CCP); rheumatoid factor (RF) | |
| Causative pathogen: chikungunya virus (CHIKV) | Causative pathogen(s): Epstein-Barr virus, cytomegalovirus, human immunodeficiency virus, human T-cell lymphotropic virus type I -, hepatitis C virus, and others (implicated in the pathogenesis) | |
|
Serum cytokine profile: ↑ IL-1Ra, IL-1β, IL-6, IL-7, IL-8, IL-12, IL-15, and IFN-α (during acute arthritis); ↓ CCL5/RANTES (during acute arthritis); ↑ GM-CSF and TNF (during chronic arthritis). |
||
|
Serum cytokine profile: ↑ CCL5/RANTES correlates with disease severity. |
||
Clinical Significance.
Chikungunya fever is an arboviruse re-emergent characterized by high fever, disabling polyarthritis, and maculopapular rash.
More than 40% of the infected patients develop arthritic manifestations lasting more than 3 months after the acute illness.
In many patients, chronic chikungunya arthritis mimics rheumatoid arthritis clinically and both pathogenesis diseases may involve similar mechanisms.
Emerging evidence demonstrates that low dose methotrexate could also be effective in the treatment of chronic chikungunya arthritis.
Acknowledgments
This study has no funding source to disclose
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
J. Kennedy Amaral, Federal University of Minas Gerais, Department of Infectious Diseases and Tropical Medicine, Belo Horizonte, Minas Gerais, Brazil.
Joshua B. Bilsborrow, Section of Rheumatology, Allergy and Immunology, Yale University School of Medicine, New Haven, Connecticut, USA.
Robert T. Schoen, Section of Rheumatology, Allergy and Immunology, Yale University School of Medicine, New Haven, Connecticut, USA.
References
- 1.Wimalasiri-Yapa BM, Stassen L, Huang X, Hafner LM, Hu W, Devine GJ et al. (2019). Chikungunya virus in Asia – Pacific: a systematic review. Emerging Microbes & Infections, 8(1), 70–79. doi: 10.1080/22221751.2018.1559708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaBeaud A, Bashir F, King CH (2011). Measuring the burden of arboviral diseases: the spectrum of morbidity and mortality from four prevalent infections. Population Health Metrics, 9(1). doi: 10.1186/1478-7954-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul BJ, and Sadanand S (2018). Chikungunya Infection: A Re-emerging Epidemic. Rheumatology and Therapy, 5(2), 317–326. doi: 10.1007/s40744-018-0121-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver SC, and Lecuit M Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med 2015;372:1231–9 [DOI] [PubMed] [Google Scholar]
- 5.Thiberville S, Moyen N, Dupuis-Maguiraga L, Nougairede A, Gould EA, Roques P, et al. (2013). Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Research, 99(3), 345–370. doi: 10.1016/j.antiviral.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodríguez-Morales AJ, Cardona-Ospina JA, Fernanda Urbano-Garzón S, Sebastian Hurtado-Zapata J (2016). Prevalence of Post-Chikungunya Infection Chronic Inflammatory Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care & Research, 68(12), 1849–1858. doi: 10.1002/acr.22900 [DOI] [PubMed] [Google Scholar]
- 7.Javelle E, Ribera A, Degasne I, Gaüzère B, Marimoutou C, Simon F (2015). Specific Management of Post-Chikungunya Rheumatic Disorders: A Retrospective Study of 159 Cases in Reunion Island from 2006–2012. PLOS Neglected Tropical Diseases, 9(3), e0003603. doi: 10.1371/journal.pntd.0003603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouquillard É, and Combe B (2009). A report of 21 cases of rheumatoid arthritis following Chikungunya fever. A mean follow-up of two years. Joint Bone Spine, 76(6), 654–657. doi: 10.1016/j.jbspin.2009.08.005 [DOI] [PubMed] [Google Scholar]
- 9.Amaral JK, Bingham CO, Schoen RT (2018). Successful Methotrexate Treatment of Chronic Chikungunya Arthritis. JCR: Journal of Clinical Rheumatology, 1. doi: 10.1097/00124743900000000-99120 [DOI] [PubMed] [Google Scholar]
- 10.Amaral JK, Taylor PC, Teixeira MM, Morrison TE, Schoen RT The Clinical Features, Pathogenesis and Methotrexate Therapy of Chronic Chikungunya Arthritis. Viruses. 2019; 11(3):289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaid A, Gérardin P, Taylor A, Mostafavi H, Malvy D, Mahalingam S (2018). Review: Chikungunya Arthritis: Implications of Acute and Chronic Inflammation Mechanisms on Disease Management. Arthritis & Rheumatology, 70(4), 484–495. doi: 10.1002/art.40403 [DOI] [PubMed] [Google Scholar]
- 12.Chaaithanya IK, Muruganandam N, Sundaram SG, Kawalekar O, Sugunan AP, Manimunda SP, et al. (2011). Role of Proinflammatory Cytokines and Chemokines in Chronic Arthropathy in CHIKV Infection. Viral Immunology, 24(4), 265–271. doi: 10.1089/vim.2010.0123 [DOI] [PubMed] [Google Scholar]
- 13.Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, et al. Persistent chronic inflammation and infection by chikungunya arthritogenic alphavirus in spite of a robust host immune response. J. Immunol. 2010, 184, 5914–5927. [DOI] [PubMed] [Google Scholar]
- 14.Chow A, Her Z, Ong EK, Chen J, Dimatatac F, Kwek DJ, et al. Persistent Arthralgia Induced by Chikungunya Virus Infection is Associated with Interleukin-6 and Granulocyte Macrophage Colony-Stimulating Factor. J. Infect Dis. 2011, 203, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiwari V, Bergman MJ. Viral Arthritis [Updated 2019 Jun 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019. January-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK531507 [PubMed] [Google Scholar]
- 16.Perl A (1999). Mechanisms of viral pathogenesis in rheumatic disease. Annals of the Rheumatic Diseases, 58(8), 454–461. doi: 10.1136/ard.58.8.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marks M, and Marks JL (2016). Viral arthritis. Clinical Medicine, 16(2), 129–134. doi: 10.7861/clinmedicine.16-2-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vassilopoulos D, and Calabrese LH (2008). Virally associated arthritis 2008: clinical, epidemiologic, and pathophysiologic considerations. Arthritis Research & Therapy, 10(5), 215. doi: 10.1186/ar2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albert LJ, and Inman RD (1999). Molecular Mimicry and Autoimmunity. New England Journal of Medicine, 341(27), 2068–2074. doi: 10.1056/nejm199912303412707 [DOI] [PubMed] [Google Scholar]
- 20.Tsokos G (2004). Overview of cellular immune function in systemic lupus erythematosus. Systemic Lupus Erythematosus, 29–92. doi: 10.1016/b9-78-012433-9/01950-0053 [DOI] [Google Scholar]
- 21.Clerici M, and Shearer GM (1994). The Th1–Th2 hypothesis of HIV infection: new insights. Immunology Today, 15(12), 575–581. doi: 10.1016/0167-5699(94)90220-8 [DOI] [PubMed] [Google Scholar]
- 22.Naides SJ (1998). Rheumatic manifestations of parvovirus b19 infection. Rheumatic Disease Clinics of North America, 24(2), 375–401. doi: 10.1016/s0889-857x(05)70014-4 [DOI] [PubMed] [Google Scholar]
- 23.Sayiner ZA, Haque U, Malik MU, Gurakar A. Hepatitis C virus infection and its rheumatologic implications. Gastroenterol Hepatol 2014;10:287–93. [PMC free article] [PubMed] [Google Scholar]
- 24.Nishioka KJ (1996). HTLV-I Arthropathy and Sjögren Syndrome. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology, 13, S57–S62. doi: 10.1097/00042560199600001-00011 [DOI] [PubMed] [Google Scholar]
- 25.Draborg AH, Duus K, Houen G (2013). Epstein-Barr Virus in Systemic Autoimmune Diseases. Clinical and Developmental Immunology, 2013, 1–9. doi: 10.1155/2013/535738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalden JR, and Gay S (2008). Retroviruses and autoimmune rheumatic diseases. Clinical & Experimental Immunology, 98(1), 1–5. doi: 10.1111/j.1365-2249.1994.tb06597.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McInnes IB, and Schett G (2011). The Pathogenesis of Rheumatoid Arthritis. New England Journal of Medicine, 365(23), 2205–2219. doi: 10.1056/nejmra1004965 [DOI] [PubMed] [Google Scholar]
- 28.Scott DL, Wolfe F, Huizinga TW (2010). Rheumatoid arthritis. The Lancet, 376(9746), 1094–1108. doi: 10.1016/s0140-6736(10)60826-4 [DOI] [PubMed] [Google Scholar]
- 29.Bach JF (2005). Infections and autoimmune diseases. J. Autoimmun. 25(Suppl.), 74–80. 10.1016/j.jaut.2005.09.024 [DOI] [PubMed] [Google Scholar]
- 30.Arleevskaya MI, Kravtsova OA, Lemerle J, Renaudineau Y, Tsibulkin AP (2016). How Rheumatoid Arthritis Can Result from Provocation of the Immune System by Microorganisms and Viruses. Frontiers in Microbiology, 7. doi: 10.3389/fmicb.2016.01296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffiths SJ, Riddell NE, Masters J, Libri V, Henson SM, Wertheimer A, et al. (2013). Age-Associated Increase of Low-Avidity Cytomegalovirus-Specific CD8+ T Cells That ReExpress CD45RA. The Journal of Immunology, 190(11), 5363–5372. doi: 10.4049/jimmunol.1203267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halenius A, and Hengel H (2014). Human Cytomegalovirus and Autoimmune Disease. BioMed Research International, 2014, 1–15. doi: 10.1155/2014/472978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taneja V (2015). Cytokines pre-determined by genetic factors are involved in pathogenesis of Rheumatoid arthritis. Cytokine, 75(2), 216–221. doi: 10.1016/j.cyto.2014.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saal JG, Krimmel M, Steidle M, Gerneth F, Wagner S, Fritz P, et al. (1999). Synovial Epstein-Barr virus infection increases the risk of rheumatoid arthritis in individuals with the shared HLA-DR4 epitope. Arthritis & Rheumatism, 42(7), 1485–1496. doi: [DOI] [PubMed] [Google Scholar]
- 35.Fleming A, Dodman S, Crown JM, Corbett M (1976). Extra-articular features in early rheumatoid disease. BMJ, 1(6020), 1241–1243. doi: 10.1136/bmj.1.6020.1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleming A, Benn RT, Corbett M, Wood PH (1976). Early rheumatoid disease. II. Patterns of joint involvement. Annals of the Rheumatic Diseases, 35(4), 361–364. doi: 10.1136/ard.35.4.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Aken J (2006). Comparison of long term outcome of patients with rheumatoid arthritis presenting with undifferentiated arthritis or with rheumatoid arthritis: an observational cohort study. Annals of the Rheumatic Diseases, 65(1), 20–25. doi: 10.1136/ard.2005.038471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Firestein GS, Budd R, Gabriel SE, McInnes IB, O’Dell JR (2012). Clinical Features of Rheumatoid Arthritis In Kelley’s Textbook of Rheumatology E-Book (9th ed., p. 1111). St. Louis, MO: Elsevier Health Sciences, 1109–1135. [Google Scholar]
- 39.Borgherini G, Poubeau P, Jossaume A, Gouix A, Cotte L, Michault A, et al. (2008). Persistent Arthralgia Associated with Chikungunya Virus: A Study of 88 Adult Patients on Reunion Island. Clinical Infectious Diseases, 47(4), 469–475. doi: 10.1086/590003 [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Morales AJ, Gil-Restrepo AF, Ramírez-Jaramillo V, Montoya-Arias CP, Acevedo-Mendoza WF, Bedoya-Arias JE, et al. (2016). Post-chikungunya chronic inflammatory rheumatism: results from a retrospective follow-up study of 283 adult and child cases in La Virginia, Risaralda, Colombia. F1000Research, 5, 360. doi: 10.12688/f1000research.8235.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schilte C, Staikovsky F, Couderc T, Madec Y, Carpentier F, Kassab S, et al. (2013). Chikungunya Virus-associated Long-term Arthralgia: A 36-month Prospective Longitudinal Study. PLoS Neglected Tropical Diseases, 7(3), e2137. doi: 10.1371/journal.pntd.0002137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manimunda SP, Vijayachari P, Uppoor R, Sugunan AP, Singh SS, Rai SK, et al. (2010). Clinical progression of chikungunya fever during acute and chronic arthritic stages and the changes in joint morphology as revealed by imaging. Transactions of the Royal Society of Tropical Medicine and Hygiene, 104(6), 392–399. doi: 10.1016/j.trstmh.2010.01.011 [DOI] [PubMed] [Google Scholar]
- 43.Chaaitanya IK, Muruganandam N, Sundaram SG, Kawalekar O, Sugunan AP, Manimunda SP, et al. Role of pro-inflammatory cytokines and chemokines in chronic arthropathy in CHIKV infection. Viral Immunol 2011;24:265–71 [DOI] [PubMed] [Google Scholar]
- 44.Chow A, Her Z, Ong EK, Chen J, Dimatatac F, Kwek DJ, et al. (2011). Persistent Arthralgia Induced by Chikungunya Virus Infection is Associated with Interleukin-6 and Granulocyte Macrophage Colony-Stimulating Factor. J Infect Dis, 203(2), 149–157. doi: 10.1093/infdis/jiq042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dupuis-Maguiraga L, Noret M, Brun S, Le Grand R, Gras G, & Roques P (2012). Chikungunya Disease: Infection-Associated Markers from the Acute to the Chronic Phase of Arbovirus-Induced Arthralgia. PLoS Negl Trop Dis, 6(3), e1446. doi: 10.1371/journal.pntd.0001446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng LF, Chow A, Sun Y, Kwek DJ, Lim P, Dimatatac F, et al. IL-1b, IL-6, and RANTES as biomarkers of chikungunya severity. PLoS One 2009;4:e4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaller Raad J, Segura Rosero A, Vidal Martínez J, Parody A, Jaller Raad R, Tovar Caballero, et al. Respuesta inmunitaria de una población del Caribe colombiano infectada con el virus chikungunya. Revista Colombiana de Reumatología 2016;23:85–91. [Google Scholar]
- 48.Chang AY, Martins KAO, Encinales L, et al. A cross-sectional analysis of chikungunya arthritis patients 22-months post-infection demonstrate no detectable viral persistence in synovial fluid. Arthritis Rheumatol 2017; doi: 10.1002/art.40383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med 2009; 361:888–898. [DOI] [PubMed] [Google Scholar]
- 50.Interleukin Noret M. 6, RANKL, and osteoprotegerin expression by chikungunya virusinfected human osteoblasts. J Infect Dis 2012; 206:455–457. [DOI] [PubMed] [Google Scholar]
- 51.Hochberg MC. Systemic lupus erythematosus. Rheum Dis Clin North Am 1990;16:617–39. [PubMed] [Google Scholar]
- 52.Thanapati S, Ganu MA, Tripathy AS (2017). Differential inhibitory and activating NK cell receptor levels and NK/NKT-like cell functionality in chronic and recovered stages of chikungunya. PLOS ONE, 12(11), e0188342. doi: 10.1371/journal.pone.0188342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amaral JK, Sutaria R, Schoen RT (2018). Treatment of chronic chikungunya arthritis with methotrexate: a systematic review. Arthritis Care Res. doi: 10.1002/acr.23519 [DOI] [PubMed] [Google Scholar]
- 54.Blettery M, Brunier L, Polomat K, Moinet F, Deligny C, Arfi S, et al. (2016). Brief Report: Management of Chronic Post-Chikungunya Rheumatic Disease: The Martinican Experience. Arthritis & Rheumatology, 68(11), 2817–2824. doi: 10.1002/art.39775 [DOI] [PubMed] [Google Scholar]
- 55.Cutolo M Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann. Rheum. Dis. 2001, 60, 729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wessels JA, Huizinga TW, Guchelaar H Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford). 2007, 47, 249–255. [DOI] [PubMed] [Google Scholar]
- 57.Tian H, and Cronstein B Understanding the mechanisms of action of methotrexate: Implications for the treatment of rheumatoid arthritis. Bull NYU Hosp. Jt. Dis. 2007, 65, 168–173. [PubMed] [Google Scholar]
- 58.Amaral JK, Bilsborrow JB, Schoen RT (2019). Brief report: the disability of chronic chikungunya arthritis. Clinical Rheumatology, 38(7), 2011–2014. doi: 10.1007/s10067-019-04529-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bedoui Y, Giry C, Jaffar-Bandjee M, Selambarom J, Guiraud P, Gasque P (2018). Immunomodulatory drug methotrexate used to treat patients with chronic inflammatory rheumatisms post-chikungunya does not impair the synovial antiviral and bone repair responses. PLOS Neglected Tropical Diseases, 12(8), e0006634. doi: 10.1371/journal.pntd.0006634 [DOI] [PMC free article] [PubMed] [Google Scholar]