ABSTRACT
Blood culture negative endocarditis (BCNE) accounts for up to 20% of infective endocarditis. While the most common cause of BCNE remains the initiation of antibiotics prior to culture, intracellular organisms such as Coxiella and Bartonella spp account for a significant proportion of cases. Identifying the infecting organism remains important to ensure optimal antimicrobial treatment. However, these organisms can be difficult to diagnose. We outline a systematic approach to BCNE. Over half of patients with infective endocarditis now undergo early surgery and 16S ribosomal ribonucleic acid (rRNA) polymerase chain reaction (PCR) of excised tissue can be vitally important to secure a diagnosis. Molecular testing is likely to become a key tool in improving outcomes from BCNE and contribute to an improved understanding of the aetiology. We advocate modifying the Duke criteria to incorporate organisms identified on molecular testing, including 16S rRNA PCR, in particular from explanted tissue.
KEYWORDS: Endocarditis, culture negative, 16S rRNA, PCR, Bartonella henselae
Case presentation
A 34-year-old man who worked as a delivery driver and was usually fit and well, was admitted with a two-week history of breathlessness on exertion and a dry cough. He was afebrile, had signs of severe aortic regurgitation and pulmonary congestion. There were no peripheral stigmata of endocarditis. Blood tests showed white blood cells 7.2×109/L, C-reactive protein 33 mg/L, haemoglobin 92 g/L and creatinine 151 μmol/L. He was started on diuretics and transthoracic echocardiography demonstrated a bicuspid aortic valve with severe aortic regurgitation and several vegetations up to 1.8 cm in length. The patient underwent an urgent aortic valve replacement on haemodynamic grounds.
Several sets of peripheral blood cultures were negative. There was no history of antibiotic administration in the community. The patient was treated with high dose intravenous amoxicillin and gentamicin and clinically improved. Coxiella burnetii serology was negative and the patient was negative for HIV infection. Currently there are no serological tests for Bartonella spp in the UK. A diagnosis of blood culture negative endocarditis was made and the excised valve tissue was referred for molecular sequencing. 16S ribosomal ribonucleic acid (rRNA) bacterial polymerase chain reaction (PCR) analysis was positive for Bartonella henselae, the causative pathogen of cat scratch disease. The antibiotic regimen was changed to doxycycline 100 mg twice daily and gentamicin. This regimen has increased efficacy in treating Bartonella spp endocarditis and is advised by the European Society of Cardiology guidelines.1 The patient was discharged on oral doxycycline to complete treatment and made a full recovery. We discovered that the patient's partner worked for a cat rescue centre and occasionally they would care for abandoned cats at home.
Bartonella spp are a recognised cause of blood culture negative endocarditis (BCNE).2,3 Although six Bartonella spp are known to cause infective endocarditis in humans, Bartonella hensalae and Bartonella quintana (transmitted by human body lice and historically called ‘trench fever’, now increasingly found in the homeless population) account for 95% of cases.4–6 Bartonella is increasingly being recognised as a cause of endocarditis in the UK.7 Most patients have pre-existing valvular disease as in this case and infection with Bartonella spp typically results in destructive endocarditis with a high incidence of valve replacement, as high as 80% in one series.3,4,6,8 This case highlights the importance of history taking even in the modern era as being scratched by a cat, or coming into contact with cat fleas are important risk factors for Bartonella hensalae infection.3,4,8–10 The serodiagnostic service for Bartonella was withdrawn in the UK in 2015 following a review by Public Health England (personal communication with Dr Colin Brown, Reference Lab 2019). At least one large centre sends blood samples for serology to a French research laboratory, as they see a relatively large number of cases of Bartonella spp in the homeless population. The contemporary diagnosis of Bartonella endocarditis in the UK is made predominantly through molecular sequencing of infected tissue or from culture. The latter being lengthy, difficult and thus rarely performed. Bartonella specific PCR has a high sensitivity from valve tissue of up to 92%.11
Introduction
Despite advances in medicine endocarditis continues to be associated with significant morbidity and mortality. Timely diagnosis for a condition with protean presentation remains challenging. Delayed diagnosis prevents active management with optimal antimicrobial therapy, early involvement from a multidisciplinary endocarditis team and individualised patient management including early surgery which have all been shown to significantly improve outcomes.12
Most cases of endocarditis are caused by bacterial infection and usually the diagnosis is made by culture-dependent methods. Uncertainty about the causative organism may result in inadequate treatment, exposure of the patient to potentially toxic empirical treatment and ultimately affect outcome. A systematic approach to BCNE is needed.
Modern practice, using conventional automated blood culture systems and conventional media subculture for the standard duration (7 days in endocarditis), will yield organisms historically perceived as fastidious or slow-growing including the HACEK (Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, Kingella) group organisms, nutritionally variant Streptococci and Candida species.13,14
Prevalence and aetiology
Despite there being a near constant bacteraemia before initiation of appropriate antibiotics, approximately 12–20% patients remain culture negative with no growth after 7 days incubation.15,16 The prevalence of BCNE varies in the literature, largely reflecting the populations investigated and in particular, whether cases where prior antibiotics were administered are included.
It is widely recognised that the most common cause of BCNE is the initiation of antibiotics prior to culture. In one UK series, looking specifically at patients with BCNE found, ultimately, to have Gram-positive cocci causing infection, two-thirds had received antibiotics prior to culture.16
True BCNE is caused by infection with a variety of bacteria including Coxiella burnetii (Q fever), Bartonella spp, Brucella spp, Tropheryma whipplei, Mycobacteria species and non-candidal fungi (Table 1). These bacteria are either intracellular organisms, or are unlikely to grow on conventional media. Many of the true BCNE organisms are associated with specific risk factors or exposures and careful history taking is of paramount importance (Table 2). Non-infective thrombotic endocarditis is rare, accounting for 2.2% of BCNE in one French study from a BCNE reference centre and is associated with certain cancers and autoimmune diseases such as systemic lupus erythematosus (Table 2).17
Table 1.
Causes of blood culture negative endocarditis
| Antibiotics given prior to taking blood cultures | True BCNE | Non-infective BCNE |
|---|---|---|
|
Coxiella Bartonella spp Brucella Tropheryma whipplei Mycobacteria spp (M chimera following cardiac surgery) Fungi (Aspergillus) May be diagnosed by:
|
Behçet's disease Systemic lupis erythematosus (Libman–Sacks endocarditis) Marantic (breast/lung/prostate/colon cancer) |
BCNE = blood culture negative endocarditis.
Table 2.
Pathogens causing blood culture negative endocarditis
| Organism | Risk factors for infection |
|---|---|
|
Coxiella burnetii (intracellular) 28–37% of BCNE15,17 |
Occupational exposure to farm animals (sheep, cattle, goats)18 Living downwind from farms and infected farm material (straw manure) Abattoir workers Laboratory exposure to pathogens Immunosuppression possibly including HIV19 |
| Bartonella henselae (intracellular) | Contact with cats (higher bacteraemia in kittens)20 |
|
Bartonella quintana (intracellular) Bartonella spp collectively 12–28% of BCNE15,17 |
Contact with human body lice / homeless shelters Chronic alcoholism Travel to north Africa |
|
Brucella (fastidious Gram-negative bacilli) Only in endemic areas |
Occupational exposure to farm animals (sheep, cattle, goats) Ingesting unpasteurised milk/cheese / undercooked meat Travel to Middle East |
| Fungi 1–2% of BCNE15,17 |
HIV positive Indwelling venous catheter Prior cardiac surgery |
|
Tropheryma whipplei Up to 6% of BCNE15,17,21 |
Occupational exposure to soil / farm animals |
| Tuberculosis and other mycobacteria | Tuberculosis contact or exposure Mycobacterium chimera following cardiac surgery |
BCNE = blood culture negative endocarditis.
Identification of the causative organism in BCNE allows refinement of empirical antibiotic regimens. Standard empirical treatment for BCNE risks exposure of the patient to unnecessary toxicity and sub-optimal antimicrobial treatment.
Presentation
Evidence suggests that culture positive and culture negative endocarditis present similarly although heart failure is more prevalent (likely reflecting the delay in diagnosis) and overt sepsis less common in BCNE as in the case presented.17,22,23 Echocardiography is less likely to be diagnostic in some BCNE infections eg cases of Coxiella burnetii endocarditis, where vegetations are often small.22,24,25
Identifying the causative organism in BCNE
We suggest the following pragmatic approach to determining the causative organism, in line with current guidelines and evidence (Fig 1). History taking may identify recognised host and epidemiological factors associated with different aetiologies of BCNE (Table 2). Determining these factors, including immune status eg HIV status informs further diagnostic testing.
Fig 1.
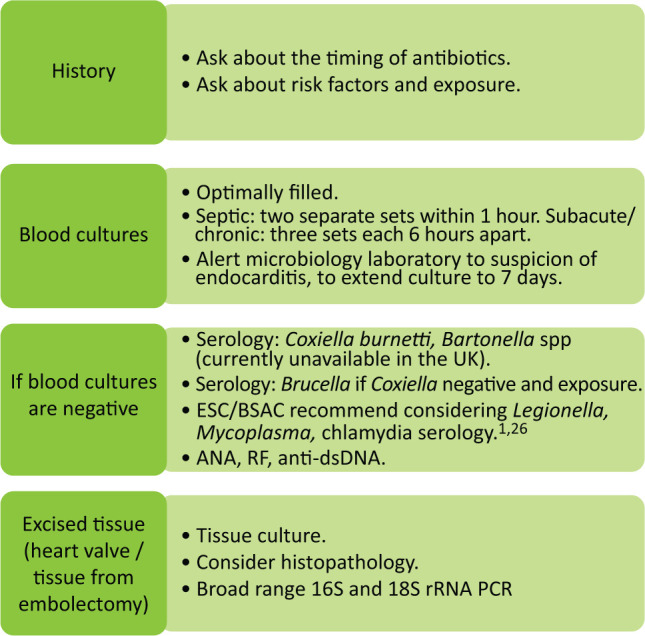
A pragmatic approach to determining the causative organism in suspected endocarditis. ANA = anti-nuclear antibody; anti-dsDNA = anti-double-stranded deoxyribonucleic acid; BSAC = British Society for Antimicrobial Chemotherapy; ESC = European Society of Cardiology; PCR = polymerase chain reaction; RF rheumatoid factor; rRNA = ribosomal ribonucleic acid.
Serology
Serology for Coxiella burnetii is first line in the investigation of BCNE; Coxiella phase 1 immunoglobulin G (IgG) ≥800 is diagnostic of Coxiella burnetii infection and qualifies as a major Duke criterion.27 Although Bartonella spp IgG ≥800 is diagnostic for Bartonella spp infection, serological testing as stated is not currently available in the UK.28 In countries where serology is available, it should be recognised that cross-reactivity occurs between Bartonella spp and Coxiella burnetii and it is notoriously difficult to interpret serology in this setting.14
The British Society for Antimicrobial Chemotherapy (BSAC) advise considering Brucella serology in the setting of dietary or travel-related exposure (Table 2).26 Mycoplasma, Legionella and Mycobacteria endocarditis are exceptionally rare.17,29 Routine serological testing for Legionella, Mycoplasma and Chlamydia spp is not recommended.26 Previous serological Chlamydia spp diagnoses may reflect cross-reactivity with Bartonella spp (with no valve positives to date).29,30 In two large studies evaluating a range of serological tests in BCNE, 624 patients were diagnosed by serology (from a total of 1,093), and only four cases BCNE would have been missed if serology testing had been restricted to testing for Coxiella burnetii and Bartonella spp only.15,17,31
Examination of explanted tissue
Explanted valve tissue or embolectomy material is routinely sent for microscopy and culture, in part to guide duration of postoperative therapy (with a longer duration of postoperative antibiotic therapy recommended if the organism is grown from the valve).32 Histopathological examination of the valve can be valuable. Specific culture media or histopathological stains can be used to look for Mycobacteria spp (alcohol and acid-fast bacilli), Tropheryma whipplei (periodic acid-Schiff positive macrophages), fungi (hyphae / silver stain) and Bartonella spp (Warthin–Starry stain).14
Molecular
Serum testing with PCR is not recommended due to low sensitivity and no additional cases of infection were diagnosed following initial serology in a large study.17
Molecular techniques to diagnose endocarditis from explanted tissue have been available for over 20 years and are becoming increasingly important in the diagnostic workup of endocarditis.30,33 Currently they remain excluded from the modified Duke criteria. One molecular technique, the pan-bacterial 16S rRNA PCR, uses PCR on homogenised tissue to amplify bacterial genetic material from the 16S rRNA. This genetic material is conserved in all bacteria and hypervariable segments can allow for further identification of bacteria down to a species level. The technique is highly sensitive and specific for identifying the causative organism although clinical correlation is required and it is important to avoid cross-contamination of tissue. Microbial deoxyribonucleic acid can persist for months following infection and the presence of bacteria from PCR analysis does not necessarily imply ongoing infection.
The technique detects the causative organism in the majority of BCNE cases and represents a major advance in the management of cases of endocarditis where antibiotics were given prior to culture, in patients with equivocal serological results, in cases where culture and serology have been negative or, as in the case illustrated, where serological testing is unavailable.17,33 Furthermore, and importantly, molecular sequencing offers improved understanding of the true aetiology of endocarditis in different countries and is a major advance in the diagnosis and management of this devastating disease.34,35 Modification of the Duke criteria to include tissue PCR has been acknowledged in the latest update from the British Society for Antimicrobial Chemotherapy, however, this has not been formalised.26,36 Current international guidelines on endocarditis reference Duke criteria, excluding molecular testing.1
Take home messages
Endocarditis remains challenging to diagnose and manage as it is associated with a wide diversity of presentation and variable clinical course. The incidence of endocarditis is increasing, particularly in the elderly. All clinicians should have a low threshold to suspect the diagnosis in the setting of unexplained sepsis, a murmur and/or evidence of systemic embolisation particularly in those at increased risk such as native valve disease, prosthetic valve replacement or the presence of an intracardiac device.
The case highlights the need to act quickly when the patient is acutely unwell. Recognition of severe aortic regurgitation prompted early discussion with the endocarditis team and urgent cardiac surgery. Over recent years, there has been increasing recognition of the importance of an experienced endocarditis team perspective on tailored medical, surgical and antimicrobial management. Patients should be discussed urgently if there is haemodynamic instability, heart failure, recurrent systemic embolisation or uncontrolled infection.
A significant proportion of endocarditis is blood culture negative. This review highlights a pragmatic approach to these patients. In the vignette, BCNE was caused by Bartonella henselae infection and diagnosis of the causative organism was delayed by the current absence of serological testing in the UK. Although with hindsight there were epidemiological clues when an extended history was taken, 16S rRNA PCR analysis of tissue from the explanted valve was ultimately diagnostic and changed antimicrobial management.
Consider endocarditis early.
Management of endocarditis with a multidisciplinary endocarditis team improves outcome through individualised care and early surgery.
Blood culture negative endocarditis is common. In patients who undergo surgery, excised tissue should be sent for 16S rRNA PCR. This can diagnose the causative organism and will inform optimal antimicrobial treatment and duration. This is of increasing relevance given that early surgical intervention is associated with a lower risk of mortality in infective endocarditis, and over 55% of patients undergo surgery early in their clinical course.37,38
We advocate modifying the Duke criteria to include organisms detected on explanted tissue using molecular testing. This valuable test is likely to become a key tool in improving outcomes from BCNE and better understanding the aetiology.
Acknowledgements
The authors thank the patient for taking the time to discuss the presentation and management of his illness, and for his consent to submit this report for publication. We would like to thank Mr Ishtiaq Ahmed for the surgical management of the patient.
References
- 1.Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075–128. [DOI] [PubMed] [Google Scholar]
- 2.Breathnach AS, Hoare JM, Eykyn SJ. Culture-negative endocarditis: contribution of bartonella infections. Heart 1997;77:474–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raoult D, Fournier PE, Vandenesch F, et al. Outcome and treatment of Bartonella endocarditis. Arch Intern Med 2003;163:226–30. [DOI] [PubMed] [Google Scholar]
- 4.Raoult D, Fournier PE, Drancourt M, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med 1996;125:646–52. [DOI] [PubMed] [Google Scholar]
- 5.Avidor B, Graidy M, Efrat G, et al. Bartonella koehlerae, a new cat-associated agent of culture-negative human endocarditis. J Clin Microbiol 2004;42:3462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raoult D, Roblot F, Rolain JM, et al. First isolation of Bartonella alsatica from a valve of a patient with endocarditis. J Clin Microbiol 2006;44:278–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaloner GL, Harrison TG, Birtles RJ. Bartonella species as a cause of infective endocarditis in the UK. Epidemiol Infect 2013;141:841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fournier PE, Lelievre H, Eykyn SJ, et al. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine (Baltimore) 2001;80:245–51. [DOI] [PubMed] [Google Scholar]
- 9.Spach DH, Kanter AS, Daniels NA, et al. Bartonella (Rochalimaea) species as a cause of apparent “culture-negative” endocarditis. Clin Infect Dis 1995;20:1044–7. [DOI] [PubMed] [Google Scholar]
- 10.Spach DH, Callis KP, Paauw DS, et al. Endocarditis caused by Rochalimaea quintana in a patient infected with human immunodeficiency virus. J Clin Microbiol 1993;31:692–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edouard S, Nabet C, Lepidi H, et al. Bartonella, a common cause of endocarditis: a report on 106 cases and review. J Clin Microbiol 2015;53:824–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davierwala PM, Marin-Cuartas M, Misfeld M, et al. The value of an “Endocarditis Team”. Ann Cardiothorac Surg 2019;8:621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liesman RM, Pritt BS, Maleszewski JJ, et al. Laboratory Diagnosis of Infective Endocarditis. J Clin Microbiol 2017;55:2599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore) 2005;84:162–73. [DOI] [PubMed] [Google Scholar]
- 16.Lamas CC, Eykyn SJ. Blood culture negative endocarditis: analysis of 63 cases presenting over 25 years. Heart 2003;89:258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fournier PE, Thuny F, Richet H, et al. Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis 2010;51:131–40. [DOI] [PubMed] [Google Scholar]
- 18.de Rooij MM, Borlee F, Smit LA, et al. Detection of Coxiella burnetii in ambient air after a large Q fever outbreak. PLoS One 2016;11:e0151281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein A, Raoult D. Q fever endocarditis. Eur Heart J 1995;16 Suppl B:19–23. [DOI] [PubMed] [Google Scholar]
- 20.Fleischman DA, Chomel BB, Kasten RW, et al. Bartonella Infection among Cats Adopted from a San Francisco Shelter, Revisited. Appl Environ Microbiol 2015;81:6446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geissdorfer W, Moos V, Moter A, et al. High frequency of Tropheryma whipplei in culture-negative endocarditis. J Clin Microbiol 2012;50:216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrera C, Vilacosta I, Fernandez C, et al. Reassessment of blood culture-negative endocarditis: its profile is similar to that of blood culture-positive endocarditis. Rev Esp Cardiol (Engl Ed) 2012;65:891–900. [DOI] [PubMed] [Google Scholar]
- 23.Lamas CC, Fournier PE, Zappa M, et al. Diagnosis of blood culture-negative endocarditis and clinical comparison between blood culture-negative and blood culture-positive cases. Infection 2016;44:459–66. [DOI] [PubMed] [Google Scholar]
- 24.Maurin M, Raoult D. Q fever. Clin Microbiol Rev 1999;12:518–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houpikian P, Habib G, Mesana T, et al. Changing clinical presentation of Q fever endocarditis. Clin Infect Dis 2002;34:E28–31. [DOI] [PubMed] [Google Scholar]
- 26.Gould FK, Denning DW, Elliott TS, et al. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 2012;67:269–89. [DOI] [PubMed] [Google Scholar]
- 27.Million M, Walter G, Bardin N, et al. Immunoglobulin G anticardiolipin antibodies and progression to Q fever endocarditis. Clin Infect Dis 2013;57:57–64. [DOI] [PubMed] [Google Scholar]
- 28.Raoult D, Casalta JP, Richet H, et al. Contribution of systematic serological testing in diagnosis of infective endocarditis. J Clin Microbiol 2005;43:5238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subedi S, Jennings Z, Chen SC. Laboratory Approach to the Diagnosis of Culture-Negative Infective Endocarditis. Heart Lung Circ 2017;26:763–71. [DOI] [PubMed] [Google Scholar]
- 30.Brouqui P, Raoult D. Endocarditis due to rare and fastidious bacteria. Clin Microbiol Rev 2001;14:177–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tattevin P, Watt G, Revest M, et al. Update on blood culture-negative endocarditis. Med Mal Infect 2015;45:1–8. [DOI] [PubMed] [Google Scholar]
- 32.Baddour LM, Wilson WR, Bayer AS, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications. Circulation 2015;132:1435–86. [DOI] [PubMed] [Google Scholar]
- 33.Goldenberger D, Kunzli A, Vogt P, et al. Molecular diagnosis of bacterial endocarditis by broad-range PCR amplification and direct sequencing. J Clin Microbiol 1997;35:2733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moter A, Musci M, Schmiedel D. Molecular methods for diagnosis of infective endocarditis. Curr Infect Dis Rep 2010;12:244–52. [DOI] [PubMed] [Google Scholar]
- 35.Calabrese F, Carturan E, Thiene G. Cardiac infections: focus on molecular diagnosis. Cardiovasc Pathol 2010;19:171–82. [DOI] [PubMed] [Google Scholar]
- 36.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8. [DOI] [PubMed] [Google Scholar]
- 37.Anantha Narayanan M, Mahfood Haddad T, Kalil AC, et al. Early versus late surgical intervention or medical management for infective endocarditis: a systematic review and meta-analysis. Heart 2016;102:950–7. [DOI] [PubMed] [Google Scholar]
- 38.Chu VH, Park LP, Athan E, et al. Association between surgical indications, operative risk, and clinical outcome in infective endocarditis: a prospective study from the International Collaboration on Endocarditis. Circulation 2015;131:131–40. [DOI] [PubMed] [Google Scholar]


