Abstract
ΔNp63 is a transcription factor of the p53 family and has crucial functions in normal development and disease. The expression pattern of ΔNp63 in human cancer suggests dynamic regulation of this isoform during cancer progression and metastasis. Many primary and metastatic tumors express high levels of ΔNp63, while ΔNp63 loss is crucial for tumor dissemination, indicating an oscillatory expression of ΔNp63 during cancer progression. Here we use genetically engineered orthotopic mouse models of breast cancer to show that while depletion of ΔNp63 inhibits primary mammary adenocarcinoma development, oscillatory expression of ΔNp63 in established tumors is crucial for metastatic dissemination in breast cancer. A TGFβ-regulated microRNA network acted as upstream regulators of this oscillatory expression of ΔNp63 during cancer progression. This work sheds light on the pleiotropic roles of ΔNp63 in cancer and unveils critical functions of TGFβ in the metastatic process.
Keywords: oscillatory ΔNp63, metastasis, breast cancer, TGFβ signaling, microRNAs
INTRODUCTION
The TP63 gene encodes multiple isoforms categorized into two groups, TAp63 and ΔNp63, which are expressed in different cellular compartments and have distinct functions in many biological processes including cancer (1). ΔNp63, which lacks the full-length transactivation domain (TA domain), is the predominant isoform found in the basal layers of stratified epidermis and is essential for terminal differentiation and maintenance of basal epidermal cells. Both TAp63 and ΔNp63 transcripts can be transcribed into proteins with at least three different C-termini termed α, β and γ via alternative splicing (2). In cancer, ΔNp63α is overexpressed in various primary tumors, such as lung squamous cell carcinomas, head and neck squamous cell carcinomas, basal-like bladder cancer and ovarian cancer (3–6). ΔNp63 exhibits oncogenic functions through its antagonistic effects on the transcriptional activities of p53, TAp63 and TAp73 (1,7–10). Importantly, tumor cells with high invasion capacity express low levels of ΔNp63, which induces an epithelial to mesenchymal transition (EMT) program in these cells (11–13). On the contrary, metastases at distant organs have high expression of ΔNp63 (14,15), indicating a dynamic oscillatory expression of ΔNp63 during cancer progression.
Rare cells within a primary tumor lose contact with surrounding cells due to the loss of cell-cell adhesion, enter into the blood circulation, and successfully infiltrate and form metastases at distant organs. The multistep metastatic process is orchestrated by a complex network of signaling pathways, not only within primary tumor cells, but also between tumor cells and the surrounding microenvironment (16). Even though there are numerous studies that have focused on the characterization of metastatic progression, defined mechanistic insights into metastatic progression are still unknown. Interestingly, while ΔNp63 is found to be overexpressed in primary tumors and metastases of multiple cancers (14,15,17), there is mounting evidence that ΔNp63 is a suppressor of the epithelial to mesenchymal transition (EMT), cell migration and invasion, and potentially cancer metastasis (12,18,19). In particular, the loss of ΔNp63 in epithelial-like bladder cancer cells promotes EMT and enhance invasion, while the induction of ΔNp63 in mesenchymal-like bladder cancer cells triggers the mesenchymal to epithelial transition (MET) and suppresses invasion (19). This oscillatory expression of ΔNp63 expression from primary tumor to tumor dissemination to distant metastasis resembles the spatiotemporal regulation of Twist1 during metastasis (20), suggesting a potential dynamic regulation of ΔNp63 in cancer metastasis. More importantly, we have recently demonstrated pleiotropic functions of ΔNp63 during tumor development and progression using a global pan-cancer approach to examine various types and stages of cancers in TCGA (21). Together, these data indicate an intricate regulation of ΔNp63 in tumor development and metastatic progression.
To understand the biological consequences of spatiotemporal expression of ΔNp63 on cancer metastasis, we modulated ΔNp63 expression during breast cancer metastasis in a genetically engineered orthotopic mouse model of breast cancer. By generating an inducible shRNA knockdown of ΔNp63 to modulate its expression in vivo, we demonstrated that an oscillatory expression of ΔNp63 is required for efficient metastatic colonization of breast cancer. We found that only breast cancer cells with an oscillatory expression of ΔNp63 (i.e. these cells initially express ΔNp63 followed by a silencing of ΔNp63 and then a re-expression of ΔNp63) efficiently metastasized and colonized the lungs of mice with mammary tumors. Further, we identified a novel network of four TGFβ–Smad3 regulated microRNAs, including miR-22–3p, miR-30a-5p, miR-203a-3p and miR-222–3p, which target ΔNp63 and efficiently silence it. These TGFβ-Smad3-dependent microRNAs cooperatively regulate the expression of ΔNp63, not only in breast cancer, but also in other types of cancers where ΔNp63 is overexpressed. This novel TGFβ/microRNA/ΔNp63 regulatory axis provides new insights into the pleiotropic functions of ΔNp63 and TGFβ in tumorigenesis and cancer metastasis.
MATERIALS AND METHODS
Cell lines and culture conditions
Human mammary epithelial MCF10A, human breast cancer MCF10DCIS.com and MCF10CA1D.cl1 cell lines were obtained from Barbara Ann Karmanos Cancer Institute, and cultured as previously described (22). All the cells lines were regularly authenticated via STR profiling by the Molecular Genomics Core at the H. Lee Moffitt Cancer Center.
Animal studies
Mouse studies were approved by the IACUC at Moffitt Cancer Center. Female athymic nu/nu mice were purchased from Envigo and used at 6–8 weeks of age. Age-matched mice were used for all experiments.
Generation of inducible shRNA against ΔNp63
An shRNA specifically targeting ΔNp63 (shΔNp63: GAGGGACTTGAGTTCTGTTAT) was designed and cloned into pLV-H1TetO-GFP-Puro lentiviral vector (SORT-CO1, Biosettia). 3 μg of the inducible shΔNp63 lentiviral (pLV-i-shΔNp63) vector were transfected into HEK 293T cells along with 3 μg of lentivirus packaging vectors, pCMV-VSVG and pRSV-REV, using X-tremeGENE HP (Roche) in accordance with the manufacturer’s protocol. 48 hours after the transfection, supernatants were collected, filtered and added to MCF10DCIS cells for 48 hours in the presence of 8 μg/ml Polybrene (Santa Cruz). 2 μg/ml of puromycin was added to the media 48 hours after infection for 2 days to select for stable clones. Doxycycline (1 μg/ml) was added to the stable cells for 3 days, then cell pellets were collected for western blot to determine knockdown efficiency of ΔNp63 protein.
In vivo mammary fat pad injection
2×106 cells mixed 1:1 ratio with growth factor reduced matrigel (BD Biosciences) were injected into the left and right mammary fat pads of nude mice. Two weeks after the injection, the tumors became palpable and the mice were fed with either control diet or doxycycline (dox) diet (200 mg/ kg, Bio-Serv) to induce the expression of the shΔNp63. At the end of the experiments, all the mice were euthanized and necropsy performed to collect tumors and internal organs for further analysis. Tumor sizes (length and width) were measured using a caliper. Tumor volume was calculated using the following formula: Tumor volume = 1/2(length × width2)
NanoString human microRNA panel to identify differential expressed microRNAs
2×105 MCF10DCIS cells were treated with TGFβ1 (10 ng/mL) for 72 hours, then collected for total RNA extraction using miRNaesy Kit (Qiagen) following manufacturer’s protocol. Experiments were done in quadruplicate. Then, RNA samples were run in the NanoString human microRNA panel (NanoString) following manufacturer’s protocol.
Statistical analysis
For statistical comparison between two groups, an unpaired two-tailed t-test was used. A p-value of less than 0.05 was considered significant. Statistical analyses were performed in Graphpad Prism 7.
Data availability
The NanoString human microRNA data and the ChIP-seq data were deposited to NCBI Gene Expression Omnibus (GEO) repository: GSE134681 and GSE144995.
RESULTS
Oscillatory expression of ΔNp63 in breast cancer is associated with invasion and progression
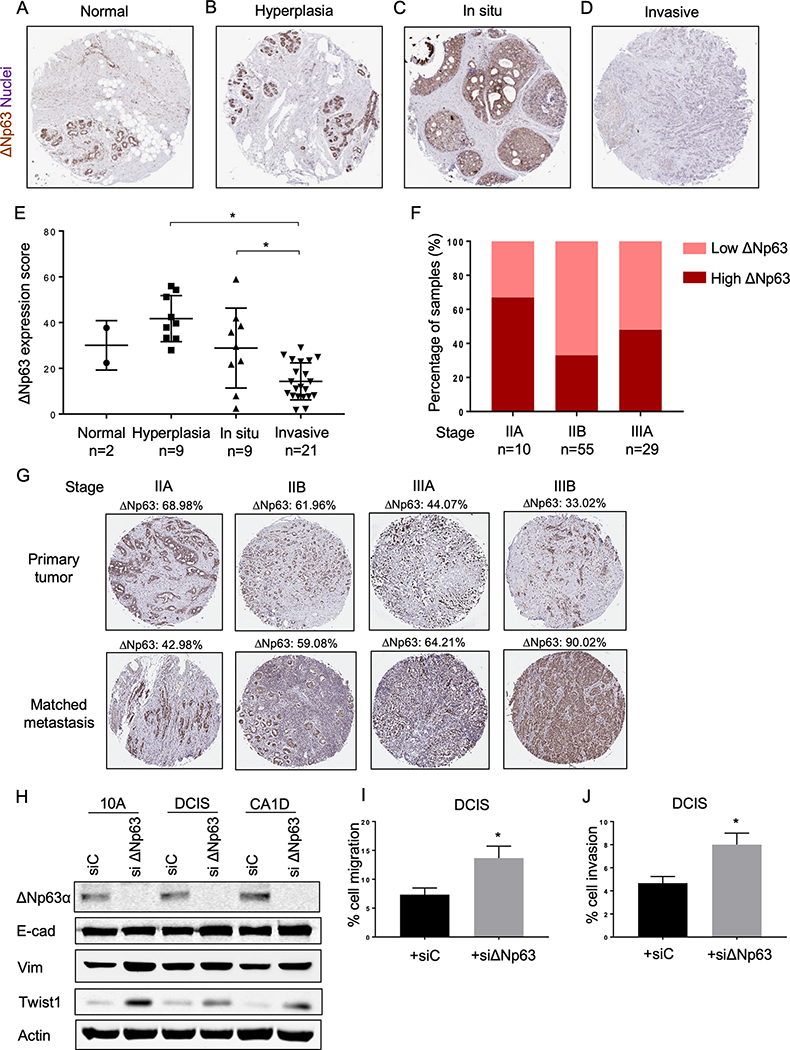
To determine the pattern of expression of ΔNp63 during the progression of breast cancer in human patients, we examined ΔNp63 expression in a tissue microarray (TMA) including samples from normal breast tissue, lobular hyperplasia, ductal carcinoma in situ (DCIS), and invasive breast cancer biopsies (BR480, US Biomax). We performed ΔNp63 immunostaining and revealed a significantly decreased expression of ΔNp63 in invasive breast cancer samples compared to hyperplasia and carcinoma in situ (Fig. 1A–E), indicating that ΔNp63 must be lost for breast cancer invasion to occur. We then determined the expression level of ΔNp63 in invasive primary tumors at different stages of disease progression using a distinct breast cancer TMA (BR20837a, US Biomax). In this TMA, we found a dramatic increase in the number of samples with low ΔNp63 expression in stage IIB and IIIA compared to stage IIA (Fig. 1F), indicating that loss of ΔNp63 is associated with tumor progression. Paradoxically, we found that the decrease in ΔNp63 expression observed in the primary tumors progressing from stage IIA to IIIB was associated with an increase in the levels of ΔNp63 in the matched lymph node metastases (Fig. 1G).
Figure 1.
Oscillatory expression ΔNp63 in breast cancer progression. A-D, Representative immunohistochemistry staining images for ΔNp63 in breast cancer tissue microarray. ΔNp63 positive nuclei are brown. Hematoxylin was used as a nuclear counterstain. 5X magnification. E, Quantification of ΔNp63 expression (ΔNp63 expression score) in breast cancer tissue microarray. Score of each sample was determined by multiplying the number of positive nuclei by the average staining intensity. Asterisk indicates p < 0.005. F, Percentage of primary tumors with either low or high expression of ΔNp63 at different stages of breast cancer. G, Immunohistochemistry staining images for ΔNp63 in invasive primary human breast tumors at different stages and matched lymph node metastases. Percentage of ΔNp63-positive tumor cells is indicated. H, Representative western blots of MCF10A (10A), MCF10DCIS (DCIS) and MCF10CA1D (CA1D) transfected with control siRNA (siC) or siRNA against ΔNp63 (siΔNp63). Immunoblots were probed with the indicated antibodies. Actin was used as a loading control. I-J, Quantification of cell migration (I) and invasion (J) assays of MCF10DCIS cells transfected with siC or siΔNp63. Data are mean ± SD. n = 3. Asterisk indicates p < 0.05.
The association of ΔNp63 loss and the induction of cancer cell migration and invasion has been reported in other cancers (12,18,19). To determine whether these features were also induced in breast cancer upon loss of ΔNp63, we made use of the MCF-10A breast cancer progression model (22–24). In this model, we depleted ΔNp63 in normal human mammary epithelial cells (MCF10A), the DCIS cells (MCF10DCIS), and metastatic cells (MCF10CA1D), and found increased expression of EMT-associated factors, vimentin and Twist1 (Fig. 1H). E-cadherin expression did not significantly change upon the depletion of ΔNp63, suggesting the induction of a partial EMT (Fig. 1H). Importantly, we also observed a significant increase in the cell migration and invasion of MCF10DCIS cells when ΔNp63 was knocked down (Fig. 1I and J), indicating that the downregulation of ΔNp63 is associated with the migratory and invasive potential of breast cancer cells similarly to what has been observed in other cancers.
Oscillatory expression of ΔNp63 enhances metastatic dissemination
To decipher the activities of ΔNp63 on different stages of the metastatic cascade in breast cancer, we mimicked the oscillatory expression of ΔNp63 during breast cancer progression by generating a doxycycline-inducible shRNA against ΔNp63 (i-shΔNp63). In the presence of doxycycline (dox), protein expression of ΔNp63 in MCF10DCIS cells harboring the inducible shΔNp63 (MCF10DCIS-i-shΔNp63) was dramatically decreased after 3 days (Supplementary Fig. S1A). Withdrawing doxycycline from the culture media for 2 days restored expression of ΔNp63 with a complete recovery of ΔNp63 expression after 4 days without doxycycline in the culture media (Supplementary Fig. S1A). Loss of ΔNp63 expression in MCF10DCIS cells dramatically reduced primary tumor growth in vivo by more than two fold (Supplementary Fig. S1B and S1C). These data are in accordance with previous studies demonstrating the essential oncogenic roles of ΔNp63 for the establishment of primary tumors in thymic lymphoma and cutaneous squamous cell carcinoma (9,10).
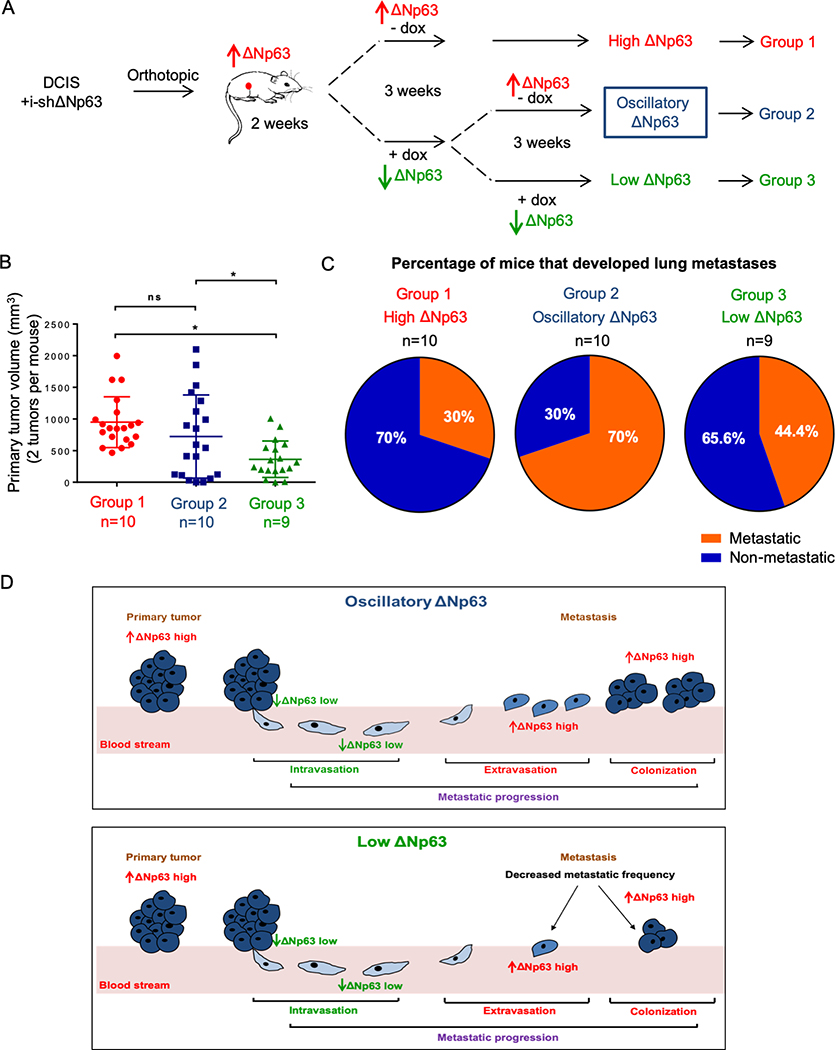
Since p63 was previously reported to be overexpressed in both primary tumors and metastases of cutaneous squamous cell carcinomas (14,15), we wanted to further investigate how ΔNp63 modulates the complete process of metastatic dissemination, from primary tumor formation to lung colonization using metastatic breast cancer as a model system. As described in Figure 2A, MCF10DCIS-i-shΔNp63 cells were injected into mammary fat pads of nude mice. After two weeks tumors became palpable, and one cohort of 10 mice was fed with control diet to maintain a “high ΔNp63” expression (Group 1). The second cohort was on doxycycline diet for 3 weeks, then switched to control diet for 3 more weeks to oscillate the expression of ΔNp63 “low then high ΔNp63 expression” (Group 2). The last cohort was continuously fed a doxycycline diet to keep a “low ΔNp63” expression (Group 3). Consistent with our previous results shown in figure S1C, downregulation of ΔNp63 in established tumors caused reduction in tumor growth (Fig. 2B). Additionally, primary tumors from group 3 with “low ΔNp63” showed decreased expression of ΔNp63 and Ki67 compared to group 1 and group 2 (Supplementary Fig. S2A and S2B), indicating that the downregulation of ΔNp63 resulted in a decrease in tumor cell proliferation which in turn led to reduced tumor growth. All the mice were also assessed for spontaneous metastasis to the lungs without removal of the primary tumors. Strikingly, tumor cells with “oscillatory ΔNp63” (Group 2) exhibited the highest capacity to metastasize and colonize the lungs, with 70% of mice developing lung metastases compared to 30% from group 1 (Fig. 2C). Tumor cells with “low ΔNp63” (Group 3) also showed an increase in metastatic frequency to the lungs compared to “high ΔNp63” group (Group 1). Interestingly, lung metastases from all three groups were positive for ΔNp63 staining (Supplementary Fig. S2C–E), suggesting that subsets of cells with high expression of ΔNp63 may possess molecular features that ultimately favor metastatic colonization.
Figure 2.
Oscillatory expression of ΔNp63 in a mouse model of mammary adenocarcinoma enhances metastatic dissemination. A, Experimental design to investigate effects of oscillatory expression of ΔNp63 in breast cancer metastasis using an inducible shRNA for ΔNp63 and an orthotopic model of metastatic mammary adenocarcinoma. B, Primary mammary tumor volume (mm3) of mice injected with DCIS-i-shΔNp63 cells and fed with different diet schedules of doxycycline (dox) as described in (A). Asterisk indicates p <0.05. NS is non-statistically significant using a two-tailed t test. C, Pie graphs indicating the percentage of mice with lung metastases among the experimental groups as described in (A). D, Working model demonstrating the effects of oscillatory expression of ΔNp63 in breast cancer metastasis.
In summary, these data revealed that the oscillatory expression of ΔNp63 facilitates metastatic dissemination of tumor cells to distant organs. The depletion of ΔNp63 in primary tumor cells, while inhibiting cell proliferation, also triggered a cell migration and invasion program that could potentially increase intravasation into the blood circulation (Fig. 2D). This mechanism of action sheds new lights on varying expression of ΔNp63 during mammary adenocarcinoma disease progression.
ΔNp63 is essential for breast cancer cell extravasation and lung colonization of circulating tumor cells
The ability of tumor cells to disseminate and proliferate in distant organs is crucial for the establishment of micro-metastatic lesions and subsequent colonization (16,25). Since our data show that ΔNp63 promotes cancer cell proliferation and formation of primary tumors, we further determined whether the expression of ΔNp63 is also required for the establishment of distant metastases to the lungs, thus explaining why an oscillatory expression of this isoform enhances metastasis as shown previously in this study. There are two critical steps involved in the formation of overt metastatic colonies at distant sties: extravasation and colonization. To investigate contribution of ΔNp63 in extravasation of circulating tumor cells into the lungs, we performed an extravasation assay in which fluorescence-labelled tumor cells are injected into nude mice via tail vein, and extravasated cells into the lungs after 48 hours are quantified using flow cytometry (20). Specifically, MCF10DCIS-i-shΔNp63 cells were labeled with red fluorescence protein RFP and cultured in doxycycline for 3 days to silence ΔNp63 prior to tail vein injection into nude mice receiving control diet or doxycycline diet as described in Figure 3A. The untreated MCF10DCIS-i-shΔNp63 cells were injected into nude mice on control diet as “high ΔNp63” (Group 1). After 48 hours, the number of RFP-positive cells successfully extravasating into the lungs was quantified. The results showed that depletion of ΔNp63 in circulating tumor cells (“low ΔNp63”, Group 3) resulted in a significant decrease in the number of RFP-positive cells in the lungs compared to control tumor cells with “high ΔNp63” expression (Group 1). Notably, tumor cells with “oscillatory ΔNp63” (Group 2) had similar percentage of extravasated RFP-positive cells to “high ΔNp63” control cells (Group 1) (Fig. 3B and C), indicating that ΔNp63 expression is essential for circulating cells to extravasate into the lungs at the early stage of lung colonization.
Figure 3.
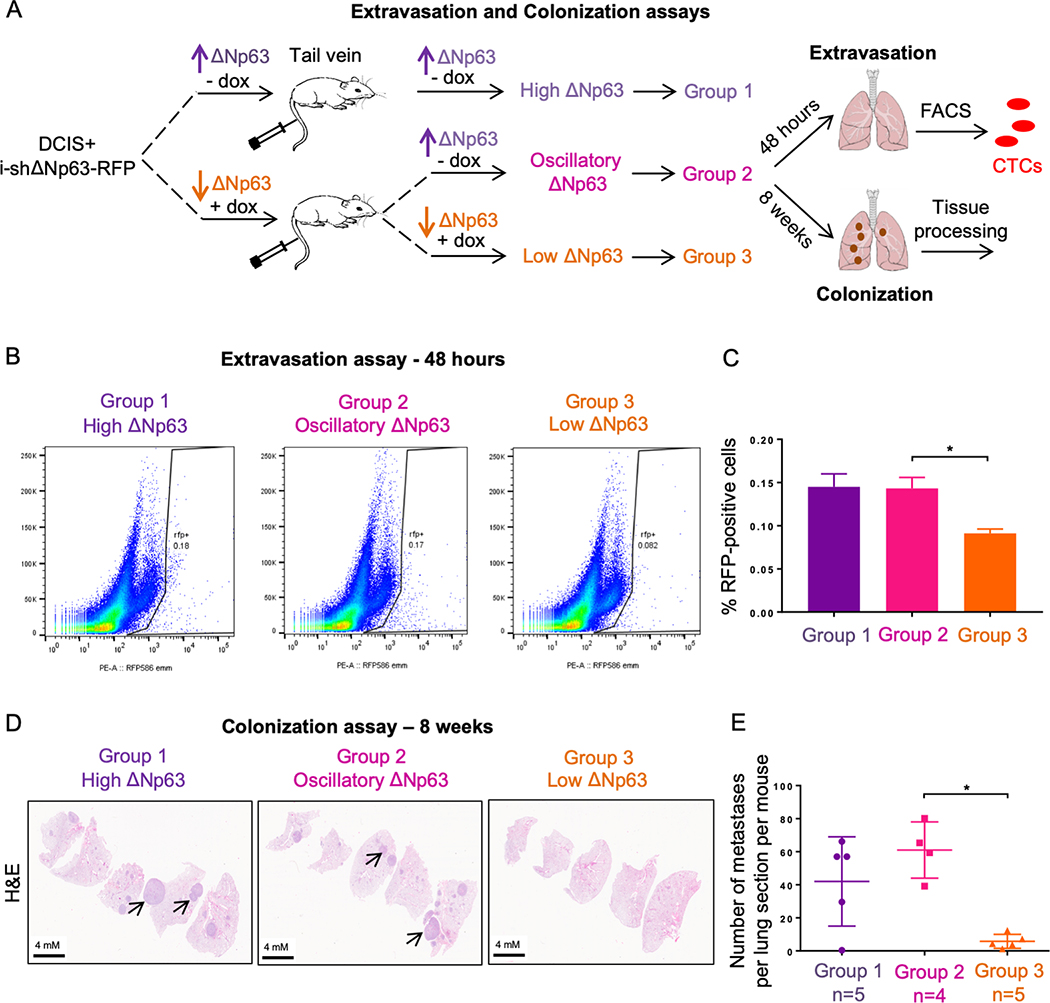
ΔNp63 re-expression is required for lung extravasation and colonization of breast cancer MCF10DCIS cells. A, Experimental design to investigate roles of ΔNp63 in extravasation (48 hours) and colonization (8 weeks) to the lungs of mice that have been treated with MCF10DCIS cells expressing an inducible shRNA against ΔNp63. B-C, Flow cytometry assay (B) and quantification (C) of the percentage of RFP-positive MCF10DCIS cells in the lungs. Data are mean ± SD. n = 6. Asterisk indicates p < 0.05. D-E, Representative hematoxylin and eosin (H&E) cross-sections of lungs from the indicated groups (D) and quantification of metastatic colonies per lung section per mouse (E). Data are mean ± SD. n = 4 (group 1 and group 2) or n = 5 (group 3). Asterisk indicates p < 0.0005.
We next determined the ability of these 3 groups with different ΔNp63 expression patterns to colonize the lungs. To do this, we repeated the above assay by injecting MCF10DCIS-i-shΔNp63 cells treated with doxycycline for 3 days to silence ΔNp63 into nude mice fed with either control diet or doxycycline diet as described in Figure 3A. In this experiment, the mice were aged for 8 additional weeks post-injection and the lungs were collected and quantified for the number of metastatic lesions (Fig. 3A). In line with our previous results, “oscillatory ΔNp63” (Group 2) had the greatest number of visible nodules in the lungs similar to “high ΔNp63” (Group 1), whereas “low ΔNp63” (Group 3) exhibited a dramatic reduction in overt metastasis formation (Supplementary Fig. S2F and G). The quantification data from lung cross sections stained with hematoxylin and eosin (H&E) confirmed the highest number of metastatic lesions in “oscillatory ΔNp63” (Group 2) and “high ΔNp63” (Group 1) compared to “low ΔNp63” (Group 3) (Fig. 3D and E). These data indicate that expression of ΔNp63 is critical for efficient lung colonization of breast cancer cells. Surprisingly, metastases in all three groups showed high expression of ΔNp63 regardless of our experimental manipulation of ΔNp63 during the course of the study (Supplementary Fig. S2H), indicating that cancer cells expressing ΔNp63 exhibit favorable characteristics to colonize distant lungs and that only cells that escape ΔNp63 depletion are capable of colonization. Taken together, these in vivo data demonstrated that ΔNp63 is required for both extravasation and colonization of breast tumor cells at distant lungs.
To determine whether ΔNp63 regulates genes and pathways relevant to cancer progression and dissemination in our in vivo model, we injected MCF10DCIS-i-shΔNp63 cells into the mammary fat pads of nude mice and fed them with either control or dox diet. After 5 weeks, the primary tumors were collected to perform ChIP-seq analyses with ΔNp63 and RNA Polymerase II (Pol II) antibodies as described in Figure S3A. Differential binding profiles of ΔNp63 and Pol II between the MCF10DCIS-control and MCF10DCIS-shΔNp63 tumors were compared to identify ΔNp63-induced and -repressed gene signatures. Then, pathway analyses were performed. We found enrichment of pathways involved in development, cell metabolism, and cell motility, including some TGFβ/EMT-related pathways in (Supplementary Fig. S3B–C and Supplementary Table S1). This data is in accordance with previously reported roles of ΔNp63 in developmental processes and cell motility regulation (12,18,19,26–28), indicating that ΔNp63 regulates a large network of downstream target genes during cancer progression.
TGFβ inhibits expression of ΔNp63 via canonical Smad-dependent signaling in breast cancer
Given that the regulation of ΔNp63 plays pivotal roles in metastatic dissemination, we aimed to identify upstream regulators of ΔNp63 during this process. TGFβ signaling has been shown to have duplicitous roles in breast cancer development and metastasis. In normal cells and early stage tumors, TGFβ exerts its tumor suppressive functions to inhibit tumorigenesis via inducing apoptosis and cell growth arrest. On the contrary, in advanced cancer, TGFβ functions as a pro-metastatic factor by inducing EMT and promoting breast cancer cell migration and invasion, which ultimately favors metastatic dissemination (29,30). Interestingly, a previous study revealed that constitutive activation of TGFβ signaling promotes single cell invasion and intravasation in primary tumors, yet fails to facilitate tumor growth of distant metastases, whereas a localized and reversible TGFβ signaling is required for sufficient metastatic dissemination of breast cancer (31). This mechanism of action resembles our observations on how ΔNp63 exerts its function during the multistep process of metastasis, suggesting a potential regulatory network connecting TGFβ signaling and ΔNp63.
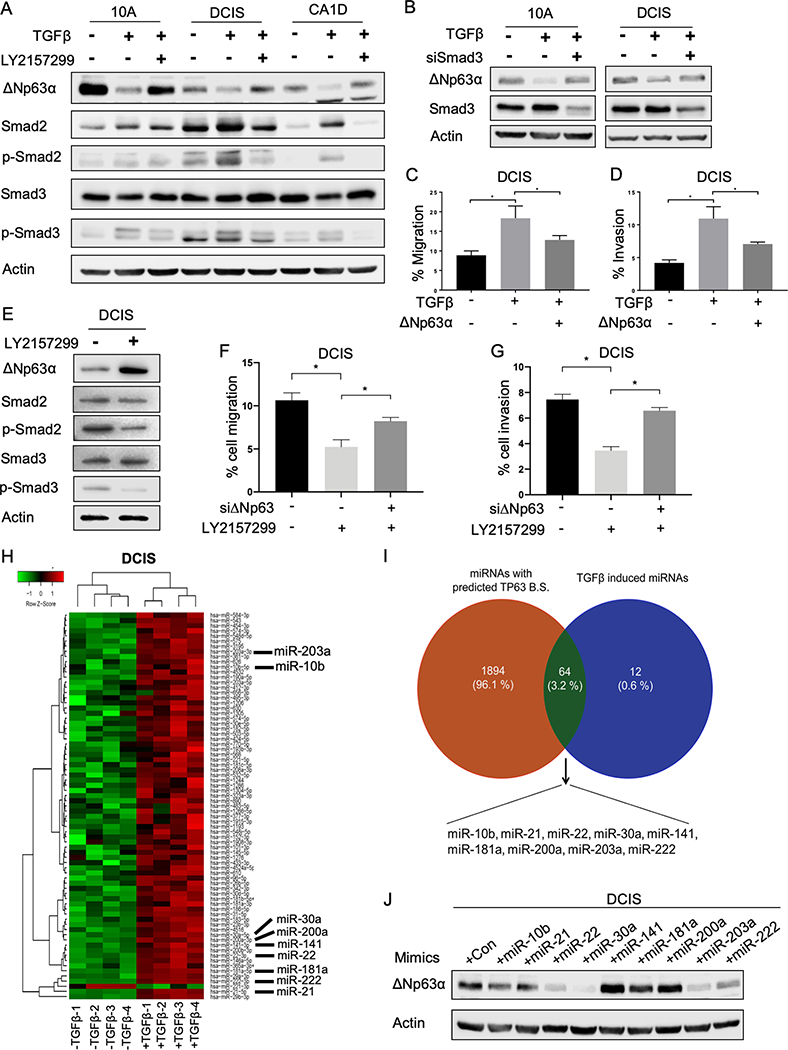
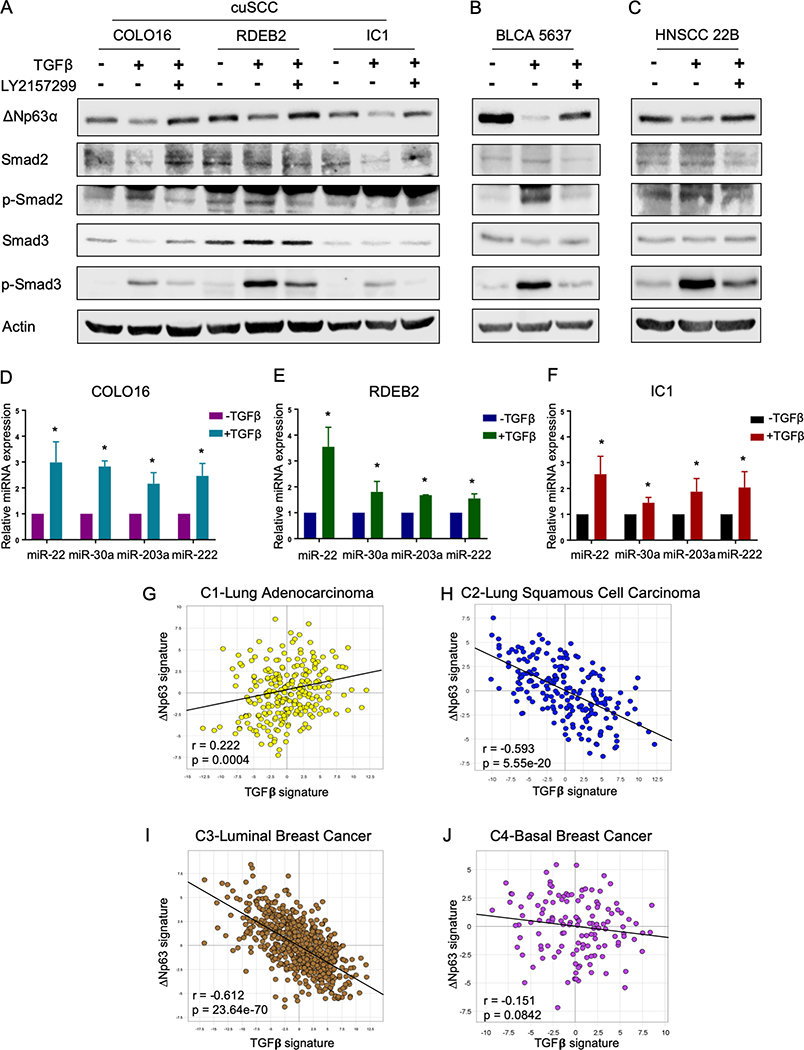
Indeed, in the presence of TGFβ, MCF10DCIS cells shared similar morphology to ΔNp63-depleted cells (Supplementary Fig. S4A). Remarkably, ΔNp63 expression was dramatically reduced when MCF10A, MCF10DCIS and MCF10CA1D cells were treated with TGFβ (Fig. 4A and Supplementary Fig. S4B). We also observed increased phosphorylation of Smad2/3 in all three cell lines upon TGFβ treatment (Fig. S4B), demonstrating the activation of TGFβ downstream signaling. To determine whether TGFβ signaling regulated ΔNp63, we treated MCF10A, MCF10DCIS, and MCFCA1D cells with LY2157299, an inhibitor of TGFβ-receptor I (TGFβRI), to inactivate the TGFβ signaling cascade. We found that the inhibitory effect of TGFβ on ΔNp63 was rescued by the addition of LY2157299 inhibitors (Fig. 4A). Additionally, the overexpression of the active form of TGFβ, but not the latent form, reduced the expression of ΔNp63 (Supplementary Fig. S4C). MCF10DCIS cells cultured in conditioned media from these cells exhibited decreased level of ΔNp63, suggesting that endogenous TGFβ secreted by tumor cells can also reduce ΔNp63 expression (Supplementary Fig. S4D). TGFβ signaling is transduced through either canonical Smad-dependent pathways or non-canonical Smad-independent pathways (32). To understand ΔNp63 regulation by TGFβ, we knocked down Smad3 in the presence of TGFβ to determine whether the regulation of TGFβ on ΔNp63 is Smad-dependent. The ablation of Smad3 in MCF10A and MCF10DCIS cells restored ΔNp63 expression (Fig. 4B), demonstrating that TGFβ reduces ΔNp63 expression via Smad2/3-dependent transcriptional activities. Moreover, while TGFβ enhanced migration and invasion in MCF10DCIS cells, the overexpression of exogenous ΔNp63α in TGFβ-treated cells largely counteracted these effects (Fig. 4C–D and Supplementary Fig. S4E). Next, we assessed whether endogenous TGFβ can modulate ΔNp63 expression similarly to exogenous TGFβ. To do this, we inhibited the activation of the signaling cascade using LY2157299 in MCF10DCIS cells, which had high level of p-Smad2/3 compared to MCF10A and MCF10CA1D (Fig. 4A), and found that inibition of the endogenous TGFβ signaling increased ΔNp63 level in these cells (Fig. 4E). In addition, downregulation of ΔNp63 in the presence of the inhibitor partially restored the cell migration and invasion ability of MCF10DCIS cells (Fig. 4F–G). Together, these findings indicate that ΔNp63 is regulated downstream of canonical Smad2/3-dependent TGFβ signaling and affects TGFβ-dependent biological processes.
Figure 4.
TGFβ inhibits expression of ΔNp63 via canonical Smad-dependent signaling in MCF10A progression model. A-B, Representative western blots of MCF-10A, DCIS and CA1D cells treated with TGFβ (10 ng/mL) or with TGFβ and the TGFBRI inhibitor, LY2157299 (1μM) for 72 hours (A) or with TGFβ and siSmad3 (B). Immunoblots were probed with the indicated antibodies. Actin was used as a loading control. C-D, Quantification of cell migration (C) and invasion (D) assay of MCF10DCIS cells overexpressing ΔNp63α in the presence of TGFβ (10 ng/mL). Asterisk indicates p < 0.05. E, Representative western blots of MCF10DCIS cells treated with or without LY2157299 (1μM) for 72 hours. Immunoblots were probed with the indicated antibodies. Actin was used as a loading control. F-G, Quantification of cell migration (F) and invasion (G) assay of MCF10DCIS cells treated with siΔNp63 and/or LY2157299 (1μM). Asterisk indicates p < 0.05. H, Heatmap of differentially expressed microRNAs between TGFβ-treated (+TGFβ −1,2,3,4) and untreated (-TGFβ −1,2,3,4) DCIS cells. I. Venn diagram showing 64 TGFβ-induced microRNAs that are predicted to bind to the 3’UTR of TP63. J, Representative western blot of DCIS cells transfected with the indicated microRNA mimics. Immunoblots were probed with the indicated antibodies. Actin was used as a loading control.
Novel TGFβ-induced microRNAs, including miR-22–3p, miR-30a-5p, miR-203a-3p and miR-222–3p, regulate expression of ΔNp63
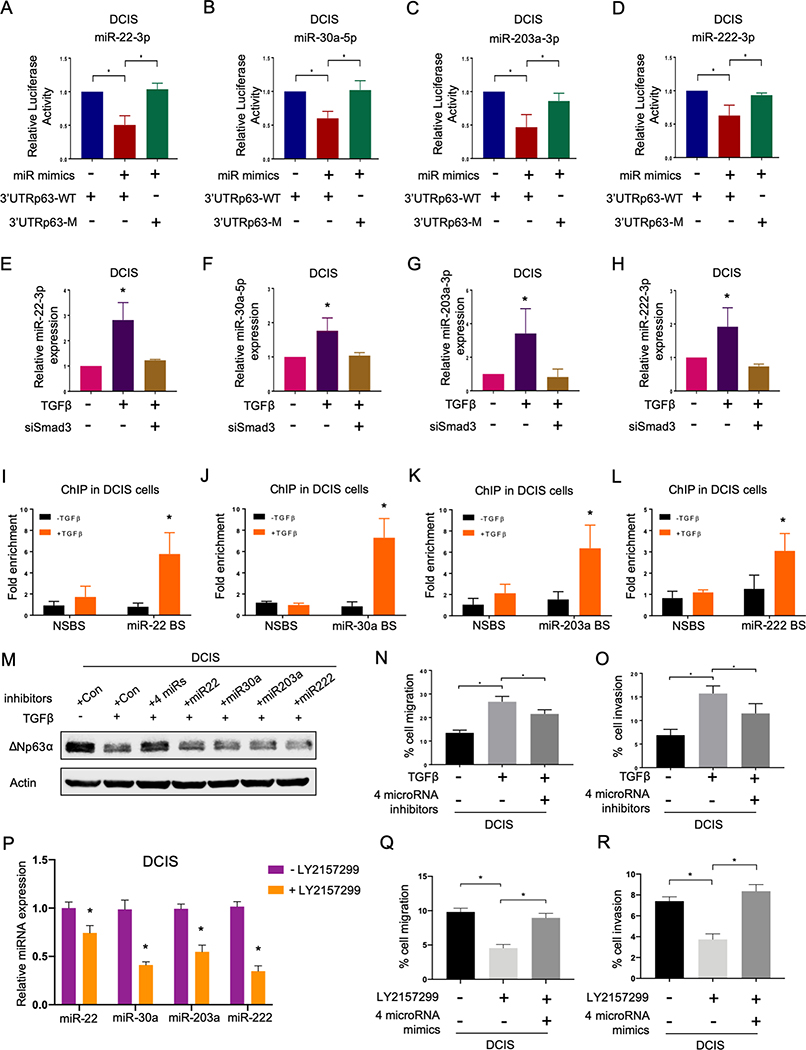
To understand whether there is a direct transcriptional repression of Smad2/3 complex on the expression of ΔNp63, we examined mRNA level of ΔNp63 upon TGFβ signaling activation. However, no significant reduction of ΔNp63 mRNA was observed in the presence of TGFβ (Supplementary Fig. S4F). This data suggested a post-transcriptional regulation of Smad2/3 on ΔNp63 expression. MicroRNAs have been intensively studied for their roles in post-transcriptional regulations of gene expression. Crosstalk between TGFβ and microRNA machinery have been reported in multiple cancers (33). Hence, we used the NanoString nCounter human microRNA platform to profile differentially expressed-microRNAs in MCF10DCIS cells upon TGFβ treatment in order to identify TGFβ-regulated microRNAs that potentially target ΔNp63. Using this method, we identified differentially expressed-microRNAs upon TGFβ treatment including several known targets of TGFβ signaling such as miR-181, miR-145 and miR-21 (33), further confirming the activation of TGFβ signaling in these cells (Fig. 4H). Our screen identified 74 up-regulated microRNAs upon TGFβ treatment (Fig. 4H and Supplementary Table S2). We then merged these identified microRNAs with a list of microRNAs predicted to have binding sites to the 3’UTR of TP63 gene using the miRWalk prediction algorithm since both TAp63 and ΔNp63 isoforms share the same 3’UTR of TP63. This approach resulted in 64 microRNAs that can potentially bind to the 3’UTR of TP63 and regulate ΔNp63 expression upon activation of TGFβ signaling (Supplementary Table S3). Among the 64 microRNAs, 9 microRNAs, including miR-10b-5p, miR-21–5p, miR-22–3p, miR-30a-5p, miR-141–3p, miR-181a-5p, miR-200a-3p, miR-203a-3p and miR-222–3p, were selected for further validation based on predicted binding affinity to the 3’UTR of TP63 and previously reported connections with breast cancer and/or p63 (Fig. 4I). We then overexpressed mimics of these 9 microRNAs and found that miR-22–3p, miR-30a-5p, miR-203a-3p and miR-222–3p downregulated ΔNp63 protein level in MCF10DCIS cells (Fig. 4J). A similar reduction in ΔNp63 expression was obtained in MCF10A and MCF10CA1D cells (Supplementary Fig. S5A). Additionally, these 4 microRNAs caused reduced luciferase activity when transfected together with the 3’UTR of ΔNp63 mRNA cloned into a luciferase reporter vector (Fig. 5A–D). We also generated mutated versions of the 3’UTR of ΔNp63 at the binding sites of miR-22–3p (Supplementary Fig. S5B), miR-30a-5p (Supplementary Fig. S5C), miR-203a-3p (Supplementary Fig. S5D) and miR-222–3p (Supplementary Fig. S5E) by replacing these binding sites on the 3’UTR with the seed sequences of the corresponding microRNAs. Mutations in the binding sites of miR-22–3p (Fig. 5A), miR-30a-5p (Fig. 5B), miR-203a-3p (Fig. 5C) and miR-222–3p (Fig. 5D) on the 3’UTR abolished microRNA-mediated repressive activities. These results indicate that these 4 TGFβ−regulated microRNAs control ΔNp63 expression in breast cancer cells and could provide a mechanism for the spatiotemporal regulation of ΔNp63.
Figure 5.
Novel TGFβ-induced microRNAs (miR-22–3p, miR-30a-5p, miR-203a-3p and miR-222–3p) regulate expression of ΔNp63. A-D, Relative luciferase expression in MCF10-DCIS cells transfected with 3’UTR of either human TP63 wild type mRNA or mutated versions of the 3’UTR at binding sites for miR-22–3p (A), miR-30a-5p (B), miR-203a-3 (C), miR-222–3p (D) and indicated microRNA mimics. Data are mean ± SD. n = 3. Asterisk indicates p <0.05. E-H, qRT-PCR of miR-22–3p (E), miR-30a-5p (F), miR-203a-3p (G) and miR-222–3p (H) in MCF10-DCIS cells treated with TGFβ (10 ng/mL) alone or TGFβ and siSmad3. Data are mean ± SD. n = 3. Asterisk indicate p < 0.05. I-L, Chromatin immunoprecipitation (ChIP) to detect binding of Smad2/3 to the promoter of miR-22–3p (I), miR-30a-5p (J), miR-203a-3p (K) and miR-222–3p (L) upon TGFβ treatment. Data are mean ± SD. n = 3. Asterisk indicates p <0.05. M, Representative immunoblots of MCF10-DCIS cells treated with TGFβ alone or TGFβ and the indicated microRNA inhibitors. Immunoblots were probed with the ΔNp63 antibody. Actin was used as a loading control. N-O, Quantification of cell migration (N) and invasion (O) assay of MCF10DCIS cells treated with a combination of 4 microRNA inhibitors for miR-22–3p, miR-30a-5p, miR-203a-3p and miR-222–3p in the presence of TGFβ (10 ng/mL). Data are mean ± SD. n = 3. Asterisk indicates p < 0.05. P, qRT-PCR of the indicated microRNAs in MCF10-DCIS cells treated with or without LY2157299 (1μM). Data are mean ± SD. n = 3. Asterisk indicates p <0.05. Q-R, Quantification of cell migration (Q) and invasion assay showing migration (Q) and invasion (R) capacity of MCF10DCIS cells treated with a combination of 4 microRNA mimics for miR-22–3p, miR-30a-5p, miR-203a-3p and miR-222–3p in the presence of LY2157299 (1μM). Data are mean ± SD. n = 3. Asterisk indicates p < 0.05.
We further determined whether these 4 microRNAs are regulated downstream of canonical TGFβ signaling. Our data showed that TGFβ treatment upregulated the expression of these 4 microRNAs in MCF10DCIS cells, whereas the addition of TGFβRI inhibitor prevented increased expression of the microRNAs triggered by TGFβ activation (Supplementary Fig. S5F–S5I). Likewise, we found that microRNA upregulation by TGFβ was rescued by knocking down Smad3 in MCF10DCIS cells (Fig. 5E–H). We then performed chromatin immunoprecipitation (ChIP) to assess Smad2/3 binding to the promoters of the 4 identified miRNAs in MCF10DCIS cells. Our results revealed that Smad2/3 indeed binds to these 4 promoter regions, demonstrating that these 4 microRNAs are direct targets of canonical Smad-dependent TGFβ signaling (Fig. 5I–L). Next, we asked whether inhibition of the microRNAs can rescue inhibitory effects of TGFβ on ΔNp63 expression. Interestingly, inhibition of all 4 microRNAs, but not any single microRNA, restored the expression of ΔNp63 in the presence of TGFβ (Fig. 5M), indicating the importance of all 4 in ΔNp63 silencing. The inhibition of these 4 microRNAs also reduced migration and invasion capacity of MCF10DCIS cells treated with TGFβ (Fig. 5N and O), thus indicating their relevance for TGFβ-dependent biological activities. We also observed a decrease in Smad3 phosphorylation level in the presence of the four microRNA inhibitors and TGFβ (Supplementary Fig. S5J), suggesting a possible feedback regulation among the four microRNAs and TGFβ signaling to modulate ΔNp63. In addition, the inhibition of endogenous TGFβ signaling by LY2157299 in MCF10DCIS cells also reduced the expression of all 4 microRNAs (Fig. 5P). The overexpression of all 4 microRNAs using microRNA mimics in the presence of LY2157299 rescued the migration and invasion capacity of MCF10DCIS cells (Fig. 5Q and R). Together, these data demonstrate that canonical TGFβ signaling modulates the expression of ΔNp63 via a network of 4 TGFβ-induced microRNAs, including miR-22–3p, miR-30a-5p, miR-203a-3p and miR-222–3p.
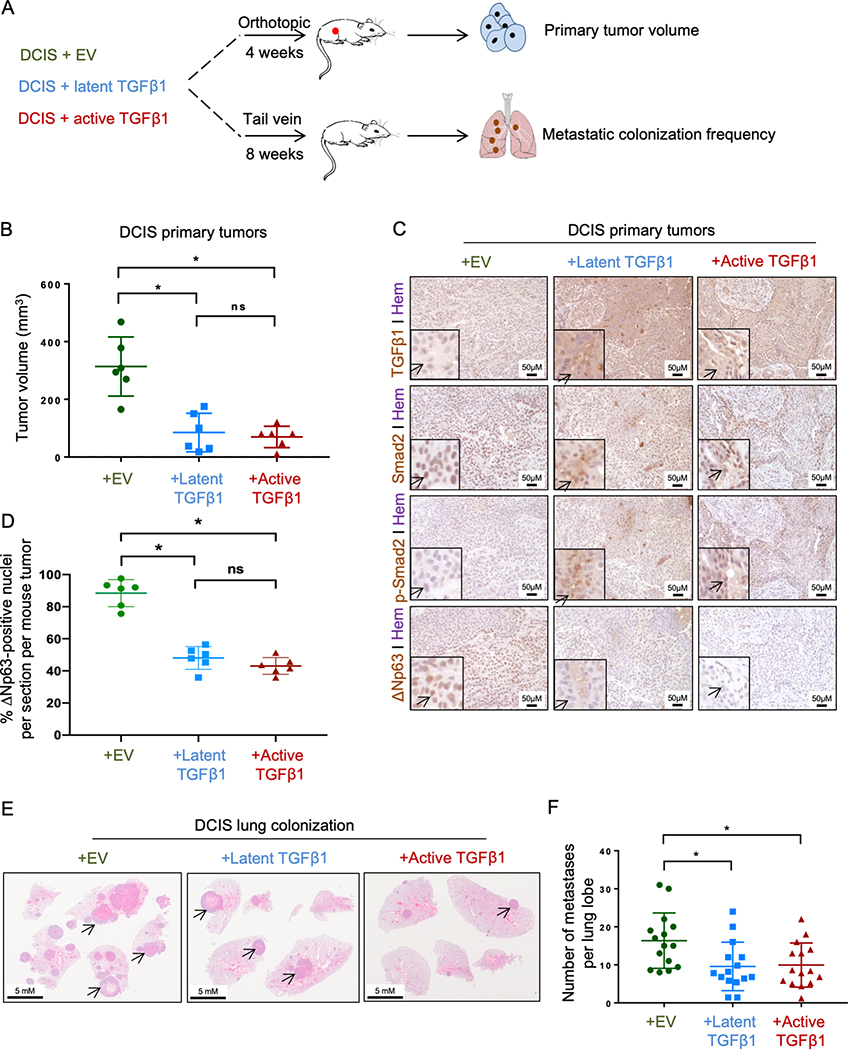
In vivo TGFβ overexpression reduces primary tumor growth and metastatic colonization
To demonstrate the in vivo effects of the TGFβ/ΔNp63 axis on primary tumor growth and lung colonization, we injected MCF10DCIS cells expressing either a latent or an active form of TGFβ1 into mammary fat pads and tail veins of nude mice (Fig. 6A). Since TGFβ is produced in a biologically inactive (or latent) form, then gets activated by various extracellular matrix components (34), and we did not observe the activation of the signaling cascade when overexpressing the latent TGFβ in vitro (Figure S4C), we wanted to examine whether the overexpression of this latent form in vivo would activate the cascade. Indeed, the overexpression of both latent and active form of TGFβ1 significantly reduced DCIS primary tumor volume (Fig. 6B). Immunostaining results confirmed high expression of TGFβ1 together with the activation of the downstream signaling and downregulation of ΔNp63 in these tumors (Fig. 6C and D). Similar to the depletion of ΔNp63 (Fig. 3D and E), TGFβ1 overexpression also hindered the formation of overt metastatic colonies in the lungs (Fig. 6E and F). Taken together, these data further emphasize the contribution of TGFβ in reducing primary tumor growth and metastatic progression in vivo through the modulation of ΔNp63.
Figure 6.
TGFβ overexpression reduces mammary adenocarcinoma primary tumor growth and metastatic lung colonization. A, Experimental design to investigate effects of TGFβ on mammary tumor development and metastatic lung colonization using orthotopic mouse models and tail vein injections. B, Primary mammary adenocarcinoma volume (mm3) of mice injected with MCF10-DCIS cells expressing either empty vector (EV), latent TGFβ1 or active TGFβ1 as described in (A). Data are mean ± SD. n = 6. Asterisk indicates p <0.05. C, Immunohistochemistry to detect TGFβ1, Smad2, p-Smad2 and ΔNp63 in cross sections of MCF10-DCIS primary tumors overexpressing TGFβ1. D, Quantification of the percentage of ΔNp63-positive cells in DCIS primary tumors in (C). E-F, Representative hematoxylin and eosin (H&E) stained cross sections of lung tissues (E) and quantification of metastases per lung lobe (F). Data are mean ± SD. n = 15. Asterisk indicates p < 0.05.
TGFβ/microRNAs axis is required for the downregulation of ΔNp63 in squamous cell carcinomas and bladder cancer
Because ΔNp63 is overexpressed in many cancers (14,15,35,36), we investigated the importance of the TGFβ/microRNAs/ΔNp63 axis in other types of cancers with high expression of ΔNp63. To do this, we treated a panel of human cancer cell lines from various sites, including cutaneous squamous cell carcinoma (cuSCC), bladder cancer (BLCA), head and neck squamous cell carcinomas (HNSCC), and lung squamous cell carcinoma (LUSC), with TGFβ and examined ΔNp63 protein expression. We found that TGFβ signaling activation decreased expression of ΔNp63 in three cuSCC lines, including COLO16, RDEB2 and IC1, one BLCA line 5637 and one HNSCC line 22B. In accordance with our previous findings, treatment with TGFβRI inhibitor, LY2157299, also restored ΔNp63 expression in these cells (Fig. 7A–C). Surprisingly, TGFβ did not exhibit inhibitory effects on ΔNp63 expression of one cuSCC line, SRB12, and one LUSC line, HCC95, despite the fact that the signaling cascade was activated indicated by the phosphorylation of Smad3 (Supplementary Fig. S6A and S6B). Notably, although ΔNp63 is a known marker for LUSC, we found that out of five LUSC cell lines ΔNp63 expression was high only in the HCC95 cell line (Supplementary Fig. S6C). This prompted us to ask whether the four microRNAs identified in breast cancer were up-regulated by TGFβ signaling in these cancer cell lines. Indeed, miR-22–3p, miR-30a-5p, miR-203a-3p and miR-222–3p expression was increased in COLO16, RDEB2 and IC1 cells treated with TGFβ (Fig. 7D–F). However, we did not observe significant induction of these 4 microRNAs in both SRB12 and HCC95 (Supplementary Fig. S6D and S6E). We further asked whether the LUSC cell lines with undetectable or low levels of ΔNp63 actually have high expression of the 4 microRNAs of our interest. Indeed, H226, H1703 and H2286 lines showed significantly higher expression of miR-22–3p, miR-30a-5p and miR-222–3p compared to HCC95 (Supplementary Fig. S6F–S6H), indicating that high levels of these microRNAs decrease expression of ΔNp63, leading to its low steady state level in these cell lines. Moreover, treatment of LY2157299 in H226, H170 and H2286 lines not only induced expression of ΔNp63 (Supplementary Fig. S6I), but decreased level of the 4 microRNAs (Supplementary Fig. S6J–L), indicating that the high level of the 4 microRNAs and low expression of ΔNp63 in these cells are TGFβ-dependent. In summary, these data indicate that elevated expression of these microRNAs is essential to modulate ΔNp63 levels downstream of TGFβ signaling cascade in various types of cancer and is critical to the spatiotemporal expression of ΔNp63.
Figure 7.
TGFβ/microRNAs axis is required for the down-regulation of ΔNp63 in other cancers including squamous cell carcinomas and bladder cancer. A-C, Representative immunoblots of cutaneous squamous cell carcinoma (cuSCC) lines COLO16, RDEB2, IC1 (A), bladder cancer cells (BLCA) 5637 (B), and head and neck squamous cell carcinoma (HNSCC) 22B (C) treated with TGFβ or with TGFβ plus TGFBRI inhibitor, LY2157299. Immunoblots were probed with the indicated antibodies. Actin was used as a loading control. D-F, qRT-PCR of the indicated microRNAs in COLO16 (D), RDEB2 (E) and IC1 (F) cells treated with or without TGFβ. Data are mean ± SD. n = 3. Asterisk indicates p <0.05. G-J, PCA analyses comparing a ΔNp63-transcriptional signature and a TGFβ-transcriptional signature in the indicated clusters, including C1-Lung Adenocarcinoma (G), C2-Lung Squamous Cell Carcinoma (H), C3-Luminal Breast Cancer (I) and C4-Basal Breast Cancer (J) subtypes.
Bioinformatic analyses revealed an anti-correlation between TGFβ-driven transcriptional signature and ΔNp63-driven transcriptional signature in different cancer molecular subtypes
Our results showing that TGFβ signaling affects ΔNp63 in cell lines of different origin, including breast and lung cancer, prompted us to determine whether the crosstalk between TGFβ and ΔNp63 is also occurring in tumors of breast and lung cancer patients. Therefore, we utilized a TGFβ signature (37) and a ΔNp63 signature (21) as readouts of the activity of TGFβ and ΔNp63, respectively, to analyze four clusters of TGCA datasets (36): C1 (enriched in LUAD – lung adenocarcinomas), C2 (enriched in LUSC – lung squamous cell carcinomas), C3 (enriched in luminal-type breast cancers), and C4 (enriched in basal-type breast cancers). Principal component analysis (PCA) was performed using the TGFβ and ΔNp63 signatures in each of these four clusters. Notably, in C2 (LUSC) but not in C1 (LUAD) there is a negative correlation between the two signatures (Fig. 7G and H and Supplementary Table S4). This is in line with ΔNp63 being a crucial marker in LUSC (38) and with our data showing that ΔNp63 levels increase in LUSC cell lines if TGFβ signaling is inhibited (Supplementary Fig. S6I). In the case of the two breast cancer clusters, there is a negative correlation between the TGFβ and ΔNp63 signatures in C3 (luminal subtype) but not in C4 (basal subtype) (Fig. 7I and J and Supplementary Table S4). This suggests that the control of ΔNp63 by TGFβ could be more relevant in breast cancers with low levels of ΔNp63 (luminal subtype) than in those with high levels of ΔNp63 (basal subtype) (39–42). In summary, these data demonstate a negative correlation between TGFβ and ΔNp63 signatures in subtypes of breast and lung tumors, further providing evidence for the regulatory axis of TGFβ/ΔNp63 in human cancers.
DISCUSSION
Given its pivotal roles in the maintenance and proliferation of epithelial cells, ΔNp63 is highly expressed in primary tumors and metastases across multiple epithelial cancers (35,36,43). Work from our laboratory and others have unveiled tumor-promoting activities of ΔNp63 in various cancer types (9,10,21). However, the suppressive function of ΔNp63 on the cell adhesion and motility through regulation of different transcription factors and microRNAs has been demonstrated in multiple cell lines and cancers (11–13,18,19,44), indicating tumor suppressive activities of ΔNp63 in cell migration and invasion. In this study, we found that ΔNp63 expression is essential for breast primary tumor development, consistent with its role as an oncogene in multiple cancers, and that this is achieved via the ΔNp63-dependent regulation of numerous pathways, including in development, metabolism, signal transduction, and TGFβ-regulated epithelial to mesenchymal transition (EMT). More interestingly, our in vivo data demonstrated an indispensable role of ΔNp63 for circulating tumor cells to extravasate and establish new colonies at distant organs. This is in line with the fact that ΔNp63 expression is crucial for the maintenance of epithelial characteristics and that the reversion to epithelial state of circulating tumor cells arrested at distant organs is important for cell proliferation and establishment of metastatic lesions (16,45). Importantly, we showed that an oscillatory expression of ΔNp63 is essential for efficient metastatic progression of breast cancer both in genetically engineered orthotopic mouse models and in breast cancer patients. This dynamic expression of ΔNp63 is modulated by TGFβ and a novel network of four TGFβ-regulated microRNAs in various ΔNp63-expressing cancers. Together, our findings support a model in which spatiotemporal regulation of ΔNp63 and its oscillatory expression is crucial for metastasis.
The overexpression of p63/ΔNp63 has been implicated as a molecular feature of various squamous cell carcinomas (SCCs) (35,36). The most common mechanistic explanation for high p63 in these cancers is the amplification of chromosome 3q (35). Here, we provide a detailed mechanism through canonical TGFβ signaling and its microRNA targets for the regulation of ΔNp63. Previously, multiple studies have focused on characterizing molecular pathways downstream of ΔNp63 that contribute to cancer progression and metastasis (9,13,18,19,44,46,47). In this study, we aimed to elucidate the regulatory network upstream of ΔNp63 and emphasize its importance in cancers. Our work demonstrated that TGFβ signaling represses ΔNp63 expression at the post-transcriptional level in both normal mammary epithelial cells and breast cancer cells via canonical Smad3-dependent pathway and downstream miRNAs. Activated TGFβ signaling has been strongly associated with increased metastatic dissemination via induction of an EMT program in tumor cells (30,32). Yet, impaired TGFβ signaling cascade also resulted in enhanced metastasis (48,49). TGFβ has also been shown to function as a molecular switch between collective and single cell invasion programs in breast cancer (31), further emphasizing pleiotropic roles of TGFβ in metastatic dissemination, which is similar to our proposed model on the contributions of ΔNp63 in metastasis. Of note, TGFβ has also been previously reported to have a positive regulatory effect on ΔNp63 (50,51), even though these studies were performed in other tumor types (51) or did not distinguish between the different p63 isoforms (50), which have opposing effects on cell motility and cancer metastasis (19,46,52).
The four TGFβ-induced microRNAs identified as upstream regulators of ΔNp63, including miR-22–3p, miR-30a-5p, miR-203a-3p and miR-222–3p, exhibit inhibitory effects on the protein expression of ΔNp63. Interestingly, the inhibition of 4 microRNAs, but not any single microRNA, rescued the inhibitory effects of TGFβ on ΔNp63 expression, suggesting cooperative functions of those microRNAs in modulating ΔNp63 downstream of TGFβ signaling. This TGFβ/microRNAs/ΔNp63 axis was further validated in several cancers that highly express ΔNp63, such as bladder cancers, cutaneous and lung SCCs. Among the four microRNAs, miR-203a-3p is a known modulator of ΔNp63 during terminal differentiation of the epidermis (53). The identification of the negative regulatory effect of this microRNA on ΔNp63 downstream of TGFβ signaling indicates its consistent role as a regulator of ΔNp63 across different cell types. In breast cancer, the roles of miR-203 are not clear. Studies have shown its function as either a suppressor or promoter of breast cancers (54,55), suggesting a perplexing role of this microRNA in breast cancer. Similarly, tumor-promoting and tumor-suppressive roles of miR-22–3p have also been documented (56,57). miR-30a-5p, on the other hand, has been consistently demonstrated to be a suppressor of breast cancer cell growth and metastasis (58,59). Conversely, miR-222–3p has been implicated as oncogenes in breast cancer metastasis by promoting EMT in basal-like breast cancer (60,61). These contradictory findings indicate a more intricate regulatory network involving these microRNAs in breast cancer and can be due to context dependent effects likely regulated by TGFβ and further by ΔNp63.
Taken together, our data have unveiled oscillatory expression of ΔNp63 during breast cancer progression. Moreover, we identified a novel regulatory mechanism for ΔNp63 downstream of TGFβ signaling and miRNAs in modulating metastatic dissemination. Our data provide key information of the upstream regulation of ΔNp63 oscillatory expression during breast cancer metastasis that is key to designing ΔNp63-targeted therapies to treat cancer.
Supplementary Material
SIGNIFICANCE.
This study unveils TGFβ signaling and a network of 4 microRNAs as upstream regulators of ΔNp63, providing key information for the development of therapeutic strategies to treat cancers that commonly overexpress ΔNp63.
Acknowledgements
We thank X. Su, R. Checker, X. Li and I. Grammatikakis for technical advice, J. Yao for the analysis of the NanoString data and the ChIP-seq data, and E. A. Welsh for the PCA analysis of the TCGA datasets. This work has been supported in part by the Molecular Genomics Core, the Flow Cytometry Core, the Biostatistics and Bioinformatics Core, the Vivarium services Core, the Analytic Microscopy Core and the Tissue Core at Moffitt Cancer Center, an NCI-designated Comprehensive Cancer Center (P30-CA076292).
Financial Support: This work was supported by R35CA197452 to E.R.F. E.R.F. is a National Cancer Institute Outstanding Investigator, Moffitt Distinguished Scholar, and Scholar of the Leukemia and Lymphoma Society, the Rita Allen Foundation, and the V Foundation for Cancer Research. N.H.B.B. is a Vietnam Education Foundation Fellow and a recipient of the Andrew Sowell-Wade Huggins Scholarship in Cancer Research.
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest.
REFERENCES
- 1.Su X, Chakravarti D, Flores ER. p63 steps into the limelight: crucial roles in the suppression of tumorigenesis and metastasis. Nat Rev Cancer 2013;13:136–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakravarti D, Su X, Cho MS, Bui NH, Coarfa C, Venkatanarayan A, et al. Induced multipotency in adult keratinocytes through down-regulation of DeltaNp63 or DGCR8. Proc Natl Acad Sci U S A 2014;111:E572–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchini S, Marabese M, Marrazzo E, Mariani P, Cattaneo D, Fossati R, et al. DeltaNp63 expression is associated with poor survival in ovarian cancer. Ann Oncol 2008;19:501–7 [DOI] [PubMed] [Google Scholar]
- 4.Crook T, Nicholls JM, Brooks L, O’Nions J, Allday MJ. High level expression of deltaN-p63: a mechanism for the inactivation of p53 in undifferentiated nasopharyngeal carcinoma (NPC)? Oncogene 2000;19:3439–44 [DOI] [PubMed] [Google Scholar]
- 5.Flores ER. The roles of p63 in cancer. Cell Cycle 2007;6:300–4 [DOI] [PubMed] [Google Scholar]
- 6.Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer cell 2014;25:152–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, et al. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 1998;2:305–16 [DOI] [PubMed] [Google Scholar]
- 8.Flores ER, Tsai KY, Crowley D, Sengupta S, Yang A, McKeon F, et al. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature 2002;416:560–4 [DOI] [PubMed] [Google Scholar]
- 9.Napoli M, Venkatanarayan A, Raulji P, Meyers BA, Norton W, Mangala LS, et al. DeltaNp63/DGCR8-Dependent MicroRNAs Mediate Therapeutic Efficacy of HDAC Inhibitors in Cancer. Cancer cell 2016;29:874–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkatanarayan A, Raulji P, Norton W, Chakravarti D, Coarfa C, Su X, et al. IAPP-driven metabolic reprogramming induces regression of p53-deficient tumours in vivo. Nature 2015;517:626–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbieri CE, Tang LJ, Brown KA, Pietenpol JA. Loss of p63 leads to increased cell migration and up-regulation of genes involved in invasion and metastasis. Cancer Res 2006;66:7589–97 [DOI] [PubMed] [Google Scholar]
- 12.Olsen JR, Oyan AM, Rostad K, Hellem MR, Liu J, Li L, et al. p63 attenuates epithelial to mesenchymal potential in an experimental prostate cell model. PLoS One 2013;8:e62547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao W, Wang H, Han X, Ma J, Zhou Y, Chen Z, et al. DeltaNp63alpha attenuates tumor aggressiveness by suppressing miR-205/ZEB1-mediated epithelial-mesenchymal transition in cervical squamous cell carcinoma. Tumour Biol 2016 [DOI] [PubMed] [Google Scholar]
- 14.Kanitakis J, Chouvet B. Expression of p63 in cutaneous metastases. Am J Clin Pathol 2007;128:753–8 [DOI] [PubMed] [Google Scholar]
- 15.Reis-Filho JS, Torio B, Albergaria A, Schmitt FC. p63 expression in normal skin and usual cutaneous carcinomas. J Cutan Pathol 2002;29:517–23 [DOI] [PubMed] [Google Scholar]
- 16.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell 2011;147:275–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noszczyk BH, Majewski ST. p63 expression during normal cutaneous wound healing in humans. Plast Reconstr Surg 2001;108:1242–7; discussion 8–50 [DOI] [PubMed] [Google Scholar]
- 18.Tucci P, Agostini M, Grespi F, Markert EK, Terrinoni A, Vousden KH, et al. Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc Natl Acad Sci U S A 2012;109:15312–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran MN, Choi W, Wszolek MF, Navai N, Lee IL, Nitti G, et al. The p63 protein isoform DeltaNp63alpha inhibits epithelial-mesenchymal transition in human bladder cancer cells: role of MIR-205. J Biol Chem 2013;288:3275–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer cell 2012;22:725–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbas HA, Bui NHB, Rajapakshe K, Wong J, Gunaratne P, Tsai KY, et al. Distinct TP63 Isoform-Driven Transcriptional Signatures Predict Tumor Progression and Clinical Outcomes. Cancer Res 2018;78:451–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santner SJ, Dawson PJ, Tait L, Soule HD, Eliason J, Mohamed AN, et al. Malignant MCF10CA1 cell lines derived from premalignant human breast epithelial MCF10AT cells. Breast Cancer Res Treat 2001;65:101–10 [DOI] [PubMed] [Google Scholar]
- 23.Miller FR, Santner SJ, Tait L, Dawson PJ. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J Natl Cancer Inst 2000;92:1185–6 [DOI] [PubMed] [Google Scholar]
- 24.Miller FR, Soule HD, Tait L, Pauley RJ, Wolman SR, Dawson PJ, et al. Xenograft model of progressive human proliferative breast disease. J Natl Cancer Inst 1993;85:1725–32 [DOI] [PubMed] [Google Scholar]
- 25.Kang Y, Pantel K. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer cell 2013;23:573–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 1999;398:708–13 [DOI] [PubMed] [Google Scholar]
- 27.Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev 2004;18:126–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carroll DK, Carroll JS, Leong CO, Cheng F, Brown M, Mills AA, et al. p63 regulates an adhesion programme and cell survival in epithelial cells. Nat Cell Biol 2006;8:551–61 [DOI] [PubMed] [Google Scholar]
- 29.Lebrun JJ. The Dual Role of TGFbeta in Human Cancer: From Tumor Suppression to Cancer Metastasis. ISRN Mol Biol 2012;2012:381428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colak S, Ten Dijke P. Targeting TGF-beta Signaling in Cancer. Trends Cancer 2017;3:56–71 [DOI] [PubMed] [Google Scholar]
- 31.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol 2009;11:1287–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer 2010;10:415–24 [DOI] [PubMed] [Google Scholar]
- 33.Butz H, Racz K, Hunyady L, Patocs A. Crosstalk between TGF-beta signaling and the microRNA machinery. Trends Pharmacol Sci 2012;33:382–93 [DOI] [PubMed] [Google Scholar]
- 34.Ballesteros A, Mentink-Kane MM, Warren J, Kaplan GG, Dveksler GS. Induction and activation of latent transforming growth factor-beta1 are carried out by two distinct domains of pregnancy-specific glycoprotein 1 (PSG1). J Biol Chem 2015;290:4422–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell JD, Yau C, Bowlby R, Liu Y, Brennan K, Fan H, et al. Genomic, Pathway Network, and Immunologic Features Distinguishing Squamous Carcinomas. Cell Rep 2018;23:194–212 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoadley KA, Yau C, Wolf DM, Cherniack AD, Tamborero D, Ng S, et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell 2014;158:929–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordian E, Welsh EA, Gimbrone N, Siegel EM, Shibata D, Creelan BC, et al. Transforming growth factor beta-induced epithelial-to-mesenchymal signature predicts metastasis-free survival in non-small cell lung cancer. Oncotarget 2019;10:810–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cancer Genome Atlas Research N. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 2004;10:5367–74 [DOI] [PubMed] [Google Scholar]
- 40.Badowska-Kozakiewicz AM, Budzik MP. Immunohistochemical characteristics of basal-like breast cancer. Contemp Oncol (Pozn) 2016;20:436–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuroda N, Ohara M, Inoue K, Mizuno K, Fujishima N, Hamaguchi N, et al. The majority of triple-negative breast cancer may correspond to basal-like carcinoma, but triple-negative breast cancer is not identical to basal-like carcinoma. Med Mol Morphol 2009;42:128–31 [DOI] [PubMed] [Google Scholar]
- 42.Kamarlis RK, Lubis MN, Hernowo BS, Kar AS. Immunoexpression of P63 and SOX2 in triple-negative breast cancers, Indonesia. F1000Res 2017;6:1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lo Muzio L, Santarelli A, Caltabiano R, Rubini C, Pieramici T, Trevisiol L, et al. p63 overexpression associates with poor prognosis in head and neck squamous cell carcinoma. Hum Pathol 2005;36:187–94 [DOI] [PubMed] [Google Scholar]
- 44.Urist MJ, Di Como CJ, Lu ML, Charytonowicz E, Verbel D, Crum CP, et al. Loss of p63 expression is associated with tumor progression in bladder cancer. Am J Pathol 2002;161:1199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging Biological Principles of Metastasis. Cell 2017;168:670–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergholz J, Zhang Y, Wu J, Meng L, Walsh EM, Rai A, et al. DeltaNp63alpha regulates Erk signaling via MKP3 to inhibit cancer metastasis. Oncogene 2014;33:212–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Franco S, Turdo A, Benfante A, Colorito ML, Gaggianesi M, Apuzzo T, et al. DeltaNp63 drives metastasis in breast cancer cells via PI3K/CD44v6 axis. Oncotarget 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu SL, Herrington H, Reh D, Weber S, Bornstein S, Wang D, et al. Loss of transforming growth factor-beta type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev 2006;20:1331–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forrester E, Chytil A, Bierie B, Aakre M, Gorska AE, Sharif-Afshar AR, et al. Effect of conditional knockout of the type II TGF-beta receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res 2005;65:2296–302 [DOI] [PubMed] [Google Scholar]
- 50.Vasilaki E, Morikawa M, Koinuma D, Mizutani A, Hirano Y, Ehata S, et al. Ras and TGF-beta signaling enhance cancer progression by promoting the DeltaNp63 transcriptional program. Sci Signal 2016;9:ra84. [DOI] [PubMed] [Google Scholar]
- 51.Hu L, Liu J, Li Z, Wang C, Nawshad A. Transforming growth factor-beta1 activates DeltaNp63/c-Myc to promote oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol 2016;122:460–82 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su X, Chakravarti D, Cho MS, Liu L, Gi YJ, Lin YL, et al. TAp63 suppresses metastasis through coordinate regulation of Dicer and miRNAs. Nature 2010;467:986–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature 2008;452:225–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He S, Zhang G, Dong H, Ma M, Sun Q. miR-203 facilitates tumor growth and metastasis by targeting fibroblast growth factor 2 in breast cancer. Onco Targets Ther 2016;9:6203–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao S, Han J, Zheng L, Yang Z, Zhao L, Lv Y. MicroRNA-203 Regulates Growth and Metastasis of Breast Cancer. Cell Physiol Biochem 2015;37:35–42 [DOI] [PubMed] [Google Scholar]
- 56.Xu D, Takeshita F, Hino Y, Fukunaga S, Kudo Y, Tamaki A, et al. miR-22 represses cancer progression by inducing cellular senescence. J Cell Biol 2011;193:409–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song SJ, Poliseno L, Song MS, Ala U, Webster K, Ng C, et al. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell 2013;154:311–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fu J, Xu X, Kang L, Zhou L, Wang S, Lu J, et al. miR-30a suppresses breast cancer cell proliferation and migration by targeting Eya2. Biochem Biophys Res Commun 2014;445:314–9 [DOI] [PubMed] [Google Scholar]
- 59.Li L, Kang L, Zhao W, Feng Y, Liu W, Wang T, et al. miR-30a-5p suppresses breast tumor growth and metastasis through inhibition of LDHA-mediated Warburg effect. Cancer Lett 2017;400:89–98 [DOI] [PubMed] [Google Scholar]
- 60.Stinson S, Lackner MR, Adai AT, Yu N, Kim HJ, O’Brien C, et al. TRPS1 targeting by miR-221/222 promotes the epithelial-to-mesenchymal transition in breast cancer. Sci Signal 2011;4:ra41. [DOI] [PubMed] [Google Scholar]
- 61.Shah MY, Calin GA. MicroRNAs miR-221 and miR-222: a new level of regulation in aggressive breast cancer. Genome Med 2011;3:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The NanoString human microRNA data and the ChIP-seq data were deposited to NCBI Gene Expression Omnibus (GEO) repository: GSE134681 and GSE144995.