Summary
Amphibians are known for their skin rich in glands containing toxins employed in passive chemical defense against predators, different from, for example, snakes that have active chemical defense, injecting their venom into the prey. Caecilians (Amphibia, Gymnophiona) are snake-shaped animals with fossorial habits, considered one of the least known vertebrate groups. We show here that amphibian caecilians, including species from the basal groups, besides having cutaneous poisonous glands as other amphibians do, possess specific glands at the base of the teeth that produce enzymes commonly found in venoms. Our analysis of the origin of these glands shows that they originate from the same tissue that gives rise to teeth, similar to the venom glands in reptiles. We speculate that caecilians might have independently developed mechanisms of production and injection of toxins early in their evolutionary history.
Subject Areas: Biological Sciences, Evolutionary Biology, Zoology
Graphical Abstract
Highlights
-
•
Amphibian caecilians have tooth-related glands in both upper and lower jaws
-
•
The glands have the same origin of reptile venom glands
-
•
The secretion contains proteins with enzymatic activities commonly found in venoms
-
•
Caecilians might have developed the ability to inject oral toxins early in evolution
Biological Sciences; Evolutionary Biology; Zoology
Introduction
Caecilian amphibians have around 250 million years of evolutionary history apart from anurans and salamanders (Pyron, 2011, Roelants et al., 2007, San Mauro, 2010, Zhang and Wake, 2009). Anurans and salamanders have developed a variety of feeding systems, whereas caecilians are exclusively jaw-feeders (Bemis et al., 1983, Duellman and Trueb, 1994) utilizing a powerful bite (Measey and Herrel, 2006, Summers and Wake, 2005) and a series of curved sharp-pointed teeth, which act on apprehension and ingestion (Wake, 1976) of invertebrates such as earthworms and subterranean arthropods, or even larger prey, such as anurans (Kupfer et al., 2005, Ngo et al., 2014), lizards (Moll and Smith, 1967), and snakes (Greef, 1884, Presswell et al., 2002).
The production of toxins for prey subjugation or defense has evolved many times in animals (Arbuckle, 2015, Casewell et al., 2013). Amphibians produce (or sequester) toxins in their skin glands (Jeckel et al., 2015) and are considered poisonous instead of venomous owing to their passive defense, lacking a system for toxin injection (Jared et al., 2015, Mailho-Fontana et al., 2014).
In this study we evaluated the morphology of the head of the South American caecilian Siphonops annulatus and found a series of tooth-related glands whose secretion composition was biochemically examined. We also studied the predatory behavior of this species in captivity in order to observe the possible participation of secretion from these glands during bites. Additionally, the tissue origin of these glands was evaluated and their presence in species belonging to different families was investigated.
Results
Caecilians Have Tooth-Related Glands
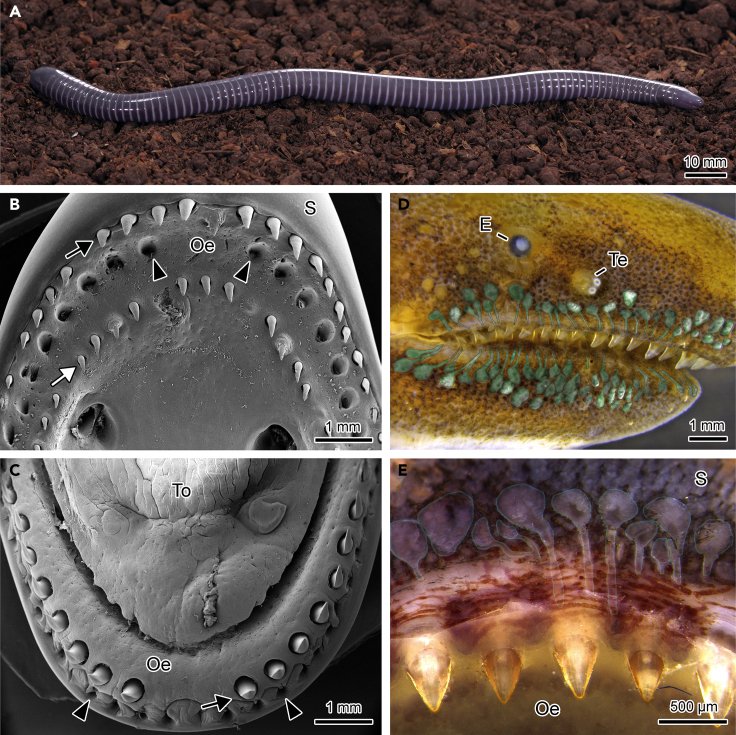
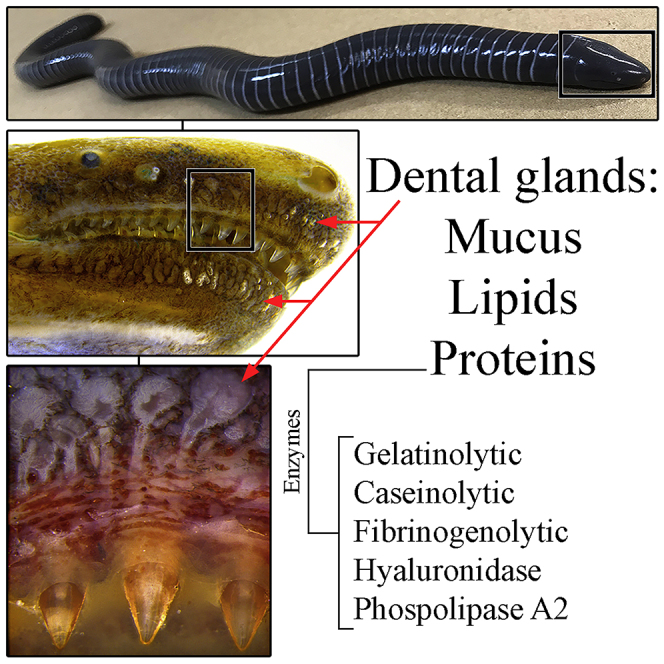
In S. annulatus (Figure 1A) two tooth rows are present in the upper jaw (Figure 1B) with the lower jaw having only a single row (Figure 1C). When the mouth is closed, the upper jaw outer teeth remain individually housed in a row of cavities located just in front of the mandibular teeth in a more external position (Figure 1C). The mandibular teeth are housed in cavities present in the oral upper jaw epithelium in between the two tooth rows (Figures 1B and S1). When the skin is partially removed, a series of glands characterized by long ducts opening at the base of the teeth is observed in both labial regions (Figures 1D and 1E).
Figure 1.
Structure of the Upper Jaw and Lower Jaw of S. annulatus
(A–D) (A) S. annulatus. (B) Upper jaw. (C) Lower jaw. (D) Head after partial skin corrosion showing the tooth-related glands digitally enhanced in green.
(E) Section of the upper labial region showing the glands and glandular ducts. Black arrows (outer tooth row); white arrow (inner tooth row); arrowheads (cavities that accommodate the teeth when the mouth is closed); To, tongue; S, skin; E, eye; Te, tentacle; Oe, oral epithelium.
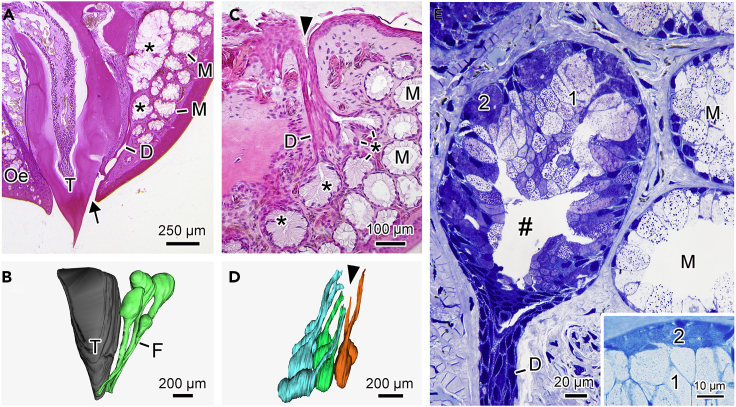
In the upper jaw, the glandular ducts open exclusively at the base of the outer tooth row (Figures 2A, 2B, and S1), whereas in the lower jaw, the ducts can also open in the interior of the cavities that accommodate the upper jaw teeth when the mouth is closed (Figures 2C, 2D, and S1). Each of the tooth-related glands is provided with a single duct, which may fuse with the ducts of adjacent glands (Figure 2B).
Figure 2.
Structure of Tooth-Related Glands in S. annulatus
(A) Upper jaw.
(B) 3D reconstruction of a set of glands associated with one upper jaw tooth.
(C) Lower jaw.
(D) 3D reconstruction of a set of glands associated with one lower jaw cavity.
(E) Upper jaw tooth-related gland, showing the presence of two types of cells. The insert shows the abundance of protein in type 2 cells. Asterisk, tooth-related glands; T, tooth; D, duct; arrow, duct opening; arrowhead, cavity that accommodates an upper jaw tooth; F, fusion of the glandular ducts; #, lumen; M, mucous skin glands; 1, type 1 cells; 2, type 2 cells.
Each gland is acinar in shape with a well-defined central lumen (Figure 2E). The glandular body is composed of two cell types (Figure 2E). Type 1 cells are more abundant (Figure 2E) and primarily contain mucous substances (Figure S2), whereas type 2 cells are less abundant and show high protein concentration (Figure 2E insert and S2). The morphology and histochemistry in both cell types are different from those observed in the ordinary cutaneous mucous glands (Figures 2E and S2). Moreover, the secretory epithelium of the tooth-related glands is in direct contact with the surrounding dermal connective tissue, without a myoepithelial layer and with no relationship with muscles (Figures 2A, 2C, 2E, and S1).
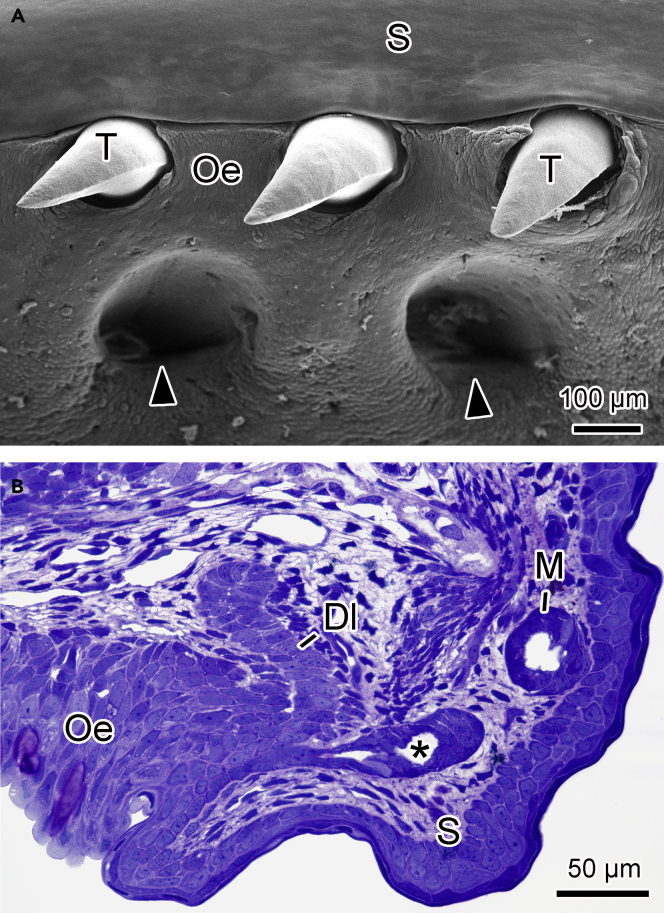
Teeth are conical and sharply pointed with a slight lateral flattening. However, they do not show grooves or slits to facilitate the flow of glandular secretion (Figure 3A). When the animal opened its mouth before attacking the prey, a highly viscous secretion covering the teeth was also observed. The teeth in the upper jaw composing the outer row are interconnected through a continuous groove that contours the whole upper lip (Figure 3A) and seems to play an important role in conducting and homogeneously distributing the secretion during prey apprehension (Figure S3).
Figure 3.
S. annulatus Teeth and the Development of Associated Glands
(A) Outer tooth row of the upper jaw. Note a continuous groove between the skin and the oral epithelium.
(B) Histological section of a recently hatched young. S, skin; Oe, oral epithelium; arrowheads, cavities that accommodate the mandibular teeth; asterisk, dental gland; Dl, dental lamina; M, skin mucous glands.
Among all species we examined (except for the aquatic Typhlonectes compressicauda [family Typhlonectidae], lacking tooth-related glands in the upper jaw), all species, including the basal genus Rhinatrema (family Rhinatrematidae), revealed dental gland arrangements very similar to those observed in S. annulatus (Figure S4), with, however, variation in the number of glands (Table S1).
The analysis of the jaws of newly hatched S. annulatus showed that these glands are developed from the dental lamina, the same tissue that usually gives rise to teeth (Figure 3B).
Dental Secretion Contains Enzymes
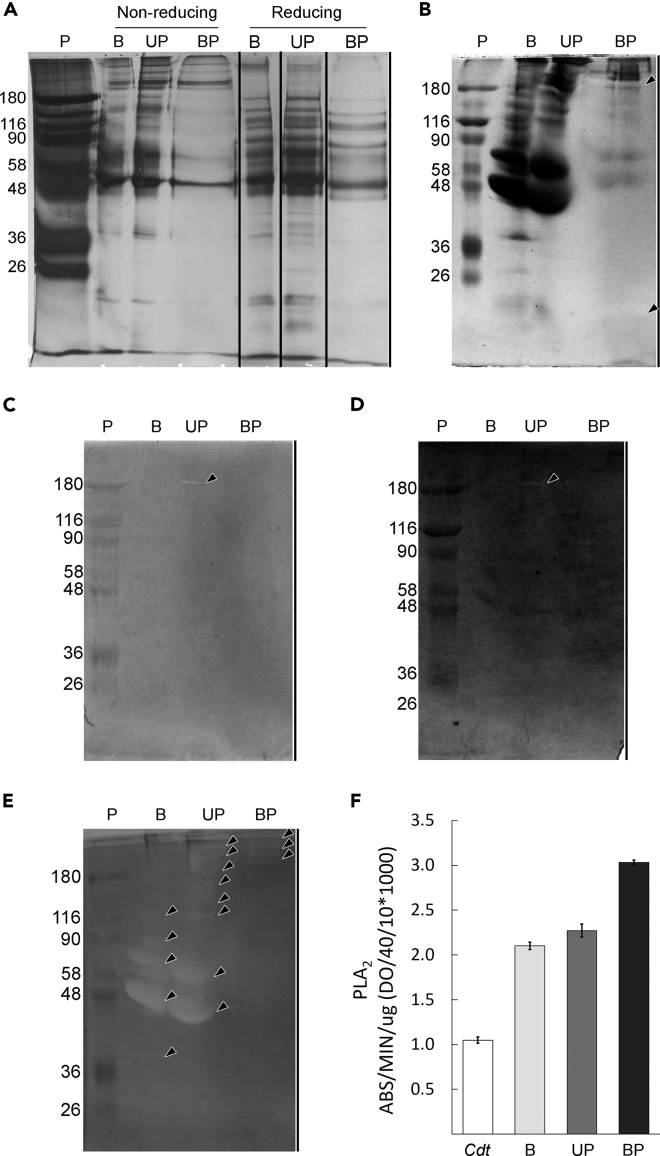
In order to remove the low-molecular-mass molecules (non-protein components) and to concentrate and optimize the analysis of the proteins, the buffered supernatant dental gland secretion was submitted to an ion exchange solid phase extraction procedure, resulting in two additional samples (unbound and bound proteins). SDS-PAGE of the tooth-related glands secretion of S. annulatus revealed several bands between 40 and 200 kDa (Figure 4A). The analysis of enzymatic activities demonstrated gelatinolytic (Figures 4B and S5), caseinolytic (Figures 4C and S5), and fibrinogenolytic proteins (Figures 4D and S5) in the preparations processed by ion-exchange chromatography besides a pronounced hyaluronidasic activity (Figures 4E and S5) in all preparations. Phospholipase A2 activity was also detected in all preparations tested (Figure 4F) and was higher than that detected in Crotalus durissus terrificus venom.
Figure 4.
Protein Profile and Enzymatic Activity of S. annulatus Dental Gland Secretion
(A) SDS-PAGE 12%.
(B) Gelatinolytic activity.
(C) Caseinolytic activity.
(D) Fibrinogenolytic activity.
(E) Hyaluronidase activity.
(F) Phospholipase activity. P, molecular mass markers (in kDa); B, buffered secretion supernatant; UP, unbound proteins; BP, bound proteins (BP); Cdt, Crotalus durissus terrificus venom. Results were expressed as mean ± SE.
Discussion
Caecilians are amphibians with a serpentine and ringed body primarily adapted to the life underground. Possibly originated in the Late Carboniferous-Early Permian (San Mauro, 2010), most of the current species occur in lands originated from the supercontinent Gondwana (Duellman and Trueb, 1994). With 214 species, the order Gymnophiona is divided into 10 families (Frost, 2020), which represents only 2.65% of all amphibians. Owing to their distribution and fossorial habits, caecilians consist in one of the least known vertebrate groups (Torres-Sánchez et al., 2019), especially with regard to their biology, ecology, natural history, and behavior (Jared et al., 2019).
It seems quite evident that such negligence in the study of caecilians is due to their fossoriality, making them secretive, quite inaccessible animals. The active collection of specimens is only possible through manual excavation, which undertakes great energy. It is estimated that 4–20 h of excavation is necessary, depending on the season, to localize one single specimen (Jared et al., 2019). Moreover, during aleatory manual excavation of the soil, severe injuries or even death of specimens are frequent (Jared et al., 2019). Because of the difficulty in collecting data on caecilian biology, the restrict scientific knowledge available, in general, comes from sparse reports, indirectly obtained mainly through specimens deposited in zoological museums. Investments in the study of life underground in groups such as snakes, amphisbaenians, and caecilians, may be very enlightening, giving an effective contribution to the knowledge of vertebrate morphological and physiological adaptations to the fossorial world.
All current groups of amphibians (Lissamphibia) share a skin characterized by the presence of mucous and granular (or venom) glands distributed throughout the body (Duellman and Trueb, 1994, Toledo and Jared, 1995). Despite that, very few studies are devoted to the morphological and biochemical characterization of such glands and their secretion. It thus remains unknown whether the structure and biochemical properties of caecilian cutaneous glands are similar to those of other amphibians or if they diversified along their evolutionary history. Despite this lack of information, there seems to be a consensus that caecilians do not have any type of structure capable of inoculating the poison produced by the cutaneous glands. Unlike animals with active chemical defense, such as snakes, which actively inoculate the venom through bites, in caecilians as well as in the vast majority of anurans and salamanders, the aggressors (or predators) themselves are responsible for causing their poisoning (Heiss et al., 2010, Jared et al., 2018, Jared et al., 2015, Jared et al., 2009, Mailho-Fontana et al., 2014, Nowak and Brodie, 1978, Regis-Alves et al., 2017).
It was previously shown by our group that the caecilian S. annulatus has three basic types of cutaneous glands with a heterogeneous distribution pattern through the body, possibly in response to fossoriality (Jared et al., 2018); such characteristic seems to be shared by other species within the group. However, while doing this study, when analyzing S. annulatus cranial morphology, especially the labial region, we were surprised by the presence of a fourth type of gland associated with the upper jaw teeth and with the cavities that accommodate the lower jaw teeth. Given the presence of such glands in adult males and females and newly hatched individuals, we proceeded to investigate the glandular morphology, as well as the role of the secretion they produce for predation.
Although in S. annulatus we verified quantitative differences in the tooth-related glands when comparing the upper and lower jaws, all glands show very similar morphology, basically composed of cells secreting a mixture of mucus, lipids, and proteins. However, at the periphery of the glandular tissue, a clear distinct cell type was also identified, whose histochemistry indicated a higher protein content, similar to what was described for the oral venom glands identified in the lizard Gerrhonotus infernalis (Fry et al., 2010).
Unlike amphibian ordinary cutaneous glands, the tooth-related glands of S. annulatus are not involved by a myoepithelial cell monolayer (Toledo and Jared, 1995) or compressor muscles present in snake venom glands (Weinstein et al., 2010). Consequently, secretion release seems to depend on prolonged bites that compress the glands located in the labial area. Additionally, the behavior of biting and holding the prey while pushing it against the substrate (Jared et al., 1999) together with powerful long-axis body rotations during predation (Measey and Herrel, 2006), besides facilitating prey tearing (Bemis et al., 1983), may play an important role in gland secretion release.
Unlike what is observed in snakes and some lizards (Weinstein et al., 2010), S. annulatus teeth have no slots or grooves able to conduct secretion. On the other hand, the presence of secretion covering the teeth surface during predation suggests that the teeth can inoculate secretion at the moment of the bite. Moreover, the groove that circles the upper jaw interconnecting the teeth appears to play an important role in the uniform distribution of secretion during bites.
(Mang 1935) reported the presence of tooth-related glands in the caecilian Hypogeophis rostratus (family Indotyphidae) but considered them mere differentiated cutaneous mucous glands. Differently, our analysis of teeth of newly hatched S. annulatus clearly showed that these glands are developed from the dental lamina, the same tissue that usually gives rise to teeth, and not from the epidermis, as ordinarily seen in amphibian cutaneous glands (Toledo and Jared, 1995). Owing to the common ontogenetic origin of the glandular tissue and teeth, these glands are named “dental glands” in reptiles (Tucker, 2010). By evolutionary viewpoint, our finding is particularly important since in snakes the venom glands (including Duvernoy glands) also originate from the dental lamina (Tucker, 2010, Vonk et al., 2008). As far as we could verify, this is the first time that dental glands have been found within Class Amphibia.
Analyzing caecilians of different families, including basal groups, a glandular arrangement similar to that of S. annulatus was found, showing that the presence of dental glands seems to be a characteristic shared by all Gymnophiona. On the other hand, the absence of glands in the maxilla of the caecilian Typhlonectes compressicauda indicates that their presence and arrangement inside the mouth may be related to the aquatic environment in which they live. Absence (or regression) of oral glands has also been observed in some aquatic snakes, as they do not need to moisten the food to facilitate swallowing (Arbuckle, 2015). In this sense, a study of the dental glands in different species of Gymnophiona is necessary in order to analyze the relationship between the presence of such glands and the development and biology of different species. The characteristics of the secretion in aggressive species able of biting when harassed would be another interesting aspect to be investigated.
SDS-PAGE of S. annulatus dental gland secretion revealed distinct characteristics when compared with the cutaneous secretion of S. annulatus (Jared et al., 2018), once more highlighting its peculiarities when compared with the ordinary cutaneous glands. The enzymatic activities, usually considered typical of proteins composing animal venoms, specially snakes (Jared et al., 2015, Mackessy, 2010), evidence the potential of dental gland secretion to promote disruption in prey physiology and initial digestive processes concomitant to prey swallowing. Among these results, the type A2 phospholipase activity is undeniable and even more evident than that of the venom of the South American rattlesnake Crotalus durissus terrificus. Despite our promising results, a more complete study still is needed in order to identify such proteins and to compare them with proteins composing snake venoms. Also, the analysis of caecilian dental glands by RNA sequencing would be a key point for the elucidation of the protein classes secreted by these glands in order to reveal their toxic potential. It is worth mentioning that amphibians are known by their variety of extremely toxic non-protein molecules in the skin (Duellman and Trueb, 1994, Jeckel et al., 2015, Toledo and Jared, 1995). Therefore, one cannot rule out the possibility of caecilian dental glands containing molecules from other classes, such as lipids, widely found in toads (Mailho-Fontana et al., 2018) and already identified by histochemistry in S. annulatus tooth-glands.
In reptiles, vertebrates whose evolution of the oral glands has been extensively studied, the basal condition for the venom glands is considered the occurrence of incipient glands, both in the mandibular and maxillary regions (Fry et al., 2013, Fry et al., 2010, Fry et al., 2006). Particularly in snakes, evolution seems to have favored the venom glands located in the maxilla, causing, secondarily, the loss of the mandibular venom glands (Fry et al., 2013, Fry et al., 2006). In lizards, on the other hand, the path seems to have been exactly the opposite (Fry et al., 2010). Some lineages of snakes and lizards, however, have functional glands in both jaws (Fry et al., 2013, Fry et al., 2010, Fry et al., 2006). Thus, the presence of dental glands both in upper and lower jaws as seen in caecilians would represent a plesiomorphic state in relation to the reptile venomous system. It seems quite possible that the selective pressure imposed by the underground environment was a key factor for the development of the dental glands, since anurans and salamanders, which constitute Gymnophiona's sister group (San Mauro, 2010), do not have such structures.
Venom is assumed to be a substance produced by an organism in specialized structures, either a tissue or a gland, that interferes in physiological and biochemical processes when injected into another organism through an injury (Arbuckle, 2015). Venom action does not necessarily lead the prey to rapid death (Arbuckle, 2015, Jackson et al., 2017). It is important to note that the medical consequences to humans are not taken into consideration in this definition (Arbuckle, 2015).
Despite the differences in glandular structure between Gymnophiona and Squamata and taking in consideration the recognition of Lissamphibia as monophyletic (Pyron, 2011, San Mauro, 2010), the dental glands seem to be homologous structures that have evolved independently in the two groups. Based on our data we suggest that caecilians developed the ability to actively inoculate toxins through their teeth early in their evolutionary history, probably representing one of the first terrestrial vertebrates having an oral venom system.
Limitations of the Study
In this work, we showed the presence of dental glands in caecilians (order Gymnophiona), the group of amphibians considered the least known within vertebrates in several biological aspects. Our analyses have clearly shown that the caecilian dental glands secrete a mixture of molecules of different classes, such as proteins with enzymatic activities, widely found in venomous animals in general. Although we have shown the presence of dental glands and their possible role during predation, more evidence is still needed about the precise identity of the proteins present in the secretion, as well as data about the toxic potential of these compounds. However, for such studies, additional specimens are necessary. Finally, future comparisons of caecilian dental glands with samples from other glands of S. annulatus and/or from venom glands of other animals will be useful in order to determine their differences and similarities and conclude whether caecilians can be considered venomous animals.
Resource Availability
Lead Contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Carlos Jared (carlos.jared@butantan.gov.br).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate or analyze datasets or code.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Daiane Laise da Silva, Emídio Beraldo Neto, Beatriz Maurício, Luciana Almeida Sato, Simone Gonçalves Silva Jared, and Mariza Valsechi dos Santos for their technical assistance. We thank National Council for Scientific and Technological Development (CNPq) processes #308178/2014–9 to C.J., #303792/2016–7 to M.M.A., and #303792/2016–7 to D.C.P., São Paulo Research Foundation (FAPESP) process #2017/10488-1 to P.L.M.-F. and #2018/03265-9 to C.J., and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) Finance Code 001 to P.L.M.-F. and C.A.
Author Contributions
P.L.M.-F., M.M.A., and C.J. conceived the research. All authors collated the data and contributed with ideas. P.L.M.-F. conducted the analysis and writing with input from all authors.
Declaration of Interests
The authors declare no competing interests.
Published: July 3, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101234.
Supplemental Information
References
- Arbuckle K. Springer; 2015. Evolution of venomous animals and their toxins; pp. 1–23. [Google Scholar]
- Bemis W.E., Schwenk K., Wake M.H. Morphology and function of the feeding apparatus in Dermophis mexicanus (Amphibia: Gymnophiona) Zool. J. Linn. Soc. 1983;77:75–96. [Google Scholar]
- Casewell N.R., Wüster W., Vonk F.J., Harrison R.A., Fry B.G. Complex cocktails: the evolutionary novelty of venoms. Trends Ecol. Evol. 2013;28:219–229. doi: 10.1016/j.tree.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Duellman W.E., Trueb L. Second Edition. The Johns Hopkins University Press; 1994. Biology of Amphibians. [Google Scholar]
- Frost D.R. Am. Museum Nat. Hist; 2020. Amphibian Species of the World: an Online Reference. Version 6.0 Database.http://research.amnh.org/herpetology/amphibia/index.html [Google Scholar]
- Fry B.G., Undheim E.A.B., Ali S.A., Jackson T.N.W., Debono J., Scheib H., Ruder T., Morgenstern D., Cadwallader L., Whitehead D. Squeezers and leaf-cutters: differential diversification and degeneration of the venom system in toxicoferan reptiles. Mol. Cell. Proteomics. 2013 doi: 10.1074/mcp.M112.023143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry B.G., Vidal N., Norman J.A., Vonk F.J., Scheib H., Ramjan S.F.R., Kuruppu S., Fung K., Blair Hedges S., Richardson M.K. Early evolution of the venom system in lizards and snakes. Nature. 2006;439:584–588. doi: 10.1038/nature04328. [DOI] [PubMed] [Google Scholar]
- Fry B.G., Winter K., Norman J.A., Roelants K., Nabuurs R.J.A., Van Osch M.J.P., Teeuwisse W.M., Van Der Weerd L., Mcnaughtan J.E., Kwok H.F. Functional and structural diversification of the anguimorpha lizard venom system. Mol. Cell. Proteomics. 2010;9:2369–2390. doi: 10.1074/mcp.M110.001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greef R. Über Siphonops thomensis Barboza du Bocage. Naturwiss Z. 1884;184:15–32. [Google Scholar]
- Heiss E., Natchev N., Salaberger D., Gumpenberger M., Rabanser A., Weisgram J. Hurt yourself to hurt your enemy: new insights on the function of the bizarre antipredator mechanism in the salamandrid Pleurodeles waltl. J. Zool. 2010;280:156–162. [Google Scholar]
- Jackson T.N.W., Young B., Underwood G., McCarthy C.J., Kochva E., Vidal N., van der Weerd L., Nabuurs R., Dobson J., Whitehead D. Endless forms most beautiful: the evolution of ophidian oral glands, including the venom system, and the use of appropriate terminology for homologous structures. Zoomorphology. 2017;136:107–130. [Google Scholar]
- Jared C., Antoniazzi M.M., Jordão A.E.C., Silva J.R.M.C., Greven H., Rodrigues M.T. Parotoid macroglands in toad (Rhinella jimi): their structure and functioning in passive defence. Toxicon. 2009;53:197–207. doi: 10.1016/j.toxicon.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Jared C., Mailho-Fontana P.L., Antoniazzi M.M., Mendes V.A., Barbaro K.C., Rodrigues M.T., Brodie E.D. Venomous frogs use heads as weapons. Curr. Biol. 2015;25:2166–2170. doi: 10.1016/j.cub.2015.06.061. [DOI] [PubMed] [Google Scholar]
- Jared C., Mailho-Fontana P.L., Marques-Porto R., Sciani J.M., Pimenta D.C., Brodie E.D., Antoniazzi M.M. Skin gland concentrations adapted to different evolutionary pressures in the head and posterior regions of the caecilian Siphonops annulatus. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-22005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jared C., Mailho-Fontana P.L., Jared S.G.S., Kupfer A., Delabie J.H.C., Wilkinson M., Antoniazzi M.M. Life history and reproduction of the neotropical caecilian Siphonops annulatus (Amphibia, Gymnophiona, Siphonopidae), with special emphasis on parental care. Acta Zool. 2019;100:292–302. [Google Scholar]
- Jared C., Navas C.A., Toledo R.C. An appreciation of the physiology and morphology of the Caecilians (Amphibia: Gymnophiona) Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1999;123:313–328. [Google Scholar]
- Jeckel A.M., Grant T., Saporito R.A. Sequestered and synthesized chemical defenses in the poison frog Melanophryniscus moreirae. J. Chem. Ecol. 2015;41:505–512. doi: 10.1007/s10886-015-0578-6. [DOI] [PubMed] [Google Scholar]
- Kupfer A., Nabhitabhata J., Himstedt W. From water into soil: trophic ecology of a caecilian amphibian (Genus Ichthyophis) Acta Oecol. 2005;28:95–105. [Google Scholar]
- Mackessy S.P. First Edition. CRC Press; 2010. Handbook of Venoms and Toxins of Reptiles. [Google Scholar]
- Mailho-Fontana P.L., Antoniazzi M.M., Sciani J.M., Pimenta D.C., Barbaro K.C., Jared C. Morphological and biochemical characterization of the cutaneous poison glands in toads (Rhinella marina group) from different environments. Front. Zool. 2018 doi: 10.1186/s12983-018-0294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailho-Fontana P.L., Antoniazzi M.M., Toledo L.F., Verdade V.K., Sciani J.M., Barbaro K.C., Pimenta D.C., Rodrigues M.T., Jared C. Passive and active defense in toads: the parotoid macroglands in Rhinella marina and Rhaebo guttatus. J. Exp. Zool. A Ecol. Genet. Physiol. 2014;321:65–77. doi: 10.1002/jez.1838. [DOI] [PubMed] [Google Scholar]
- Mang A. Beitrage zur Kenntnis der Gymnophionen XXII. Uber die Drusen der Haut und der Mundhole van Hypogeophis. Morphol. Jahrb. 1935;75:296–314. [Google Scholar]
- Measey G.J., Herrel A. Rotational feeding in caecilians: putting a spin on the evolution of cranial design. Biol. Lett. 2006;2:485–487. doi: 10.1098/rsbl.2006.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll E.O., Smith H.M. Lizards in the diet of an American caecilian. Nat. Hist. Misc. 1967;187:1–2. [Google Scholar]
- Ngo B.V., Hoang N.T., Ngo C.D. Diet of the Bannan Caecilian Ichthyophis bannanicus (Amphibia: Gymnophiona: Ichthyophiidae) in the Mekong Delta, Vietnam. J. Herpetol. 2014;48:506–513. [Google Scholar]
- Nowak R.T., Brodie E.D. Rib penetration and associated antipredator adaptations in the salamander Pleurodeles waltl (Salamandridae) Copeia. 1978;1978:424–429. [Google Scholar]
- Presswell B., Gower D.J., Oomen O.V., Measey G.J., Wilkinson M. Scolecophidian snakes in the diets of south Asian caecilian amphibians. Herpetol. J. 2002;12:123–126. [Google Scholar]
- Pyron R.A. Divergence time estimation using fossils as terminal taxa and the origins of lissamphibia. Syst. Biol. 2011;60:466–481. doi: 10.1093/sysbio/syr047. [DOI] [PubMed] [Google Scholar]
- Regis-Alves E., Jared S.G.S., Maurício B., Mailho-Fontana P.L., Antoniazzi M.M., Fleury-Curado M.C., Brodie E.D., Jared C. Structural cutaneous adaptations for defense in toad (Rhinella icterica) parotoid macroglands. Toxicon. 2017;137:128–134. doi: 10.1016/j.toxicon.2017.07.022. [DOI] [PubMed] [Google Scholar]
- Roelants K., Gower D.J., Wilkinson M., Loader S.P., Biju S.D., Guillaume K., Moriau L., Bossuyt F. Global patterns of diversification in the history of modern amphibians. Proc. Natl. Acad. Sci. U S A. 2007;104:887–892. doi: 10.1073/pnas.0608378104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Mauro D. A multilocus timescale for the origin of extant amphibians. Mol. Phylogenet. Evol. 2010;56:554–561. doi: 10.1016/j.ympev.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Summers A.P., Wake M.H. The retroarticular process, streptostyly and the caecilian jaw closing system. Zoology. 2005;108:307–315. doi: 10.1016/j.zool.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Toledo R.C., Jared C. Cutaneous granular glands and amphibian venoms. Comp. Biochem. Physiol. A Physiol. 1995;111:1–29. [Google Scholar]
- Torres-Sánchez M., Creevey C.J., Kornobis E., Gower D.J., Wilkinson M., San Mauro D. Multi-tissue transcriptomes of caecilian amphibians highlight incomplete knowledge of vertebrate gene families. DNA Res. 2019;26:13–20. doi: 10.1093/dnares/dsy034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A.S. Salivary gland adaptations: modification of the glands for novel uses. In: Tucker A.S., Miletich I., editors. Salivary Glands: Development, Adaptations and Disease. Karger; 2010. pp. 21–31. [DOI] [PubMed] [Google Scholar]
- Vonk F.J., Admiraal J.F., Jackson K., Reshef R., De Bakker M.A.G., Vanderschoot K., Van Den Berge I., Van Atten M., Burgerhout E., Beck A. Evolutionary origin and development of snake fangs. Nature. 2008;454:630–633. doi: 10.1038/nature07178. [DOI] [PubMed] [Google Scholar]
- Wake M.H. The development and replacement of teeth in viviparous caecilians. J. Morphol. 1976;148:33–63. doi: 10.1002/jmor.1051480104. [DOI] [PubMed] [Google Scholar]
- Weinstein S.A., Smith T.L., Kardong K.V. Reptile venom glands: form, function, and future. In: Mackessy S.P., editor. Handbook of Reptile Venoms and Toxins. Taylor Francis; 2010. pp. 65–91. [Google Scholar]
- Zhang P., Wake D.B. Higher-level salamander relationships and divergence dates inferred from complete mitochondrial genomes. Mol. Phylogenet. Evol. 2009;53:492–508. doi: 10.1016/j.ympev.2009.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate or analyze datasets or code.