Abstract

The
adsorption of 2-mercaptobenzothiazole (2-MBT) vapor on a Cu(111)
surface under ultra-low pressure was investigated. For an exposure
of 45 L at 150 °C, a Moiré pattern was observed as a result
of the superposition of an underlying 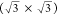 R30° structure and an outer
layer compressed
by 18% and rotated by 1.2°. The Moiré pattern was rich
in S bonded to Cu as a result of molecular decomposition and partial
desorption and was transformed to a
R30° structure and an outer
layer compressed
by 18% and rotated by 1.2°. The Moiré pattern was rich
in S bonded to Cu as a result of molecular decomposition and partial
desorption and was transformed to a  R19.1° structure when the
sample temperature
was increased above 250 °C during deposition. This pre-adsorbed
Moiré structure led to the sharp decrease of the oxidation
kinetics, which better protects copper against corrosion than the
non-ordered 2-MBT monolayer formed at room temperature. Upon further
exposure to 2-MBT at room temperature, an equivalent monolayer of
the molecule was adsorbed on the Moiré structure at saturation
whereas a multilayer was formed for the direct deposition on Cu(111)
at room temperature.
R19.1° structure when the
sample temperature
was increased above 250 °C during deposition. This pre-adsorbed
Moiré structure led to the sharp decrease of the oxidation
kinetics, which better protects copper against corrosion than the
non-ordered 2-MBT monolayer formed at room temperature. Upon further
exposure to 2-MBT at room temperature, an equivalent monolayer of
the molecule was adsorbed on the Moiré structure at saturation
whereas a multilayer was formed for the direct deposition on Cu(111)
at room temperature.
Introduction
2-Mercaptobenzothiazole (2-MBT) is used commercially in oils, greases, and cooling fluids as a corrosion inhibitor for copper and its alloys. The molecule may be heated during application, so it is of great importance to elucidate the adsorption of 2-MBT not only at room temperature but also at higher temperatures. However, the interaction of 2-MBT with the copper surface is not well understood, since in practice empirical tests are performed to evaluate the effectiveness of different compounds for industrial applications. Several studies have been carried out in solution to investigate the inhibiting effect of 2-MBT on copper corrosion.1−3 It is believed that 2-MBT forms a complex layer on copper through bonding with sulfur or nitrogen or both atoms, which can protect the copper from corrosion. However, the inhibition mechanism of the molecular layer is still debated.
The structural aspects
of the molecular adsorption at model interfaces
is thus of great interest. Our previous study on 2-MBT adsorption
by sublimation at ultra-low pressure on Cu(111) at room temperature
presented the formation of a non-ordered monolayer followed by flat-lying
multilayers at saturation.4 It has been
shown that 2-MBT forms a self-assembled monolayer from the liquid
phase with flat-lying molecules on the Au(111) surface.5−7 A Moiré pattern was observed for the adsorption of sulfate
anions on the Cu(111) surface, which was accompanied by a reconstruction
of the Cu(111) substrate.8 Moreover, the
adsorption of S on Cu(111) under ultra-high vacuum (UHV) has been
widely studied, with the formation of various structures, including 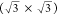 R30° and
R30° and 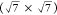 R19.1° structures, depending
on the
S coverage.9−11 A reconstruction to form a pseudo-(100) structure
was also observed for the adsorption of nitrogen on Cu(111).12,13
R19.1° structures, depending
on the
S coverage.9−11 A reconstruction to form a pseudo-(100) structure
was also observed for the adsorption of nitrogen on Cu(111).12,13
In this work, 2-MBT exposure on a Cu(111) surface by sublimation at ultra-low pressure and 150 °C and the resulting formation of an interfacial layer characterized by a Moiré pattern (hereafter denoted as a “Moiré structure”) were investigated. The inhibition efficiency of the Moiré structure was studied by exposure to gaseous oxygen and ambient air at room temperature as well as its effect on the buildup of a 2-MBT mutilayer at room temperature. The sample surfaces were characterized by scanning tunneling microscopy (STM), X-ray photoelectron spectroscopy (XPS), Auger electron spectroscopy (AES), and low-energy electron diffraction (LEED). The influence of temperature on the molecular deposition was also studied. The results provide new insight on the chemical and structural interaction between the inhibitor molecule and the copper surface and contribute to the design of new inhibitors that better protect the metals.
Experimental Section
Experiments were carried out in an UHV system with base pressure in the 10–10 mbar that was equipped with a reactor for 2-MBT evaporation. The following surface analytical techniques were used: STM (Omicron, STM1 with a SCALA system), AES (Omicron, CMA-100), and LEED (Omicron, SpectaLEED). The sample was a high purity (99.999%) Cu(111) single crystal. It was mechanically polished to 0.25 μm (diamond paste), electropolished in phosphoric acid, and then prepared in the UHV system by cycles of ion sputtering (PAr = 1 × 10–5 mbar, 600 V, and 20 mA for 30 min) and annealing (600 °C for 30 min) until a clean copper surface was obtained, i.e., no contamination was shown by AES, a sharp (1 × 1) pattern was observed by LEED, and large and flat terraces were observed by STM.
The clean metallic Cu(111) surface was exposed to the 2-MBT vapor at ultra-low pressure (2 × 10–9 mbar) in the UHV reactor. Exposures are expressed in langmuirs (1 L = 1 × 10–6 Torr·s). The 2-MBT powder (99% purity, Sigma-Aldrich) was placed in a vacuum-sealed glass mounted on the reactor. The pressure in the reactor was limited by the vapor pressure of 2-MBT at room temperature. The Cu(111) sample was heated to 150 °C during molecule deposition. The temperature was selected to avoid the total desorption of molecules from the surface and to allow the reorganization of the molecular layer.
The STM characterization was carried out at room temperature and in the constant current mode. A W tip was prepared by electrochemical attack in a NaOH solution followed by voltage pulses under UHV. Image processing was performed using WSxM software (5.0 develop 9.1).14 No filtering was applied. All images were corrected by plane subtraction.
Quantitative chemical analysis was performed by quickly transferring the sample to another UHV system equipped with a spectrometer for XPS analysis (Thermo Electron Corporation, ESCALAB 250) with a base pressure of 10–10 mbar. A monochromatic Al Kα source (1486.6 eV) was used. The survey spectra were recorded with a pass energy of 100 eV corresponding to an overall resolution of 1.8 eV, and the high resolution spectra were recorded with a pass energy of 20 eV corresponding to an overall resolution of 360 meV. The XPS measurements were performed at a takeoff angle of 90°, and the data analysis was preformed using the CasaXPS software (ver. 2.3.19).15
In order to verify the inhibition properties of the adsorbed Moiré layer formed at 150 °C, further exposure to 2-MBT at room temperature and 2 × 10–9 mbar was performed as well as exposure of the surface with the Moiré structure to oxygen at a low pressure (1 × 10–5 mbar) for up to 1 h 30 min and to ambient air at atmospheric pressure. Finally, to investigate the influence of temperature on the adsorption of 2-MBT, exposure was also performed at a higher temperature.
Results and discussion
Exposure at 150 °C: Moiré Structure Formation
STM images of the Cu(111) surface before and after exposure to 2-MBT at 150 °C are shown in Figure 1. On clean Cu(111), the step edges were aligned according to the disorientation of the single-crystal, with multiatomic steps (1–3 atomic planes), as shown in Figure 1a. The step edges appear to be fuzzy because of the high mobility of copper atoms at room temperature. After exposure to 2-MBT at 150 °C (45 L), as shown in Figure 1b, the step edges became straight, suggesting a reduced surface mobility of Cu atoms after exposure to 2-MBT. Moreover, they were reoriented in three directions, forming a 120° angle with each other. Large terraces are favored by the formation of higher multiatomic steps (1–6 atomic planes). The terraces remained atomically flat, suggesting the formation of an organized layer after exposure to 2-MBT.
Figure 1.

STM images of (a) the clean Cu(111) surface (100 × 100 nm2, V = 0.7 V, and I = 0.2 nA) and (b) the Cu(111) surface exposed to 45 L of 2-MBT at 150 °C (100 × 100 nm2, V = 0.1 V, and I = 2.5 nA).
By zooming in on the
terraces obtained after 2-MBT exposure, a
Moiré structure was observed with the presence of two hexagonal
structures, as indicated in Figure 2a and modeled in Figure 2b. The short-range hexagonal unit cell is marked in
blue with a lattice parameter of 3.5 ± 0.5 Å and a rotation
of 31 ± 4° with respect to the directions of the substrate.
This was also confirmed by fast Fourier transform (FFT) (Figure 2c) and LEED (Figure 2d) measurements,
which show the same direction and gives a lattice parameter of 3.7
Å. Moreover, two overlapped mirror domains were observed by LEED.
The long-range hexagonal lattice (Moiré structure) is indicated
in black with a lattice parameter 5.6 times larger (19.6 ± 0.6
Å) than that of the short-range structure and a rotation of 6.4°
(25 ± 5° with respect to the substrate). According to Moiré
pattern mathematical formalism,16 we found
that the observed Moiré structure may be explained by the superposition
of the following two molecular layers: the 3.5 Å lattice observed
in the STM image and an underlying hexagonal lattice not observed
in the STM image, with a lattice parameter mismatch of 18% and a rotation
of 1.2° between the two, as shown in Figure 2b. The lattice parameter for the underlying
layer is 4.1 ± 0.6 Å, corresponding to 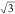 times
the lattice constant of Cu(111),
with a rotation of 30 ± 4° with respect to the substrate.
It thus forms a
times
the lattice constant of Cu(111),
with a rotation of 30 ± 4° with respect to the substrate.
It thus forms a 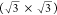 R30°
structure.
R30°
structure.
Figure 2.

Moiré structure for 45 L of 2-MBT deposited on the Cu(111) surface at 150 °C. (a) STM image (10 × 10 nm2, V = 0.05 V, and I = 4.0 nA); (b) model of the Moiré structure consisting of two superimposed molecular layers marked with the unit cells observed in the STM image (the substrate is not shown); (c) FFT of the image in (a) with the Moiré structure and top molecular layer structure marked; and (d) the LEED pattern (Ep = 55 eV).
AES peak-to-peak height ratios of S (151 eV) and N (380 eV) to C (271 eV) signals were calculated for the Moiré structure, and their relative ratios were corrected using the relative sensitivities of the Auger transitions. The results give a S:C atomic ratio of 3.57, which is about 12.5 times the theoretical (molecule stoichiometry) value (2:7), and a N:C atomic ratio of 0.05, which is about 0.4 times the theoretical value (1:7). These differences suggest that the Moiré structure is rich in S as the result of a partial decomposition of 2-MBT by the cleavage of the C=S or C–S bonds during exposure at 150 °C, probably due to the high affinity of sulfur to copper.17−21 The Moiré structure also appears to be poor in N, suggesting the desorption of molecular fragments after decomposition.
The
surface with this Moiré structure was then transferred
to the XPS system for quantitative analysis, and the results are shown
in Figure 3. For the
peak fitting of S 2p spectra, spin–orbit doublets S 2p1/2 and S 2p3/2 with a branching ratio of 0.5 and
a spin–orbit splitting of 1.18 eV were considered.23,24 The Moiré structure is mainly composed of a S3 component with S 2p3/2 at 161.7 eV. This component is
slightly shifted to a higher binding energy (0.2 eV) compared to that
of the 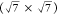 R19.1° S/Cu(111) surface obtained
by
exposing the clean Cu(111) surface to H2S, which may be
explained by an interaction with oxygen during the transfer in air.
The S3 component is assigned to the S bonded to the metallic
copper.22 A N 1s component at 399.0 eV
was observed, corresponding to N in the non-bonded molecule.22 XPS spectra obtained from the 2-MBT monolayer
formed on Cu(111) at room temperature are also shown for comparison.
The intensity of the S 2p spectrum was found to be 0.6 times that
obtained from the Moiré structure, and the intensity of the
N 1s spectrum was found to be 1.3 times that obtained from the Moiré
structure, suggesting that the Moiré structure is richer in
S than the 2-MBT monolayer formed at room temperature.
R19.1° S/Cu(111) surface obtained
by
exposing the clean Cu(111) surface to H2S, which may be
explained by an interaction with oxygen during the transfer in air.
The S3 component is assigned to the S bonded to the metallic
copper.22 A N 1s component at 399.0 eV
was observed, corresponding to N in the non-bonded molecule.22 XPS spectra obtained from the 2-MBT monolayer
formed on Cu(111) at room temperature are also shown for comparison.
The intensity of the S 2p spectrum was found to be 0.6 times that
obtained from the Moiré structure, and the intensity of the
N 1s spectrum was found to be 1.3 times that obtained from the Moiré
structure, suggesting that the Moiré structure is richer in
S than the 2-MBT monolayer formed at room temperature.
Figure 3.

XPS spectra of the S
2p, N 1s, and C 1s core levels for Cu(111)
that was exposed to 45 L of 2-MBT at 150 °C (Moiré structure)
and 15 L of 2-MBT at RT (2-MBT monolayer22) for comparison. The S 2p spectrum for a 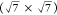 R19.1° S/Cu(111) surface is
also shown
for comparison.22
R19.1° S/Cu(111) surface is
also shown
for comparison.22
The atomic ratios of S and N vs C were calculated for the Moiré structure. We found a S to C atomic ratio 1.4 times the theoretical value for the stoichiometry of the molecule (2:7) and a N to C atomic ratio 0.4 times the theoretical value (1:7), confirming the decomposition of the molecule. We assumed three types of carbon, C2, C3, and C4, for the peak fitting of the C 1s spectrum. The full width at half maximum (FWHM) values (1.3 eV) of the C2 and C3 components were set to be the same, and the fit is in agreement with the measured spectrum. The C4 component at a binding energy of 288.2 eV indicates the presence of carboxylic groups (air contamination),25 which explains the difference in the atomic ratios of S and N to C obtained by AES. The C2 and C3 components at binding energies of 285.8 ± 0.2 and 284.7 ± 0.1 eV correspond to C–N bond and the remaining C atoms in the benzene ring, respectively.22 The intensity of C3 is 5–6 times that of C2. This confirms the decomposition of 2-MBT by the cleavage of the C=S and C–S bonds.
In order to study the influence
of temperature on the adsorption
of 2-MBT, exposures were carried out on Cu(111) at different temperatures,
and the surface structure was characterized by LEED. The Moiré
structure formed at 150 °C was transformed to a 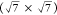 R19.1° structure when the
deposition
temperature was increased to 250 °C.
R19.1° structure when the
deposition
temperature was increased to 250 °C.
Growth of the 2-MBT Layer on a Pre-Adsorbed Moiré Structure at Room Temperature
The surface with the Moiré structure was then exposed to 2-MBT under ultra-low pressure (2 × 10–9 mbar) at room temperature, and the AES peak-to-peak height ratios of S (151 eV), C (271 eV), and N (380 eV) to the Cu (920 eV) signals were calculated and plotted as a function of the exposure (Figure 4). The values at 0 L correspond to those for the Moiré structure.
Figure 4.
Growth of the 2-MBT layer on a pre-adsorbed Moiré structure at 2 × 10–9 mbar and RT. Change in the AES peak-to-peak height ratios of hS/hCu, hC/hCu, and hN/hCu as a function of the 2-MBT exposure.
After exposure to 65 L of 2-MBT, a slight decrease of the S signal was first observed. This may be explained by the attenuation of the Moiré structure (rich in S) by the adsorbing 2-MBT molecules. On the contrary, N and C signals were observed to increase, confirming the adsorption of molecules on the surface. Further exposure of 2-MBT up to 4000 L showed no obvious increase in the S, C, and N signals. By comparing the AES peak-to-peak height ratios to those obtained for 2-MBT deposited on the clean Cu(111) surface,4 we estimated that only one equivalent monolayer of 2-MBT was adsorbed; meanwhile, 2-MBT multilayers were grown on the clean Cu(111) surface.
The sample was also analyzed by XPS to obtain information on the chemical composition (Figure 5). The S 2p spectrum shows that the growth of 2-MBT on the Moiré structure at room temperature gives rise to two other components, S1 and S2, with 2p3/2 peaks at 164.2 and 162.8 eV corresponding to the endocyclic and exocyclic S atoms in the 2-MBT molecule not bonded to copper, respectively.22,24 This confirms the adsorption of molecules on the Moiré structure. The comparison of the intensity of the XPS spectra with those obtained from Cu(111) with the pre-adsorbed 2-MBT monolayer22 confirmed the formation of one equivalent 2-MBT monolayer on the Moiré structure at room temperature. The binding energies of the S2 and S3 components were found to be slightly superior to those found in our previous study on 2-MBT,22 which may be explained in this case by the influence of the exposure to air during the transfer to XPS. An increase in the intensities of C and N was also observed, with a slight shift to a higher binding energy (0.3 eV) and a decrease in the FWHM value (1.5 eV) of about 25% for the N 1s spectrum. A component C1 at a binding energy of 286.4 eV is also observed, which was assigned to the C=S bond.22 Similarly, the C4 component at 288.2 eV was assigned to carboxylic groups (air contamination).25
Figure 5.

XPS spectra of the S 2p, N 1s, and C 1s core levels for the pre-adsorbed Moiré structure exposed to 65 L of 2-MBT at RT.
The topography of the surface of the Moiré structure obtained after exposure at room temperature to 2-MBT was characterized by STM and is shown in Figures 6 and 7. After an exposure of 65 L (Figure 6), the surface was homogeneous, with the presence of multiatomic steps with heights of about 7 Å (3–4 atomic planes). The step edges remained straight. By zooming in on the terraces (Figure 6b), we can see that the adsorbed molecular layer is almost complete and is relatively flat with a maximal height difference of around 1.5 Å. The size of the protrusions is 1–2 nm, which could be assigned to the adsorption of 2-MBT molecules. FFT measurements showed no ordered structure.
Figure 6.

STM images of the Moiré pattern exposed at RT to 65 L of 2-MBT at (a) 100 × 100 nm2, V = 1.0 V, and I = 0.1 nA and (b) 25 × 25 nm, V = 0.5 V, and I = 0.5 nA.
Figure 7.

STM images of the Moiré pattern exposed at RT to 4000 L of 2-MBT at (a) 50 × 50 nm2, V = 1.0 V, and I = 0.2 nA and (b) 30 × 30 nm, V = 1.0 V, and I = 0.3 nA.
At higher exposures up to 4000 L, Figure 7a shows that the step edges are reorganized and the surface is always flat and homogeneous, as confirmed by Figure 7b. The molecular layer is very similar to that observed at 65 L, with a maximal height difference of around 1.7 Å and protrusions of 1–2 nm; no ordered local structure was revealed by FFT.
Inhibition Effect of the Pre-Adsorbed Moiré Structure on the Oxidation of Cu(111)
In order to clarify the inhibition properties of the interfacial Moiré structure, the Cu(111) surface with the pre-adsorbed Moiré structure was exposed to oxygen under a low pressure (until 10–5 mbar) at room temperature. The oxidation kinetics were followed by AES, as shown in Figure 8. When the copper surface is pre-covered by the Moiré structure there is almost no uptake of oxygen, in contrast to the oxidation kinetics on the clean Cu(111) surface.26 This confirms the effective protection of copper by the Moiré structure at a low O2 pressure and room temperature. Because of the well-organized pre-adsorbed structure, no residual uptake of oxygen was observed as in the case of Cu(111) with a non-ordered pre-adsorbed 2-MBT monolayer formed at room temperature,22 suggesting a better protection of copper by the Moiré structure against corrosion.
Figure 8.
Oxidation kinetics of the Moiré structure showing the effect of pre-adsorbed 2-MBT (45 L at 150 °C prior to oxidation).
When exposed to ambient air, the sample surface was slightly oxidized, as indicated by the XPS spectra shown in Figure 9. The results were compared to those obtained on the metallic Cu(111) surface after exposure to air. First, we can see that the intensities of the Cu 2p, O 1s, and Cu LMM core levels were largely decreased in the presence of the pre-adsorbed Moiré structure. The decrease of the Cu 2p intensity can be explained by a strong attenuation of the signal by the pre-adsorbed Moiré structure. Moreover, weak satellite structures are observed between the 2p1/2 and 2p3/2 peaks, indicating the formation of Cu(II) in two cases.27,28 Analysis of the O 1s spectrum obtained on metallic Cu(111) gives two components O1 and O2 at binding energies of 531.5 and 530.3 eV, respectively. The O1 component may be attributed to the interface OH groups resulting from the dissociative adsorption of water or oxygenated contaminations, while the O2 component is usually assigned to the bulk oxide.29,30 The decrease in the O 1s spectrum (both O1 and O2 components) with the Moiré structure indicates that the metallic Cu(111) sample is more oxidized and contaminated. This confirms that the Moiré pattern offers good protection of copper against oxidation even in ambient air.
Figure 9.

XPS spectra of the Cu 2p, O 1s, and Cu LMM core levels for metallic Cu(111) and the Moiré structure exposed to air.
In order to elucidate the nature of the oxide formed on the sample surface, decomposition of the Cu LMM spectrum was also carried out using three reference spectra obtained by analyzing the metallic Cu sample and copper oxides.31 The results show the formation of 34% cuprous oxide (Cu2O) and 19% cupric oxide (CuO) on the metallic Cu(111). In the presence of the Moiré structure, the intensities of the Cu(I) and Cu(II) components decrease; however, the relative proportion of the Cu(I) component increases to 76%. This can be explained by a preferential Cu(I) oxidation occurring upon the formation of the the Moiré structure, with the Cu(I) component assigned to the oxidized copper bonded to S in the Moiré structure. The results confirm that the Moiré structure can protect the copper substrate from oxidation when exposed to air.
Conclusions
The adsorption of 2-MBT
on Cu(111) at ultra-low pressure was investigated
at mild temperature by STM, XPS, AES, and LEED. The STM images show
that a Moiré structure was formed from exposure at 150 °C
with a short-range unit cell and an underlying superimposed 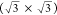 R30° structure. XPS spectra
show that
the Moiré structure is rich in S bonded to Cu as a result of
2-MBT decomposition and partial desorption. When the deposition temperature
was increased, the Moiré structure formed at 150 °C was
transformed to a
R30° structure. XPS spectra
show that
the Moiré structure is rich in S bonded to Cu as a result of
2-MBT decomposition and partial desorption. When the deposition temperature
was increased, the Moiré structure formed at 150 °C was
transformed to a 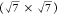 R19.1°
structure at 250 °C, which
was assigned to atomic S bonded to Cu.
R19.1°
structure at 250 °C, which
was assigned to atomic S bonded to Cu.
The corrosion inhibition efficiency of the interfacial Moiré structure was determined by dosing oxygen to the pre-adsorbed Moiré structure. No uptake of oxygen was observed until an oxygen partial pressure of 10–5 mbar, and the sample was only slightly oxidized when exposed to ambient air. This indicates the good protection by the pre-adsorbed Moiré structure against oxidation of the Cu(111) surface, which is more efficient than a 2-MBT monolayer formed at room temperature.
Upon further exposure of the Moiré structure to 2-MBT at 2 × 10–9 mbar and room temperature, an equivalent monolayer of molecules was formed, which was flat and homogeneous. However, no further growth was shown until an exposure of 4000 L, suggesting that the pre-adsorbed Moiré structure does not enable the formation of multilayers of 2-MBT that was observed on the clean Cu(111) surface.
Acknowledgments
This project received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (ERC Advanced Grant CIMNAS 741123). We also thank Région Île-de-France for the partial funding of the XPS equipment.
The authors declare no competing financial interest.
References
- Ohsawa M.; Suëtaka W. Spectro-electrochemical studies of the corrosion inhibition of copper by mercaptobenzothiazole. Corros. Sci. 1979, 19, 709–722. 10.1016/S0010-938X(79)80142-0. [DOI] [Google Scholar]
- Li J.; Du C. W.; Liu Z. Y.; Li X. G.; Liu M. Inhibition Film Formed by 2-mercaptobenzothiazole on Copper Surface and Its Degradation Mechanism in Sodium Chloride Solution. Int. J. Electrochem. Sci. 2016, 11, 10690–10705. 10.20964/2016.12.46. [DOI] [Google Scholar]
- Kazansky L.; Selyaninov I.; Kuznetsov Y. Adsorption of 2-mercaptobenzothiazole on copper surface from phosphate solutions. Appl. Surf. Sci. 2012, 258, 6807–6813. 10.1016/j.apsusc.2012.03.097. [DOI] [Google Scholar]
- Wu X.; Wiame F.; Maurice V.; Marcus P. Adsorption and thermal stability of 2-mercaptobenzothiazole corrosion inhibitor on metallic and pre-oxidized Cu (111) model surfaces. Appl. Surf. Sci. 2020, 508, 145132. 10.1016/j.apsusc.2019.145132. [DOI] [Google Scholar]
- Whelan C. M.; Smyth M. R.; Barnes C. J.; Brown N. M. D.; Anderson C. A. An XPS study of heterocyclic thiol self-assembly on Au (111). Appl. Surf. Sci. 1998, 134, 144–158. 10.1016/S0169-4332(98)00204-9. [DOI] [Google Scholar]
- Whelan C. M.; Smyth M. R.; Barnes C. J. The influence of heterocyclic thiols on the electrodeposition of Cu on Au(111). J. Electroanal. Chem. 1998, 441, 109–129. 10.1016/S0022-0728(97)00415-4. [DOI] [Google Scholar]
- Zerulla D.; Uhlig I.; Szargan R.; Chassé T. Competing interaction of different thiol species on gold surfaces. Surf. Sci. 1998, 402–404, 604–608. 10.1016/S0039-6028(98)00015-6. [DOI] [Google Scholar]
- Wilms M.; Broekmann P.; Stuhlmann C.; Wandelt K. In-situ STM investigation of adsorbate structures on Cu(111) in sulfuric acid electrolyte. Surf. Sci. 1998, 416, 121–140. 10.1016/S0039-6028(98)00550-0. [DOI] [Google Scholar]
- Domange J.-L.; Oudar J. Structure et conditions de formation de la couche d’adsorption du soufre sur le cuivre. Surf. Sci. 1968, 11, 124–142. 10.1016/0039-6028(68)90043-5. [DOI] [Google Scholar]
- Ruan L.; Stensgaard I.; Besenbacher F.; Laegsgaard E. A scanning tunneling microscopy study of the interaction of S with the Cu(111) surface. Ultramicroscopy 1992, 42–44, 498–504. 10.1016/0304-3991(92)90313-9. [DOI] [Google Scholar]
- Campbell C. T.; Koel B. E. H2S/Cu(111): A model study of sulfur poisoning of water-gas shift catalysts. Surf. Sci. 1987, 183, 100–112. 10.1016/S0039-6028(87)80337-0. [DOI] [Google Scholar]
- Soon A.; Wong L.; Lee M.; Todorova M.; Delley B.; Stampfl C. Nitrogen adsorption and thin surface nitrides on Cu(111) from first-principles. Surf. Sci. 2007, 601, 4775–4785. 10.1016/j.susc.2007.07.011. [DOI] [Google Scholar]
- Skelly J. F.; Bertrams T.; Munz A. W.; Murphy M. J.; Hodgson A. Nitrogen induced restructuring of Cu(111) and explosive desorption of N2. Surf. Sci. 1998, 415, 48–61. 10.1016/S0039-6028(98)00460-9. [DOI] [Google Scholar]
- Horcas I.; Fernández R.; Gómez-Rodríguez J. M.; Colchero J.; Gómez-Herrero J.; Baro A. M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. 10.1063/1.2432410. [DOI] [PubMed] [Google Scholar]
- CasaXPS Manual 2.3.15; Casa Software, Ltd., 2009. [Google Scholar]
- Hermann K. Periodic overlayers and moire patterns: theoretical studies of geometric properties. J. Phys.: Condens. Matter 2012, 24, 314210. 10.1088/0953-8984/24/31/314210. [DOI] [PubMed] [Google Scholar]
- Vericat C.; Vela M. E.; Corthey G.; Pensa E.; Cortes E.; Fonticelli M. H.; Ibanez F.; Benitez G.; Carro P.; Salvarezza R. C. Self-assembled monolayers of thiolates on metals: a review article on sulfur-metal chemistry and surface structures. RSC Adv. 2014, 4, 27730–27754. 10.1039/C4RA04659E. [DOI] [Google Scholar]
- Ertl G. Reactions at surfaces: from atoms to complexity (Nobel Lecture). Angew. Chem., Int. Ed. 2008, 47, 3524–3535. 10.1002/anie.200800480. [DOI] [PubMed] [Google Scholar]
- Wiame F.; Maurice V.; Marcus P. Reactivity to sulphur of clean and pre-oxidised Cu(111) surfaces. Surf. Sci. 2006, 600, 3540–3543. 10.1016/j.susc.2005.11.062. [DOI] [Google Scholar]
- Keller H.; Simak P.; Schrepp W.; Dembowski J. Surface chemistry of thiols on copper: an efficient way of producing multilayers. Thin Solid Films 1994, 244, 799–805. 10.1016/0040-6090(94)90574-6. [DOI] [Google Scholar]
- Issa R. M.; Awad M. K.; Atlam F. M. Quantum chemical studies on the inhibition of corrosion of copper surface by substituted uracils. Appl. Surf. Sci. 2008, 255, 2433–2441. 10.1016/j.apsusc.2008.07.155. [DOI] [Google Scholar]
- Wu X.; Wiame F.; Maurice V.; Marcus P. 2-Mercaptobenzothiazole corrosion inhibitor deposited at ultra-low pressure on model copper surfaces. Corros. Sci. 2020, 166, 108464. 10.1016/j.corsci.2020.108464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J.; Kara A.; Pasquali L.; Bendounan A.; Sirotti F.; Esaulov V. A. On sulfur core level binding energies in thiol self-assembly and alternative adsorption sites: An experimental and theoretical study. J. Chem. Phys. 2015, 143, 104702. 10.1063/1.4929350. [DOI] [PubMed] [Google Scholar]
- Finsgar M.; Kek Merl D. An electrochemical, long-term immersion, and XPS study of 2-mercaptobenzothiazole as a copper corrosion inhibitor in chloride solution. Corros. Sci. 2014, 83, 164–175. 10.1016/j.corsci.2014.02.016. [DOI] [Google Scholar]
- Finšgar M. 2-Mercaptobenzimidazole as a copper corrosion inhibitor: Part II. Surface analysis using X-ray photoelectron spectroscopy. Corros. Sci. 2013, 72, 90–98. 10.1016/j.corsci.2013.03.010. [DOI] [Google Scholar]
- Wiame F.; Maurice V.; Marcus P. Initial stages of oxidation of Cu(111). Surf. Sci. 2007, 601, 1193–1204. 10.1016/j.susc.2006.12.028. [DOI] [Google Scholar]
- Klein J. C.; Li C. P.; Hercules D. M.; Black J. F. Decomposition of Copper Compounds in X-Ray Photoelectron Spectrometers. Appl. Spectrosc. 1984, 38, 729–734. 10.1366/0003702844555016. [DOI] [Google Scholar]
- Poulston S.; Parlett P. M.; Stone P.; Bowker M. Surface Oxidation and Reduction of CuO and Cu2O Studied Using XPS and XAES. Surf. Interface Anal. 1996, 24, 811–820. . [DOI] [Google Scholar]
- Fleisch T.H.; Mains G.J. Reduction of copper oxides by UV radiation and atomic hydrogen studied by XPS. Appl. Surf. Sci. 1982, 10, 51–62. 10.1016/0378-5963(82)90134-9. [DOI] [Google Scholar]
- Wiame F.; Jasnot F.-R.; Swiatowska J.; Seyeux A.; Bertran F.; Le Fevre P.; Taleb-Ibrahimi A.; Maurice V.; Marcus P. Oxidation of α-brass: A photoelectron spectroscopy study. Surf. Sci. 2015, 641, 51–59. 10.1016/j.susc.2015.05.013. [DOI] [Google Scholar]
- Torres Bautista B. E.; Wikieł A. J.; Datsenko I.; Vera M.; Sand W.; Seyeux A.; Zanna S.; Frateur I.; Marcus P. Influence of extracellular polymeric substances (EPS) from Pseudomonas NCIMB 2021 on the corrosion behaviour of 70Cu-30Ni alloy in seawater. J. Electroanal. Chem. 2015, 737, 184–197. 10.1016/j.jelechem.2014.09.024. [DOI] [Google Scholar]




