Abstract
Background and aims:
To the best of our knowledge, no studies have investigated the metabolic control of patients with premature coronary artery disease (CAD). The present study analyzes the metabolic control, defined as the simultaneous target in blood pressure, low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol and hemoglobin A1c, as well as the factors associated with its achievement in patients with premature CAD.
Methods:
The study included 1206 patients with CAD diagnosed before the age of 55 and 65 years in men and women, respectively. Sociodemographic, clinical and biochemical data were collected to know the prevalence of cardiovascular risk factors, including individual components of metabolic control plus smoking cessation and body mass index (BMI) <25 kg/m2. Non-strict and strict targets were used to evaluate metabolic control.
Results:
Participants were 54 ± 8 years old, 19.7% were women and had a median CAD evolution of 2.4 years. Non-strict and strict metabolic control was achieved by 18.4% and 6.2% of patients, respectively. Moreover, 79.8% and 67.6% met a composite of three or more cardiovascular risk factor goals using both criteria. BMI <25 kg/m2 was independently associated with 1.734 (95% confidence interval: 1.207–2.492) and 2.541 (95% confidence interval: 1.608–4.014) higher probabilities to meet non-strict or strict metabolic control.
Conclusion:
Our results show that 18.4% and 6.2% of subjects with premature CAD achieved non-strict and strict metabolic control, respectively. BMI <25 kg/m2 was found to be associated with the achievement of metabolic control. Multidisciplinary strategies including healthy lifestyle changes and pharmacological therapies could decrease the socioeconomic and clinical impact of premature CAD.
Keywords: blood pressure, dyslipidemia, metabolic control, premature coronary artery disease
Coronary artery disease (CAD) is the leading cause of mortality and disability around the world.1,2 and the years of life lost due to premature cardiovascular death have gradually increased since the end of the last century.1 The American Heart Association (AHA) has established a new concept of cardiovascular health that promotes healthier lifestyles.3 This concept includes the control of environmental risk factors such as diet, physical activity, smoking cessation and body mass index (BMI), in addition to the control of total cholesterol, blood pressure and fasting plasma glucose, which have been considered as classical clinical risk factors. Improvement of these risk factors helps to reduce the development of incident and recurrent cardiovascular events.3 Likewise, and based on scientific evidence, several clinical practice guidelines have been developed for the control of cardiovascular risk factors in patients with established CAD;4–6 these recommendations have been updating constantly in the last few years. Although the AHA and the American College of Cardiology (ACC) previously recommended blood pressure <140/90 mmHg in patients with CAD, they have recently reduced its goal to <130/80 mmHg.6 In addition, the AHA/ACC suggests that low-density lipoprotein cholesterol (LDL-C) should be kept less than 70 mg/dL and non-high-density lipoprotein cholesterol (non-HDL-C) less than 100 mg/dL to reduce the onset of new and/or recurrent cardiovascular events.7 On the other hand, in 2017, the American Association of Clinical Endocrinologists (AACE) proposed values of LDL-C <55 mg/dL and non-HDL-C <80 mg/dL, in patients with premature CAD considered as subjects with extreme cardiovascular risk.4 Concerning patients with CAD and diabetes, the American Diabetes Association recommends that hemoglobin A1c (HbA1c) should be kept below 8% as a goal to reduce morbidity and mortality in patients with macrovascular complications.8 According to the AHA/ACC recommendations (non-strict criteria for blood pressure, LDL-C and non-HDL-C), it has been reported that less than half of patients with secondary prevention reached an adequate metabolic control.9,10 However, currently, there are no studies that: (a) have used the AACE recommendations (strict criteria for LDL-C and non-HDL-C) and the stringent blood pressure goal (<130/80 mmHg) to analyze the metabolic control in patients with CAD; (b) have analyzed metabolic control in patients with premature CAD; and (c) have investigated the association of clinical, biochemical and environmental risk factors with metabolic control of premature CAD patients. Hence, the objective of the present study was to analyze the prevalence of non-strict and strict metabolic control (defined as blood pressure, LDL-C, non-HDL-C and HbA1c goals achieved), as well as the independent variables associated with this metabolic control, in patients with premature CAD.
Methods
Patients
The population studied was selected from the Genetics of Atherosclerotic Disease (GEA) study database. The GEA is an observational transversal study designed to examine the genomic basis of CAD, and to determine the association of traditional and emerging cardiovascular risk factors with clinical and subclinical CAD, in a sample of Mestizo-Mexican subjects.11 The study included 1206 patients with a previous diagnosis of premature CAD, 35–74 years old, treated, and enrolled from 2008 to 2013 at the Instituto Nacional de Cardiología Ignacio Chávez (INCICh). Premature CAD was defined as documented history of stable or unstable angina pectoris, or acute myocardial infarction for more than 3 months before enrollment, history of percutaneous coronary intervention, coronary artery by-pass grafting, or coronary stenosis >50% detected by angiography, diagnosed before 55 and 65 years of age in men and women, respectively. Patients with current use of corticosteroids, established chronic kidney disease, hepatic, thyroid or malignant disease were excluded from the study. The GEA study was approved by the INCICh Ethics Committee of Research on humans (approval number: 09646) and was conducted according to the 1975 Declaration of Helsinki. Written informed consent was obtained from each patient included in the study.
All participants completed a medical examination in which previously trained staff interviewed them and applied standardized questionnaires to collect sociodemographic information, history of CAD, current use of drug therapy, and alcohol or tobacco consumption. CAD evolution of each patient was calculated as the time from the first cardiovascular event until enrollment in the study. Optimal pharmacological compliance (at least 85%) was considered when the patient self-reported quitting treatment for 1 day or less per week. Current smoking status was defined when subjects self-reported that they had smoked tobacco in the previous 12 months and included those who had quit within the past year. Former smokers were defined as those who had quit more than a year earlier.12 The physical activity index was calculated using the Baecke questionnaire,13 the total physical activity was obtained from the sum of the physical activity during work, sports and recreational time. The questionnaire and physical activity indexes have been previously validated in an adult population.14 The height was measured with a rigid stadiometer that identifies up to 1 cm difference and the weight was assessed with a calibrated scale that reaches 0.1 kg differences. The BMI was calculated as the weight in kilograms divided by the square of body height in meters (m2); overweight was defined as BMI between 25 and 29.9 kg/m2 and obesity as BMI ⩾30 kg/m2. Systolic/diastolic blood pressure was measured after the patients remained seated for at least 10 min and the average of the second and third measurements was used for the analysis. Hypertension was defined as blood pressure values >140/90 mmHg or the use of antihypertensive drug therapy. Blood pressure goals were defined as <130/80 and <140/90 mmHg for strict and non-strict metabolic control cut-off points.6 Type 2 diabetes mellitus was defined when fasting plasma glucose values were ⩾126 mg/dL or when the patient self-reported a previous diagnosis or current hypoglycemic drug use.15 As suggested for patients with macrovascular complications, HbA1c <8% was considered as the goal for glycemic control in patients with diabetes.8
Biochemical analysis
Blood samples from the patients were collected after at least 10 h of fasting. The glucose blood concentration, total cholesterol, HDL-C, apolipoproteins AI and B100, as well as triglycerides, were evaluated in fresh samples, using standardized enzymatic procedures in a Hitachi autoanalyzer 902 (Hitachi Ltd, Tokyo, Japan). The precision and accuracy of lipid measurements are evaluated regularly by the Centers for Disease Control and Prevention (Atlanta, GA, USA). LDL-C was calculated using the De Long et al. formula.16 Adequate control of total cholesterol was considered when its blood concentration was <200 mg/dL. Non-HDL-C was calculated as total cholesterol minus HDL-C. According to the AACE, strict cut-off points for LDL-C and non-HDL-C were defined as serum levels <55 mg/dL and <80 mg/dL, respectively.4 According to the AHA/ACC, non-strict cut-off points were considered when those values were <70 mg/dL and <100 mg/dL, respectively.7 The percentage of HbA1c was assessed by an immunoassay in an autoanalyzer for clinical chemistry (COBAS C501; Roche Diagnostics GmbH, Mannheim, Germany). The simultaneous achievement of blood pressure, LDL-C, non-HDL-C, and HbA1c <8.0% (only in subjects with diabetes) goals was considered as metabolic control, according to either non-strict or strict criteria (Table 1).
Table 1.
Cardiovascular risk factor targets to achieve non-strict or strict metabolic controla in patients with premature coronary artery disease.
| Cardiovascular risk factor | Non-strict cut-off point | Strict cut-off point |
|---|---|---|
| Systolic/diastolic blood pressure, mmHg | <140/90 | <130/80 |
| Low density lipoprotein cholesterol, mg/dL | <70 | <55 |
| Non-high-density lipoprotein cholesterol, mg/dL | <100 | <80 |
| Hemoglobin A1c, % | <8 | <8 |
Metabolic control includes the simultaneous achievement of all cardiovascular risk factors, except for hemoglobin A1c, which was included only if the patient had a previous diagnosis of diabetes. Non-strict metabolic control defined according to the American Heart Association and the American College of Cardiology.6 Strict metabolic control defined according to the American Association of Clinical Endocrinologists.4 Hemoglobin A1c cut-off point was based on The American Diabetes Association statement for patients with diabetes and coronary artery disease.8
Statistical analysis
Values are expressed as mean ± standard deviation, median (interquartile range) or number of subjects (%). Means and medians were compared using ANOVA (Scheffe test as post hoc) or Kruskal–Wallis tests (Mann–Whitney U test for individual comparison) when needed, and frequencies were compared with chi-squared test. Multiple logistic regression analysis was used to evaluate independent factors associated with the achievement of strict or non-strict metabolic control. Variables that showed statistical differences among patients with or without metabolic control, as well as those with biological plausibility, were used as co-variables. Analyses were performed using the SPSS versus 15.0 statistical package (SPSS Chicago, IL, USA). All values with p < 0.05 were considered statistically significant.
Results
The clinical, sociodemographic and biochemical characteristics of patients with premature CAD are shown in Table 2. The participants had a mean age of 54 ± 8 years and one-fifth were women (19.7%). Most subjects had a diagnosis of prior myocardial infarction (one event: 78.8%, two events: 8.4%, three events: 0.5%) and only 12.3% had angina pectoris (stable angina: 8.9%, unstable angina: 3.4%). The prevalence in women was different according to the diagnosis of CAD (stable angina: 41.7%, unstable angina: 22.0%, one myocardial infarction event: 18.3%, two myocardial infarction events: 8.9%, three myocardial infarction events: 0%; p < 0.001). The median evolution of cardiovascular disease was 2.4 (0.7–6.4) years and despite the significant difference in CAD diagnoses [stable angina: 1.4 (0.6–4.0); unstable angina: 2.4 (0.6–7.6), one myocardial infarction event: 1.8 (0.6–5.5), two myocardial infarction events: 7.0 (4.0–11.1), three myocardial infarction events: 11.2 (6.2–15.3)], the time elapsed since the last coronary event was similar for all subjects [1.7 (0.6–5.3) years]. The prevalence of percutaneous coronary intervention with stenting and coronary artery by-pass grafting surgery showed a gradual increase when patients were stratified by the severity of the disease (stable angina: 13.1% and 1.9%, unstable angina: 42.5% and 17.1%, one myocardial infarction event: 45.9%, 9.9%, two myocardial infarction events: 69.0% and 14.9%, three myocardial infarction events: 83.3% and 16.7%; respectively, p < 0.05 for both). Sociodemographic data indicate that about 80% of patients lived as a couple, more than half of them had an education beyond primary school, about 30% were unemployed, and more than 20% had incomes below the minimum Mexican wage. Dietary-healthy habits showed that they consumed more than 2000 kcal/day, median alcohol consumption was 1.34 g/day and the median of the total physical activity index was 7.5. On average, blood pressure values were below 120/80 mmHg and nearly 100% of the subjects were under antihypertensive therapy. Although 94% of patients were under lipid-lowering treatment and mean total cholesterol was below 200 mg/dL, LDL-C and non-HDL-C were above the recommended goals.
Table 2.
Clinical, sociodemographic and biochemical characteristics of patients with premature coronary artery disease.
| Total N = 1206 |
|
|---|---|
| Age, years | 54 ± 8 |
| Female sex, % | 19.7 |
| Coronary artery disease evolution, years | 2.4 (0.7–6.4) |
| Coronary artery disease type, % (n) | |
| Stable angina | 8.9 (108) |
| Unstable angina | 3.4 (41) |
| Myocardial infarction | |
| One event | 78.8 (950) |
| Two events | 8.4 (101) |
| Three events | 0.5 (6) |
| Time since last event, years | 1.7 (0.6–5.3) |
| PCI with stenting, % | 45.0 |
| CABG, % | 9.9 |
| Married, % | 79.0 |
| Education, % | |
| Less than high school | 44.0 |
| High school | 14.9 |
| Some college | 39.0 |
| Completed college | 2.1 |
| Working status, % | |
| Full time | 33.6 |
| Part time | 37.4 |
| Unemployed | 28.9 |
| Monthly income | |
| ⩾US$485 | 11.5 |
| US$242–424 | 22.6 |
| US$121–176 | 43.9 |
| US$⩽60.5 | 21.9 |
| Smoking status, % | |
| Current | 11.4 |
| Former | 62.8 |
| Never | 25.8 |
| Total physical activity index | 7.50 (6.75–8.38) |
| Total kilocalories per day index | 2048 (1656–2534) |
| Alcohol intake, g/day | 1.34 (0.38–4.80) |
| BMI, kg/m2 | 29 ± 4 |
| Normal weight, % | 17.0 |
| Overweight, % | 46.9 |
| Obesity, % | 36.1 |
| Systolic blood pressure, mmHg | 119 ± 19 |
| Diastolic blood pressure, mmHg | 72 ± 10 |
| Antihypertensive treatment, % | 97.8 |
| Treatment adherence ⩾85%, % | 94 |
| Cholesterol, mg/dL | |
| Total | 165 ± 45 |
| Low-density lipoprotein | 96 ± 39 |
| High-density lipoprotein | 39 ± 10 |
| Non-high-density lipoprotein | 119 (92–149) |
| Triglycerides, mg/dL | 160 (116–214) |
| Apolipoprotein B100, mg/dL | 84 ± 31 |
| Apolipoprotein AI, mg/dL | 121 ± 26 |
| Lipid-lowering treatment, % | 94.1 |
| Treatment adherence ⩾85%, % | 87 |
| Fasting plasma glucosea, mg/dL | 91 ± 9 |
| Type 2 diabetes, % | 36.2 |
| Hypoglycemic treatment, % | 92.9 |
| Fasting plasma glucose, mg/dL | 148 ± 55 |
| HbA1c, % | 7.89 ± 2.03 |
| Insulin, IU/dL | 19.0 (13.8–26.5) |
| Antiplatelet treatment, % | 97.3 |
| Anticoagulant treatment, % | 6.1 |
| Lipid-lowering treatment, % | 94.1 |
Normal weight = BMI <25 kg/m2, overweight = BMI 25–29.9 kg/m2, obesity = BMI ⩾30 kg/m2; current smoking was defined as tobacco consumption ⩾1per day.
Only patients without diabetes.
BMI, body mass index; CABG, coronary artery bypass grafting; HbA1c, hemoglobin A1c; PCI, percutaneous coronary intervention.
The prevalence of non-strict metabolic control was 18.4%, whereas strict metabolic control was achieved by 6.2% of the total population. The individual goals achieved for blood pressure, LDL-C and non-HDL-C (strict and non-strict metabolic control), as well as for smoking cessation, BMI <25 kg/m2, and HbA1c <8.0% (in subjects with diabetes) are shown in Figure 1. Overall, data indicate that more than half of the subjects reached the target for blood pressure, smoking cessation and glycemic control. Conversely, less than one-third of patients achieved the goals for lipid and weight control. Importantly, strict lipid control was achieved by ~12% to ~14% of the subjects. Compared with those that did not reach any lipid targets, lipid-lowering treatment prevalence was higher among subjects who achieved goals in LDL-C (92.5% versus 98.4%; p < 0.001) and non-HDL-C (96.6% versus 97.9%; p = 0.044), regardless of the cut-off point. On the other hand, the prevalence of hypoglycemic treatment was higher among subjects with uncontrolled diabetes (97.9% versus 92.0%; p = 0.017). The analysis of the cumulative number of clinical, biochemical and behavioral cardiovascular risk factor goals achieved reveals that using the non-strict cut-off points, 79.8% of the subjects had ⩾3 well-controlled risk factors, but only 4.5% had six risk factors on target. When the strict cut-off points were considered, the prevalence was reduced to 67.6% and 2.1%, respectively (Figure 2).
Figure 1.
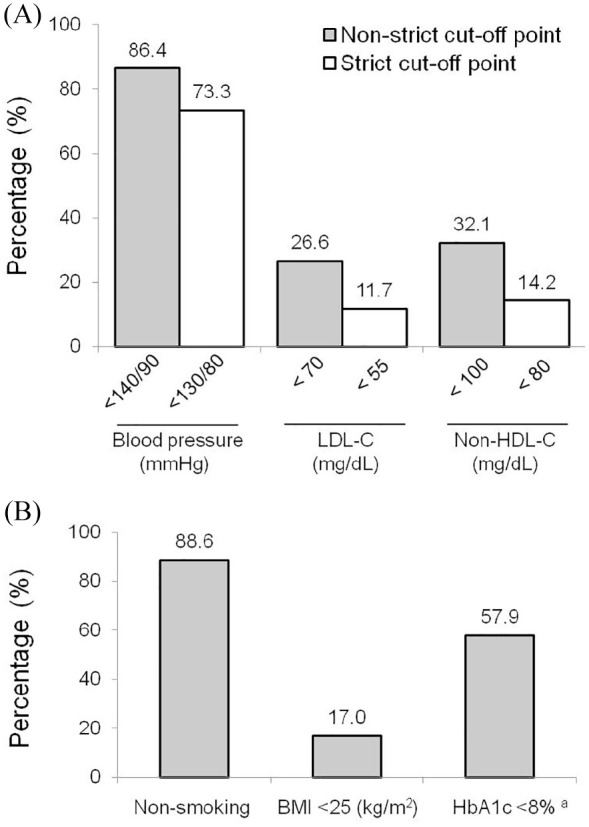
Prevalence of cardiovascular risk factors goals achieved in patients with premature coronary artery disease. (A) Non-strict and strict cut-off points for blood pressure, low-density lipoprotein cholesterol (LDL-C) and non-high-density lipoprotein cholesterol (non-HDL-C). (B) Prevalence of goals achieved in smoking, body mass index (BMI) and hemoglobin A1c (HbA1c).
aOnly patients with diabetes.
Figure 2.
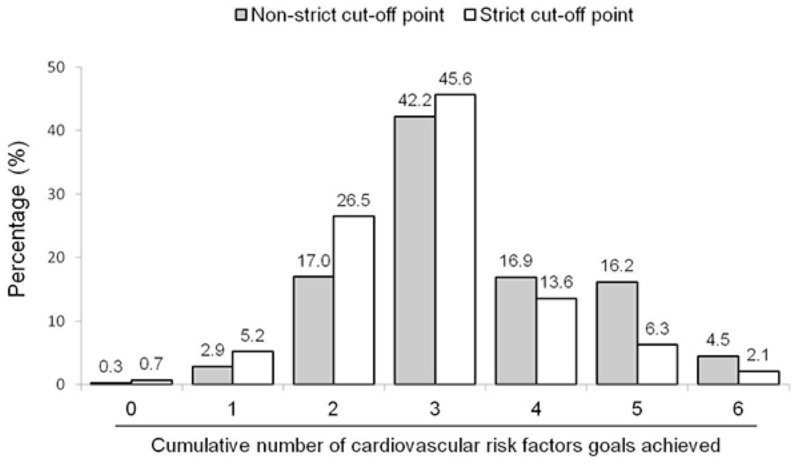
Prevalence of the cumulative number of cardiovascular risk factors goals achieved in patients with premature coronary artery disease. Non-strict cut-off points: blood pressure <140/90 mmHg, low-density lipoprotein cholesterol (LDL-C) <70 mg/dL, non-high-density lipoprotein cholesterol (non-HDL-C) <100 mg/dL, absence of tobacco consumption, body mass index <25 kg/m2, hemoglobin A1c <8% (only patients with diabetes). Strict cut-off points consider the same values except for blood pressure (<130/80 mmHg), LDL-C (<55 mg/dL) and non-HDL-C (<80 mg/dL).
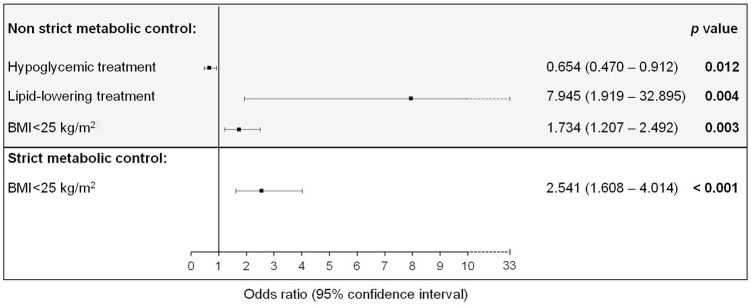
Univariate analysis showed that smoking cessation [odds ratio: 1.828; (95% confidence interval: 1.062–3.146) p = 0.029], BMI <25 mg/m2 [1.828 (1.062–2.603); p < 0.001] and lipid-lowering therapy [1.206 (1.149–1.267); p < 0.001] were associated with non-strict metabolic control achievement; whereas hypoglycemic treatment was associated with poor achievement [0.937 (0.888–0.989); p = 0.023]. On the other hand, BMI <25 kg/m2 was the only variable that was associated with strict metabolic control achievement [2.541 (1.608–4.014); p < 0.001]. The multivariate analysis showed that except from smoking cessation, all these variables were independently associated with metabolic control achievement (Figure 3).
Figure 3.
Multiple regression analysis to identify factors independently associated with composite control in blood pressure, low-density lipoprotein cholesterol (LDL-C), non-high-density lipoprotein cholesterol (non-HDL-C) and hemoglobin A1c (HbA1c) in patients with premature coronary artery disease. Age, gender, smoking status, lipid-lowering treatment, hypoglycemic treatment, body mass index (BMI) <25 kg/m2, living status, unemployment and monthly income were included as covariates. Non-strict metabolic control was defined as blood pressure <140/90 mmHg, LDL-C <70 mg/dL, non-HDL-C <100 mg/dL and HbA1c <8% in patients with diabetes. Strict metabolic control was defined as blood pressure <130/80 mmHg, LDL-C <55 mg/dL, non-HDL-C <80 mg/dL and HbA1c <8% in patients with diabetes.
Discussion
Reports published during the last decade have shown that the socioeconomic impact of premature CAD has gradually increased in high-, middle- and low-income countries.1,2 As noted, recommendation-based guidelines on cardiovascular secondary prevention worldwide have been constantly updated with the aim of reducing cardiovascular morbidity and mortality and decreasing the high costs represented by CAD.4–8 In this context, several studies have reported that simultaneous achievement of cardiovascular risk prevention goals is poor.9,10,17,18 To the best of our knowledge, the present study is the first to analyze the composite cardiovascular risk factors control in premature CAD patients, using former and recent stringent criteria. Our results highlight that less than 20% and 10% of premature CAD patients achieved non-strict or strict metabolic control, respectively. Likewise, 4.5% and 2.1% from the studied population achieved goals of blood pressure, LDL-C, non-HDL-C, smoking cessation, BMI <25 kg/m2 and HbA1c <8.0% (in those with diabetes) using non-strict and strict criteria, respectively. Moreover, 79.8% and 67.6% met a composite of ⩾3 behavioral and clinical risk factor goals using both criteria. In addition, the data show that, independently from the pharmacological therapy, BMI <25kg/m2 was consistently associated with higher rates of composite cardiovascular risk factors met. The aforementioned suggests that improvement in behavioral changes could enhance quality of life and reduce socioeconomic burden of CAD, as previously reported by epidemiological studies.12,18–20
One of the major modern challenges for cardiometabolic control around the world in secondary prevention is the lack of multiple cardiovascular risk factors control. In a recent report, Blom et al. described that only 12% of patients with high- and very high-cardiovascular risk, including those with stable CAD, achieved goals in LDL-C, HbA1c and blood pressure.17 The authors highlighted that their findings are similar to other European and American studies showing low attainment goal rates of metabolic control.18–24 In other American cohorts of patients with stable CAD, researchers found that nearly 7.6% achieved simultaneous control of LDL-C, non-HDL-C, blood pressure, HbA1c, BMI, smoking and physical activity.9,10 Compared with those reports, the present study shows that less than 5% of subjects with premature CAD met a composite of six cardiovascular risk factor goals, including behavioral and clinical risk factors, using both non-strict and strict criteria. Moreover, we found that 18.4% and 6.2% of patients met non-strict and strict metabolic control, respectively. Likewise, by design of the GEA study, selected subjects were younger in order to analyze the natural history of premature CAD. In addition, this analysis shows higher rates of smoking history (74.2%) and BMI <30 kg/m2 (63.9%), and a lesser prevalence of type 2 diabetes mellitus (36.2%).17–19,21 Although most previous studies did not include physical activity and kilocalories consumption, their results suggest a poor balance between energy consumption and expenditure in patients with very high cardiovascular risk. These unhealthy lifestyles adversely impact the control of major cardiovascular risk factors such as hypertension, raised LDL-C and glycemic control.18 Our results support this hypothesis, showing a low total physical activity index (normal ranges: 9.1–14.9 in adult population).14 Furthermore, we found that 68.3% of the participants were consuming more kilocalories per day than they required, according to the Harris–Bennedict formula (data not shown).25 Our data also show that a BMI <25 kg/m2 was consistently and positively associated with the achievement of non-strict and strict metabolic control (Figure 3). This finding enhances the hypothesis about a poor balance between energy consumption and expenditure among patients with established CAD, and is supported by studies showing that obesity is associated with poorer glycemic, blood pressure and lipid control.18,26–32
Following the growing evidence from the last decades about secondary prevention in patients with CAD, guidelines have updated their criteria to prevent recurrent events.4–8 Despite that stringent control has shown further cardiovascular benefits, their achievement has not been widely met.15,17–19,22 Noteworthy, participants from the GEA study were selected from 2008 to 2013, when recommendation-based guidelines were not yet as tight. Although more than 90% of subjects were under blood pressure- and lipid-lowering treatment, 73.3%, 14.2% and 11.7% met strict goals in blood pressure, non-HDL-C and LDL-C, respectively. Although previous studies have found that socioeconomic status was negatively associated with poor metabolic control,19–23,26,27 the present analysis shows that sociodemographic data were not different among subjects with premature CAD that met or did not meet metabolic control. Of note, most of the subjects selected in the GEA study come from low socioeconomic status; therefore, results from sociodemographic data could not be fully reliable. On the other hand, lipid-lowering treatment was independently associated with an eight-fold higher probability to meet non-strict metabolic control, whereas hypoglycemic treatment was inversely related to its achievement. A plausible explanation for the last controversial finding is that patients with diabetes had a worse cardiovascular profile. This is supported by the fact that, although almost 60% of the subjects with diabetes met HbA1c <8.0%, patients with uncontrolled diabetes had higher prevalence of glucose-lowering drugs prescription (97.9% versus 92.0%; p = 0.017). In addition, compared with subjects with HbA1c <8%, those with uncontrolled diabetes were characterized by having higher prevalence of insulin treatment (5.0% versus 15.9%; p < 0.001). Overall, the cluster of these findings, regarding weight control, unhealthy diet, poor physical activity, and pharmacological treatment, supports the fact that metabolic control of patients with CAD requires modern preventive programs. Recent studies have pointed out that multidisciplinary teams of healthcare professionals, including nutritionists, dietitians, cardiovascular physiotherapists, psychologists and medical specialists, should address all these aspects of lifestyle changes to improve the cardiovascular perspectives in patients with established CAD.18,19,21,22
An important strength of our analysis is that we studied the composite impact of sociodemographic, clinical and biochemical data in patients with premature CAD, which has not been widely studied yet. Likewise, we further compared prior evidence-based guidelines for metabolic target goals with the recent goals proposed lately. A limitation of the present study is that our population was extracted from a single tertiary center of cardiology in Mexico, and this may interfere with the interpretation of the results from the whole premature CAD population due to possible bias of the same medical inertia of our center. Furthermore, it is important to point out that the selected sample was of Mexican-Mestizo origin and results may not apply for other ethnic groups. Although the small number of women could be considered a limitation of the present study, it is consistent with observations in previous cohorts where the prevalence of women with CAD has been found to be 20–35%.9,10,18,21 Another limitation is the cross-sectional design of the present study, which does not allow knowing the causality of the associations found. In addition, the short follow-up of some patients after the first cardiovascular event could also impact on the metabolic control achievement. Finally, it was not possible to know accurately what drugs and the amount the patients were taking through direct evaluation; however, we applied standardized questionnaires to collect information about pharmacological prescriptions to properly analyze the association of therapies with metabolic goals achievement. As noted, mechanism of action, doses and intensity of blood pressure-, lipid- and glucose-lowering drugs were not evaluated in this work because this will be described in more detail elsewhere.
In summary, the present study indicates that 18.4% and 6.2% of subjects with premature CAD achieved non-strict and strict metabolic control, respectively. In addition, BMI <25 kg/m2 was found to be an independent factor and consistently associated with the achievement of metabolic control. The results suggest that healthy lifestyle changes, added to current pharmacological therapies, should be implemented through multidisciplinary strategies to reduce the socioeconomic and clinical impact on subjects with premature CAD.
Acknowledgments
The authors thank the Consejo Nacional de Ciencia y Tecnología by funding the GEA study. We also thank the participation of Cesar Iridiani Javier-Montiel for his contribution to the drafting of the manuscript.
Footnotes
Author contribution(s): Aida X Medina-Urrutia: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Supervision; Writing-original draft; Writing-review & editing.
Froylan David Martínez-Sánchez: Conceptualization; Formal analysis; Writing-original draft; Writing-review & editing.
Carlos Posadas-Romero: Conceptualization; Investigation; Methodology; Project administration; Resources; Writing-original draft; Writing-review & editing.
Esteban Jorge-Galarza: Investigation; Methodology; Writing-original draft; Writing-review & editing.
María del Rocío Martínez-Alvarado: Investigation; Writing-original draft; Writing-review & editing.
María del Carmen González-Salazar: Investigation; Methodology; Writing-original draft; Writing-review & editing.
Horacio Osorio-Alonso: Formal analysis; Writing-original draft; Writing-review & editing.
Juan Gabriel Juárez-Rojas: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Writing-original draft; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This study was partially supported by Mexico’s Consejo Nacional de Ciencia y Tecnología (Project No. SALUD-2010-2-150537).
Data accessibility statement: Juárez-Rojas Juan Gabriel is custodian of the research data and guarantees its availability upon request.
ORCID iD: Juárez-Rojas Juan Gabriel  https://orcid.org/0000-0001-8864-2304
https://orcid.org/0000-0001-8864-2304
Contributor Information
Aida X Medina-Urrutia, Department of Endocrinology, Instituto Nacional de Cardiología Ignacio Chávez, Tlalpan, Mexico City, Mexico.
Froylan D Martínez-Sánchez, Department of Endocrinology, Instituto Nacional de Cardiología Ignacio Chávez, Tlalpan, Mexico City, Mexico.
Carlos Posadas-Romero, Department of Endocrinology, Instituto Nacional de Cardiología Ignacio Chávez, Tlalpan, Mexico City, Mexico.
Esteban Jorge-Galarza, Department of Endocrinology, Instituto Nacional de Cardiología Ignacio Chávez, Tlalpan, Mexico City, Mexico.
María del Rocío Martínez-Alvarado, Department of Endocrinology, Instituto Nacional de Cardiología Ignacio Chávez, Tlalpan, Mexico City, Mexico.
María del Carmen González-Salazar, Department of Endocrinology, Instituto Nacional de Cardiología Ignacio Chávez, Tlalpan, Mexico City, Mexico.
Horacio Osorio-Alonso, Department of Cardio-Renal Physiopathology, Instituto Nacional de Cardiología Ignacio Chávez, Tlalpan, Mexico City, Mexico.
Juan Gabriel Juárez-Rojas, Department of Endocrinology, Instituto Nacional de Cardiología Ignacio Chávez, Juan Badiano 1, Sección XVI, C.P., Tlalpan, Mexico City, 14080, Mexico.
References
- 1. Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380: 2197–2223. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2015. Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016; 388: 1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation 2019; 139: e56–e528. [DOI] [PubMed] [Google Scholar]
- 4. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American association of clinical endocrinologists and American college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract 2017; 23(Suppl. 2): 1–87. [DOI] [PubMed] [Google Scholar]
- 5. Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016; 37: 2999–3058. [DOI] [PubMed] [Google Scholar]
- 6. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. J Am Coll Cardiol 2019; 73: e285–e350. [DOI] [PubMed] [Google Scholar]
- 7. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J Am Coll Cardiol 2018; 71: e127–e248. [DOI] [PubMed] [Google Scholar]
- 8. American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2019. Diabetes Care 2019; 42: S61–S70. [DOI] [PubMed] [Google Scholar]
- 9. Farkouh ME, Boden WE, Bittner V, et al. Risk factor control for coronary artery disease secondary prevention in large randomized trials. J Am Coll Cardiol 2013; 61: 1607–1615. [DOI] [PubMed] [Google Scholar]
- 10. Brown TM, Voeks JH, Bittner V, et al. Achievement of optimal medical therapy goals for U.S. adults with coronary artery disease. J Am Coll Cardiol 2014; 63: 1626–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Villarreal-Molina T, Posadas-Romero C, Romero-Hidalgo S, et al. The ABCA1 gene R230C variant is associated with decreased risk of premature coronary artery disease: the genetics of atherosclerotic disease (GEA) study. PLoS One 2012; 7: e49285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004; 364: 937–952. [DOI] [PubMed] [Google Scholar]
- 13. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982; 36: 936–942. [DOI] [PubMed] [Google Scholar]
- 14. Hertogh EM, Monninkhof EM, Schouten EG, et al. Validity of the modified Baecke questionnaire: comparison with energy expenditure according to the doubly labeled water method. Int J Behav Nutr Phys Act 2008; 5: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care 2019; 42: S13–S28. [DOI] [PubMed] [Google Scholar]
- 16. DeLong DM, DeLong ER, Wood PD, et al. A comparison of methods for the estimation of plasma low- and very low-density lipoprotein cholesterol. The lipid research clinics prevalence study. JAMA 1986; 256: 2372–2377. [PubMed] [Google Scholar]
- 17. Blom DJ, Santos RD, Daclin V, et al. The challenge of multiple cardiovascular risk factor control outside Western Europe: findings from the international ChoLesterol management practice study. Eur J Prev Cardiol 2019; 204748731987173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kotseva K, De Backer G, De Bacquer D, et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: results from the European society of cardiology ESC-EORP EUROASPIRE V registry. Eur J Prev Cardiol 2019; 26: 824–835. [DOI] [PubMed] [Google Scholar]
- 19. Fan W, Song Y, Inzucchi SE, et al. Composite cardiovascular risk factor target achievement and its predictors in US adults with diabetes: the diabetes collaborative registry. Diabetes Obes Metab 2019; 21: 1121–1127. [DOI] [PubMed] [Google Scholar]
- 20. Schroeder EB, Hanratty R, Beaty BL, et al. Simultaneous control of diabetes mellitus, hypertension, and hyperlipidemia in 2 health systems. Circ Cardiovasc Qual Outcomes 2012; 5: 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bertoni AG, Clark JM, Feeney P, et al. Suboptimal control of glycemia, blood pressure, and LDL cholesterol in overweight adults with diabetes: the look AHEAD study. J Diabetes Complications 2008; 22: 1–9. [DOI] [PubMed] [Google Scholar]
- 22. Jaffiol C, Thomas F, Bean K, et al. Impact of socioeconomic status on diabetes and cardiovascular risk factors: results of a large French survey. Diabetes Metab 2013; 39: 56–62. [DOI] [PubMed] [Google Scholar]
- 23. Rarau P, Pulford J, Gouda H, et al. Socio-economic status and behavioural and cardiovascular risk factors in Papua New Guinea: a cross-sectional survey. PLoS One 2019; 14: e0211068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song Y, Liu X, Zhu X, et al. Increasing trend of diabetes combined with hypertension or hypercholesterolemia: NHANES data analysis 1999–2012. Sci Rep 2016; 6: 36093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McKeown NM, Troy LM, Jacques PF, et al. Whole- and refined-grain intakes are differentially associated with abdominal visceral and subcutaneous adiposity in healthy adults: the Framingham heart study. Am J Clin Nutr 2010; 92: 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Williams J, Allen L, Wickramasinghe K, et al. A systematic review of associations between non-communicable diseases and socioeconomic status within low- and lower-middle-income countries. J Glob Health 2018; 8: 020409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yusuf S, Rangarajan S, Teo K, et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med 2014; 371: 818–827. [DOI] [PubMed] [Google Scholar]
- 28. Aucott L, Poobalan A, Smith WC, et al. Effects of weight loss in overweight/obese individuals and long-term hypertension outcomes: a systematic review. Hypertension 2005; 45: 1035–1041. [DOI] [PubMed] [Google Scholar]
- 29. Maggio CA, Pi-Sunyer FX. Obesity and type 2 diabetes. Endocrinol Metab Clin North Am 2003; 32: 805–822. [DOI] [PubMed] [Google Scholar]
- 30. Norris SL, Zhang X, Avenell A, et al. Long-term effectiveness of lifestyle and behavioral weight loss interventions in adults with type 2 diabetes: a meta-analysis. Am J Med 2004; 117: 762–774. [DOI] [PubMed] [Google Scholar]
- 31. Safford MM, Russell L, Suh DC, et al. How much time do patients with diabetes spend on self-care? J Am Board Fam Pract 2005; 18: 262–270. [DOI] [PubMed] [Google Scholar]
- 32. Wing RR, Goldstein MG, Acton KJ, et al. Behavioral science research in diabetes: lifestyle changes related to obesity, eating behavior, and physical activity. Diabetes Care 2001; 24: 117–123. [DOI] [PubMed] [Google Scholar]