Abstract
Objective
Many lung diseases are associated with changes in autophagic activity. The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling pathway plays a key regulatory role in autophagy. Our aim was to explore the function of PI3K/AKT/mTOR pathway on autophagy in chronic obstructive pulmonary disease (COPD) caused by particulate matter with a diameter <2.5 µm (PM2.5).
Methods
Male C57BL/6 mice were randomly divided into sham, model, and PI3K inhibitor groups. Mice were exposed to PM2.5 for 4 weeks to establish an in vivo COPD model. Alveolar epithelial cells were stimulated with PM2.5 to establish an in vitro COPD model.
Results
In mice with COPD induced by PM2.5, the PI3K inhibitor PF-04979064 suppressed protein expression of PI3K, p-AKT, and p-mTOR to increase apoptosis of alveolar epithelial cells and reduce autophagy. Short interfering PI3K suppressed the PI3K/AKT/mTOR pathway to induce apoptosis and reduce autophagy of alveolar epithelial cells in an in vitro model of COPD. Activation of PI3K induced the PI3K/AKT/mTOR pathway to reduce apoptosis of alveolar epithelial cells in the in vitro model of COPD by promoting autophagy.
Conclusions
These data demonstrate that PI3K/AKT/mTOR pathway regulates autophagy to induce apoptosis of alveolar epithelial cells in COPD.
Keywords: Chronic obstructive pulmonary disease, particulate matter, PI3K/AKT/mTOR pathway, autophagy, alveolar epithelial cells, lung disease
Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic respiratory system disease with high disability rate and high mortality. It is characterized by an incompletely reversible airway limitation.1 COPD frequently leads to gradual loss of labor capacity in patients.2 As a result, COPD patients cannot take care of themselves, severely affecting their quality of life. Research suggests that COPD ranks fourth among the current causes of death.2 It is estimated to rank fifth in global economic burden of disease by 2020.2 It has been reported that the highest morbidity associated with COPD is observed in the Western Pacific, while adult deaths mainly occur in China.1,3
During autophagy, the autophagosome binds with a lysosome to form the autophagic lysosome (or autophagosome).4 It can degrade damaged and degenerated macromolecules and organelles in the cytoplasm.5 The degradation products can be recycled, providing the raw materials for normal cellular survival and metabolism.5 However, excessive autophagy will induce excess cell damage, resulting in programmed cell death.4
The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling pathway plays a key regulatory role in autophagy.6 mTOR is the target molecule as well as the confluence of the cell autophagy upstream pathway.7 It is one of two complexes regulating autophagy and is the major inhibitory signal of autophagy.8 Its mechanism is to phosphorylate autophagy-related protein 13 (ATG13), preventing its binding with ATG1 to form the autophagosome.6 It can also promote adhesion of the ribosome to the endoplasmic reticulum to suppress endoplasmic reticulum detachment and form the autophagosome membrane.8 The PI3K/AKT cell survival signaling pathway is one of two upstream pathways regulating mTOR. Suppression of PI3K can greatly block the downstream signaling pathways AKT and mTOR.7
Atmospheric particulate matter is well-recognized risk factor for human health.9 Multiple epidemiological surveys and animal experiments have shown that a high concentration of particulate matter with a diameter <2.5 µm (PM2.5) in the atmosphere is closely related to high morbidity and mortality in many diseases such as lung cancer and cardiovascular disease.10 The respiratory tract is the key pathway for atmospheric particulate matter to enter the body, and lung is the primary affected organ. Air pollution is serious in China and has a great impact on public health.10 However, the pathogenic mechanism of a high PM2.5 concentration remains poorly understood. Oxidative stress in the body is considered the foundation of the toxicological effect of atmospheric particulate matter.11 Experiments in vitro on A549 cells suggest that PM2.5 can activate the PIK3/AKT signaling pathway and induce the nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated defense mechanism to resist cell oxidative stress.9 The aim of the present study was to explore the effect of PI3K/AKT/mTOR pathway on autophagy of COPD induced by PM2.5.
Materials and methods
Animal model
Male C57BL/6 (body weight 18–20 g, 4–5 weeks old) were housed at 22 to 23°C and 55% to 60% humidity. The mice were randomly divided into three groups: sham, model, and PI3K inhibitor groups. In the model and PI3K inhibitor groups, mice were exposed to PM2.5 (1.46 ± 0.034 mg/m3) for 2 hours a day for 4 weeks (hereafter, COPD model mice). In the sham group, mice were exposed to fresh air for 2 hours a day for 4 weeks. In addition, in week 4 of induction, mice in the PI3K inhibitor group were treated with PI3K inhibitor. All of the animal experiments were approved by the ethics committee of Gansu Province People Hospital.
Lung wet/dry weight ratio
Lung tissue was collected and weighed (lung wet weight). Then, the tissue was dried at 80°C for 48 hours and weighed again (lung dry weight). The ratio of lung wet and dry weight was calculated as lung wet weight/lung dry weight × 100%.
Histological determination
Lung tissues samples were collected in phosphate-buffered saline (PBS) and fixed with 10% formaldehyde overnight. Lung tissues samples were embedded in paraffin and cut into 4-μm-thick sections. Lung tissue samples were stained with hematoxylin and eosin (H&E) for 15 minutes and examined using a Leica confocal microscope (Solms, Germany) at 100× magnification.
In vitro model and transfection
Human bronchial epithelial cells (HBECs) were cultured in 2 mL of Dulbecco’s modified Eagle medium (DMEM; Sigma Chemical Co., St. Louis, MO, USA) containing 10% fetal bovine serum (Sigma Chemical Co.) at 37°C in the presence of 95% O2 and 5% CO2. Cells were transfected with short interfering (si)-PI3K, PI3K plasmid, and negative mimics using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). In the in vitro model, HBECs were subsequently stimulated with 20 μg/mL PM2.5 in medium for 24, 48, and 72 hours after transfection for 4 hours.
Cell viability assay and lactate dehydrogenase measurement
To measure viability, 10 μL of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was added to HBECs for 4 hours at 37°C. Then, old DMEM was removed and 120 μL of dimethyl sulfoxide (DMSO) was added to cells for 20 minutes at 37°C to dissolve formazan. Absorbance was measured at 490 nm using a microplate reader (Thermo Scientific, Waltham, MA, USA). Lactate dehydrogenase (LDH) activity was measured in HBECs using a kit (Beyotime, Jiangsu, China) and the results were measured at 490 nm using a micro-plate reader (Thermo Scientific).
Apoptosis rate
Cells were washed in PBS and stained with fluorescein isothiocyanate and propidium iodide (Pharmingen, Becton Dickinson Co., San Diego, CA, USA) for 15 minutes under darkness. Cell apoptosis was analyzed by flow cytometry (FACSCalibur; Becton-Dickinson Co.) using Flowjo 7.6.1 (FlowJo LLC/Becton-Dickinson Co.).
Caspase-3 and caspase-9 staining
Cells were collected and the concentration of protein estimated using the bicinchoninic acid assay. Protein (10 µg) was loaded and used to measure caspase-3/caspase-9 activity levels using caspase-3 and caspase-9 activity ELISA kits.
Western blot analysis
Total protein was extracted using radioimmunoprecipitation assay (RIPA) lysis buffer on ice for 30 minutes, and the concentration of protein was estimated using the bicinchoninic acid assay. Protein (50 μg) was loaded onto 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels for electrophoresis and transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% nonfat milk in Tris-buffered saline-Tween (TBST) for 1 hour, and incubated with primary anti-bodies: PI3K (ab32089, Abcam, Cambridge, UK), p-AKT (ab8805, Abcam), p-mTOR (ab109268, Abcam), LC3 (ab48394, Abcam), ATG5 (ab228668, Abcam), and GAPDH (1:5,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. The membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies (1:5,000, Santa Cruz Biotechnology) for 1 hour and washed with TBST for 15 minutes. Protein blanks were visualized by chemiluminescent horseradish peroxidase substrate and analyzed using Image Lab 3.0 (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Immunofluorescence
Cells were washed with PBS and fixed with 10% formaldehyde for 30 minutes. Cells were incubated with 5% bovine serum albumin in TBST for 1 hour at 37°C and incubated with LC3 (1:100, Santa Cruz Biotechnology) at 4°C overnight. Then, cells were incubated with anti-rabbit antibody for 1 hour at 37°C and stained with 4′,6-diamidino-2-phenylindole (DAPI) for 15 minutes under darkness. Immunofluorescence was examined using fluorescence microscopy (Axiovert 200 M; Carl Zeiss, Jena, Germany) at 200× magnification.
Statistical analysis
All values are expressed as the mean ± standard deviation. Student’s t-test or one-way analysis of variance and Tukey’s post-test were performed to evaluate differences among multiple groups. The level of significance was set to P < 0.05.
Results
Inhibition of PI3K increased apoptosis of alveolar epithelial cells in COPD mice
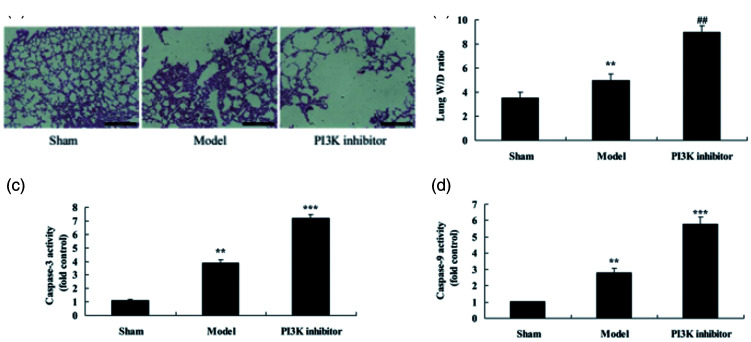
To study the function of PI3K in COPD by PM2.5, COPD mice were treated with the PI3K inhibitor PF-04979064. As shown in Figure 1a, apoptosis of alveolar epithelial cells was increased in COPD mice compared with those in the sham control group. Lung wet/dry weight rate (P = 0.0352), protein concentration (P = 0.0217) and caspase-3/9 activity (P = 0.0134 and 0.0208) were increased in COPD mice compared with mice in the sham control group (Figure 1b–1e). PI3K inhibitor promoted apoptosis of alveolar epithelial cells (P = 0.0072) and increased lung wet/dry weight ratio (P = 0.0174), protein concentration (P = 0.0131), and caspase-3/9 activity (P = 0.0091) in COPD mice (Figure 1a–1e).
Figure 1.
Inhibition of PI3K increased alveolar epithelial cells apoptosis in a COPD mouse model induced by PM2.5. Hematoxylin and eosin staining of lung tissue samples (a), lung wet weight/dry weight rate (b), and caspase-3/9 activity (c and d). **P < 0.01 compared with sham group; ##P < 0.01 compared with COPD model group. PI3K, phosphatidylinositol 3-kinase; COPD, chronic obstructive pulmonary disease; PM2.5, particulate matter with a diameter <2.5 µm; Sham, sham control mice group; Model, mouse model with COPD induced by PM2.5; PI3K inhibitor, treatment with PI3K inhibitor group.
Inhibition of PI3K suppressed PI3K/AKT/mTOR pathway in COPD mice
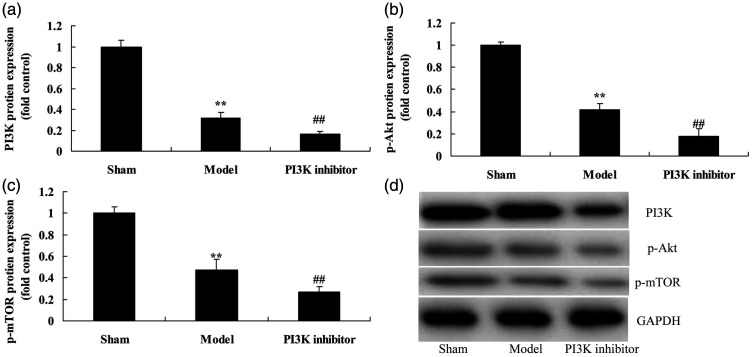
We explored the function of PI3K inhibitor on the PI3K/AKT/mTOR pathway in COPD. Protein expression of PI3K (P = 0.0092), p-AKT (P = 0.0176), and p-mTOR (P = 0.0231) was reduced in COPD mice compared with that in the sham group (Figure 2). We found that PI3K inhibitor suppressed protein expression of PI3K (P = 0.0288), p-AKT (P = 0.0131), and p-mTOR (P = 0.0347) compared with mice in the COPD group (Figure 2).
Figure 2.
Inhibition of PI3K suppressed the PI3K/AKT/mTOR pathway in a COPD mouse model induced by PM2.5. Protein expression of PI3K, p-AKT, and p-mTOR by statistical analysis (a, b, and c) and western blotting assays of PI3K, p-AKT, and p-mTOR protein level (d). **P < 0.01 compared with sham group; ##P < 0.01 compared with COPD model group. COPD, chronic obstructive pulmonary disease; PM2.5, particulate matter with a diameter <2.5 µm; PI3K, phosphatidylinositol 3-kinase; AKT, protein kinase B; mTOR, mammalian target of rapamycin; Sham, sham control mice group; Model, mouse model with COPD induced by PM2.5; PI3K inhibitor, treatment with PI3K inhibitor group.
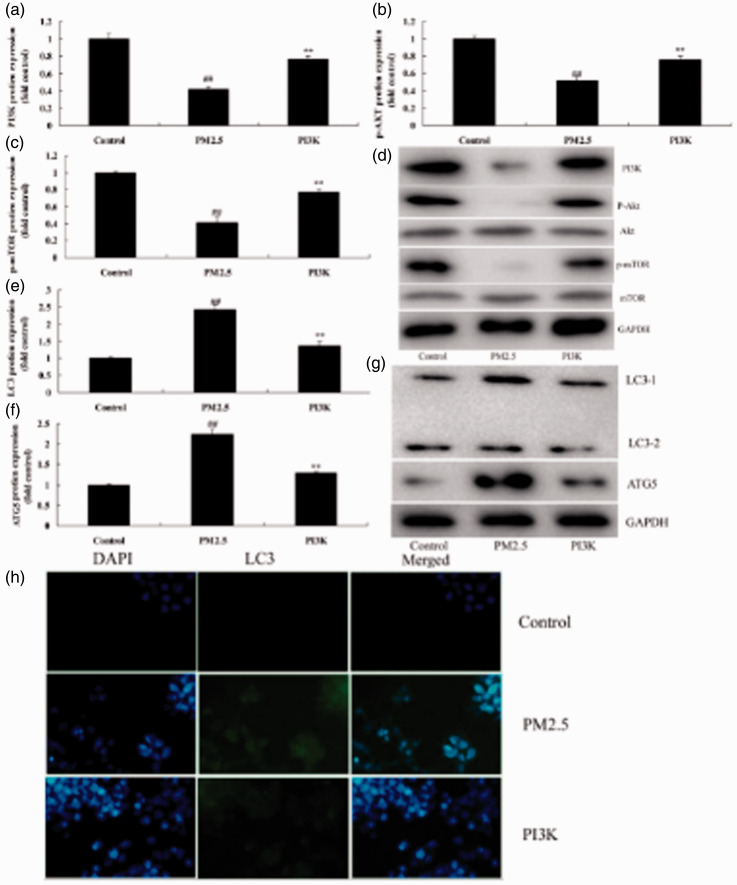
Inhibition of PI3K reduced autophagy in COPD mice
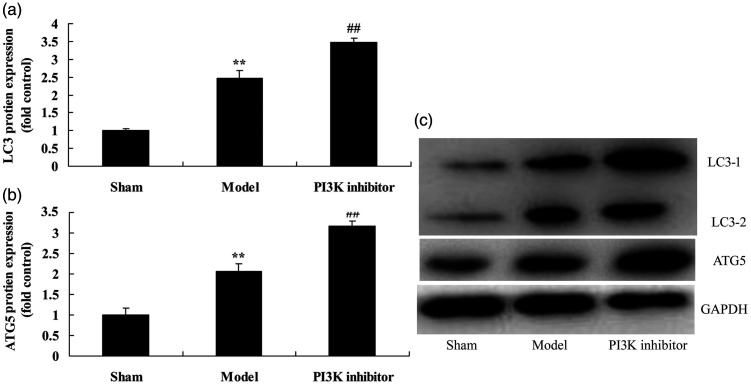
Next, we explored the function of PI3K on autophagy in COPD mice. Protein expression of the markers of autophagy LC3 (P = 0.0201) and ATG5 (P = 0.0125) were increased in COPD mice compared with mice in the sham group (Figure 3). However, treatment with the PI3K inhibitor PF-04979064 suppressed expression of LC3 (P = 0.0357) and ATG5 (P = 0.0186) compared with that of mice in the COPD group (Figure 3).
Figure 3.
Inhibition of PI3K reduced autophagy in a COPD mouse model induced by PM2.5. Protein expression of LC3 and ATG5 by statistical analysis (a and b) and western blotting assay of LC3 and ATG5 protein level (c). **P < 0.01 compared with sham group; ##P < 0.01 compared with COPD model group. COPD, chronic obstructive pulmonary disease; PI3K, phosphatidylinositol 3-kinase; PM2.5, particulate matter with a diameter <2.5 µm; Sham, sham control mice group; Model, mouse model with COPD induced by PM2.5; PI3K inhibitor, treatment with PI3K inhibitor group.
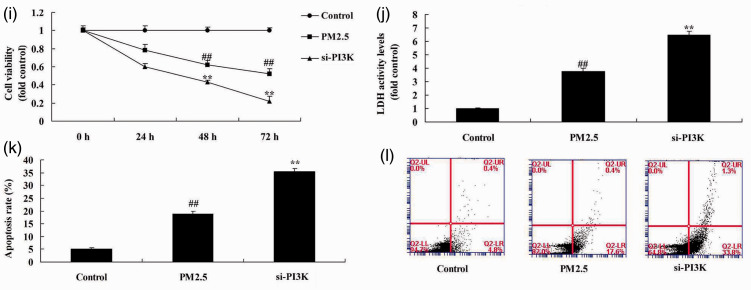
Si-PI3K suppressed PI3K/AKT/mTOR pathway in the COPD in vitro model
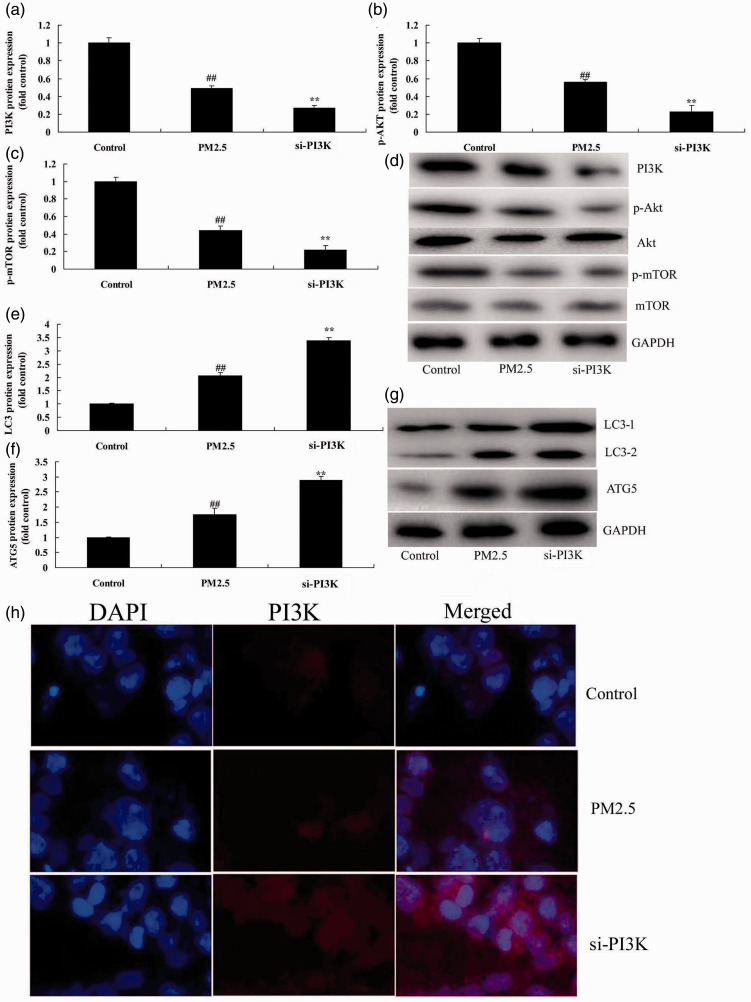
We studied the function of PI3K/AKT/mTOR pathway in the in vitro COPD model (HBECs). Protein expression of PI3K (P = 0.0093), p-AKT (P = 0.0124), and p-mTOR (P = 0.0077) was reduced in the in vitro COPD model compared with the negative control group (Figure 4a–4d). Si-PI3K suppressed the PI3K/AKT/mTOR pathway (P = 0.0362, 0.0451, and 0.0398) in the in vitro COPD model compared with control group (Figure 4a–4d). Protein expression of LC3 (P = 0.0172) and ATG5 (P = 0.0073) was increased in the in vitro COPD model compared with the control group (Figure 4e–4g), whereas si-PI3K suppressed protein expression of LC3 (P = 0.0113) and ATG5 (P = 0.0092) in the in vitro COPD model compared with control group (Figure 4e–4g). Immunofluorescence showed that si-PI3K suppressed expression of PI3K compared with the COPD group without si-PI3K (Figure 4h). Cell viability (P = 0.0132 and 0.0076) was reduced and LDH activity level (P = 0.0045) and apoptosis rate (P = 0.0065) were increased in the in vitro COPD group compared with the control group (Figure 4i–4l). Treatment with si-PI3K reduced cell viability (P = 0.0375 and 0.0231) and promoted LDH activity (P = 0.0127) and apoptosis rate (P = 0.0313) compared with the in vitro COPD group (Figure 4i–4l). These results showed that the PI3K/AKT/mTOR pathway regulated autophagy to induce apoptosis of alveolar epithelial cells in an in vitro COPD model.
Figure 4.
Si-PI3K suppressed PI3K/AKT/mTOR pathway in an in vitro model of COPD induced by PM2.5. Protein expression of PI3K, p-AKT, and p-mTOR by statistical analysis (a, b, and c) and western blotting assays of PI3K, p-AKT, and p-mTOR protein level (d); protein expression of LC3 and ATG5 by statistical analysis (e and f) and western blotting assay of LC3 and ATG5 protein level (g), immunofluorescence for PI3K protein expression (h), cell growth (i), LDH activity (j), and apoptosis rate (k and l). **P < 0.01 compared with negative control group; ##P < 0.01 compared with HBECs cells induced by PM2.5. COPD, chronic obstructive pulmonary disease; PM2.5, particulate matter with a diameter <2.5 µm; PI3K, phosphatidylinositol 3-kinase; AKT, protein kinase B; mTOR, mammalian target of rapamycin; LDH, lactate dehydrogenase; Control, negative control group; PM2.5 group, human bronchial epithelial cells (HBECs) with COPD induced by PM2.5; si-PI3K group, HBECs induced by PM2.5 and treated with short interfering (si)-PI3K.
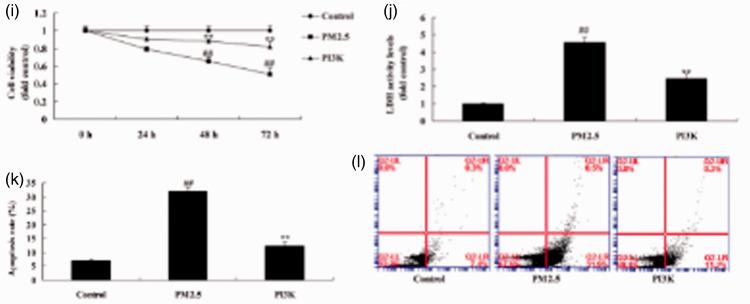
Activation of PI3K reduced apoptosis of alveolar epithelial cells in COPD in vitro model
To further determine whether activation of PI3K reduced apoptosis of alveolar epithelial cells in the in vitro COPD model, PI3K plasmid was used to induce expression of PI3K (P = 0.0092), p-AKT (P = 0.0156), and p-mTOR (P = 0.0143) (Figure 5a–5d). As shown in Figure 5e–5g, activation of PI3K reduced protein expression of LC3 (P = 0.0203) and ATG5 (P = 0.0288) in the in vitro COPD model, and si-PI3K suppressed PI3K protein expression in the in vitro COPD model (Figure 5h). Last, activation of PI3K decreased cell viability (P = 0.0114 and 0.0021) and reduced LDH activity levels (P = 0.0312) and apoptosis rate (P = 0.0162) in the in vitro COPD model (Figure 5i–5l).
Figure 5.
Activation of PI3K reduced apoptosis of alveolar epithelial cells in an in vitro model of COPD induced by PM2.5. Protein expression of PI3K, p-AKT, and p-mTOR by statistical analysis (a, b, and c) and western blotting assays of PI3K, p-AKT, and p-mTOR protein level (d); protein expression of LC3 and ATG5 by statistical analysis (e and f) and western blotting assay of LC3 and ATG5 protein level (g), immunofluorescence for PI3K protein expression (h), cell growth (i), LDH activity (j), and apoptosis rate (k and l). **P < 0.01 compared with negative control group; ##P < 0.01 compared with HBECs cells induced by PM2.5. COPD, chronic obstructive pulmonary disease; PM2.5, particulate matter with a diameter <2.5 µm; PI3K, phosphatidylinositol 3-kinase; AKT, protein kinase B; mTOR, mammalian target of rapamycin; LDH, lactate dehydrogenase; Control, negative control group; PM2.5 group, human bronchial epithelial cells (HBECs) induced by PM2.5; PI3K group, HBECs induced by PM2.5 and treated with PI3K.
Discussion
COPD is a progressive lung disease often caused by smoking; it is characterized by pulmonary emphysema, bronchitis, and fibrosis, and it induces tissue injury and airflow obstruction.12 Pulmonary emphysema is an abnormal and persistent dilation in the distal air space of the terminal bronchiole in the lung. It is accompanied by destruction of the alveolar wall and bronchiole with no obvious pulmonary fibrosis. Few existing treatments are effective for COPD; drug therapy mainly includes bronchodilators, anti-inflammatory agents, antioxidants, and protease inhibitors.13 Recent research has demonstrated the role of autophagy in chronic lung disease. Regulating the autophagic signaling pathway might provide a new approach to prevent and treat human diseases.14 In the present study, we demonstrated that inhibition of PI3K increased apoptosis of alveolar epithelial cells in a mouse model of COPD induced by PM2.5.
During cell autophagy, the free LC3-I in cytoplasm gradually locates on the surface of the autophagic vesicle in the form of LC3-II.15 Therefore, the level of conversion to LC3-II can reflect the degree of autophagy in cells. Under physiological conditions, autophagy is maintained at a low level to degrade and recycle intracellular substances.16 Thus, it plays a role in recycling and supplying nutrition to cells. However, under pathological conditions, autophagic reactivity is elevated.15 In the present study, we observed that inhibition of PI3K reduced autophagy in COPD mice, similar to results of others. Zhou et al.17 showed that autophagy played a protective role against PM2.5-induced cytotoxicity.
Metabolism, inflammatory response, neurodegenerative disease, and therapeutic pressure all induce a stress environment in cells.18 Under growth conditions, mTOR can regulate cell growth and survival. However, in nutritional deficiency, the mTOR pathway is suppressed and autophagy can be induced. Specific growth factors, amino acids, and glucose can negatively regulate autophagy through the mTOR signaling pathway. AKT is the central molecule in the PI3K/AKT/mTOR pathway; it can activate and regulate multiple downstream targets. For instance, AKT can suppress TSC1/2 and activate mTOR to promote protein synthesis and cell growth.18 It can also regulate cell proliferation by inactivating cell cycle inhibitor.19 We found that the PI3K/AKT/mTOR pathway regulates autophagy to induce apoptosis of alveolar epithelial cells in a mouse model of COPD induced by PM2.5.
In this study, we showed that the PI3K/AKT/mTOR pathway regulates autophagy to induce apoptosis of alveolar epithelial cells in COPD induced by PM2.5. Our findings suggest that autophagy might be a potential therapeutic target for COPD via the PI3K/AKT/mTOR pathway.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Fang Zhang https://orcid.org/0000-0002-2615-2251
References
- 1.Liu DY, Wang ZG, Gao Y, et al. Effect and safety of roflumilast for chronic obstructive pulmonary disease in Chinese patients. Medicine (Baltimore) 2018; 97: e9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heslop K, Newton J, Baker C, et al. Effectiveness of cognitive behavioural therapy (CBT) interventions for anxiety in patients with chronic obstructive pulmonary disease (COPD) undertaken by respiratory nurses: the COPD CBT CARE study: (ISRCTN55206395). BMC Pulm Med 2013; 13: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohbayashi H. Comparison of the rapid effects of single inhalations of formoterol and tiotropium bromide on respiratory function and COPD symptoms in a randomized crossover study. Respir Investig 2017; 55: 348–356. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Mao B, Chen C. Xiaoqinglong decoction attenuates chronic obstructive pulmonary disease in rats via inhibition of autophagy. Evid Based Complement Alternat Med 2018; 2018: 6705871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y, Xu B, He X, et al. Correlation between autophagy levels in peripheral blood mononuclear cells and clinical parameters in patients with chronic obstructive pulmonary disease. Mol Med Rep 2018; 17: 8003–8009. [DOI] [PubMed] [Google Scholar]
- 6.Wu N, Zhu Y, Xu X, et al. The anti-tumor effects of dual PI3K/mTOR inhibitor BEZ235 and histone deacetylase inhibitor Trichostatin A on inducing autophagy in esophageal squamous cell carcinoma. J Cancer 2018; 9: 987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito K, Caramori G, Adcock IM. Therapeutic potential of phosphatidylinositol 3-kinase inhibitors in inflammatory respiratory disease. J Pharmacol Exp Ther 2007; 321: 1–8. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Kong Y, Sun Y, et al. Bone marrow mesenchymal stem cells conditioned medium protects VSC4.1 cells against 2,5-hexanedione-induced autophagy via NGF-PI3K/Akt/mTOR signaling pathway. Brain Res 2018; 1696: 1–9. [DOI] [PubMed] [Google Scholar]
- 9.Deng X, Rui W, Zhang F, et al. PM2.5 induces Nrf2-mediated defense mechanisms against oxidative stress by activating PIK3/AKT signaling pathway in human lung alveolar epithelial A549 cells. Cell Biol Toxicol 2013; 29: 143–157. [DOI] [PubMed] [Google Scholar]
- 10.Wan Q, Liu Z, Yang Y. Puerarin inhibits vascular smooth muscle cells proliferation induced by fine particulate matter via suppressing of the p38 MAPK signaling pathway. BMC Complement Altern Med 2018; 18: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei T, Tang M. Biological effects of airborne fine particulate matter (PM2.5) exposure on pulmonary immune system. Environ Toxicol Pharmacol 2018; 60: 195–201. [DOI] [PubMed] [Google Scholar]
- 12.McNicholas W, Hansson D, Schiza S, et al. Sleep in chronic respiratory disease: COPD and hypoventilation disorders. Eur Respir Rev 2019; 28: 190064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neunhauserer D, Steidle-Kloc E, Weiss G, et al. Supplemental oxygen during high-intensity exercise training in nonhypoxemic chronic obstructive pulmonary disease. Am J Med 2016; 129: 1185–1193. [DOI] [PubMed] [Google Scholar]
- 14.Faul J, Schoors D, Brouwers S, et al. Creation of an iliac arteriovenous shunt lowers blood pressure in chronic obstructive pulmonary disease patients with hypertension. J Vasc Surg 2014; 59: 1078–1083. [DOI] [PubMed] [Google Scholar]
- 15.Racanelli AC, Kikkers SA, Choi AMK, et al. Autophagy and inflammation in chronic respiratory disease. Autophagy 2018; 14: 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Zhang M, Zhang L, et al. Klotho regulates cigarette smoke-induced autophagy: implication in pathogenesis of COPD. Lung 2017; 195: 295–301. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Z, Shao T, Qin M, et al. The effects of autophagy on vascular endothelial cells induced by airborne PM2.5. J Environ Sci (China) 2018; 66: 182–187. [DOI] [PubMed] [Google Scholar]
- 18.Joassard OR, Amirouche A, Gallot YS, et al. Regulation of Akt-mTOR, ubiquitin-proteasome and autophagy-lysosome pathways in response to formoterol administration in rat skeletal muscle. Int J Biochem Cell Biol 2013; 45: 2444–2455. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Liu J, Zhou JS, et al. MTOR suppresses cigarette smoke-induced epithelial cell death and airway inflammation in chronic obstructive pulmonary disease. J Immunol 2018; 200: 2571–2580. [DOI] [PubMed] [Google Scholar]