Abstract
This review focuses on the pathogenic role of sodium glucose cotransporter (SGLT)-2 in the development of renal dysfunction and heart failure in patients with diabetes, by emphasizing the concept of reno-cardiac syndrome (kidney injury worsens cardiac condition) and by substantiating the deleterious effect of sympathetic overdrive in this context. Furthermore, the review proposes a mechanistic hypothesis to explain the benefits of SGLT2 inhibitors, specifically that SGLT-2 inhibitors reduce sympathetic activation at the renal level. To illustrate this point, several examples from both animal experiments and clinical observations are introduced. The bidirectional interaction of the heart and kidney were deeply implicated as an exacerbator of heart failure and renal failure without diabetes. Renal cortical ischemia and abnormal glucose metabolism of tubular epithelial cells are likely to exist as common pathologies in nondiabetic heart failure patients. It is no wonder why SGLT-2 inhibitors are specifically being studied even in the absence of diabetes, both for heart failure and also for renal failure.
Keywords: Cardio-renal syndrome, Sodium glucose cotransporter-2, Heart failure, Diabetic kidney disease, Sympathetic nervous system
Introduction
The goal of diabetes treatment is to maintain the same quality of life and ensure longevity as a healthy person. To achieve this goal, it has been recommended that blood sugar, weight, blood pressure, and lipids be maintained in a state of good control, along with smoking cessation. However, large epidemiological studies have shown that even diabetic patients with good control of all five risk factors (levels of HbA1c, blood pressure, lipids, trace albuminuria, and smoking) are still 1.45 times more likely to develop heart failure, even though they have a similar probability of developing a myocardial infarction as healthy people.1 This means that the current practice guidelines aimed at preventing the development of myocardial infarction are not sufficient to prevent the development of heart failure. I have proposed the hypothesis that the higher risk of heart failure in diabetic patients is due to over-activation of sympathetic nerves resulting from interorgan communication that are driven by stressors that afflict the kidney.2,3 Sodium glucose cotransporter (SGLT)-2 inhibitors may prevent or treat heart failure by relieving the load on the kidneys and reducing sympathetic nerve overactivation.
Heart failure in diabetic patients: involvement of reno-cardiac syndrome
Heart failure is a pathological condition caused by an over-response of interorgan communication that regulates circulatory system dynamics determined by the autonomic nervous system, including hemodynamics, cardiac function, exercise tolerance, and regulatory mechanisms. In particular, connection between kidney disease and heart disease has attracted attention as an event often experienced in daily clinical practice. Acute or chronic dysfunction of the heart (kidney) leads to acute or chronic dysfunction of the kidney (heart); thus, the bidirectional interaction of the heart and kidney has been named cardio-renal syndrome and proposed to be classified as types 1–5.4 Type 1 is acute kidney injury (AKI) due to acute heart disease, type 2 is chronic kidney disease (CKD) due to chronic heart disease, type 3 is acute heart disease associated with AKI, type 4 is chronic and acute heart disease associated with CKD, and type 5 is concurrent heart and kidney damage due to systemic disease. Although the concept of cardio-renal syndrome is widespread, it is still in the phenomenological realm and not well understood scientifically. By definition, the cardio-renal syndrome associated with diabetes is classified as type 5, but the results of cohort studies showing that heart failure and mortality increase with decreased renal function suggest that type 4 cardio-renal syndrome is heavily involved in the development of heart failure in diabetic patients.5
SGLT2 inhibitors act on reno-cardiac syndrome to reduce heart failure hospitalizations and deaths in diabetic patients
In diabetic patients, the parameters of cardiovascular dynamics (blood pressure, heart rate, fluid volume, peripheral vascular resistance, autonomic activity, etc.) deviate from those of healthy subjects in several respects. Diabetic patients have higher blood pressure, faster heart rate, increased peripheral vascular resistance, and a tendency towards fluid retention (increased renal reabsorption of Na+ and water) compared with healthy subjects. Changes in these hemodynamic parameters impose a hemodynamic load on the heart. If this condition persists for a long time, it can lead to functional and instrumental changes in the heart, resulting in the development of heart failure.
What disrupts circulatory homeostasis in diabetic patients? SGLT2 inhibitors provide a clue to this enigma. SGLT2 inhibitors reduce heart failure hospitalizations and deaths in diabetic patients having received adequate doses of renin-angiotensin-aldosterone system inhibitors or beta-blockers.6–8 The antihypertensive effect of SGLT2 inhibitors does not induce reflex activation of the sympathetic nervous system and associated tachycardia. Rather, SGLT2 inhibitors reduce the heart rate in diabetic patients with a pre-treatment heart rate of 70 bpm or more.9 SGLT2 inhibitors do not further reduce heart rate in patients with a pre-treatment heart rate less than 70 bpm. SGLT2 inhibitors improve fluid retention tendencies but are less likely to cause dehydration or acute renal impairment due to excessive diuresis. Thus, compared with conventional heart failure agents such as heart-rate lowering agents, diuretics, renin-angiotensin-aldosterone system inhibitors or beta-blockers, SGLT2 inhibitors may act more upstream in the mechanisms of heart failure development. SGLT2 inhibitors reduce the speed of decline in renal function over time in diabetic patients with microalbuminuria, without albuminuria.10 SGLT2 inhibitors significantly delay the progression to end-stage renal failure in patients with diabetic kidney disease with overt albuminuria.11 From a reno-cardiac syndrome point of view, SGLT2 inhibitors are drugs that protect the kidneys and reduce hospitalization and death from heart failure. Therefore, it is likely that the kidney is heavily involved in the regulation of circulatory system dynamics, and the clinical utility of SGLT2 inhibitors reaffirms the importance of the reno-cardiac syndrome in the development of heart failure in diabetic patients.
Renal stress causes the activation of sympathetic nervous system
Animal studies have proven that the cause of hypertension is in the kidneys, and that an increase in central sympathetic activity is involved in the development of hypertension. When the kidneys are injured, that information is transmitted to the brain via the afferent nerve, and sympathetic output from the brain is increased. Activation of the sympathetic nervous system induces vasoconstriction, sodium and water retention, and tachycardia, resulting in an increase in blood pressure. A prolonged increase in sympathetic tone can lead to advanced atherosclerosis and reduced renal blood flow, leading to a decline in renal function. It also exacerbates heart failure.
Based on the results of animal studies, renal denervation and baroreflex activation therapy, which suppresses the increase in central sympathetic activity, has been attempted as a treatment for treatment-resistant hypertension in humans, and both have been reported to lower blood pressure and, in addition, to exert therapeutic effects on heart failure.12,13
SGLT2 inhibitors inhibit overactivation of the sympathetic nervous system
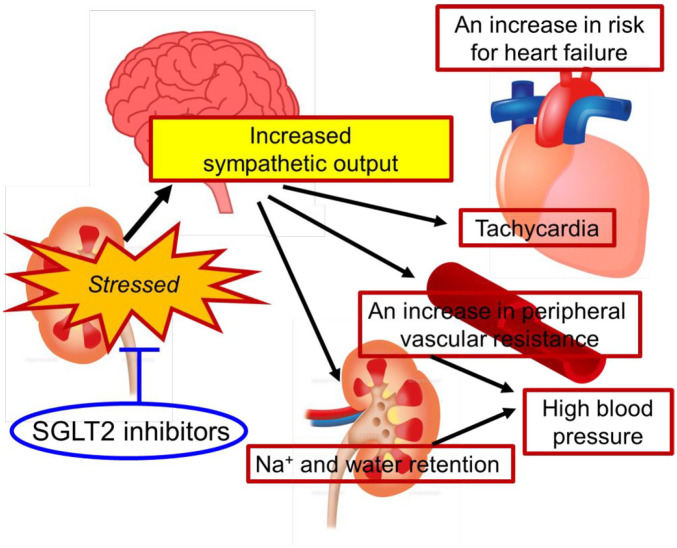
Sympathetic nervous system activation is associated with hospitalization and death in heart failure.14 It has been shown that in diabetic patients, as in hypertensive patients, there is an over-activation of the systemic sympathetic nervous system.15 SGLT2 inhibitors lower blood pressure, reduce heart rate, and improve edema. These changes in the hemodynamic parameters suggest that SGLT2 inhibitors have a sympathoinhibitory effect similar to the renal denervation therapy. In other words, the preventive and therapeutic effects of SGLT2 inhibitors on heart failure may be based on the mechanism by which they act on the kidneys and reduce the central sympathetic output (Figure 1).
Figure 1.
Hemodynamic parameters are determined by the kidney. In the context of diabetes, heart failure, and CKD, the kidneys are overloaded. This information is transmitted from the kidneys to the brain via the afferent nerve, and the sympathetic output from the brain is enhanced, resulting in the systemic sympathetic overdrive. As a consequence, the setting of the hemodynamic parameters shifts in the direction of load on the heart and kidneys. An increase in renal load further activates the sympathetic nervous system, thus creating a positive feedback mechanism. SGLT-2 inhibitors alleviate stressors afflicting the kidneys that is responsible for sympathetic activation.
CKD, chronic kidney disease; SGLT-2, sodium glucose cotransporter-2.
Studies investigating the pharmacological effects of SGLT2 inhibitors in animal models of hypertension, left ventricular (LV) remodeling after myocardial infarction, and CKD have been reported. Dapagliflozin reduced renal sympathetic activity, as evidenced by decreased innervation of tyrosine hydroxylase positive nerves and norepinephrine content, thereby promoting blood pressure reduction in neurogenic hypertensive Schlager (BPH/2J) mice fed a high-fat diet.16 In female Yorkshire pigs undergoing myocardial infarction, empagliflozin ameliorates adverse LV remodeling and enhances LV systolic function, which is coincident with a mitigation of central sympathetic overdrive as evidenced by lower plasma levels of normetanephrine (catabolite of norepinephrine).17 Luseogliflozin significantly suppressed an increase in sympathetic nerve activity and elevated blood pressure in adenine-induced CKD rats with a high-salt diet.18 These studies showing that SGLT2 inhibitors inhibit sympathetic nerve activity in both diabetic and non-diabetic animal models support my hypothesis.
What kind of stress is being placed on the kidneys of diabetic patients?
Animal research has demonstrated that renal ischemia and parenchymal disorders excite the afferent renal nerves and transmit that information to the brain.19 Consequently, changes in the brain that ultimately activate the rostral ventrolateral medulla (RVLM) neurons result in an enhancement of sympathetic output from the brain, resulting in the systemic sympathetic overdrive.20
One can image that the kidneys of diabetic patients are exposed to a variety of stressors of glomerular and tubulointerstitial origin. Diabetic kidney disease has been considered to be primarily of glomerular origin, but there is now compelling evidence that disruption of the tubulointerstitial architecture determines the outcome of diabetic nephropathy, in interplay with glomerular damage.21 First, increased SGLT2-mediated glucose and Na+ reabsorption in diabetic patients disrupts the tubular glomerular feedback mechanism and leads to glomerular hypertension.22 Second, SGLT2-mediated glucose and Na+ reabsorption couples with the function of the Na+/K+ pump (Na+, K+-ATPase). In diabetic patients, oxygen consumption in mitochondria, which are abundant in proximal tubular epithelial cells, is increased in order to maintain high activity of the Na+/K+ pump. As a result, the renal parenchyma goes into a hypoxic state.23,24 Third, proximal tubular epithelial cells have an underdeveloped glycolytic pathway and are more vulnerable to ischemia and hyperglycemia than other cells. During the process of glucose reabsorption in proximal tubular epithelial cells, glucose is taken up from SGLT2 on the tubular lumen side and released from glucose transporter (GLUT) on the vascular lumen side. When proximal tubular epithelial cells are exposed to hyperglycemia or ischemia, glucose is inversely taken up from the GLUT, which drives a glycolytic flux. Metabolites derived from the improvised glycolysis pathway exhibit cytotoxicity and induce transcriptional activation of pro-inflammatory and pro-fibrotic genes.25,26 These are candidate stressors that afflict the kidney in diabetes mellitus. Renal cortical ischemia and abnormal glucose metabolism of tubular epithelial cells are likely to exist as common pathologies in nondiabetic heart failure patients.
Conclusion
The kidney is an organ that was acquired in a dynamic evolutionary process. Biological homeostatic mechanisms have evolved as adaptive responses to changes in the environment. During the transition from aquatic to terrestrial life, the kidneys acquired the ability to filter wastes in the blood and expel them from the body as urine; to regulate the balance of water content, electrolytes, and minerals in the body by reabsorbing necessary components; and to control the number of red blood cells required for gas exchange in the lungs and peripheral tissues through the production of erythropoietin. Probably from the beginning of bipedal walking, the kidneys became the key organ responsible for the circulatory homeostasis. Thus, the kidneys are rich in mechanisms that are strongly driven when the organism is under load and maintain homeostasis through organ–organ connection. Lifestyle disruptions in modern society lead to excessive and continuous stimulation of the sympathetic nervous system based on the kidney-based interorgan connection, causing the cardiovascular setpoints (desirable values for parameters such as blood pressure, heart rate, fluid volume, peripheral vascular resistance, and autonomic activity) to deviate from their original default values. These dynamic alterations increase the hemodynamic load on the heart and increase the risk of developing heart failure. Nowadays, a major problem is the continued high rate of hospitalization and mortality for heart failure, even when the risk factors for arteriosclerosis are thoroughly controlled. SGLT2 inhibitors make up for what has been lacking in the treatment of diabetes so far. SGLT2 inhibitors can reduce the risk of heart failure by reducing the burden on the heart and kidneys by returning the cardiovascular set point to a healthy reference level.2,3,24
Future perspectives
Diabetes and heart failure are common in that they overload the kidneys and increase sympathetic activity throughout the body. The therapeutic effect of SGLT2 inhibitors on heart failure and CKD in diabetic patients has been demonstrated to be independent of pre-treatment blood glucose levels and changes in blood glucose levels due to SGLT2 inhibitors.27 Therefore, it is reasonable to test whether SGLT-2 inhibitors exert a therapeutic effect on the heart and kidney even in the absence of diabetes.28–33 Future work will explore the types of stressors that afflict the kidneys in diabetes and heart failure, and identify factors that excite afferent nerves from the kidneys that lead to activation of the sympathetic center of the brain.
Footnotes
Conflict of interest statement: The author declares that there is no conflict of interest.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Motoaki Sano  https://orcid.org/0000-0003-1771-8310
https://orcid.org/0000-0003-1771-8310
References
- 1. Rawshani A, Rawshani A, Franzén S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2018; 379: 633–644. [DOI] [PubMed] [Google Scholar]
- 2. Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol 2018; 71: 471–476. [DOI] [PubMed] [Google Scholar]
- 3. Sano M. Inter-organ communication pathway manifested by non-physiological stress to the kidney in type II diabetic patients: why are diabetic patients prone to develop heart failure? Intern Med 2020; 59: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol 2008; 52: 1527–1539. [DOI] [PubMed] [Google Scholar]
- 5. Pálsson R, Patel UD. Cardiovascular complications of diabetic kidney disease. Adv Chronic Kidney Dis 2014; 21: 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 7. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 8. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 9. Sano M, Chen S, Imazeki H, et al. Changes in heart rate in patients with type 2 diabetes mellitus after treatment with luseogliflozin: subanalysis of placebo-controlled, double-blind clinical trials. J Diabetes Invest 2018; 9: 638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cherney DZI, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2017; 5: 610–621. [DOI] [PubMed] [Google Scholar]
- 11. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 12. Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018; 391: 2335–2345. [DOI] [PubMed] [Google Scholar]
- 13. Lohmeier TE, Hall JE. Device-based neuromodulation for resistant hypertension therapy: too early for prime time? Circ Res 2019; 124: 1071–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Triposkiadis F, Karayannis G, Giamouzis G, et al. The sympathetic nervous system in heart failure: physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 2009; 54: 1747–1762. [DOI] [PubMed] [Google Scholar]
- 15. Huggett RJ, Scott EM, Gilbey SG, et al. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation 2003; 108: 3097–3101. [DOI] [PubMed] [Google Scholar]
- 16. Hyrat LY, Magno AL, Rudnicka C, et al. SGLT2 inhibitor-induced sympathoinhibition: a novel mechanism for cardiorenal protection. JACC Basic Transl Sci 2020; 29: 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Santos-Gallego CG, Requena-Ibanez JA, Antonio RS, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol 2019; 73: 1931–1944. [DOI] [PubMed] [Google Scholar]
- 18. Wan N, Fujisawa Y, Kobara H, et al. Effects of an SGLT2 inhibitor on the salt sensitivity of blood pressure and sympathetic nerve activity in a nondiabetic rat model of chronic kidney disease. Hypertens Res 2020; 43: 492–499. [DOI] [PubMed] [Google Scholar]
- 19. Ye S, Zhong H, Yanamadala S, et al. Oxidative stress mediates the stimulation of sympathetic nerve activity in the phenol renal injury model of hypertension. Hypertension 2006; 48: 309–315. [DOI] [PubMed] [Google Scholar]
- 20. Kishi T, Hirooka Y, Kimura Y, et al. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation 2004; 109: 2357–2362. [DOI] [PubMed] [Google Scholar]
- 21. Gilbert RE. Proximal tubulopathy: prime mover and key therapeutic target in diabetic kidney disease. Diabetes 2017; 66: 791–800. [DOI] [PubMed] [Google Scholar]
- 22. Persson P, Hansell P, Palm F. Tubular reabsorption and diabetes-induced glomerular hyperfiltration. Acta Physiol (Oxf) 2010; 200: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O’Neill J, Fasching A, Pihl L, et al. Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am J Physiol Renal Physiol 2015; 309: F227–F234. [DOI] [PubMed] [Google Scholar]
- 24. Sano M, Goto S. Possible mechanism of hematocrit elevation by sodium glucose cotransporter 2 inhibitors and associated beneficial renal and cardiovascular effects. Circulation 2019; 139: 1985–1987. [DOI] [PubMed] [Google Scholar]
- 25. Srivastav SP, Li J, Kitada M, et al. SIRT3 deficiency leads to induction of abnormal glycolysis in diabetic kidney with fibrosis. Cell Death Dis 2018; 9: 997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qi W, Keenan HA, Li Q, et al. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med 2017; 23: 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inzucchi SE, Kosiborod M, Fitchett D, et al. Improvement in cardiovascular outcomes with empagliflozin is independent of glycemic control. Circulation 2018; 138: 1904–1907. [DOI] [PubMed] [Google Scholar]
- 28. Packer M, Butler J, Filippatos GS, et al. Evaluation of the effect of sodium–glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR-reduced trial. Eur J Heart Fail 2019; 21: 1270–1278. [DOI] [PubMed] [Google Scholar]
- 29. Anker SD, Butler J, Filippatos GS, et al. Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-preserved trial. Eur J Heart Fail 2019; 21: 1279–1287. [DOI] [PubMed] [Google Scholar]
- 30. Herrington WG, Preiss D, Haynes R, et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 2018; 11: 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santos-Gallego CG, Garcia-Ropero A, Mancini D, et al. Rationale and design of the EMPA-TROPISM trial (ATRU-4): are the “cardiac benefits” of empagliflozin independent of its hypoglycemic activity? Cardiovasc Drugs Ther 2019; 33: 87–95. [DOI] [PubMed] [Google Scholar]
- 32. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 33. Heerspink HJL, Stefansson BV, Chertow GM, et al. Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 2020; 35: 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]