Abstract
Background:
25-Hydroxyvitamin D [25(OH)D] deficiency has been implicated as a possible risk factor for the onset and progression of diabetes kidney disease (DKD). The aim of this study was to evaluate the interaction between levels of 25(OH)D and DKD in type 2 diabetes mellitus (DM) patients.
Methods:
Cross-sectional design, outpatient type 2 DM. Glomerular filtration rate (GFR) was measured by 51Cr-EDTA and estimated by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), urinary albumin excretion (UAE) by immunoturbidimetry, and 25(OH)D by chemiluminescence. Receiver operating characteristic (ROC) curve analysis and generalized linear model (Poisson robust regression estimator) were used to assess the interaction between 25(OH)D levels and renal function.
Results:
A total of 114 type 2 DM patients aged 60 ± 10 years, 49 males (43%), DM duration 22 ± 10 years, with GFR > 60 ml/min/1.73 m2 were evaluated. Patients with GFRs 60–90 (n = 50) had significantly lower 25(OH)D levels than individuals with GFRs > 90 ml/min/1.73 m2 (n = 64), respectively 40 ± 20 versus 48 ± 20 nmol/l, p = 0.027. This difference was more pronounced for older individuals (39 ± 20 versus 54 ± 23 nmol/l, respectively), and Poisson robust regression disclosed that lower 25(OH)D [Poisson regression (PR) = 0.989, confidence interval (CI): 0.978–0.999, p = 0.034], and advanced age (PR = 1.050, CI: 1.007–1.096, p = 0.023) were significantly associated with the lower GFR category, adjusted for seasons. ROC curve analysis showed that the cutoff point of 25(OH)D of 41 nmol/l was associated with lower GFR [area under the curve (AUC) = 0.694, p = 0.009]. CKD-EPI estimated GFR (eGFR) was not associated with 25(OH)D in any analysis. There was no difference in 25(OH)D levels between patients with elevated UAE as compared with normoalbuminuric ones (44 ± 21 versus 46 ± 19 nmol/l, p = 0.587).
Conclusion:
Lower levels of 25(OH)D are associated with decreased GFR in patients with type 2 DM, especially in older patients, with no evidence of interaction with UAE levels.
Keywords: 25(OH)D, albuminuria, diabetic kidney disease, glomerular filtration rate, urinary albumin excretion, vitamin D
Introduction
A recent meta-analysis of data on diabetes mellitus (DM) patients revealed an annual incidence of elevated albuminuria of 2–8%; the incidence of estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2 was as high as 4% per year, and that of end-stage renal disease (ESRD) up to 2% per year.1 Currently, diabetes kidney disease (DKD) is responsible for about 40% of chronic kidney disease (CKD) cases worldwide.2–4 These findings indicate that DKD is a noteworthy health problem all over the world, even though data from developing countries are notably scarce.
Current data suggest that cardiovascular and all-cause rates of mortality are declining in DM, so an increased prevalence of DKD is an upcoming trend.5,6 Sharp increases of 40–700% in the incidence of ESRD in diabetes have been reported between 2002 and 2015 in Russia, Mexico, Bosnia, Australia, and several Asian countries, also reflecting the increasing prevalence of diabetes itself.2,6 Whereas these increases have been reported for the overall population, diabetic patients have tended to show a decrease in rates of micro- and macrovascular complications over the last 20 years. Notwithstanding, the smallest reductions have been described for ESRD, in contrast with the marked reductions in acute myocardial infarction, stroke, and amputations.3
Unfavorable DKD evolution stems from the fact that current treatment is not able to completely control the progression of the disease, since the pathogenesis of kidney damage has not yet been fully elucidated.4 Vitamin D deficiency has raised special interest because some cross-sectional studies in DM patients have demonstrated an association between lower levels of 25(OH)D and elevated urinary albumin excretion (UAE).7–9 25(OH)D deficiency is highly prevalent among patients with type 2 diabetes, especially in the presence of DKD, when up to 50–70% of patients are affected.7,8,10–15 The direct interaction between decreased 1,25(OH)2D levels and renal damage is expected and well established, but it is rather intriguing when it comes to 25(OH)D. Either 25(OH)D deficiency would be causing kidney injury, or occurring as a consequence of renal involvement. Alternatively, confounding factors such as obesity, high blood pressure, and hyperglycemia – usually coexisting with lower 25(OH)D levels – would be the real promoters of kidney disease.4,16 However, not all studies confirm the association between 25(OH)D levels and albuminuria,17,18 and, as a matter of fact, no previous study has properly investigated measured GFR in this scenario. Therefore, the aim of this study was to investigate the interaction between 25(OH)D levels and the degree of renal function impairment, analyzing urinary albumin excretion (UAE) and GFR measured with a reference method.
Subjects and methods
Subjects
This cross-sectional study included type 2 DM outpatients with preserved renal function (GFR > 60 ml/min/1.73 m2) from the Endocrine Division of the South University Hospital. Informed written consent was obtained from all patients, as approved by Grupo de Pesquisa e Pós-Graduação (GPPG), project number 140501, and ethical approval was obtained from the Research Ethics Committee of Hospital de Clínicas de Porto Alegre (Comitê de Ética e Pesquisa: CEP). It complied with the Declaration of Helsinki. Type 2 DM was defined by onset after 35 years of age, no insulin therapy in the first 5 years since diagnosis, no previous episodes of ketoacidosis, and compatible C-peptide levels.19 Skin color was self-defined by the patient. No patient was taking vitamin D supplements.
The patients underwent a complete physical examination. Body mass index (BMI) was determined as the weight in kilograms divided by height in meters squared. Waist circumference was measured along a line midway between the rib cage and iliac crest. Blood pressure was taken twice, after 5 min of sitting at rest, with a validated digital sphygmomanometer (OMRON, Model HEM-705CP); the mean of two measurements was used.
Methods
Serum samples were collected after 12-h fasting; glucose was determined by the glucose oxidase method, cholesterol by an enzymatic method, and HbA1c was analyzed with certified HPLC (Merck-Hitachi L-9100 Analyzer, Merck, Darmstadt, Germany).
GFR was measured by the 51Cr-EDTA single-injection method, after a single intravenous dose of 150 µCi, and calculated as: volume of distribution × 0.693 × 0.87 × 1000/t1/2, where 0.87 is a correction factor developed by Chantler.20 Blood samples were collected at 0, 2, 3, and 4 h after the injection, and the results were expressed in ml/min/1.73 m2. The coefficient of variation established in our institution is 12%.21 GFR was also estimated by the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation and expressed as eGFR (ml/min/1.73 m²), using a traceable Jaffe creatinine assay.22 Patients were categorized according to 51Cr-EDTA GFR (51Cr GFR) as ⩾90 or 60–90 ml/min/1.73 m2, according to Kidney Disease Improving Global Outcomes (KDIGO).4
UAE was measured by immunoturbidimetry in random urine samples, and values above 14 mg/l were considered elevated.23 DKD was defined by elevated UAE and/or decreased GFR.
25(OH)D was measured by chemiluminescence, which detects levels of vitamin D between 10 and 375 nmol/l. The inter-assay coefficients of variation (CV) were 7% and 6.3% for values of 45 and 92.5 nmol/l, respectively, with a 5% intra-assay CV. Vitamin D deficiency was defined by levels of 25(OH)D < 50 nmol/l.24
Statistical analysis
The results were expressed as mean ± standard deviation (SD), percentages or median [interquartile range (IQR)]. Continuous parametric variables were assessed using Student’s t test or ANOVA; variables without normal distribution were analyzed using non-parametric tests or Mann–Whitney test; qualitative variables were analyzed using the chi-square test. Receiver operating characteristic (ROC) curves were used to assess the association between GFR levels and 25(OH)D cutoff points; Youden’s test was used to check the equilibrium point. Poisson analysis was performed to assess the possible association of categorized binary GFR with 25(OH)D, adjusted for season, gender, and HbA1c.
To evaluate the relation between elevated albuminuria and 25(OH)D levels, the sample size was calculated on the website www.openepi.com; considering an alpha error of 5% and a power of 80%, 50 individuals were required in each group to find a putative difference.25 As for GFR and 25(OH)D interaction, since no studies were available in the literature, the power of our findings, and also the Cohen effect size, were calculated to analyze the impact of 25(OH)D on renal function.26
A p value < 0.05 was considered statistically significant. Statistical analyzes were performed using SPSS software, version 25 (SPSS, Chicago, IL, USA).
Results
This study included 114 type 2 DM patients, with a mean age of 60 ± 10 years, 49 males (43%), mean DM duration of 22 ± 10 years, and BMI of 30 ± 4 kg/m2.
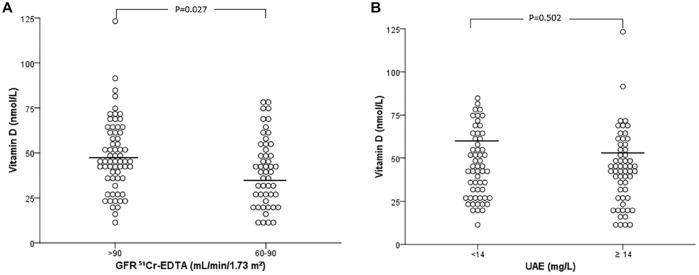
Table 1 presents the clinical and laboratory characteristics of patients classified according to GFR levels: >90 versus 60–90 ml/min/1.73 m2. Patients with GFR 60–90 were older and displayed significantly lower 25(OH)D values compared with those with GFR > 90 ml/min/1.73 m2 (Figure 1A). In contrast to 51Cr-EDTA measured GFR, estimated CKD-EPI GFR was not able to recognize any association with lower 25(OH)D values, since, when the patients were divided by estimated CKD-EPI GFR values of >90 versus 60–90 ml/min/1.73 m2, vitamin D levels were similar between the groups thus classified (45 ± 18 versus 45 ± 22 nmol/l, p = 0.911). Even when analyzed according to each stage of estimated GFR in older patients, it was also not possible to see any association, as opposed to measured GFR. Furthermore, there was a positive significant correlation between 51Cr-EDTA GFR and 25(OH)D (r = 0.22, p = 0.021), not observed for estimated GFR (r = 0.09, p = 0.356).
Table 1.
Clinical and laboratory characteristics of patients with type 2 DM according to GFR (51Cr-EDTA GFR) categorized as >90 versus 60–90 ml/min/1.73 m2.
| GFR > 90 (n = 64) |
GFR 60–90 (n = 50) |
p value | |
|---|---|---|---|
| Age (years) | 57 ± 9 | 64 ± 10 | 0.001 |
| Diabetes duration (years) | 22 ± 8 | 24 ± 10 | 0.173 |
| BMI (kg/m2) | 30 ± 4 | 30 ± 5 | 0.910 |
| Hypertension (%) | 91 | 94 | 0.995 |
| Male sex (%) | 46 | 38 | 0.446 |
| Skin color (% white/black) | 81/19 | 79/21 | 0.857 |
| Smokers (%) | 40 | 47 | 0.558 |
| Use of ACEi/ARB (%) | 69 | 78 | 0.359 |
| Collection season (%) | |||
| Summer | 8 | 10 | 0.135 |
| Spring | 20 | 20 | |
| Winter | 38 | 54 | |
| Autumn | 34 | 16 | |
| HbA1c (%) | 8.5 ± 1.9 | 8.7 ± 1.7 | 0.669 |
| 25(OH)D (nmol/l) | 48 ± 20 | 40 ± 20 | 0.027 |
| Vitamin D deficiency (%) | 57 | 72 | 0.117 |
| Cholesterol (mmol/l) | 4.6 ± 0.9 | 4.7 ± 1.3 | 0.657 |
| GFR 51Cr-EDTA (ml/min/1.73 m2) | 115 ± 22 | 73 ± 10 | By design |
| eGFR CKD-EPI (ml/min/1.73 m2) | 93 ± 17 | 74 ± 20 | <0.001 |
| UAE (mg/l) | 17 (6–56) | 20 (5–132) | 0.638 |
Data expressed as mean ± SD, percent, median (IQR).
χ2 test was used for qualitative variables and Student’s t test and Mann–Whitney test were used for quantitative variables. Statistical significance was assumed at p < 0.05.
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; 25(OH)D, hydroxyvitamin D; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate; HbA1c, glycated hemoglobin; IQR, interquartile range; SD, standard deviation; UAE, urinary albumin excretion.
Figure 1.
(A) 25(OH)D values according to GFR stages, and (B) UAE values. Student’s t test was used in (A) and Mann–Whitney test was used in (B).
25(OH)D, 25-hydroxyvitamin D; GFR, glomerular filtration rate; UAE, urinary albumin excretion.
Table 2 shows the results of patients divided by UAE levels, categorized as normo (<14 mg/l) versus micro/macroalbuminuria (⩾14 mg/l). The values of 25(OH)D were similar between patients with normal versus elevated UAE (Figure 1B). Likewise, the proportion of vitamin D deficiency was similar between the groups.
Table 2.
Clinical and laboratory characteristics of patients with type 2 DM according to UAE categorized as normo (<14 mg/l) versus micro/macroalbuminuria (⩾14 mg/l).
| Normo (n = 56) |
Micro/Macro (n = 43/15) |
p value | |
|---|---|---|---|
| Age (years) | 62 ± 10 | 59 ± 10 | 0.167 |
| Diabetes duration (years) | 23 ± 9 | 23 ± 10 | 0.876 |
| BMI (kg/m2) | 30 ± 4 | 30 ± 4 | 0.863 |
| Hypertension (%) | 91 | 92 | 0.750 |
| Male sex (%) | 34 | 51 | 0.087 |
| Skin color (% white/black) | 80/20 | 80/20 | 0.962 |
| Smokers (%) | 43 | 44 | 0.941 |
| Use of ACEi/ARB (%) | 66 | 78 | 0.223 |
| Collection season (%) | |||
| Summer | 5 | 10 | 0.227 |
| Spring | 27 | 14 | |
| Winter | 46 | 44 | |
| Autumn | 21 | 32 | |
| HbA1c (%) | 8.5 ± 1.7 | 8.7 ± 1.9 | 0.725 |
| 25(OH)D (nmol/l) | 46 ± 19 | 44 ± 21 | 0.587 |
| Vitamin D deficiency (%) | 59 | 68 | 0.294 |
| Cholesterol (mmol/l) | 4.6 ± 1.2 | 4.6 ± 1.0 | 0.896 |
| GFR 51Cr-EDTA (ml/min/1.73 m2) | 99 ± 28 | 96 ± 26 | 0.532 |
| UAE (mg/l) | 5 (3–11) | 62 (32–199) | By design |
Data expressed as mean ± SD, percent, median (IQR).
χ2 test was used for qualitative variables and Student’s t test and Mann–Whitney test were used for quantitative variables. Statistical significance was assumed at p < 0.05.
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; 25(OH)D, hydroxyvitamin D; GFR, glomerular filtration rate; HbA1c, glycated hemoglobin; IQR, interquartile range; SD, standard deviation; UAE, urinary albumin excretion.
Since age seemed to influence the association of GFR with 25(OH)D levels, we categorized the patients according to median age, which was 60 years. Figure 2 shows the levels of 25(OH)D according to GFR in patients classified by age ⩾ 60 (n = 62) versus <60 years (n = 52). Interestingly, 25(OH)D levels were lower in patients with lower GFRs, but only in patients ⩾60 years (39 ± 20; n = 34 versus 54 ± 23 nmol/l; n = 28), in contrast to patients <60 years (43 ± 17; n = 16 versus 44 ± 17 nmol/l; n = 36, in each GFR stage). The calculated power for this finding was 77%. In the same way, 25(OH)D was positively and moderately correlated with GFR only in older patients (r = 0.36, p = 0.004), as opposed to younger patients (r = 0.12, p = 0.396). Furthermore, Poisson robust analyses taking binary GFR category as the outcome, demonstrated that, in older subjects, lower 25(OH)D [ Poisson regression (PR) = 0.989, confidence interval (CI): 0.978–0.999, p = 0.034], and advanced age (PR = 1.050, CI: 1.007–1.096, p = 0.023) were significantly associated with the lower GFR category, adjusted for seasons. This association was not observed in younger patients [PR for 25(OH)D was 0.994, CI: 0.969–1.020, p = 0.628], age: PR 1.016, CI: 0.956–1.080, and season of the year, p = 0.610. These results remained the same even after removing an outlier patient that had elevated levels of 25(OH)D.
Figure 2.
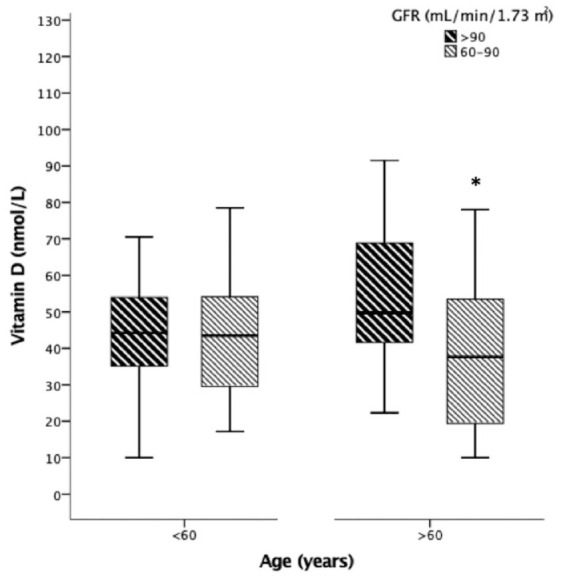
25(OH)D values according to GFR values >90 versus 60–90 ml/min/1.73 m2 in patients aged <60 versus >60 years of age. ANOVA test followed by Tukey pairwise post hoc was used. *p = 0.004.
ANOVA, analysis of variance; 25(OH)D, 25-hydroxyvitamin D; GFR, glomerular filtration rate.
To allow a more meaningful interpretation of clinical relevance of 25(OH)D levels, we also calculated the effect size of Cohen.26 The effect size of 25(OH)D levels on GFR was 0.4 for the whole group (small effect), and 0.69 for older group (intermediate effect), according to the interpretation table for different effect sizes.26
To define the cutoff of 25(OH)D capable of identifying a link between the level of GFR and 25(OH)D levels, a ROC curve was constructed. By means of the Youden test, the value of 41 nmol/l of vitamin D (Figure 3, AUC = 0.694, p = 0.009) was determined as the cutoff point that identified the presence of a decline in measured GFR, with a calculated power of 78%.
Figure 3.
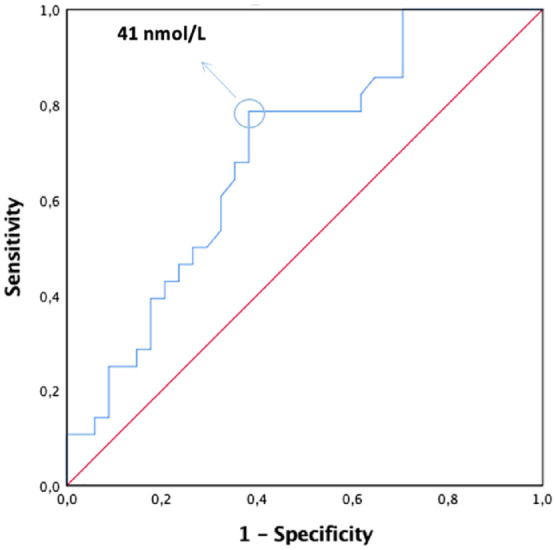
The value of 41 nmol/l of 25(OH)D was the identified Youden cutoff point that was associated with the presence of a decline in measured GFR in patients >60 years; AUC = 0.694, p = 0.009, power 78%.
AUC, area under the curve; 25(OH)D, 25-hydroxyvitamin D; GFR, glomerular filtration rate.
Discussion
In the present study, lower levels of 25(OH)D were associated with lower GFR in type 2 DM patients with preserved renal function; no evidence of interaction between 25(OH)D and increased UAE was found.
Regarding GFR, we were able to demonstrate that 25(OH)D levels were lower in patients with GFR values of 60–90 when compared with >90 ml/min/1.73 m2, primarily in older patients. As far as we know, our study was the first to use a reference GFR measurement method to demonstrate this association. Previous studies investigated only equation-based eGFR. When analyzing eGFR, some studies described a direct interaction between 25(OH)D and GFR,15,16 whereas other authors did not confirm this finding.7,8,12,15,18,27 As we and others have previously demonstrated, the accuracy of GFR estimated by equations is poor, especially in patients with diabetes, markedly underestimating true GFR.21,28–30 This finding is probably due to either analytical factors, such as interference of glucose levels on creatinine measurement, or to the lack of sensitivity of creatinine to identify glomerular hyperfiltration, a peculiar scenario in diabetes.21 Thus, the studies that determined eGFR could have failed to demonstrate such an association because of eGFR limitations. Accordingly, when GFR was evaluated by CKD-EPI equation in our study, no association between 25(OH)D and renal function was found, in contrast to the direct correlation obtained with measured 51Cr-EDTA GFR. Nevertheless, this significant correlation was limited herein to older patients, probably because age reduces renal functioning mass, making the kidney more susceptible to damage. Several factors, from genetic predisposition to environmental factors, create a condition where the renal status of elderly individuals shows different susceptibility to injury. Renal aging is a complex process expressed by anatomical and functional changes, such as impaired function of glomerular, tubular, and endocrine systems, such as 25(OH)D activation.31 Therefore, this reduced activation, on top of low levels of cholecalciferol, might explain the increased vulnerability of elderly kidneys. Through a presumed increased state of chronic inflammation, 25(OH)D deficiency would represent an additional source of aggression to the aging kidney. In a cohort of elderly patients with various degrees of renal impairment, reduced 25(OH)D levels were independent predictors of progression to dialysis and death.32 A recent investigation showed that the kidney-secreted hormone Klotho would be a central player at the ageing-inflammation interface.33
Regarding albuminuria, some studies have demonstrated that patients with elevated UAE have lower 25(OH)D levels compared with normoalbuminuric individuals.34,35 However, herein, no association between UAE and 25(OH)D was found. This divergence could be attributed to the fact that about 70% of our patients were using angiotensin converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB), thereby masking increased UAE levels. Another explanation could be that urinary albumin levels in our cohort were not as high as those of previous studies; an inverse correlation has been previously demonstrated between levels of albuminuria and 25(OH)D.7,12,35 The importance of this putative association is still a matter of debate; whereas cross-sectional and longitudinal studies suggest an unfavorable effect of vitamin D deficiency on albuminuria, studies of vitamin D supplementation show controversial effects on UAE.34,36,37 Further clarification could come from ongoing larger randomized studies.38
Typical characteristics of type 2 DM, such as obesity, high blood pressure and hyperglycemia, usually occur concomitantly with lower 25(OH)D levels.16,18 These parameters could be the real factors promoting kidney damage. Indeed, HbA1c has been found to be inversely related to serum 25(OH)D levels.39 However, whereas two recent meta-analyses showed that vitamin D supplementation improved glycemic control in vitamin D-deficient type 2 diabetes patients, two other studies showed conflicting results.40,41 In our study, there was a weak inverse correlation between HbA1c and 25(OH)D; nevertheless, this was not evident in multivariable analysis. The same dispute applies to blood pressure levels. A number of studies have demonstrated that both systolic and diastolic pressures are increased in patients with vitamin D deficiency,16,42 and a meta-analysis described that supplementation was able to reduce blood pressure in patients with diabetes.43 This question is still open to debate, and the independent roles of vitamin D deficiency and elevated blood pressure on kidney damage are still to be elucidated.
An important issue to be considered in our study is the season of the year in which the samples for vitamin D analyses were collected. The seasonal variation of ultra-violet B (UVB) solar radiation causes a variation in circulating levels of 25(OH)D. In a cohort of 4758 individuals inhabitants of northern Finland, individuals whose blood samples were collected during periods of low sunlight exposure (winter months) showed an increased risk of showing low levels of 25(OH)D.44 Likewise, vitamin D deficiency was observed in winter and spring as compared with summer and fall in a group of 11,150 Italian individuals,45 and the same pattern has been demonstrated in a German population.46 A retrospective analysis of 25(OH)D including 39,004 individuals in Brazil, aged 2–95 years, revealed that vitamin D deficiency was more evident in winter when compared with summer.47 All these studies confirm the importance of seasonality as a potential bias in determining 25(OH)D levels. In uni and multivariable analyses, the season of collection was not related to renal function in our study.
The present study has strengths and limitations. First, the patients were examined with a GFR reference method, in contrast to all previous studies, which evaluated only eGFR, which is particularly inaccurate in diabetic patients.23,28,29 Indeed, in contrast to measured GFR, CKD-EPI eGFR was not able to recognize any association with 25(OH)D values in our study. Furthermore, the use of a reference GFR method allows a more precise analysis of patients with relatively preserved renal function, to reinforce that lower 25(OH)D levels were present even in early stages of kidney damage.48 As for limitations, the cross-sectional nature of data does not allow the analysis of causality, and only speculations can be made in this sense. Another point is the relatively restricted size of the sample. Nonetheless, we carefully described our results showing a calculated power of about 80%, also describing details of Cohen effect size to improve data interpretation.
In conclusion, our study was the first to demonstrate an interrelationship between lower levels of 25(OH)D and lower GFR in older patients with type 2 DM, in a relatively preserved range of GFR function. No evidence of interaction was found with UAE, although the broad use of ACEi/ARB could have interfered with this analysis.
Footnotes
Author contribution(s): Angélica Dall’Agnol: Data curation; Formal analysis; Investigation; Methodology; Writing-original draft; Writing-review & editing.
Letícia Almeida Brondani: Formal analysis; Investigation; Writing-original draft; Writing-review & editing.
Vítor Agostim Cancelier: Data curation; Formal analysis; Investigation; Writing-original draft.
Eduardo Guimarães Camargo: Conceptualization; Data curation; Investigation; Methodology; Visualization; Writing-original draft; Writing-review & editing.
Silveiro, Sandra Pinho: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Visualization; Writing-original draft; Writing-review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Programa de Apoio a Núcleos de Excelência (PRONEX process number 16/2551-0000-476-5), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundo de Incentivo à Pesquisa e Eventos (FIPE) Hospital de Clínicas de Porto Alegre.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Sandra Pinho Silveiro  https://orcid.org/0000-0003-4891-4074
https://orcid.org/0000-0003-4891-4074
Contributor Information
Angélica Dall’Agnol, Department of Internal Medicine, Faculty of Medicine, Graduate Program in Medical Sciences: Endocrinology, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil.
Letícia de Almeida Brondani, Department of Internal Medicine, Faculty of Medicine, Graduate Program in Medical Sciences: Endocrinology, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil.
Vítor da Agostim Cancelier, Department of Internal Medicine, Faculty of Medicine, Graduate Program in Medical Sciences: Endocrinology, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil.
Eduardo Guimarães Camargo, Department of Internal Medicine, Faculty of Medicine, Graduate Program in Medical Sciences: Endocrinology, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil.
Sandra Pinho Silveiro, Endocrine Division, HCPA, Rua Ramiro Barcelos, Porto Alegre, RS Brazil; Department of Internal Medicine, Faculty of Medicine, Graduate Program in Medical Sciences: Endocrinology, Universidade Federal do Rio Grande do Sul, 2350, 4° andar, Porto Alegre, RS 90035-003, Brazil.
References
- 1. Koye DN, Shaw JE, Reid CM, et al. Incidence of chronic kidney disease among people with diabetes: a systematic review of observational studies. Diabet Med 2017; 34: 887–901. [DOI] [PubMed] [Google Scholar]
- 2. United States Renal Data System, www.usrds.org/adr.aspx (2018, accessed 4 July 2019).
- 3. Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med 2014; 370: 1514–1523. [DOI] [PubMed] [Google Scholar]
- 4. Barrett EJ, Liu Z, Khamaisi M, et al. Diabetic microvascular disease: an endocrine society scientific statement. J Clin Endocrinol Metab 2017; 102: 4343–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. International Diabetes Federation. Diabetes and cardiovascular disease report, www.idf.org/our-activities/care-prevention/cardiovascular-disease/cvd-report (2017, accessed 4 July 2019).
- 6. Harding JL, Pavkov ME, Magliano DJ, et al. Global trends in diabetes complications: a review of current evidence. Diabetologia 2019; 62: 3–16. [DOI] [PubMed] [Google Scholar]
- 7. Huang Y, Yu H, Lu J, et al. Oral supplementation with cholecalciferol 800 IU ameliorates albuminuria in Chinese type 2 diabetic patients with nephropathy. PLoS One 2012; 7: e50510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kondo M, Toyoda M, Miyatake H, et al. The prevalence of 25-hydroxyvitamin D deficiency in Japanese patients with diabetic nephropathy. Intern Med 2016; 55: 2555–2562. [DOI] [PubMed] [Google Scholar]
- 9. Ucak S, Sevim E, Ersoy D, et al. Evaluation of the relationship between microalbuminuria and 25-(OH) vitamin D levels in patients with type 2 diabetes mellitus. Aging Male. Epub ahead of print 26 June 2018. DOI: 10.1080/13685538.2018. [DOI] [PubMed] [Google Scholar]
- 10. Sipahi S, Acikgoz SB, Genc AB, et al. The association of vitamin D status and vitamin D replacement therapy with glycemic control, serum uric acid levels, and microalbuminuria in patients with type 2 diabetes and chronic kidney disease. Med Princ Pract 2017; 26: 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diaz VA, Mainous AG, III, Carek PJ, et al. The association of vitamin D deficiency and insufficiency with diabetic nephropathy: implications for health disparities. J Am Board Fam Med 2009; 22: 521–527. [DOI] [PubMed] [Google Scholar]
- 12. Sánchez-Hernández RM, García-Cantón C, Lorenzo DL, et al. The specific relationship between vitamin D deficiency and diabetic nephropathy among patients with advanced chronic kidney disease: a cross-sectional study in Gran Canaria, Spain. Clin Nephrol 2015; 83: 218–224. [DOI] [PubMed] [Google Scholar]
- 13. Peng Y, Li LJ. Serum 25-hydroxyvitamin D level and diabetic nephropathy in patients with type 2 diabetes mellitus. Int Urol Nephrol 2015; 47: 983–989. [DOI] [PubMed] [Google Scholar]
- 14. Shao Y, Lv C, Yuan Q, et al. Levels of serum 25(OH)VD3, HIF-1α, VEGF, vWf, and IGF-1 and their correlation in type 2 diabetes patients with different urine albumin creatinine ratio. J Diabetes Res 2016; 2016: 1925424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Usluogullari CA, Balkan F, Caner S, et al. The relationship between microvascular complications and vitamin D deficiency in type 2 diabetes mellitus. BMC Endocr Disord 2015; 15: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moreira JSR, de Paula TP, Sperb LF, et al. Association of plasma vitamin D status with lifestyle patterns and ambulatory blood pressure monitoring parameters in patients with type 2 diabetes and hypertension. Diabetes Res Clin Pract 2018; 139: 139–146. [DOI] [PubMed] [Google Scholar]
- 17. Thrailkill KM, Jo CH, Cockrell GE, et al. Enhanced excretion of vitamin D binding protein in type 1 diabetes: a role in vitamin D deficiency? J Clin Endocrinol Metab 2011; 96: 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yokoyama K, Nakashima A, Urashima M, et al. Interactions between serum vitamin D levels and vitamin D receptor gene fokI polymorphisms for renal function in patients with type 2 diabetes. PLoS One 2012; 7: e51171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes 2020. Diabetes Care 2020; 43(Suppl. 1): S14–S31. [DOI] [PubMed] [Google Scholar]
- 20. Chantler C, Barratt TM. Estimation of glomerular filtration rate from plasma clearance of 51-chromium edetic acid. Arch Dis Child 1972; 47: 613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Silveiro SP, Araújo GN, Ferreira MN, et al. Chronic kidney disease epidemiology collaboration (CKD-EPI) equation pronouncedly underestimates glomerular filtration rate in type 2 diabetes. Diabetes Care 2011; 34: 2353–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Incerti J, Zelmanovitz T, Camargo JL, et al. Evaluation of tests for microalbuminuria screening in patients with diabetes. Nephrol Dial Transplant 2005; 20: 2402–2407. [DOI] [PubMed] [Google Scholar]
- 24. Giustina A, Adler RA, Binkley N, et al. Consensus statement from 2nd international conference on controversies in vitamin D. Rev Endocr Metab Disord 2020; 21: 89–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xiao X, Wang Y, Hou Y, et al. Vitamin D deficiency and related risk factors in patients with diabetic nephropathy. J Int Med Res 2016; 44: 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cohen J. Statistical power analysis for the behavioral sciences 2nd ed, http://www.utstat.toronto.edu/~brunner/oldclass/378f16/readings/CohenPower.pdf (1988, accessed 13 April 2020).
- 27. Yaturu S, Youngberg B, Zdunek S. Vitamin D levels in subjects with or without chronic kidney disease among veterans with diabetes in North East United States. World J Diabetes 2017; 8: 346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. MacIsaac RJ, Ekinci EI, Premaratne E, et al. The chronic kidney disease-epidemiology collaboration (CKD-EPI) equation does not improve the underestimation of glomerular filtration rate (GFR) in people with diabetes and preserved renal function. BMC Nephrol 2015; 16: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Camargo EG, Soares AA, Detanico AB, et al. The chronic kidney disease epidemiology collaboration (CKD-EPI) equation is less accurate in patients with type 2 diabetes when compared with healthy individuals. Diabet Med 2011; 28: 90–95. [DOI] [PubMed] [Google Scholar]
- 30. Porrini E, Ruggenenti P, Luis-Lima S, et al. Estimated GFR: time for a critical appraisal. Nat Rev Nephrol 2019; 15: 177–190. [DOI] [PubMed] [Google Scholar]
- 31. Bolignano D, Mattace-Raso F, Sijbrands EJG, et al. The aging kidney revisited: a systematic review. Ageing Res Rev 2014; 14: 65–80. [DOI] [PubMed] [Google Scholar]
- 32. Ravani P, Malberti F, Tripepi G, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int 2009; 75: 88–95. [DOI] [PubMed] [Google Scholar]
- 33. Kurosu H, Ogawa Y, Miyoshi M, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 2006; 281: 6120–6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Derakhshanian H, Shab-Bidar S, Speakman JR, et al. Vitamin D and diabetic nephropathy: a systematic review and meta-analysis. Nutrition 2015; 31: 1189–1194. [DOI] [PubMed] [Google Scholar]
- 35. Fernández-Juárez G, Luño J, Barrio V, et al. 25 (OH) vitamin D levels and renal disease progression in patients with type 2 diabetic nephropathy and blockade of the renin-angiotensin system. Clin J Am Soc Nephrol 2013; 8: 1870–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao J, Dong J, Wang H, et al. Efficacy and safety of vitamin D3 in patients with diabetic nephropathy: a meta-analysis of randomized controlled trials. Chin Med J (Engl) 2014; 127: 2837–2843. [PubMed] [Google Scholar]
- 37. Lundwall K, Jacobson SH, Jörneskog G, et al. Treating endothelial dysfunction with vitamin D in chronic kidney disease: a meta-analysis. BMC Nephrol 2018; 19: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. ClinicalTrials.gov. Vitamin D and Omega-3 trial to prevent and treat diabetic kidney disease (Vital-DKD). NCT01684722, https://clinicaltrials.gov/ct2/show/NCT01684722 (Accessed 10 June 2019)
- 39. Santos RKF, Brandão-Lima PN, Tete RMDD, et al. Vitamin D ratio and glycemic control in individuals with type 2 diabetes mellitus: a systematic review. Diabetes Metab Res Rev. Epub ahead of print 21 December 2017. DOI: 10.1002/dmrr.2969. [DOI] [PubMed] [Google Scholar]
- 40. Wu C, Qiu S, Zhu X, et al. Vitamin D supplementation and glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Metabolism 2017; 73: 67–76. [DOI] [PubMed] [Google Scholar]
- 41. Mirhosseini N, Vatanparast H, Mazidi M, et al. The effect of improved serum 25-hydroxyvitamin D status on glycemic control in diabetic patients: a meta-analysis. J Clin Endocrinol Metab 2017; 102: 3097–3110. [DOI] [PubMed] [Google Scholar]
- 42. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a Mendelian randomisation study. Lancet Diabetes Endocrinol 2014; 2: 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Paula TP, Kramer CK, Viana LV, et al. Effects of individual micronutrients on blood pressure in patients with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. Sci Rep 2017; 7: 40751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Palaniswamy S, Hyppönen E, Williams DM, et al. Potential determinants of vitamin D in Finnish adults: a cross-sectional study from the Northern Finland birth cohort 1966. BMJ Open 2017; 7: e013161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bonelli P, Buonocore R, Aloe R, et al. Blood sampling seasonality as an important preanalytical factor for assessment of vitamin D status. J Med Biochem 2016; 35: 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schramm S, Lahner H, Jöckel KH, et al. Impact of season and different vitamin D thresholds on prevalence of vitamin D deficiency in epidemiological cohorts-a note of caution. Endocrine 2017; 56: 658–666. [DOI] [PubMed] [Google Scholar]
- 47. Eloi M, Horvath DV, Szejnfeld VL, et al. Vitamin D deficiency and seasonal variation over the years in São Paulo, Brazil. Osteoporos Int 2016; 27: 3449–3456. [DOI] [PubMed] [Google Scholar]
- 48. Soveri I, Berg UB, Björk J, et al. Measuring GFR: a systematic review. Am J Kidney Dis 2014; 64: 411–424. [DOI] [PubMed] [Google Scholar]