Abstract
Objective:
This study aimed to investigate the correlation of polo-like kinase 4 with clinicopathological features and survival profiles in patients with gastric cancer.
Methods:
This retrospective study was conducted based on the clinical data from 289 eligible patients with gastric cancer who received resection. Polo-like kinase 4 expression in adjacent tissue and tumor tissue was determined by immunohistochemical assay and semiquantified scoring method using immunohistochemical score by staining intensity score multiplying staining density score. Based on the total immunohistochemical score (ranged from 0 to 12), the polo-like kinase 4 expression was classified as low expression (immunohistochemical: 0-3) and high expression (immunohistochemical: 4-12); furthermore, high expression was divided into high+ expression (immunohistochemical: 4-6), high++ expression (immunohistochemical: 7-9), and high+++ expression (immunohistochemical: 10-12).
Results:
Polo-like kinase 4 expression was elevated in tumor tissue compared with adjacent tissue. Tumor polo-like kinase 4 high expression correlated with increased T stage and Tumor, Node, Metastasis (TNM) stage, while, it did not correlate with age, gender, current smoke, current drink, chronic complications, Helicobacter pylori infection, tumor location, pathological grade, or N stage. Besides, higher tumor polo-like kinase 4 expression correlated with shorter disease-free survival and overall survival. Subsequently, multivariate Cox proportional hazards regression analysis showed that higher tumor polo-like kinase 4 expression was an independent predictive factor for worse disease-free survival but not for overall survival.
Conclusion:
Polo-like kinase 4 possesses the clinical significance as a biomarker for aiding prognostication and facilitating postoperative tumor management in patients with gastric cancer.
Keywords: gastric cancer, polo-like kinase 4, T stage, TNM stage, prognosis
Introduction
Gastric cancer (GC), the most common upper gastrointestinal malignancy, constitutes a notable proportion of global cancer mortality.1,2 Meanwhile, the incidence of GC is on the rise in East Asia countries, particularly in China, Japan, and Korea.3,4 Surgical resection is the primary treatment for patients with GC, while, more than half of the resected patients have recurrent disease within 5 years of surgery, and two-thirds of patients who have inoperable disease at diagnosis accompanied by malignant proliferation, excessive invasion, and distal visceral metastasis.1,5 As a result, the 5-year survival rate for all stages remains unsatisfied.1 To combat the dismal prognosis, discovery of efficient biomarkers will potentially benefit prognostic evaluation and facilitate the comprehensive management of GC.
Polo-like kinases (PLKs) are a family of serine/threonine protein kinases, which consist of 5 members (PLK1-PLK5).6 Polo-like kinases regulate several intracellular processes including DNA replications, mitosis, and stress responses, which are closely implicated in the tumorigenesis of various cancers.6 For instance, PLK1 correlated with tumor differentiation, invasion, advanced Tumor, Node, Metastasis (TNM) stage, and worse prognosis in patients with GC.7 Regarding PLK4, it facilitates colorectal cancer cell proliferation, invasion, and metastasis in vitro, and it is associated with increased tumor size, lymph node metastasis, and raised TNM stage in patients with colorectal cancer.8 Another study illuminates that knocking down PLK4 impedes migration and invasion while accelerates apoptosis of neuroblastoma cells, and PLK4 high expression correlates with higher serum lactate dehydrogenase, recurrence, increased expression level of Ki-67, and advanced tumor stage in patients with neuroblastoma.9 As for GC, PLK4 expression is elevated in multiple GC cell lines, and its overexpression triggers centrosome amplification and chromosome instability.10 Furthermore, PLK4 is modified by released proteins from Helicobacter pylori (H pylori), which then contributes to the pathogenesis of GC.11 In the context of the above evidence, we hypothesized that PLK4 exhibits the potency as a biomarker for disease management and prognosis of GC. Therefore, the present study aimed at investigating tumor expression of PLK4, and its correlation with clinical/pathological features as well as the survival profiles in patients with GC, to assess its value for GC monitoring in clinical setting.
Materials and Methods
Patients
A total of 289 eligible patients with GC who underwent resection between February 2015 and November 2019 in our hospital were retrospectively reviewed in this study, among which 283 (97.9%) cases underwent standard D2 gastrectomy and 6 (2.10%) cases underwent D1 gastrectomy. The inclusion criteria were (1) pathological diagnosis of primary GC, (2) aged from 18 to 80 years, (3) tumor tissue resected from surgery was accessible and available for immunohistochemical (IHC) assay, (4) had complete clinical data, and (5) had complete survival data. The exclusion criteria included (1) metastatic or secondary GC, (2) history of other cancers, (3) severe liver and kidney dysfunction before surgery, and (4) history of gastric surgeries. This study was approved by the Ethics Committee of our hospital. The written informed consents were provided by patients or their family members.
Data Collection
Information of demographics, preoperative tumor features, extent of surgery, and postoperative treatments was collected from the database of our hospital, which included age, gender, current smoke, current drink, chronic complications, H pylori infection status, tumor location, pathological grade, T stage, N stage, TNM stage, adjuvant chemotherapy, and adjuvant radiotherapy. The survival data were acquired from follow-up records, and the last follow-up date was December 11, 2019. The disease-free survival (DFS) was calculated from the date of surgery to the date of relapse, progression or death and the overall survival (OS) were calculated from the date of surgery to the date of death.
Polo-Like Kinase 4 Detection
Formalin-fixed paraffin-embedded adjacent tissue and tumor tissue were obtained from the storeroom of pathology department in our hospital. And the expression of PLK4 in tumor tissue and adjacent tissue was detected by IHC staining. Primary antibody Rabbit polyclonal to PLK4 and the secondary antibody Goat AntiRabbit IgG H&L were purchased from Abcam. Standard procedures of IHC were carried out referring to the application manual of the antibodies. A semiquantitative scoring method was used to assess the IHC staining result, which included staining intensity score and staining density score.12 The total IHC score (staining intensity score × staining density score) was ranging from 0 to 12. The IHC score ≤3 was defined as PLK4 low expression, and the IHC score >3 was defined as PLK4 high expression.13,14
Statistical Analysis
McNemar test was used to compare the proportions of PLK4 high expression and PLK4 low expression between tumor tissue and adjacent tissue. Comparison of clinical features between PLK4 low group and PLK4 high group was determined by χ2 test or Wilcoxon rank sum test. Disease-free survival and OS were displayed using Kaplan-Meier curves. Comparison of DFS and OS between PLK4 low group and PLK4 high group was determined by log-rank test. For further analysis regarding the correlation of DFS and OS with PLK4 expression, the PLK4 high patients was classified into PLK4 high+ group (IHC score 4-6), PLK4 high++ group (IHC score 7-9), and PLK4 high+++ group (IHC score 10-12). And comparison of DFS and OS among 4 groups was also determined by log-rank test. Factors predicting DFS and OS were analyzed by univariate Cox proportional hazard regression model, and the factors with a P value <.05 in the univariate Cox regression were further included in multivariate Cox regression. All statistical analyses were performed using SPSS version 22.0 (IBM), and all figures were plotted using GraphPad Prism version 7.00 (GraphPad Software). P value <.05 was considered as significant.
Results
Gastric Cancer Patients’ Features
The mean age was 60.0 ± 11.6 years, and the median age was 61.0 (51.0-70.0) years (Table 1). There were 138 (47.8%) females and 151 (52.2%) males. Furthermore, 101 (34.9%) patients were complicated with H pylori infection. Regarding pathological grade, 43 (14.9%), 212 (73.3%), and 34 (11.8%) patients presented with pathological grade G1, G2, and G3, respectively. The number (percentage) of patients at TNM stage I, II, and III were 38 (13.1%), 150 (51.9%), and 101 (34.9%), respectively. As for treatment history, 186 (64.4%) and 34 (11.8%) patients received adjuvant chemotherapy and adjuvant radiotherapy, respectively. Other detailed characteristics are displayed in Table 1.
Table 1.
Clinical Features.
| Items | GC patients (N = 289) |
|---|---|
| Age (years) | |
| Mean ± SD | 60.0 ± 11.6 |
| Median (IQR) | 61.0 (51.0-70.0) |
| Gender, no. (%) | |
| Female | 138 (47.8) |
| Male | 151 (52.2) |
| Current smoke, no. (%) | |
| No | 201 (69.6) |
| Yes | 88 (30.4) |
| Current drink, no. (%) | |
| No | 192 (66.4) |
| Yes | 97 (33.6) |
| Hypertension, no. (%) | |
| No | 191 (66.1) |
| Yes | 98 (33.9) |
| Hyperlipidemia, no. (%) | |
| No | 209 (72.3) |
| Yes | 80 (27.7) |
| Diabetes, no. (%) | |
| No | 245 (84.8) |
| Yes | 44 (15.2) |
| H pylori infection, no. (%) | |
| Negative | 188 (65.1) |
| Positive | 101 (34.9) |
| Tumor location, no. (%) | |
| Cardia | 77 (26.6) |
| Gastric body | 24 (8.3) |
| Gastric antrum | 188 (65.1) |
| Pathological grade, no. (%) | |
| G1 | 43 (14.9) |
| G2 | 212 (73.3) |
| G3 | 34 (11.8) |
| T stage, no. (%) | |
| T1 | 9 (3.1) |
| T2 | 29 (10.0) |
| T3 | 248 (85.9) |
| T4 | 3 (1.0) |
| N stage, no. (%) | |
| N0 | 104 (36.0) |
| N1 | 88 (30.4) |
| N2 | 84 (29.1) |
| N3 | 13 (4.5) |
| TNM stage, no. (%) | |
| I | 38 (13.1) |
| II | 150 (51.9) |
| III | 101 (34.9) |
| Adjuvant chemotherapy, no. (%) | |
| No | 103 (35.6) |
| Yes | 186 (64.4) |
| Adjuvant radiotherapy, no. (%) | |
| No | 255 (88.2) |
| Yes | 34 (11.8) |
| Extent of surgery, no. (%) | |
| D1 | 6 (2.10%) |
| D2 | 283 (97.9%) |
Abbreviations: GC, gastric carcinoma; H pylori, Helicobacter pylori; IQR, interquartile range; SD, standard deviation.
Polo-Like Kinase 4 Expression in Tumor Tissue and Adjacent Tissue
The PLK4 expression in tumor tissue and adjacent tissue of patients with GC was examined by IHC staining. According to the IHC score, the PLK4 expression was classified as low expression (IHC score 0-3) and high expression (IHC score 4-12); meanwhile, the PLK4 expression was divided into low expression (IHC score 0-3), high+ expression (IHC score 4-6), high++ expression (IHC score 7-9), and high+++ expression (IHC score 10-12). Immunohistochemical staining image examples of tumor PLK4 low, high+, high++, and high+++ expressions are exhibited in Figure 1A, and IHC staining image examples of adjacent PLK4 low, high+, high++, and high+++ expressions are shown in Figure 1B. In tumor tissue, 133 (46.0%) cases and 156 (54.0%) cases exhibited tumor PLK4 low expression and high expression, respectively. In adjacent tissue, 63 (73.3%) and 23 (26.7%) cases were with adjacent PLK4 low expression and high expression, respectively. Meanwhile, the percentage of PLK4 high expression was increased in tumor tissue compared with adjacent tissue (Figure 1C). Additionally, 133 (46.1%), 96 (33.2%), 41 (14.2%), and 19 (6.6%) tumor tissues presented with tumor PLK4 low, high+, high++, and high+++ expressions, while 63 (73.3%), 16 (18.6%), 5 (5.8%), and 2 (2.3%) adjacent tissues were with tumor PLK4 low, high+, high++, and high+++ expressions; further comparison analysis disclosed that the PLK4 expression was elevated in tumor tissues compared with adjacent tissues (Figure 1D).
Figure 1.
PLK4 expression in tumor tissue and adjacent tissue. Representative IHC staining images of tumor tissue with PLK4 low expression, PLK4 high+ expression, PLK4 high++ expression, and PLK4 high+++ expression (A), and adjacent tissue with PLK4 low expression, PLK4 high+ expression, PLK4 high++ expression, and PLK4 high+++ expression (B). Comparison of the percentage of PLK4 low expression and the percentage of PLK4 high expression between tumor tissue and adjacent tissue (C). Comparison of the percentage of PLK4 low expression, the percentage of PLK4 high+ expression, the percentage of PLK4 high++ expression, and the percentage of PLK4 high+++ expression between tumor tissue and adjacent tissue (D). Comparisons were assessed by McNemar test. P < .05 was considered significant. IHC indicates immunohistochemical; PLK4, polo-like kinase 4.
Correlation of Tumor PLK4 Expression With Clinicopathological Features
In regard to tumor features, tumor PLK4 expression correlated with more advanced T stage (P < .001; Figure 2C) and TNM stage (P = .002; Figure 2E), while, there was no correlation of tumor PLK4 expression with tumor location (P = .292; Figure 2A), pathological grade (P = .112; Figure 2B) or N stage (P = .057; Figure 2D) in patients with GC. As for demographic features, chronic complications and H pylori infection status, no correlation of tumor PLK4 expression with age (P = .139), gender (P = .908), current smoke (P = .522), current drink (P = .276), hypertension (P = .204), hyperlipidemia (P = .961), diabetes (P = .163), or H pylori infection (P = .172) was disclosed in patients with GC (Table 2). In terms of adjuvant treatments, tumor PLK4 expression did not correlate with adjuvant chemotherapy (P = .375) or adjuvant radiotherapy (P = .546) in patients with GC (Supplementary Table 1).
Figure 2.
Correlation of PLK4 expression with tumor features. Comparisons of tumor location (A), pathological grade (B), T stage (C), N stage (D), and TNM stage (E) between patients with tumor PLK4 high expression and patients with tumor PLK4 low expression. Comparisons between patients with tumor PLK4 high expression and patients with tumor PLK4 low expression were assessed by χ2 test or Wilcoxon rank sum test. P < .05 was considered significant. PLK4 indicates polo-like kinase 4.
Table 2.
Correlation of PLK4 Expression With Clinical Features Apart From Tumor Features.a
| Items | PLK4 low (n = 133) | PLK4 high (n = 156) | P value |
|---|---|---|---|
| Age, no. (%) | .139 | ||
| ≤60 years | 60 (45.1) | 84 (53.8) | |
| >60 years | 73 (54.9) | 72 (46.2) | |
| Gender, no. (%) | .908 | ||
| Female | 64 (48.1) | 74 (47.4) | |
| Male | 69 (51.9) | 82 (52.6) | |
| Current smoke, no. (%) | .522 | ||
| No | 95 (71.4) | 106 (67.9) | |
| Yes | 38 (28.6) | 50 (32.1) | |
| Current drink, no. (%) | .276 | ||
| No | 84 (63.2) | 108 (69.2) | |
| Yes | 49 (36.8) | 48 (30.8) | |
| Hypertension, no. (%) | .204 | ||
| No | 93 (69.9) | 98 (62.8) | |
| Yes | 40 (30.1) | 58 (37.2) | |
| Hyperlipidemia, no. (%) | .961 | ||
| No | 96 (72.2) | 113 (72.4) | |
| Yes | 37 (27.8) | 43 (27.6) | |
| Diabetes, no. (%) | .163 | ||
| No | 117 (88.0) | 128 (82.1) | |
| Yes | 16 (12.0) | 28 (17.9) | |
| H pylori infection, no. (%) | .172 | ||
| Negative | 81 (60.9) | 107 (68.6) | |
| Positive | 52 (39.1) | 49 (31.4) |
Abbreviations: H pylori, Helicobacter pylori; PLK4, polo-like kinase 4.
a Comparison was determined by χ2 test.
Correlation of Tumor PLK4 Expression With DFS and OS
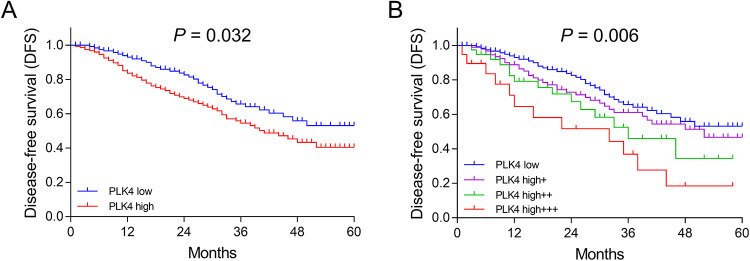
In order to verify the correlation of tumor PLK4 expression with prognosis, we adopted 2 different classified methods to divide tumor PLK4 expression and group the patients based on the corresponding tumor PLK4 expression. The first method was that tumor PLK4 expression was classified as tumor PLK4 low and tumor PLK4 high. The second method was that tumor PLK4 expression was classified as tumor PLK4 low, tumor PLK4 high+, tumor PLK4 high++, and tumor PLK4 high+++. Based on the first classified method, tumor PLK4 high expression correlated with worse DFS (P = .032) and OS (P = .016; Figures 3A and 4A). Based on the second classified method, increased grade of PLK4 expression correlated with shorter DFS (P = .006) and OS (P = .016; Figures 3B and 4B). Both methods indicate that tumor PLK4 expression negatively correlated with prognosis.
Figure 3.
Negative correlation of PLK4 expression with DFS. Comparison of DFS between patients with tumor PLK4 high expression and patients with tumor PLK4 low expression (A). Comparison of DFS among patients with tumor PLK4 high+++ expression, patients with tumor PLK4 high++ expression, patients with tumor PLK4 high+ expression, and patients with tumor PLK4 low expression (B). Kaplan-Meier curve and log-rank test were used to compare DFS between patients with tumor PLK4 high expression and patients with tumor PLK4 low expression. P < .05 was considered significant. DFS indicates disease-free survival; PLK4, polo-like kinase 4.
Figure 4.
Negative correlation of PLK4 expression with OS. Comparison of OS between patients with tumor PLK4 high expression and patients with tumor PLK4 low expression (A). Comparison of OS among patients with tumor PLK4 high+++ expression, patients with tumor PLK4 high++ expression, patients with tumor PLK4 high+ expression, and patients with tumor PLK4 low expression (B). Kaplan-Meier curve and log-rank test were used to compare OS between patients with tumor PLK4 high expression and patients with tumor PLK4 low expression. P < .05 was considered significant. OS indicates overall survival; PLK4, polo-like kinase 4.
Correlation of Tumor PLK4 Expression With OS
Overall survival was worse in patients with tumor PLK4 high expression compared to patients with tumor PLK4 low expression (P = .016; Figure 4A). Furthermore, patients with tumor PLK4 high+++ expression presented with the lowest OS, followed by patients with tumor PLK4 high++ expression and patients with tumor PLK4 high+ expression, and then patients with tumor PLK4 low expression (P = .016; Figure 4B).
Factors Predicting DFS
Univariate Cox proportional hazard regression analysis revealed that higher PLK4 expression (P = .001, hazard ratio [HR] = 1.395), increased pathological grade (G3 vs G2/G1; P = .001, HR = 2.388), and elevated TNM stage (III vs II/I; P < .001, HR = 3.269) predicted worse DFS in patients with GC (Table 3). Further multivariate Cox proportional hazard regression analysis showed that higher PLK4 expression (P = .027, HR = 1.261), increased pathological grade (G3 vs G2/G1; P = .042, HR = 1.727), and elevated TNM stage (III vs II/I; P < .001, HR = 2.838) independently predicted worse DFS.
Table 3.
Analysis of Factors Predicting DFS.a
| Items | Cox proportional hazard regression model | |||
|---|---|---|---|---|
| P value | HR | 95% CI | ||
| Lower | Higher | |||
| Univariate Cox regression | ||||
| Higher PLK4 expressionb | .001 | 1.395 | 1.140 | 1.707 |
| Age (>60 years) | .580 | 0.894 | 0.601 | 1.330 |
| Male | .640 | 0.910 | 0.612 | 1.352 |
| Current smoke | .622 | 0.896 | 0.578 | 1.388 |
| Current drink | .426 | 1.185 | 0.781 | 1.798 |
| Hypertension | .117 | 1.383 | 0.922 | 2.073 |
| Hyperlipidemia | .142 | 1.366 | 0.901 | 2.072 |
| Diabetes | .485 | 1.211 | 0.708 | 2.071 |
| H pylori positive | .575 | 0.884 | 0.575 | 1.359 |
| Tumor location | ||||
| Gastric antrum | Reference | - | - | - |
| Cardia | .594 | 1.135 | 0.713 | 1.806 |
| Gastric body | .235 | 1.475 | 0.776 | 2.802 |
| Pathological grade (G3 vs. G2/G1) | .001 | 2.388 | 1.430 | 3.987 |
| TNM stage (III vs II/I) | <.001 | 3.269 | 2.185 | 4.891 |
| Adjuvant chemotherapy | .348 | 0.822 | 0.546 | 1.238 |
| Adjuvant radiotherapy | .066 | 0.507 | 0.246 | 1.046 |
| Multivariate Cox regression | ||||
| Higher PLK4 expressionb | .027 | 1.261 | 1.026 | 1.550 |
| Pathological grade (G3 vs G2/G1) | .042 | 1.727 | 1.020 | 2.925 |
| TNM stage (III vs II/I) | <.001 | 2.838 | 1.869 | 4.311 |
Abbreviations: DFS, disease-free survival; H pylori, Helicobacter pylori; HR, hazard ratio; PLK4, polo-like kinase 4.
a Factors with P value <.05 in univariate Cox regression were included in the multivariate Cox regression.
b PLK4 expression was categorized as low = 0, high+ = 1, high++ = 2, high+++ = 3.
Factors Predicting OS
Univariate Cox proportional hazard regression analysis revealed that higher PLK4 expression (P = .002, HR = 1.489), raised pathological grade (G3 vs G2/G1; P = .004, HR = 2.582), and more advanced TNM stage (III vs II/I; P < .001, HR = 5.895) predicted shorter OS in patients with GC (Table 4). Further multivariate Cox proportional hazard regression analysis showed that more advanced TNM stage (III vs II/I; P < .001, HR = 5.130) independently predicted shorter OS, while, higher PLK4 expression (P = .067, HR = 1.275) or pathological grade (G3 vs G2/G1; P = .167, HR = 1.589) could not independently predict OS.
Table 4.
Analysis of Factors Predicting OS.a
| Items | Cox proportional hazard regression model | |||
|---|---|---|---|---|
| P value | HR | 95% CI | ||
| Lower | Higher | |||
| Univariate Cox regression | ||||
| Higher PLK4 expressionb | .002 | 1.489 | 1.156 | 1.917 |
| Age (>60 years) | .652 | 0.888 | 0.529 | 1.490 |
| Male | .529 | 0.848 | 0.506 | 1.419 |
| Current smoke | .887 | 0.960 | 0.545 | 1.690 |
| Current drink | .097 | 1.565 | 0.922 | 2.659 |
| Hypertension | .701 | 1.112 | 0.647 | 1.913 |
| Hyperlipidemia | .715 | 0.898 | 0.504 | 1.599 |
| Diabetes | .119 | 1.662 | 0.878 | 3.149 |
| H pylori positive | .731 | 0.905 | 0.513 | 1.597 |
| Tumor location | ||||
| Gastric antrum | Reference | - | - | - |
| Cardia | .180 | 1.479 | 0.835 | 2.620 |
| Gastric body | .406 | 1.445 | 0.607 | 3.443 |
| Pathological grade (G3 vs. G2/G1) | .004 | 2.582 | 1.365 | 4.884 |
| TNM stage (III vs II/I) | <.001 | 5.895 | 3.310 | 10.497 |
| Adjuvant chemotherapy | .109 | 0.653 | 0.388 | 1.099 |
| Adjuvant radiotherapy | .218 | 0.561 | 0.224 | 1.406 |
| Multivariate Cox regression | ||||
| Higher PLK4 expressionb | .067 | 1.275 | 0.983 | 1.654 |
| Pathological grade (G3 vs G2/G1) | .167 | 1.589 | 0.824 | 3.067 |
| TNM stage (III vs II/I) | <.001 | 5.130 | 2.836 | 9.282 |
Abbreviations: H pylori, Helicobacter pylori; HR, hazard ratio; OS, overall survival; PLK4, polo-like kinase 4.
a Factors with P value <.05 in univariate Cox regression were included in the multivariate Cox regression.
b PLK4 expression was categorized as low = 0, high+ = 1, high++ = 2, high+++ = 3.
Discussion
The carcinogenesis of GC is a multistep, slowly progressive, and multifactorial pathology process, among which genetic mutations, epigenetic alternation, and abnormal molecular signaling pathways largely contribute to the tumor formation, invasion, metastasis, tumor recurrence, and drug resistance.15,16 Up-to-date, no effective biomarkers have been established for tracking progression and prognosis of GC.1 Further exploration of novel biomarkers is needed for tumor management and surveillance of GC.
Polo-like kinase 4, mapped to chromosome 4q27-28, is a vital modulator in cell progression and response to DNA damage, which has been implicated in the oncogenesis of various cancers.17-21 Mechanically, PLK4 inhibition hinders cell proliferation and tumor-initiating cells in lung cancer and pancreatic cancer.18,19 Clinically, PLK4 expression is related to larger tumor size, lymph node metastasis, and increased TNM stage in non-small cell lung cancer.20 In breast cancer, an overexpression of PLK4 correlates with raised incidence of lymph node metastasis, distal metastasis, or surrounding recurrence.21 As to GC, the clinical implication of PLK4 in the tumor progression of GC has not studied yet. Two experimental studies illuminate that PLK4 overexpression mediates centrosome amplification, chromosome instability, and subsequent inhibition of primary cilia formation, and it is upregulated in GC cells by proteins secreted from H pylori, which plays a role in H pylori–associated GC pathogenesis.10,11 However, the clinical implication of PLK4 in GC has not reported yet. In the present study, PLK4 was sufficiently expressed in tumor tissue, and it correlated with elevated T stage and TNM stage in patients with GC, which was in parallel with the previous evidences in other solid tumors. Hereby, several explanations have been suggested (1) PLK4 might promote GC cell proliferation and metastasis by activating the Wnt/β-catenin signaling pathway.8 Hence, PLK4 high expression was associated with advanced T stage and TNM stage in patients with GC. (2) The PLK4 high expression probably induced centrosome amplification and chromosomal instability, which consequently accelerated proliferation, migration, invasion, and metastasis while slowed apoptosis in GC cells.17,22 Hence, PLK4 high expression patients were more vulnerable to increased tumor stage.
Accumulated researches report that PLK4 displays prognostic value in multiple cancers.9,20 As an example, in non-small cell lung cancer, patients with PLK4 high expression present favorable DFS and OS compared to patients with PLK4 low expression, and PLK4 high expression independently predicts worse DFS and OS.20 And in neuroblastoma, 3-year OS and 3-year progression-free survival are lower in patients with PLK4 high expression than that in patients with PLK4 low expression.9 Nonetheless, the correlation of PLK4 with clinical outcomes in patients with GC remains unknown. In the present study, how PLK4 affected the prognosis in patients with GC was assessed by Kaplan-Meier curve and log-rank test, which revealed that both DFS and OS were reduced in patients with PLK4 high expression compared to patients with PLK4 low expression; the higher the PLK4 expression, the shorter the DFS and OS. Notably, the subsequent Cox proportional hazard regression model analysis disclosed that higher PLK4 expression was an independent predictive factor for shorter DFS but not for OS in patients with GC. These could be explained by (1) Our previous findings showed that PLK4 correlated with increased T stage and TNM stage, thereby, higher PLK4 expression presented the potential of being a predictor for worse survival profiles in patients with GC. (2) The PLK4 possibly accelerated tumor growth via Wnt/β-catenin signaling pathway and induced the insensitivity of tumor cells to chemotherapy via PLK4-γ-tubulin axis, which led to tumor recurrent and drug resistance in patients with GC.8,21 Thereby, higher PLK4 expression was associated with shorter DFS and OS in patients with GC. (3) Although higher PLK4 expression correlated with worse OS, it could not independently predict OS in patients with GC, which was likely to be explained by that PLK4 impacted other factors (such as TNM stage) to indirectly result in worse OS in GC.
The present study was the first to unravel the clinical significance of PLK4 in the progression and prognosis of patients with GC. However, several limitations should not be neglected when interpreting the findings. First, detailed mechanism of PLK4 in the pathogenesis of GC was not explored, thereby, further in vivo and in vitro experiments are warranted. Second, the sample size of patients with GC was relatively small, which might reduce the statistic power of study, thereby further studies with larger sample size would be desirable for validating our findings. Last, only nonmetastatic primary patients with GC were included in the analysis, while metastatic or secondary patients with GC were excluded since they were not eligible for tumor resection, which lacked the generalizability of our findings.
To conclude, PLK4 correlates with advanced tumor stage and shorter survival profiles in patients with GC who underwent resection, which owns good potential as a biomarker for refining individual prognosis and guiding treatment strategy decisions.
Supplemental Material
Supplemental Material, Supplementary_table_1-revised for Clinical Significance of Polo-Like Kinase 4 as a Marker for Advanced Tumor Stage and Dismal Prognosis in Patients With Surgical Gastric Cancer by Ting Cao, Shijie Yi, Xuefeng Yang and Qing Wu in Technology in Cancer Research & Treatment
Abbreviations
- DFS
disease-free survival;
- GC
gastric cancer;
- H pylori
Helicobacter pylori;
- HR
hazard ratio;
- IHC
immunohistochemical;
- OS
overall survival;
- PLK4
polo-like kinase 4.
Footnotes
Authors’ Note: Our study was approved by the Ethics Committee of Affiliated Nanhua Hospital, University of South China (approval no. NH2019-06). The written informed consents were provided by patients or their family members.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD: Shijie Yi  https://orcid.org/0000-0003-1128-7601
https://orcid.org/0000-0003-1128-7601
References
- 1. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. [DOI] [PubMed] [Google Scholar]
- 2. Yu S, Zhang Y, Li Q, Zhang Z, Zhao G, Xu J. CLDN6 promotes tumor progression through the YAP1-snail1 axis in gastric cancer. Cell Death Dis. 2019;10(12):949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 4. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 5. Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S. Gastric adenocarcinoma. Nat Rev Dis Primers. 2017;3(1):17036. [DOI] [PubMed] [Google Scholar]
- 6. Liu X. Targeting polo-like kinases: a promising therapeutic approach for cancer treatment. Transl Oncol. 2015;8(3):185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lan B, Liu BY, Chen XH, et al. Polo like kinase 1 expression and prognostic value in gastric carcinomas [in Chinese]. Zhonghua Wei Chang Wai Ke Za Zhi. 2007;10(1):70–72. [PubMed] [Google Scholar]
- 8. Liao Z, Zhang H, Fan P, et al. High PLK4 expression promotes tumor progression and induces epithelial mesenchymal transition by regulating the Wnt/betacatenin signaling pathway in colorectal cancer. Int J Oncol. 2019;54(2):479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tian X, Zhou D, Chen L, et al. Polo-like kinase 4 mediates epithelial-mesenchymal transition in neuroblastoma via PI3K/Akt signaling pathway. Cell Death Dis. 2018;9(2):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shinmura K, Kurabe N, Goto M, et al. PLK4 overexpression and its effect on centrosome regulation and chromosome stability in human gastric cancer. Mol Biol Rep. 2014;41(10):6635–6644. [DOI] [PubMed] [Google Scholar]
- 11. Kim N, Park WY, Kim JM, et al. Gene expression of AGS cells stimulated with released proteins by Helicobacter pylori . J Gastroenterol Hepatol. 2008;23(4):643–651. [DOI] [PubMed] [Google Scholar]
- 12. Fu H, Jin C, Zhu Q, et al. Dysregulated expressions of PTEN, NF-kappaB, WWP2, p53 and c-Myc in different subtypes of B cell lymphoma and reactive follicular hyperplasia. Am J Transl Res. 2019;11(2):1092–1101. [PMC free article] [PubMed] [Google Scholar]
- 13. Wang G, Wang Z, Yu H. Kinesin family member 2A high expression correlates with advanced tumor stages and worse prognosis in non-small cell lung cancer patients. J Clin Lab Anal. 2019;34(4):e23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye Q, Zhang M, Yin Y. Katanin P80 correlates with larger tumor size, lymph node metastasis, and advanced TNM stage and predicts poor prognosis in non-small-cell lung cancer patients. J Clin Lab Anal. 2020;34(4):e23141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shan C, Zhang Y, Hao X, Gao J, Chen X, Wang K. Biogenesis, functions and clinical significance of circRNAs in gastric cancer. Mol Cancer. 2019;18(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagini S. Carcinoma of the stomach: a review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol. 2012;4(7):156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maniswami RR, Prashanth S, Karanth AV, et al. PLK4: a link between centriole biogenesis and cancer. Expert Opin Ther Targets. 2018;22(1):59–73. [DOI] [PubMed] [Google Scholar]
- 18. Lei Q, Xiong L, Xia Y, et al. YLT-11, a novel PLK4 inhibitor, inhibits human breast cancer growth via inducing maladjusted centriole duplication and mitotic defect. Cell Death Dis. 2018;9(11):1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lohse I, Mason J, Cao PM, Pintilie M, Bray M, Hedley DW. Activity of the novel polo-like kinase 4 inhibitor CFI-400945 in pancreatic cancer patient-derived xenografts. Oncotarget. 2017;8(2):3064–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou Q, Fan G, Dong Y. Polo-like kinase 4 correlates with greater tumor size, lymph node metastasis and confers poor survival in non-small cell lung cancer. J Clin Lab Anal. 2019;34(4):e23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Z, Dai K, Wang C, et al. Expression of polo-like kinase 4(PLK4) in breast cancer and its response to taxane-based neoadjuvant chemotherapy. J Cancer. 2016;7(9):1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao Y, Wang X. PLK4: a promising target for cancer therapy. J Cancer Res Clin Oncol. 2019;145(10):2413–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Supplementary_table_1-revised for Clinical Significance of Polo-Like Kinase 4 as a Marker for Advanced Tumor Stage and Dismal Prognosis in Patients With Surgical Gastric Cancer by Ting Cao, Shijie Yi, Xuefeng Yang and Qing Wu in Technology in Cancer Research & Treatment