Abstract
Nitroxides are low molecular weight (150–400 Da) superoxide dismutase mimics that exhibit antioxidant, radical scavenging, and radioprotective activity. Additionally, the paramagnetic nature of nitroxides makes them viable as both spin probes for electron paramagnetic resonance imaging as well as contrast agents for magnetic resonance imaging. These imaging techniques enable in vivo monitoring of nitroxide metabolism. In biological systems, nitroxide metabolism occurs predominantly via reduction of the nitroxide to a hydroxylamine. The rate of nitroxide reduction can increase or decrease due to oxidative stress, suggesting that nitroxides can provide an imaging-based assay of tissue redox status. The current review briefly summarizes the potential clinical applications of nitroxides, and focuses on the biochemical and tumor microenvironmental factors that affect the rate of nitroxide reduction. The potential therapeutic applications and bio-reduction mechanisms are discussed in the context of their relevance to oncology.
Keywords: Nitroxides, oxidative stress, redox-imaging, magnetic resonance imaging, electron paramagnetic resonance
1. GENERAL INTRODUCTION
Exposure of cells to oxidative stress may promote carcinogenesis, cellular proliferation, and resistance to chemotherapy treatments [1–4]. Antioxidants that relieve oxidative stress are therefore a promising strategy for clinical cancer prevention and treatment [1]. The antioxidant enzyme superoxide dismutase (SOD) is particularly interesting because it appears to be upregulated or downregulated in mammalian cancer cells [2, 3], and because in some models elevated SOD levels correlate with reduced rates of malignant transformation and metastasis [3]. While these observations suggest it may be beneficial to increase the intracellular SOD activity of tumor tissue during clinical cancer therapy, the large size of the SOD enzyme (Mr ~ 32,500) renders it impermeable to cell-membranes. To overcome this problem, various low molecular weight SOD mimics have been discovered which are indeed cell permeable [1]. This report reviews nitroxides, which are cell-permeable SOD mimics. In the context of oncology, nitroxides exhibit several interesting properties including radioprotection, antioxidant activity, and paramagnetism. The later property enables imaging of their pharmacokinetics and metabolism in tumor tissue with various techniques including magnetic resonance imaging (MRI).
When nitroxides cross a cell membrane, they are subsequently reduced by intracellular antioxidants which may include thiols, antioxidant enzymes, and redox cofactors. Because nitroxide reduction can be non-invasively monitored using MRI and other spectroscopic techniques, they have been proposed as contrast agents for an imaging-based assay of the intracellular redox status of tissue. The biological and therapeutic properties of nitroxides [4, 5] and the recent developments in the field of nitroxide-based redox imaging [6] have both been recently reviewed, and the current review therefore covers these subjects only briefly. The intent of this review is to outline the biochemical factors that contribute to nitroxide reduction and metabolism, and to discuss the biological significance of redox imaging data.
2. INTRODUCTION TO NITROXIDES
Nitroxides are low molecular weight (often 155–400 Da), cyclic, paramagnetic, stable free radicals. Fig. (1) shows the molecular structure of the nitroxides that are discussed in this review. As can be seen, the molecular structure varies considerably between different nitroxides, but nearly all nitroxides have a 5- or 6- membered ring that contains a NO• radical group. In biological systems, nitroxides pass through the cellular membrane and participate in various reduction-oxidation (redox) reactions involving heme proteins, reactive oxygen species, thiols, and redox cofactors [7–11]. These redox reactions result in the cycling of nitroxides between three redox states (Fig. 2), with an equilibrium that greatly favors the hydroxylamine over the nitroxide radical and oxoammonium cation. Thus, after nitroxides are intravenously injected into a mouse, they distribute into various tissues and cells and are subsequently converted to the diamagnetic hydroxylamine by intracellular oxidizing and reducing species [6, 11, 12]. Notably, the reduction rates and biological effects of nitroxides are as variable as their structure. For example, SCCVII tumors reduce the piperidine nitroxide Tempol 11 times faster than the pyrroline nitroxide 3-carbomyl proxyl (3-CP) [12]. Furthermore, for a given ring structure, different R groups (Fig. 1) can result in substantially different radioprotective potencies [13] and metabolism (bioreduction) rates [11, 14, 15].
Fig. (1).
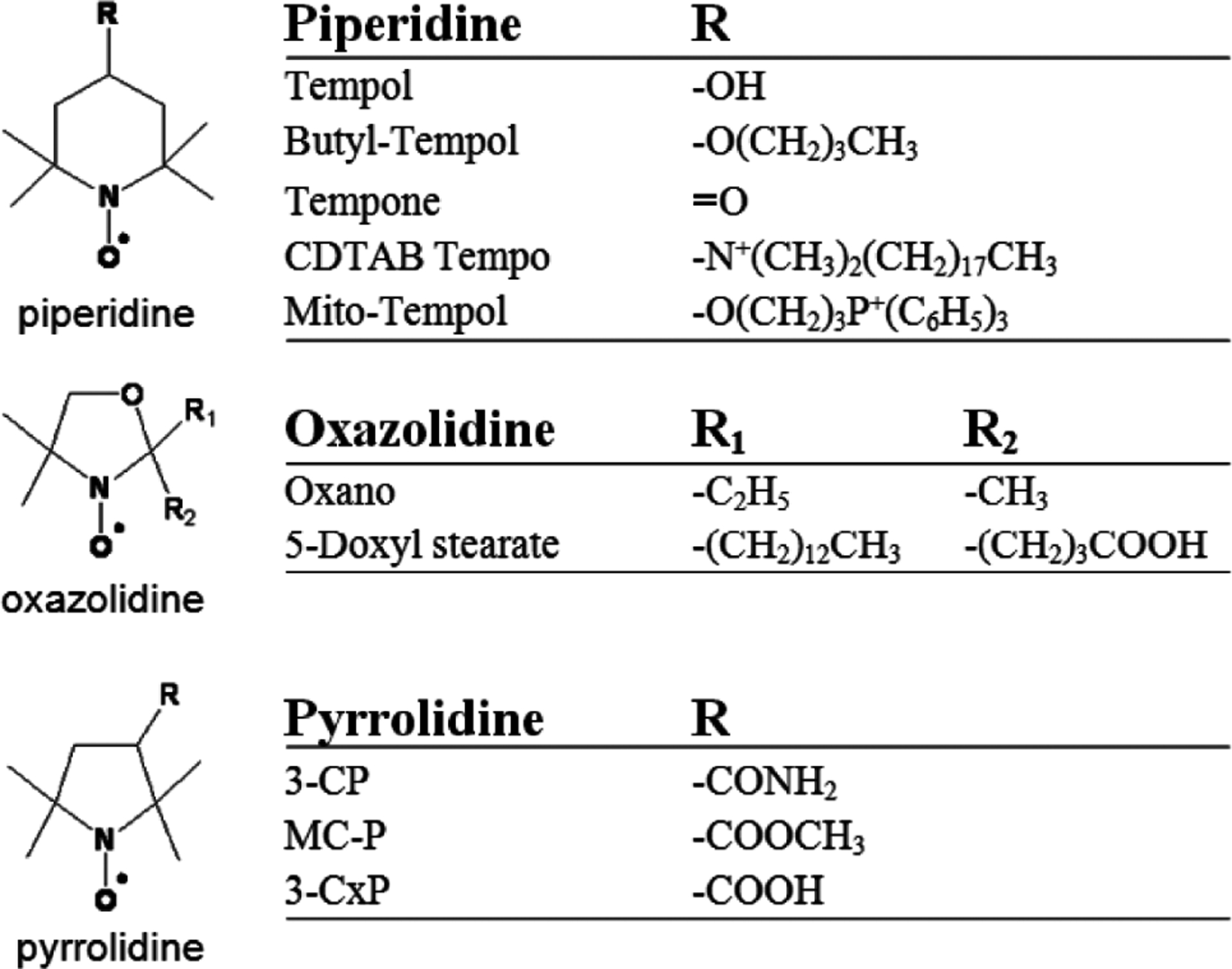
This figure depicts the classes (piperidine (top), oxazolidine (middle), pyrrolidine (bottom)) and substituents (R groups) of the nitroxides discussed in this review.
Fig. (2).
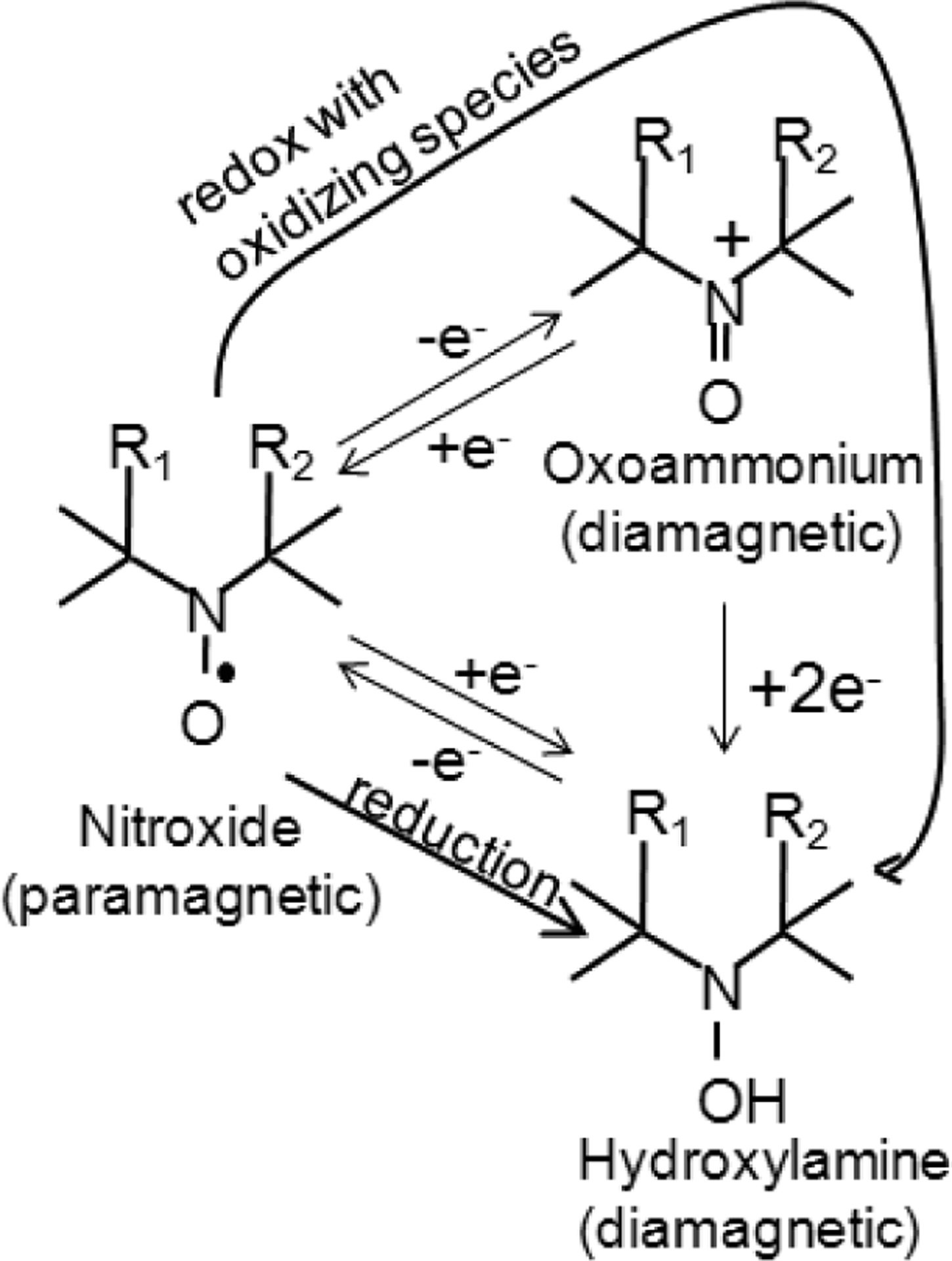
The three oxidation states of nitroxides. The diamagnetic states do not provide MRI or EPR contrast, while the paramagnetic nitroxide is detectable with both MRI and EPR. There are two paths that result in reduction of the nitroxide into the hydroxylamine. The “reduction” path is a one step process where the nitroxide is directly reduced by one electron to the hydroxylamine. The “redox with oxidizing species” path is a two-step process first involving 1e− oxidation to the oxoammonium cation then 2e− reduction to the hydroxylamine.
3. NITROXIDES AS ANTIOXIDANTS, RADIOPROTECTORS, AND SOD MIMICS.
The first nitroxide identified as an SOD mimic was Oxano (Fig. 1), and SOD mimic activity has since been found to be a general property of various piperidine and pyrrolidine nitroxides [9, 10]. The mechanism of nitroxide SOD activity involves a two-step process. In the first step, the nitroxide (RR’NO•) is oxidized to an oxoammonium cation by protonated superoxide (HOO•) [16]:
| (1) |
In the second step, the oxoammonium is then reduced by superoxide back into the nitroxide:
| (2) |
The overall result of these two sub-reactions is the dismutation of two molecules of superoxide into hydrogen peroxide and molecular oxygen:
| (3) |
The preceding reaction is the characteristic reaction of superoxide dismutase, and nitroxides are therefore SOD mimics. It is worth noting that the interaction of nitroxides with protonated superoxide (equation 1) results in increased SOD mimetic activity at lower pH.
Since the discovery that nitroxides were SOD mimics, their reaction rates with superoxide and other radicals have been characterized. Compared to SOD at pH 7.8, which has a kcat for O2•− dismutation of approximately 1 × 109 M−1s−1, and manganese porphyrins, which have a kcat as high as 4 × 108 M−1s−1 [17], piperidine nitroxides usually have a kcat in the range of 1 × 104 − 1 × 106 M−1s−1 [7, 18] at a similar pH, and are thus relatively weak superoxide dismutase mimics. Notably, at pH close to that of biological lipid membranes (pH 4–5) the kcat of O2•− dismutation increases to nearly 1×108 M−1s−1 [19], suggesting that the antioxidant effects of nitroxides vary between biological compartments. Although nitroxides react with superoxide relatively slowly, they are efficient scavengers of other radicals such as •NO2 (kcat =3 × 108 − 9 × 108 M−1s−1) and CO3•− (kcat = 2 × 108 − 6 × 108 M−1s−1). Additionally, while piperidine and pyrrolidine nitroxides do not appreciably scavenge nitric oxide and peroxynitrite [20], they rapidly scavenge glutathione (GS•), cystine (CysS•), and penicillamine (PenS•) thiyl radicals (5–7 × 108 M−1s−1 [21]). It is perhaps due to the ability of nitroxides to dismutate and scavenge radicals that they exhibit several forms of antioxidant and radioprotective activity, including the ability to inhibit DNA strand breaks in solution [22]; the ability to protect mammalian cell cultures against lethal oxidative stress initiated by superoxide [18, 22], hydrogen peroxide [13, 18, 22], and ionizing radiation [13, 23]; and the ability to protect against lethal [24–27] and sub-lethal [28–30] radiation in mice. These studies collectively suggest that nitroxides exhibit antioxidant and radioprotective effects through a variety of interactions with radicals, though not necessarily though the dismutation of superoxide. With regard to nitroxide-mediated radioprotective effects, protection occurs predominantly due to radical scavenging, chemical repair by donation of H atoms, and radical-radical interactions [4, 23]. Thus, nitroxides provide protection during radiation exposure through chemical interactions with secondary radicals generated by radiation.
4. IMAGING OF NITROXIDES: REDOX IMAGING
Nitroxides are detected in vivo using one of four techniques: electron paramagnetic resonance spectroscopy (EPRS), electron paramagnetic resonance imaging (EPRI), proton magnetic resonance imaging (MRI), and Overhauser enhanced magnetic resonance imaging (OMRI) [31]. EPRS measures the resonance absorption of microwave energy by unpaired electrons (radicals) in low strength magnetic fields (on the order of 10mT, 300MHz [32]), and thus is able to directly detect the aminoxyl group (R2NO•) on the nitroxide molecule. EPRI is similar to EPRS, except that gradient magnets are incorporated into the scanner hardware, allowing spatial mapping of the nitroxide levels. In contrast with EPRS and EPRI, MRI measures chiefly water protons, and therefore cannot directly measure the nitroxide radical. However, nitroxides are paramagnetic and they therefore shorten the longitudinal recovery time (T1) of local water protons, enabling indirect detection of the nitroxide radical with MRI. Using MRI, the presence of the nitroxide radical is thus detected with a T1-weighted gradient recalled echo pulse sequence. Finally, the OMRI technique uses both nuclear magnetic resonance and electron paramagnetic resonance. In OMRI experiments the electron paramagnetic resonances of the nitroxide are saturated, and due to the Overhauser effect [33], this saturation causes enhanced polarization of the local water protons in the sample. The polarization of the protons is then detected using a gradient recalled echo pulse sequence that is similar to the sequence used during MRI experiments [34]. In contrast with EPRI, the spatial resolution of OMRI is not dependent on the EPR linewidth, and OMRI therefore has higher spatial resolution than EPRI [35].
The endogenous radical concentration in living tissue is below the sensitivity of EPRI. This results in the inability of both EPRI and OMRI to acquire anatomical images for co-registration with the images of nitroxides. In practice, this limited the early EPRI redox imaging studies to large and easily localizable tissues such as leg muscle and leg tumors. MRI, on the other hand, provides excellent soft tissue images and has resolution sufficient to monitor nitroxide reduction in small tissues such as the salivary glands and rectum, and for these reasons nitroxides are often imaged with MRI [6, 12, 36, 37].
Fig. (3) summarizes the results of imaging experiments that illustrate the uptake and reduction of Tempol in non-cancerous and tumor tissues. These images were obtained after intravenous injection of 130 mg/kg Tempol, a dose which provides adequate signal enhancement but exhibits no toxicity [12]. Tempol tissue concentrations shown in Fig. (3a) were calculated on a voxel by voxel basis using a T1 map, a B1 field map, the longitudinal relaxivity of Tempol (0.20 mM−1s−1), and a set of dynamic (repeating) T1- weighted fast field echo images [12]. Fig. (3a) shows that 0.7 min after injection, Tempol accumulated into the leg muscle and SCCVII tumor at concentrations ranging from 0–2 mM. As time progresses, the nitroxide concentration decayed toward 0 mM in most regions of the image slice. In order to compare the dynamic Tempol concentration between leg muscle and tumor, regions of interest (ROIs) were drawn around these tissues in the concentration images (red outlines in Fig. 3a). Then, the average concentration within the ROI was plotted as a function of time after injection (Fig. 3b). Different mice were used to measure the post-injection concentration of Tempol in SCCVII, KHT, and HT-29 tumors (one tumor per mouse.) Fig. (3b) shows the concentration of Tempol in leg muscle and adjacent tumors as a function of time after injection. As can be seen on the semi-log plot (Fig. 3b), nitroxide decay resembles a single order exponential. Figs. (3a and 3b) demonstrate that the rate of Tempol signal decay varies between tumor types, and in the case of KHT (p = 0.02) and SCCVII tumors (p = 0.0004), the exponential decay rate constant kr in the legend of Fig (3b) is significantly greater in the tumor than in the adjacent leg muscle [12]. As shown in Fig. (3c), the imaging technique used in Fig. (3a and 3b) can be extended to other tissues besides tumor and muscle. Fig. (3c) shows the post-injection Tempol concentration in the jugular vein, kidney, lacrimal gland, rectum, tongue, liver, submandibular salivary gland, brain, and posterior leg muscle. Based on these data, it was found that the exponential rate constant of Tempol signal decay varied substantially between different tissues, from a minimum 0.6 min−1 in leg muscle to a maximum of 2.2 min−1 in the jugular vein [12]. Notably, muscle and tongue are the only two tissues that reduced Tempol more slowly than SCCVII tumors Fig. (3a, b); all other measured tissues in Fig. (3c) reduced Tempol more quickly than SCCVII tumors.
Fig. (3).
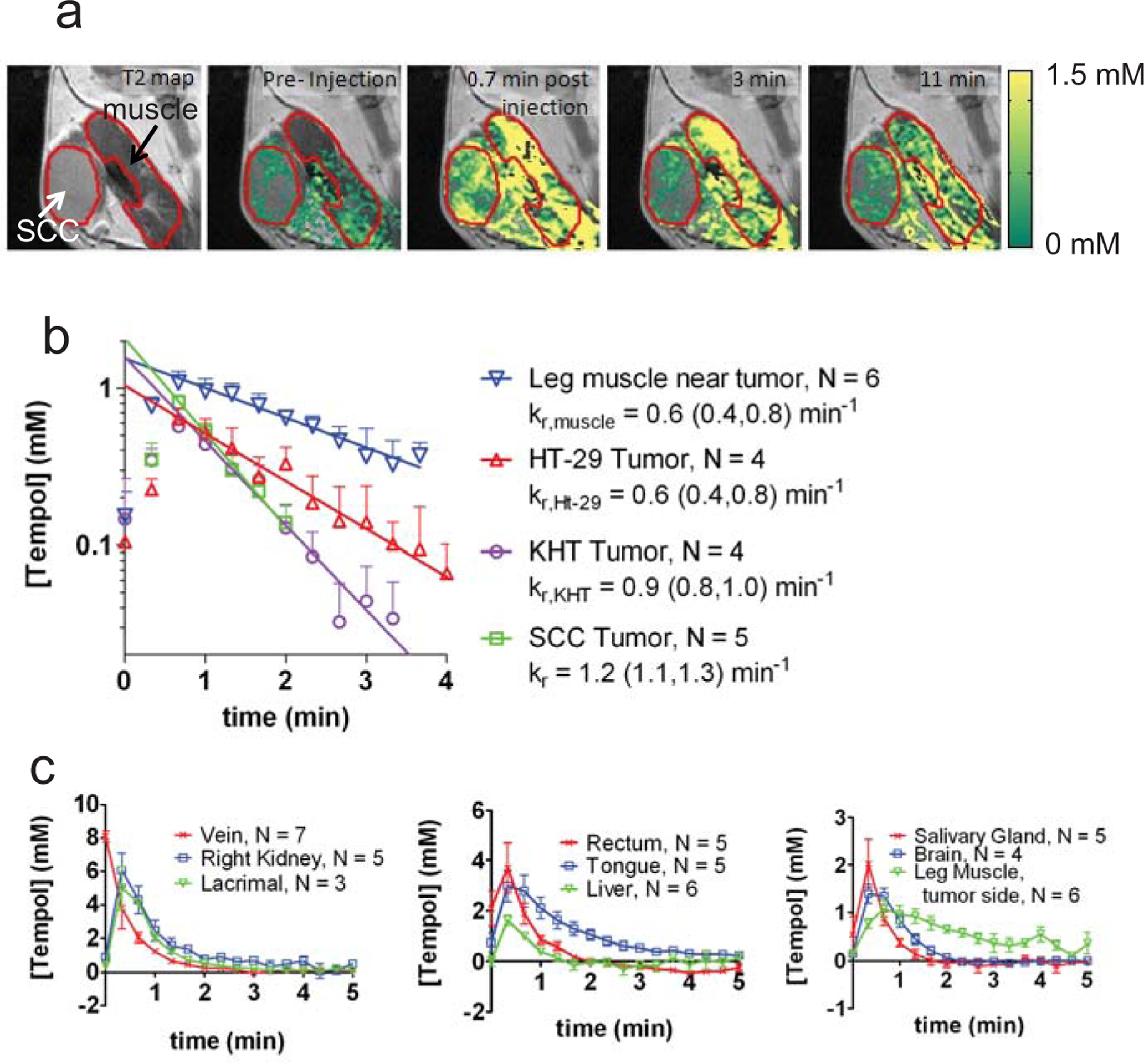
Results from a typical redox imaging experiment. a) Concentration maps overlaid on T2-weighted images corresponding to the hind leg region of a mouse. The SCCVII tumor and the adjacent leg muscle are outlined in red. b) The average Tempol concentration inside the muscle and tumor was plotted as a function of time after injection. The concentration of Tempol was determined in three different tumor models: SCCVII, KHT, and HT-29. The leg muscle, SCCVII, and KHT measurements were made in C3H mice, and the HT-29 measurements were made in nude mice. The reduction rate constant (kr) of leg muscle in nude mice is not significantly different than the reduction rate of leg muscle in C3H mice (data not shown). For each time point after injection (20 second intervals), the average concentration between 4–6 mice was determined for each tissue. Error bars are the standard error of the mean, and the lines are least-squares exponential fits to the data (t ≥ 0.7 min). c) Using the same technique as used in a-b, the concentration of Tempol was determined in 9 non-cancerous tissue compartments. The error bars represent the standard error of the mean, and the lines connect the data points. Data adapted from reference [12], with permission.
The decay of Tempol signal was initially thought to be due to a combination of bio-reduction and physical clearance of the nitroxide, but it was recently shown that in SCCVII tumor, leg muscle, and kidney, the total nitroxide plus hydroxylamine concentration remains stable at a value of approximately 1mM for up to 20 min after injection [36]. This finding suggests that the signal decay shown in Fig. (3a–c) is primarily due to reduction of the paramagnetic nitroxide into its corresponding signal non-enhancing hydroxylamine. The rate of nitroxide bioreduction has been shown to modulate due to various biological stressors including asbestosis [38], silicosis [39], hepatotoxins [40], aging [41], ischemia-reperfusion in the brain [42–44], diabetes [45], wounds [46], ultraviolet radiation [47, 48], radiation exposure [49], and cancer [6, 12, 36, 37]. With the aim of clarifying the biological significance of these disease-induced modulations in the nitroxide reduction rate, the following section of this review outlines biochemical factors that affect the rate of nitroxide reduction.
5. FACTORS AFFECTING THE RATE OF NITROXIDE REDUCTION
As outlined below, there are many biochemical systems that have been shown to reduce nitroxides under controlled experimental conditions. However, it is probable that the biochemical systems that reduce nitroxides vary between in vitro and in vivo experiments, and between different nitroxides. For example, this review shows that variations in nitroxide lipophilicity may affect the compartmentalization of nitroxides inside the tissue, which in turn affects which biochemical systems are predominantly responsible for nitroxide reduction. Because one of the most promising and interesting applications of nitroxides is the non-invasive assessment of the redox status in intact animals, this section reviews various biochemical factors that may be responsible for nitroxide reduction, with the goal of determining which factors have the largest effect on the rate of nitroxide reduction in vivo. Particular attention is paid to the nitroxides Tempol and 3-CP, which are the nitroxides most commonly used for redox imaging of cancer [6, 12, 31, 36, 37].
5.1. Mitochondrial Redox Status
Nitroxides are reduced by isolated mitochondria, and the site of nitroxide reduction on the mitochondrial respiratory chain has been determined using respiratory chain inhibitors. Specifically, addition of rotenone to mitochondria inhibits Complex I of the respiratory chain and causes downstream redox pools such as ubiquinol to become less reduced. Notably, the addition of rotenone decreases the exponential reduction rate constant (kr) of the nitroxides CDTAB [50] and MitoTempol [15] by mitochondria, suggesting that the site of nitroxide reduction is downstream of Complex I in the electron transport chain. In addition, Antimycin A or KCN, which are inhibitors of Complex III and IV respectively, both cause upstream redox pools to become more reduced. These inhibitors caused an increase in the kr of the nitroxides, suggesting that the site of nitroxide reduction was upstream of Complexes III and IV [15, 50]. Taken together, these data are consistent with reduction of nitroxides by ubiquinol, a mitochondrial membrane-bound cofactor that is essential for cellular respiration.
The role of ubiquinol in nitroxide reduction was further substantiated by isolation of mitochondria from a yeast strain (Δcoq2) which is unable to synthesize ubiquinol [15]. It was shown that unlike the wild-type yeast mitochondria that were able to reduce the nitroxide MitoTempol, the Δcoq2 mitochondria were not able to reduce the nitroxide. Upon addition of exogenous ubiquinol to the Δcoq2 mitochondria, the MitoTempol was reduced. These experiments strongly suggest that MitoTempol can be reduced by ubiquinol in the electron transport chain.
Although nitroxides are reduced by ubiquinol in isolated mitochondria, in cells the mitochondria occupy only a small fraction of the intracellular volume, and it is therefore possible that ubiquinol may not be a large contributor to nitroxide reduction in intact cells. Thus, the initial experiments showing that nitroxides can be reduced by ubiquinol in isolated mitochondria led to experiments testing the extent of nitroxide reduction by mitochondria in intact cells. Using the method of electron transport inhibition, it was found that some but not all nitroxides are reduced by ubiquinol in intact mammalian cells. Namely, in intact rat spermatozoa [51] and mouse thymus-bone marrow cells [52], the nitroxides Tempone and 5-doxyl stearate were shown to be reduced by ubiquinol. These studies support that the reduction rate of these nitroxides reflects the mitochondrial redox status. However, the redox imaging agent Tempol, unlike Tempone and 5-Doxylstearate, exhibits minimal reduction by ubiquinol in isolated mitochondria and in intact cells. Specifically, when human skin fibroblasts were incubated with the Complex IV inhibitor NaCN, Tempol did not show an increased reduction rate as would be expected if it were reduced by ubiquinol [53]. This finding is further supported by a study in isolated mitochondria showing that relative to mitochondrially targeted nitroxides such as MitoTempol and butyl-Tempol (Fig. 1), the reduction of Tempol by ubiquinol is over an order of magnitude slower [15].
The weak interaction of Tempol with ubiquinol in mitochondria may be due to its relatively low lipophilicity, which may limit its interaction with lipid-bound components of the electron transport chain such as ubiquinol. The importance of lipophilic moieties in promoting reduction of nitroxides by lipid bound reducing species is illustrated in Fig. (4). Fig. (4) shows how lipophilic moieties on a nitroxide can affect its rate of reduction by isolated mitochondria. Namely, in the analogs without a lipophilic moiety Tempamine, 2N3, and Tempol in Fig. (4), the interaction with mitochondria is diminished relative to the lipophilic analogue. In intact cells, where the mitochondria occupy only a small sub-fraction of the cellular volume, the interaction of Tempol with mitochondria does not occur to any measureable extent [53]. Thus, the in vivo reduction rate of Tempol probably does not provide information on the mitochondrial redox status of cells. In summary, in intact cells, mitochondria appear to reduce the nitroxides Tempone and 5-Doxylstearate, but do not appear to reduce Tempol.
Fig. (4).
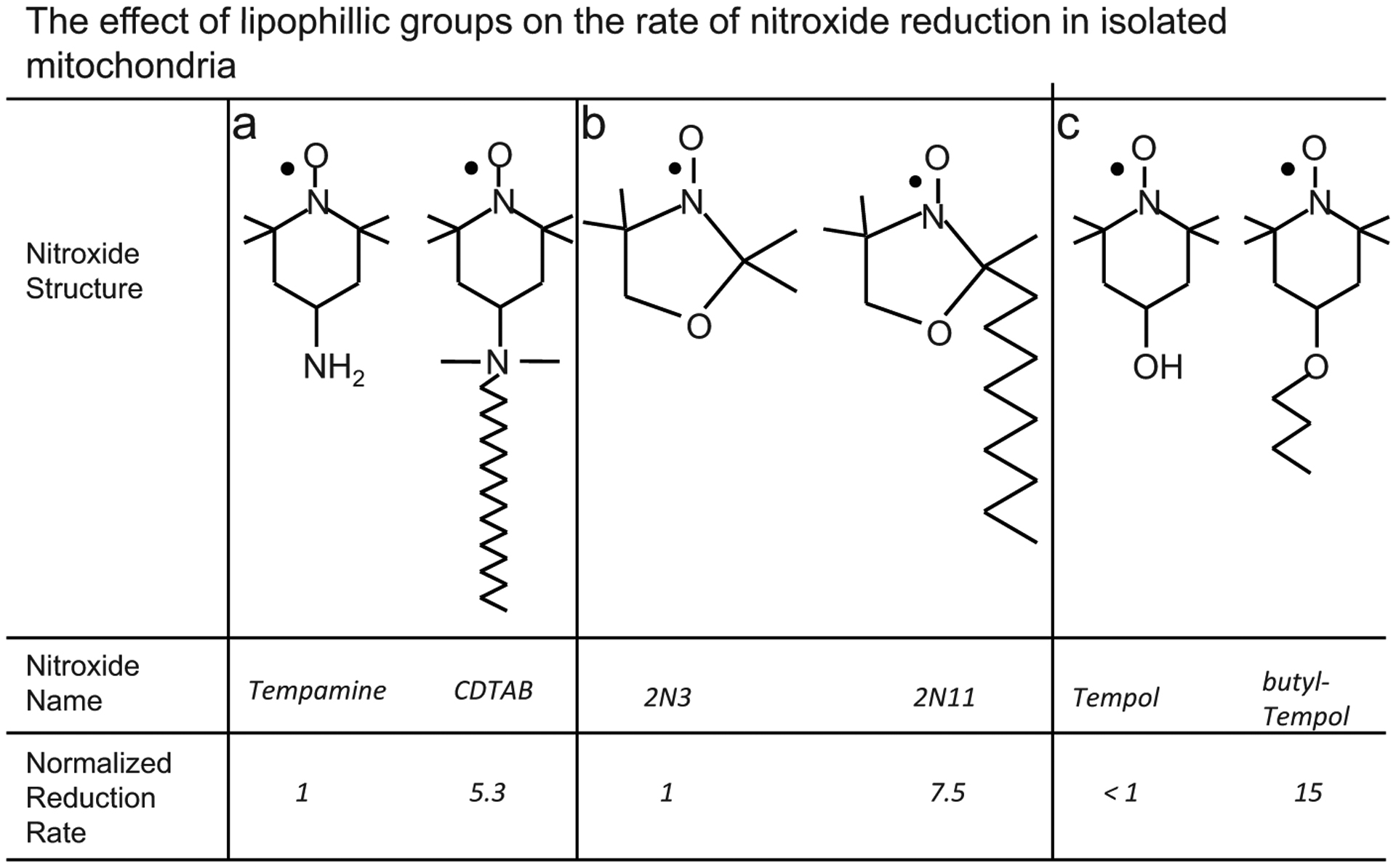
This figure shows that lipophilic groups on a nitroxide increase the rate at which the nitroxide is reduced by mitochondrial-membrane-bound reducing agents. The reducing agent that is primarily responsible for nitroxide reduction in mitochondria is the membrane-bound compound ubiquinol. A lipophilic group increases the nitroxide’s proximity to ubiquinol by enhancing the nitroxide’s compartmentalization within the lipid membrane. The spatial proximity with ubiquinol afforded by the lipophilic group causes the lipophilic analog to be reduced more rapidly than the non-lipophilic analog. The non-lipophilic analogs are a) Tempamine [50], b) 2N3 [50], c) Tempol [15].
5.2. Levels of Reactive Oxygen Species
The previous section demonstrated that in intact cells the reduction rate of Tempol probably does not depend on the redox status of the mitochondrial electron transport chain. However, in both cancerous and non-cancerous cells, the mitochondria are a source of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) and superoxide (O2−) [54–56]. In the presence of endogenously ubiquitous thiols such as glutathione and cystine, O2− promotes the reduction of nitroxides including Tempol and 3-CP to their corresponding hydroxylamine [57, 58]. Due to the ubiquity of thiol and other reducing species in vivo, it is possible that a burst of oxidative stress may cause an increase in the in vivo nitroxide reduction rate. Because this type of reduction requires both an oxidizing species and a reducing species, it is hereafter referred to as “nitroxide redox with oxidizing species” (Fig. 2). An alternate mechanism for nitroxide redox with oxidizing species involves NAD(P)H rather than thiols. This mechanism first involves one electron oxidation of the nitroxide by superoxide to the oxoammonium cation (Equation 1), and subsequently two electron reduction from the oxoammonium cation to the hydroxylamine [7]:
| (4) |
where NAD(P)H indicates that the cofactors NADH and NADPH are each capable of 2e− reduction of the oxoammonium cation. An additional mechanism for nitroxide redox by oxidizing species is the catalase-like mechanism [8]. The catalase-like mechanism for nitroxide redox commences with the oxidation of metmyoglobin (MbFeIII) to an oxo-porphyrin cation radical, •MbFeIV, radical by H2O2:
| (5) |
The next step of the catalase mechanism involves oxidation of two molecules of nitroxide for every molecule of •MbFeIV:
| (6) |
| (7) |
It is important to note that equations 5–7 do not show the exact charges of the compounds involved, and are thus not fully balanced. They rather represent overall redox pathways, which are complex and outlined in detail in reference [8]. To complete the catalase-like mechanism, the nitroxide is regenerated by reduction of the oxoammonium cation by hydrogen peroxide or superoxide:
| (8) |
| (9) |
However, in the presence of NAD(P)H, the oxoammonium cation can additionally be reduced to the hydroxylamine (Equation 4), thus completing the redox of the nitroxide to the hydroxylamine (Equations 4,6, and 7). In summary, in the presence of thiols, metmyoglobin, and redox cofactors, oxidative stress can in theory promote the redox of a nitroxide into its corresponding hydroxylamine. The remainder of this section reviews redox imaging studies suggesting that oxidative stress impacts the redox reactions of nitroxides in vivo.
There are several published sets of EPRS and EPRI data obtained in living mice that are consistent with in vivo redox reactions between nitroxides and oxidizing species. These data represent various models of oxidative stress including whole body x-ray radiation [49], asbestosis in the lung [38], streptozotocin-induced diabetes [45], ultraviolet radiation [47], ischemia-reperfusion in the brain [42, 44], and hyperoxia [59]. The studies are summarized in Table 2, and have three experimental groups: a control group, a group with a disease or treatment that causes oxidative stress (OS group), and a group identical to the OS group except that it was either exposed to an exogenous antioxidant or exhibited enhanced endogenous antioxidant activity (antioxidant group). If nitroxides are primarily reduced by antioxidants, then it is expected that the nitroxide reduction rate observed for the antioxidant group would be greater or equal to the reduction rate observed in the OS group. Contrary to this, in the studies summarized in Table 2, the OS group reduced the nitroxide faster than the antioxidant group, suggesting that the rate of nitroxide reduction correlates positively with the level of oxidative stress. In addition, for the hyperoxia, x-ray studies, ischemia-reperfusion, and diabetes studies, administration of the antioxidant in mice not experiencing oxidative stress did not cause a significant change in the nitroxide reduction rate [44, 45, 49, 59]. These results are consistent with in vivo redox reactions between nitroxides and oxidizing species.
Table 2.
Oxygen Dependence of the Rate Constant of Tempol Reduction in Various Cell Types
| Cell Type | knitrogen/kair | p(knitrogen=kair) | Reference |
|---|---|---|---|
| Mouse thymus-bone marrow cells | l;0.96 | n.s.* | [11,71] |
| Rat hepatocytes | 1.2 | n.s. | [112] |
| Adriamycin-resistant MCF7 | 1.2 | n.s. | [53] |
| Human skin fibroblast (1522) | 1.7 | p<0.05 | [53] |
| Squamous cell carcinoma VII | 1.8 | p<0.05 | [12] |
| Human breast cancer (MCF7) | 2.0 | p<0.05 | [53] |
| Radiation-induced fibrosarcoma (RIF-1) | 2.6 | p<0.05 | [53] |
n.s.: not significant. For each of these experiments, the reduction rate of Tempol was measured under ambient gas and under anoxic gas conditions. The ratio of these two values is given in the “kNitrogen/kair” column. A kNitrogen/kair = 1 suggests that the reduction rate of Tempol is not oxygen dependent in those cells.
Although the studies in Table 1 demonstrate that the rate of nitroxide reduction increases during oxidative stress, this increase may not always be due to direct interactions between the nitroxide and oxidizing species. For example, oxidative stress can trigger an antioxidant response such as increased pentose phosphate pathway activity or increased NADPH concentrations, and the resulting enhanced antioxidant activity may increase the nitroxide reduction rate. In addition, the increased reduction rate observed under oxidative stress may be blocked by an antioxidant or radical scavenger that intercepts the oxidizing species, thus attenuating the antioxidant response. Supporting this, in vitro experiments in various microorganisms and cancer cells have shown oxidative stress can induce increased NADPH production, NADPH consumption, and/or pentose phosphate shunt activity [60–65]. In one study, increased antioxidant enzyme activity was observed only 15 min after H2O2 -induced oxidative stress [61], suggesting that the antioxidant response can occur rapidly. In the context of bioreduction of nitroxides, in vitro experiments show that higher NADPH levels and higher PPP activity correlate with an increased rate of Tempol reduction (discussed in section 5.4–5.5), suggesting that in some cases oxidative stress can induce antioxidant activity that will cause an increase in the nitroxide reduction rate. In summary, the rate of nitroxide reduction tends to increase under oxidative stress. Depending on the model, this increase may be due to either ROS-induced increased antioxidant activity or direct redox reactions between nitroxides and ROS.
Table 1.
Studies Suggesting Redox of Nitroxides by Oxidative Species
| Source of Oxidative Stress (OS Group) | Treatment to Reduce Oxidative Stress (Antioxidant Group) | Nitroxide | Referi |
|---|---|---|---|
| Hyperoxia | Glutathione, uric acid | 3-CP | [59] |
| Asbestosis | Lactate dehydrogenase, catalase N-acetyl-b-D-glucoaminidase (NAG).* | Tempol | [38] |
| Diabetes (induced by streptozotocin) | Insulin, alpha-tocopherol, SOD | 3-CP | [45] |
| X-ray radiation | Cysteamine | 3-CP | [49] |
| Ultraviolet radiation | Topical sunscreen, topical antioxidants | 3-CxP | [47,48] |
| Brain ischemia-reperfusion | MCI-186 | 3-CP | [42] |
| Brain ischemia-reperfusion | Trolox | M-CP | [44] |
These antioxidants were not administered; their levels increased with the onset of asbestosis.
The current section has thus far discussed studies where oxidative stress increased the rate of nitroxide signal decay, but in some cases it is possible that oxidative stress will decrease the decay rate. In particular, one study showed a decrease in the nitroxide decay rate during oxidative stress induced by ischemia-reperfusion (IR) of rat brain [66]. The study found that relative to sham treated mice, one hour of middle cerebral artery occlusion (MCAO) followed by reperfusion caused a 45% decrease in the rate of nitroxide signal decay. As an explanation, the authors proposed that the oxidative stress induced by reperfusion depletes intracellular glutathione (GSH). Because GSH levels correlate with the rate of in vivo nitroxide reduction (section 5.4), depletion of GSH by IR may have caused the observed decrease in the nitroxide reduction rate.
5.3. Intracellular Oxygenation
Tumor hypoxia is prognostic for poor response to cancer therapy. In particular, in human head and neck [67, 68], uterine cervix [69], and squamous cell carcinoma [70] tumors, patients with higher tumor oxygenation prior to initiation of radiotherapy had a greater chance of survival than did patients with lower tumor oxygenation. For this reason, a hypoxia-sensitive contrast agent may be useful in both research and clinical settings.
In the context of redox imaging, endogenous antioxidants such as NADH, NADPH, and ubiquinol are either directly or indirectly oxidized by molecular oxygen, suggesting that the reductive capacity of these antioxidant pools may decrease at high intracellular oxygen concentrations. If these oxygen-sensitive antioxidant pools can reduce nitroxides, then it is expected that nitroxides will be reduced slower under high intracellular partial oxygen pressure (pO2), thus providing an indirect measure of tumor oxygenation.
5.3.1. In Vitro Experiments
In quantitative terms, the dependence of the nitroxide reduction rate constant on the intracellular oxygen level can be expressed as kr,nitrogen/kr,air, which is the ratio between the rate constant of cells exposed to nitrogen to the rate constant of cells exposed to air. Thus, for a given nitroxide and cell type, a kr,nitrogen/kr,air equal to one means that the rate of nitroxide reduction is not dependent on cellular oxygenation, while a kr,nitrogen/kr,air greater than one means that the cells reduced the nitroxide faster under anoxic conditions than under oxygenated conditions.
Published data demonstrate that lower cellular oxygenation often results in faster nitroxide reduction (kr,nitrogen/kr,air > 1), and that the magnitude of the kr,nitrogen/kr,air ratio varies between nitroxides and cell types. Oxygen-dependent nitroxide reduction was observed for lipophilic nitroxides such as doxyl stearates, where the kr,nitrogen/kr,air ratio is greater than 10 in intact cells [52, 71]. In the case of Tempol and 3-CP, the kr,nitrogen/kr,air ratio is approximately an order of magnitude lower [12]. Table 2 shows that the strength of oxygen dependence (i.e. the kr,nitrogen/kr,air ratio) for Tempol varies substantially between cell types, ranging from 1 – 2.6 for the cells tested. It is possible that inter-nitroxide variations in the degree of oxygen dependence are partly due to varying degrees of interaction between the nitroxide and the mitochondrial membrane. For example, doxyl stearates associate with mitochondrial membranes and exhibit a strong oxygen dependence (kr,nitrogen/kr,air > 10) in intact cells, while Tempol interacts minimally with mitochondrial membranes and exhibits relatively weak (kr,nitrogen/kr,air = 1–2.6) oxygen dependence in intact cells (Table 2).
In addition to reduction of nitroxides by ubiquinol, there are other potential mechanisms of oxygen-dependent reduction. For example, the cytochrome P450 reductase enzyme (CPR), which is a membrane-bound electron donating protein found on the endoplasmic reticulum of most cells, can reduce Tempol in an oxygen-dependent manner [72]. Specifically, in microsomes obtained from rat hepatocytes, the reduction of nitroxides including Tempol requires either NADH or NADPH, and increases for greater CPR activity. In addition, the rate constant (kr) of Tempol reduction was shown to increase at lower concentrations of oxygen in microsomes (by a factor of up to 10) as well as in aqueous solutions of CPR (by a factor of 1.3). These data show that oxygen-dependent reduction of nitroxides is not restricted to the mitochondria, and that the endoplasmic reticulum is an alternate site for in vivo nitroxide reduction.
In summary, several important points can be made about the oxygen dependence of nitroxide reduction. First, the degree of oxygen dependence (kr,nitrogen/kr,air) varies widely between nitroxides of different structure, and the dependence appears to be greater for nitroxides that are expected to interact with the mitochondria. Second, non-mitochondrial redox systems may contribute to the oxygen-dependent reduction during redox imaging experiments. Finally, the oxygen-dependent reduction rate of Tempol and other nitroxides may also depend on the presence of endogenous antioxidants such as NADH and NADPH.
5.3.2. In Vivo Experiments
As discussed in the preceding section, the nitroxides Tempol and 3-CP exhibit oxygen-dependent reduction rates in some cell lines. However, the in vitro data presented above measured reduction rates at ambient pO2 values, and it is unlikely that cells would experience ambient-level pO2 in vivo. Indeed, ambient air has a pO2 of 160 mmHg, while the pO2 of tumors and leg muscle is often less than 25 mmHg [73]. In vivo studies have therefore been conducted to test if the relatively narrow range of pO2 values that are actually observed in vivo is enough to cause variations in nitroxide reduction rate. In an EPRI study in rats bearing RIF-1 tumors, the tumors of rats breathing carbogen (95% O2, 5% CO2) reduce 3-CP 30% slower than rats breathing ambient air [74, 75]. More recently, the spatial correlation between local pO2 and the reduction rate of HM-Proxyl was found to be non-significant (r = 0.357) [76]. Finally, SCCVII, HT-29, and KHT tumors often exhibit large regions of hypoxia (pO2 < 10 mmHg) [77, 78], but these tumors reduce Tempol more slowly than most non-cancerous tissues with the exception of muscle and tongue [12]; this is opposite the effect that would be observed if tissue pO2 were the major determinant of the nitroxide reduction rate. In conclusion, for the nitroxides commonly used during redox imaging experiments, tissue oxygenation is not a major determinant of the rate of nitroxide reduction.
5.4. Thiol and Redox Cofactor Levels
The antioxidant glutathione (GSH) may play important roles in cancer, including detoxification of carcinogenic peroxides via the glutathione peroxidase enzyme [79], and regulation of resistance to cisplatin [80–82] and adriamycin [81, 83] chemotherapy. Furthermore, intracellular glutathione levels are elevated in some cancer cells [84, 85], and become depleted during apoptosis [86, 87]. Other antioxidants, such as the redox cofactors NADPH and NADH, are required for angiogenic signaling [88], replenishment of glutathione levels, and oxidative metabolism. Because the levels of these and other antioxidants change during oxidative stress [60–62] and malignant transformation [89, 90], it is likely that the bulk intracellular “reducing capacity” of a tumor is both dynamic and biologically informative. It may therefore be useful in either a clinical or research setting to non-invasively assess their levels throughout the treatment or progression of cancer.
Until recently, non-invasive assessment of the reducing capacity of tissue has been problematic. For example, the non-invasive measurement of glutathione levels requires nuclear magnetic resonance spectroscopy techniques that have low spatial resolution (for example, 3 × 3 × 3 cm3 [91]) and low sensitivity [92]. Nitroxide imaging may help overcome some limitations of current non-invasive redox assays, because it allows investigators to non-invasively measure the reductive capacity of tumors with comparatively high spatial resolution (0.3 × 0.3 × 2 mm2) [37]. Furthermore, a recently developed EPRS technique allows accurate and non-invasive measurement of glutathione concentrations in vivo [92]. The current section of this text reviews in vitro and in vivo experiments showing the collective involvement of endogenous water-soluble antioxidants in nitroxide reduction.
5.4.1. Glutathione
The rates of Tempol and 3-CP reduction correlate with GSH levels in vivo, but glutathione is not required for intracellular nitroxide reduction. In mice bearing RIF-1 tumors, depletion of tissue GSH concentration by buthionine sulphoximine (BSO) resulted in no significant change in the reduction rate constant in leg muscle, but a 35% decrease in the tumor reduction rate constant [75, 93]. In a different study also using RIF-1 tumor-bearing mice, GSH was depleted with diethyl maleate (DEM), and as a result, a 25% decrease and 35% decrease in the rate constant of 3-CP reduction was noted in the leg muscle and tumor respectively [58]. Finally, in an EPRS study in the lungs of euthanized mice, pretreatment of the mice with DEM decreased the Tempol reduction rate in a DEM-dose dependent manner [94]. This study also showed that while the reducing agent D,L- dithiothreitol (DDT) and the oxidizing agent 4,4’-dithiodipyridine (4-PDS) did not directly react with Tempol in solution, when the combination of Tempol and either DDT or 4-PDS were injected into the lung simultaneously, the reduction rate of Tempol increased for DDT and decreased for 4-PDS [94]. The above data based on GSH-depleting drugs shows that the rate of Tempol and 3-CP reduction often correlates with glutathione levels in vivo.
Because GSH is coupled to other antioxidant pools such as NADPH, the decrease in the nitroxide reduction rate observed with diminished GSH levels in vivo may be due to indirect depletion of other antioxidant pools. Supporting this, BSO-induced depletion of glutathione levels in Chinese hamster ovary cells [53] and SCCVII cells [unpublished results of corresponding author] had no effect on the rate of Tempol reduction, suggesting that in these in vitro systems there is no correlation between the rate of Tempol reduction and the intracellular GSH concentration. Thus, there appears to be a contradiction between the in vitro and in vivo studies reviewed in this section, because in vivo studies show a correlation between GSH concentration and the nitroxide reduction rate, while in vitro studies do not show a correlation. It is likely that this discrepancy relates to the coupling of GSH with other antioxidant pools [95]. In particular, GSH is present in most tissues at high concentrations, and alterations in GSH levels will almost certainly affect the levels of other antioxidants. Alterations in these non-GSH antioxidant levels by GSH depletion may in turn affect the rate of nitroxide reduction in vivo. In conclusion, reduction of redox imaging nitroxides does not necessarily require the presence of GSH, but a positive correlation does exist between tissue GSH concentration and the rate of nitroxide reduction in vivo.
More recently, a method has been introduced that enables in vivo measurement of glutathione levels with EPR using a bi-radical nitroxide [92]. These bi-radical nitroxides are composed of two standard mono-radical nitroxides that are joined by a disulfide bond, and this disulfide bond is cleaved in vivo with a rate that depends on glutathione levels:
| (10) |
Cleavage of the disulfide bond (equation 10) by glutathione results in a mono-radical (R•SH and R•SSG) spin population in addition to an un-cleaved bi-radical (R•SSR•) spin population, and each spin population has a characteristic EPR spectrum that can be measure independently of the other. Thus, using EPRS, in vivo GSH concentrations can be non-invasively measured by monitoring the rate of mono-radical formation. The toxicity of these probes is currently unknown.
5.4.2. Cytochrome P-450 Enzymes and NADPH
The Cytochrome P-450 (CYP) and Cytochrome P-450 reductase (CPR) enzymes are capable of reducing nitroxides. Reduction of nitroxides by CYP and CPR was demonstrated in microsomes, which are subcellular fractions derived from endoplasmic reticula. In isolated mammalian liver microsomes, the nitroxides Tempo [96–97], Tempol [98], and 5 doxyl-stearate [99], were rapidly reduced upon addition of NADPH to the solution. To determine which enzymes are involved in nitroxide reduction, the enzymatic activity the microsomes was modified with compounds that alter CYP or CPR activity. These compounds include Phenobarbital, 2-PrOH, TEPT, alinine, Skf-525A, and PCMA. The results from these studies demonstrate that the reduction rate of 5-doxyl stearate and Tempo correlate with CYP activity, and that the reduction rate of Tempol correlates with CPR activity. Additionally, a solution containing either purified CPR or NADPH alone did not reduce Tempol, but the combination of both did reduce Tempol [98]. Collectively, these studies suggest that CYP and CPR are able to reduce nitroxides, and that the rate of reduction depends on NADPH levels. It is currently unknown if CYP or CPR plays a substantial role in nitroxide reduction during in vivo imaging experiments.
5.5. Pentose Phosphate Shunt Activity
The pentose phosphate pathway (PPP) is a metabolic pathway that uses glucose to synthesize various biologically important molecules, including the antioxidant NADPH. The PPP is important in cancer biology because it is stimulated by anthracycline chemotherapy [64, 100, 101] and various forms of oxidative stress [60–65]. Interestingly, the rate of Tempol reduction in intact cells positively correlates with the activity of glucose 6-phosphate dehydrogenase, which is the rate limiting enzyme of the pentose phosphate shunt. Using both wild-type and G6PD-deficient Chinese hamster ovary cells, it was shown that the deficient cells reduced Tempol 30% slower than the wild-type cells [53]. In a similar study using wild-type and G6PD deficient erythrocytes, the rate of Tempol reduction was substantially slower in the G6PD deficient cells [102]. Taken together, these studies make the important suggestion that the rate of Tempol reduction correlates with the activity of the pentose phosphate shunt.
5.6. Ascorbate
This review has discussed mostly enzymatic reduction of nitroxides, but there is evidence that in living systems non-enzymatic reduction occurs as well. In particular, ascorbate is known to reduce nearly all tested nitroxides, including Tempol and 3-CP [103, 104]. In most murine tissues, ascorbate exists in concentrations ranging from 1–5mM, depending on the mouse strain or tissue [105, 106]. In solution, 5 mM ascorbate reduced 3-CP with a decay rate constant of 0.07 ± 0.01 min−1 [31]. This decay constant is at the lower end of reduction rates observed in vivo, which range from 0.04 – 0.49 min−1 depending on the tissue [12], suggesting that ascorbate is often not a major contributor to nitroxide reduction in vivo.
5.7. Nitroxide Clearance
As outlined in this review, the signal decay observed in redox imaging experiments (Fig. 3) is predominantly due to reduction of the nitroxide, but it is possible that a component of the signal decay is also due to physical clearance of the nitroxide radical from the tissue into the blood stream. Several studies have addressed the effects of clearance on the redox imaging signal decay rate, and the degree of clearance observed varies between studies, nitroxides, and tissues. Experiments comparing the reduction rate of intramuscularly injected Tempol between control mice and mice with restricted blood flow showed that the signal decay rate constant decreased by a factor of 50% in mice with restricted blood flow [107]. In an MRI experiment using the blood-brain barrier permeable nitroxide M-CP, the signal decay rate constant in dead animals was 40% less than the rate constant in living animals, suggesting that clearance of the nitroxide from the tissue is partly responsible for signal decay [66]. Finally, for intratracheal injection of the cell-impermeable nitroxide carboxy-proxyl, the signal decay rate constant in the lung was 50% less for dead mice than for living mice [108]. Although these studies suggest that perfusion is responsible for up to 50% of the observed signal decay, it is unclear if either the mode of injection (e.g. intramuscular vs. intravenous) affects nitroxide clearance or if restriction of perfusion alters tissue redox status, both of which would alter the signal decay rate independent of nitroxide clearance. To address these possible confounding factors, experiments were conducted where mice were intravenously injected with Tempol, 3-CP, or 3-CxP, followed by harvesting of the kidney, leg muscle, tumor (SCCVII), and brain tissue at various times after nitroxide injection [36, 66]. Based on total nitroxide measurements (oxidized plus reduced) in the harvested tissues, it was shown that the clearance of total nitroxide (oxidized plus reduced) from 2.5–20 min after injection is negligible compared to the rate of MRI signal loss, suggesting that nitroxide clearance plays only a minor role in signal decay. Thus, it is possible that nitroxide clearance may account for up to 50% of the signal decay rate, but recent in vivo experiments suggest that the contribution is often minor.
6. NITROXIDES AS RADIOPROTECTORS: QUANTIFYING THE DYNAMIC IN VIVO NITROXIDE CONCENTRATION WITH MRI
During cancer radiotherapy non-cancerous tissue is inevitably exposed to radiation, which may cause toxicity in healthy tissue. One way to reduce healthy tissue toxicity is to administer a radioprotector prior to radiation exposure. For a radioprotector to be useful during radiotherapy, it must exhibit at least two basic properties: it must prevent damage to biologically important molecules such as DNA, and it must prevent such damage to a greater degree in healthy tissues than in tumor tissues [109]. The compound most widely used in the clinic that exhibits both of these properties is amifostine, which has proven effective at preventing xerostomia during fractionated radiotherapy of head and neck cancer [110]. However, amifostine is ineffective in some patients [110], and can induce transient and non-lethal side effects such as hypotension, vomiting, and allergic reactions [111], suggesting that additional radioprotectors may be useful in the oncology clinic. Preclinical experiments show that Tempol protects against both lethal radiation exposure [24–27] and xerostomia [28–30] in mice, and does not protect against radiation-induced regrowth delay of SCCVII, HT-29, and RIF-1 tumors [26, 29], suggesting that Tempol selectively protects non-cancerous tissues during exposure to ionizing radiation. The selective protection of non-cancerous tissues by Tempol provides justification for the development of nitroxides as clinical radioprotectors.
A recent study used MRI to quantify the post-injection levels of Tempol in various healthy tissues and tumor types, and the results suggest that the differential radioprotection of Tempol may be partly due to low accumulation of Tempol in tumor tissue. Using MRI, Tempol and 3-CP levels were quantified in various tissues, and the difference between the healthy tissue levels and SCCVII tumor tissue levels was calculated as a function of time after nitroxide injection (Fig. 5). As can be seen, up to several minutes after injection, Tempol exists in the liver, rectum, salivary gland, tongue, leg muscle, and brain at greater concentrations than in the SCCVII tumor (Fig. 5a). It was also found that Tempol levels in these non-cancerous tissues was greater than the levels in HT-29 and KHT tumors (data not shown), suggesting that low Tempol accumulation may be a general property of tumors. Like Tempol, 3-CP levels in the liver, rectum, and salivary glands were greater than 3-CP levels in SCCVII tumors for several minutes after injection (Fig. 5). These imaging data provide additional rationale for the use of nitroxides as clinical radioprotectors, because they suggest that inherent differences in tumor perfusion and nitroxide delivery result in greater nitroxide concentrations in healthy tissue than in tumor tissue.
Fig. (5).
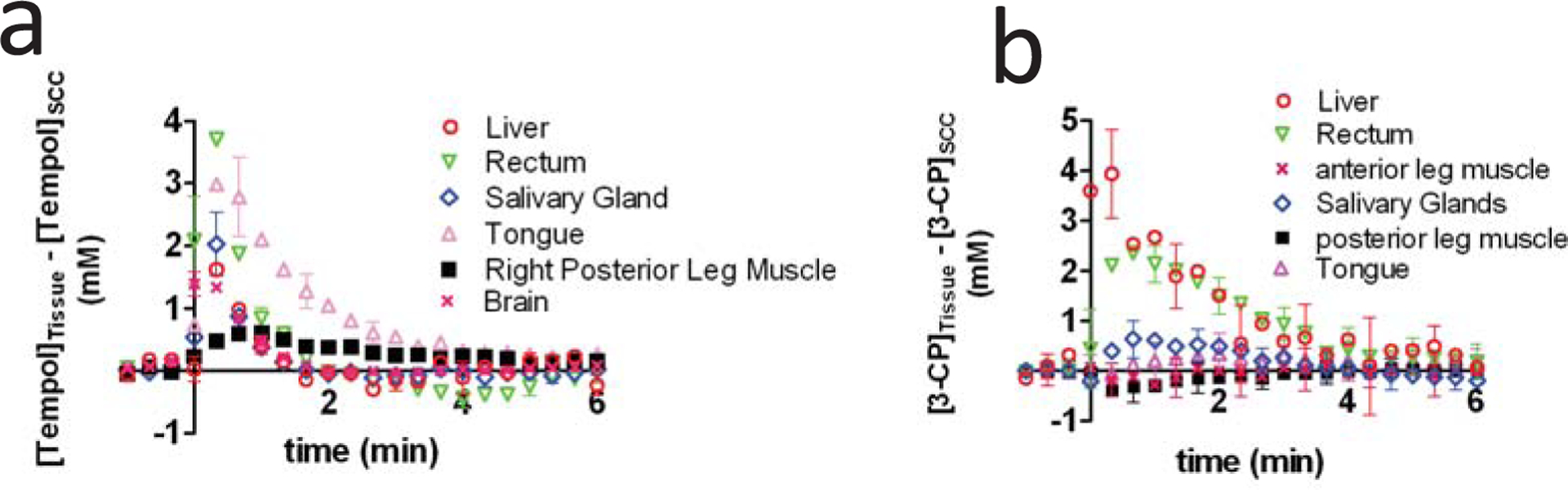
This figure shows that Tempol (a) and 3-CP (b) accumulate at greater concentrations in healthy tissues than in tumor tissues. The data were obtained by first independently measuring the dynamic post-injection concentration of nitroxide in non-cancerous tissues and SCCVII tumor. The tumor nitroxide levels were then subtracted from the non-tumor nitroxide levels at each time point, and the resulting differential concentration value was placed in the above graphs. Figure from reference [12], with permission.
7. CONCLUSION
The radical scavenging ability and paramagnetic nature of nitroxides makes them useful in areas of cancer research that include radioprotection and redox imaging. With respect to redox imaging, the in vivo bioreduction of nitroxides provides an imaging-based assay of the redox status of cells. The rate of nitroxide reduction in vivo appears to be a complex aggregate of various biochemical factors, but there are two factors that appear to be dominant. The first factor is oxidative stress, which tends to increase the rate of nitroxide reduction, possibly by promoting redox reactions between nitroxides and oxidizing species. The second is the level of intracellular glutathione, which tends to positively correlate with the rate of nitroxide reduction in in vivo experiments. Recently, nitroxide imaging has provided additional justification for the use of nitroxides as clinical radioprotectors, because MRI data show that nitroxides accumulate into healthy tissues at higher concentrations than in tumor tissue. In conclusion, the paramagnetic nature of nitroxides allows their pharmacodynamics and bio-reduction to be measured with various magnetic resonance-based techniques, and this has led to important advances in both biological imaging and radioprotector research.
ACKNOWLEDGEMENT
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH.
REFERENCES
- [1].Batinic-Haberle I; Reboucas JS; Spasojevic I Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Signal, 2010, 13 (6), 877–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Oberley LW; Buettner GR Role of superoxide dismutase in cancer: a review. Cancer Res, 1979, 39 (4), 1141–1149. [PubMed] [Google Scholar]
- [3].Kinnula VL; Crapo JD Superoxide dismutases in malignant cells and human tumors. Free Radic. Biol. Med, 2004, 36 (6), 718–744. [DOI] [PubMed] [Google Scholar]
- [4].Soule BP; Hyodo F; Matsumoto K; Simone NL; Cook JA; Krishna MC; Mitchell JB, The chemistry and biology of nitroxide compounds. Free Radic. Biol. Med, 2007, 42 (11), 1632–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].S oule BP; Hyodo F; Matsumoto K; Simone NL; Cook JA; Krishna MC; Mitchell JB, Therapeutic and clinical applications of nitroxide compounds. Antioxid. Redox. Signal, 2007, 9 (10), 1731–1743. [DOI] [PubMed] [Google Scholar]
- [6].Hyodo F; Soule BP; Matsumoto K; Matusmoto S; Cook JA; Hyodo E; Sowers AL; Krishna MC; Mitchell JB, Assessment of tissue redox status using metabolic responsive contrast agents and magnetic resonance imaging. J. Pharm. Pharmacol, 2008, 60 (8), 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Krishna MC; Grahame DA; Samuni A; Mitchell JB; Russo A Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc. Natl. Acad. Sci., USA 1992, 89 (12), 5537–5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Krishna MC; Samuni A; Taira J; Goldstein S; Mitchell JB; Russo A Stimulation by nitroxides of catalase-like activity of hemeproteins. Kinetics and mechanism. J. Biol. Chem, 1996, 271 (42), 26018–26025. [DOI] [PubMed] [Google Scholar]
- [9].Samuni A; Krishna CM; Mitchell JB; Collins CR; Russo A Superoxide reaction with nitroxides. Free Radic. Res. Commun, 1990, 9(3–6), 241–249. [DOI] [PubMed] [Google Scholar]
- [10].Samuni A; Krishna CM; Riesz P; Finkelstein E; Russo A, A novel metal-free low molecular weight superoxide dismutase mimic. J. Biol. Chem, 1988, 263 (34), 17921–17924. [PubMed] [Google Scholar]
- [11].Swartz HM; Sentjurc M; Morse PD Cellular metabolism of water-soluble nitroxides: effect on rate of reduction of cell/nitroxide ratio, oxygen concentrations and permeability of nitroxides. Biochim. Biophys. Acta, 1986, 888(1), 82–90. [DOI] [PubMed] [Google Scholar]
- [12].Davis RM; Matsumoto S; Bernardo M; Sowers A; Matsumoto KI; Krishna MC; Mitchell JB Magnetic resonance imaging of organic contrast agents in mice: Capturing the whole-body redox landscape. Free Radic. Biol. Med, 2011, 50(3), 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Krishna MC; DeGraff W; Hankovszky OH; Sar CP; Kalai T; Jeko J; Russo A; Mitchell JB; Hideg K Studies of structure-activity relationship of nitroxide free radicals and their precursors as modifiers against oxidative damage. J. Med. Chem, 1998, 41 (18), 3477–92. [DOI] [PubMed] [Google Scholar]
- [14].Chen K; Morse PD 2nd; Swartz HM Kinetics of enzyme-mediated reduction of lipid soluble nitroxide spin labels by living cells. Biochim. Biophys. Acta, 1988, 943 (3), 477–484 [DOI] [PubMed] [Google Scholar]
- [15].Trnka J; Blaikie FH; Smith RA; Murphy MP A mitochondria-targeted nitroxide is reduced to its hydroxylamine by ubiquinol in mitochondria. Free Radic. Bio. Med, 2008, 44 (7), 1406–1419. [DOI] [PubMed] [Google Scholar]
- [16].Krishna MC; Russo A; Mitchell JB; Goldstein S; Dafni H; Samuni A, Do nitroxide antioxidants act as scavengers of O2-. or as SOD mimics? J. Biol. Chem, 1996, 271 (42), 26026–26031. [DOI] [PubMed] [Google Scholar]
- [17].Batinic-Haberle I; Spasojevic I; Stevens RD; Bondurant B; Okado-Matsumoto A; Fridovich I; Vujaskovic Z; Dewhirst MW New PEG-ylated Mn(III) porphyrins approaching catalytic activity of SOD enzyme. Dalton Trans, 2006, (4), 617–624. [DOI] [PubMed] [Google Scholar]
- [18].Mitchell JB; Samuni A; Krishna MC; DeGraff WG; Ahn MS; Samuni U; Russo A Biologically active metal-independent superoxide dismutase mimics. Biochemistry, 1990, 29 (11), 2802–2807. [DOI] [PubMed] [Google Scholar]
- [19].Goldstein S; Samuni A; Hideg K; Merenyi G Structure-activity relationship of cyclic nitroxides as SOD mimics and scavengers of nitrogen dioxide and carbonate radicals. J. Phys. Chem. A 2006, 110 (10), 3679–3685. [DOI] [PubMed] [Google Scholar]
- [20].Goldstein S; Samuni A; Merenyi G Reactions of nitric oxide, peroxynitrite, and carbonate radicals with nitroxides and their corresponding oxoammonium cations. Chem. Res. Toxicol, 2004, 17 (2), 250–257. [DOI] [PubMed] [Google Scholar]
- [21].Goldstein S; Samuni A; Merenyi G, Kinetics of the reaction between nitroxide and thiyl radicals: nitroxides as antioxidants in the presence of thiols. J. Phys. Chem. A, 2008, 112 (37), 8600–8605. [DOI] [PubMed] [Google Scholar]
- [22].Xavier S; Yamada K; Samuni AM; Samuni A; DeGraff W; Krishna MC; Mitchell JB Differential protection by nitroxides and hydroxylamines to radiation-induced and metal ion-catalyzed oxidative damage. Biochim. Biophys. Acta, 2002, 1573 (2), 109–120. [DOI] [PubMed] [Google Scholar]
- [23].Mitchell JB; DeGraff W; Kaufman D; Krishna MC; Samuni A; Finkelstein E; Ahn MS; Hahn SM; Gamson J; Russo A Inhibition of oxygen-dependent radiation-induced damage by the nitroxide superoxide dismutase mimic, tempol. Arch. Biochem. Biophys, 1991, 289 (1), 62–70. [DOI] [PubMed] [Google Scholar]
- [24].Hahn SM; DeLuca AM; Coffin D; Krishna CM; Mitchell JB, In vivo radioprotection and effects on blood pressure of the stable free radical nitroxides. Int. J. Radiat. Oncol. Biol. Phys, 1998, 42 (4), 839–842. [DOI] [PubMed] [Google Scholar]
- [25].Hahn SM; Krishna MC; DeLuca AM; Coffin D; Mitchell JB Evaluation of the hydroxylamine Tempol-H as an in vivo radioprotector. Free Radic. Biol. Med 2000, 28 (6), 953–958. [DOI] [PubMed] [Google Scholar]
- [26].Hahn SM; Sullivan FJ; DeLuca AM; Krishna CM; Wersto N; Venzon D; Russo A; Mitchell JB Evaluation of tempol radioprotection in a murine tumor model. Free Radic. Biol. Med, 1997, 22 (7), 1211–1216. [DOI] [PubMed] [Google Scholar]
- [27].Hahn SM; Tochner Z; Krishna CM; Glass J; Wilson L; Samuni A; Sprague M; Venzon D; Glatstein E; Mitchell JB; Russo A Tempol, a stable free radical, is a novel murine radiation protector. Cancer Res, 1992, 52 (7), 1750–1753. [PubMed] [Google Scholar]
- [28].Cotrim AP; Sowers AL; Lodde BM; Vitolo JM; Kingman A; Russo A; Mitchell JB; Baum BJ Kinetics of tempol for prevention of xerostomia following head and neck irradiation in a mouse model. Clin. Cancer Res, 2005, 11(20), 7564–7568. [DOI] [PubMed] [Google Scholar]
- [29].Cotrim AP; Hyodo F; Matsumoto K; Sowers AL; Cook JA; Baum BJ; Krishna MC; Mitchell JB Differential radiation protection of salivary glands versus tumor by Tempol with accompanying tissue assessment of Tempol by magnetic resonance imaging. Clin. Cancer Res, 2007, 13 (16), 4928–4933. [DOI] [PubMed] [Google Scholar]
- [30].Vitolo JM; Cotrim AP; Sowers AL; Russo A; Wellner RB; Pillemer SR; Mitchell JB; Baum BJ, The stable nitroxide tempol facilitates salivary gland protection during head and neck irradiation in a mouse model. Clin. Cancer Res, 2004, 10(5), 1807–1812. [DOI] [PubMed] [Google Scholar]
- [31].Hyodo F; Murugesan R; Matsumoto K; Hyodo E; Subramanian S; Mitchell JB; Krishna MC, Monitoring redox-sensitive paramagnetic contrast agent by EPRI, OMRI and MRI. J. Magn. Reson, 2008, 190(1), 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].H Hyodo F; Matsumoto S; Devasahayam N; Dharmaraj C; Subramanian S; Mitchell JB; Krishna MC Pulsed EPR imaging of nitroxides in mice. J. Magn. Reson, 2009, 197(2), 181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Overhauser AW Polarization of Nuclei in Metals. Physical Review, 1953, 92(2), 411–415. [Google Scholar]
- [34].Krishna MC; English S; Yamada K; Yoo J; Murugesan R; Devasahayam N; Cook JA; Golman K; Ardenkjaer-Larsen JH; Subramanian S; Mitchell JB Overhauser enhanced magnetic resonance imaging for tumor oximetry: coregistration of tumor anatomy and tissue oxygen concentration. Proc. Natl. Acad. Sci., USA 2002, 99(4), 2216–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].L Li H; He G; Deng Y; Kuppusamy P; Zweier JL In vivo proton electron double resonance imaging of the distribution and clearance of nitroxide radicals in mice. Magn. Reson. Med, 2006, 55(3), 669–675. [DOI] [PubMed] [Google Scholar]
- [36].Hyodo F; Matsumoto K; Matsumoto A; Mitchell JB; Krishna MC Probing the intracellular redox status of tumors with magnetic resonance imaging and redox-sensitive contrast agents. Cancer Res, 2006, 66(20), 9921–9928. [DOI] [PubMed] [Google Scholar]
- [37].Matsumoto K; Hyodo F; Matsumoto A; Koretsky AP; Sowers AL; Mitchell JB; Krishna MC High-resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redox-sensitive contrast agents. Clin. Cancer Res, 2006, 12(8), 2455–2462. [DOI] [PubMed] [Google Scholar]
- [38].Leonard SS; Mowrey K; Pack D; Shi X; Castranova V; Kuppusamy P; Vallyathan V In vivo bioassays of acute asbestosis and its correlation with ESR spectroscopy and imaging in redox status. Mol. Cell Biochem, 2002, 234–235(1–2), 369–377. [PubMed] [Google Scholar]
- [39].Vallyathan V; Leonard S; Kuppusamy P; Pack D; Chzhan M; Sanders SP; Zweir JL, Oxidative stress in silicosis: evidence for the enhanced clearance of free radicals from whole lungs. Mol. Cell Biochem, 1997, 168(1–2), 125–132. [DOI] [PubMed] [Google Scholar]
- [40].Inaba K; Nakashima T; Shima T; Mitsuyoshi H; Sakamoto Y; Okanoue T; Kashima K; Hashiba M; Nishikawa H; Watari H Hepatic damage influences the decay of nitroxide radicals in mice--an in vivo ESR study. Free Radic. Res, 1997, 27(1), 37–43. [DOI] [PubMed] [Google Scholar]
- [41].Gomi F; Utsumi H; Hamada A; Matsuo M Aging retards spin clearance from mouse brain and food restriction prevents its age-dependent retardation. Life Sci, 1993, 52(25), 2027–2033. [DOI] [PubMed] [Google Scholar]
- [42].Yamato M; Egashira T; Utsumi H Application of in vivo ESR spectroscopy to measurement of cerebrovascular ROS generation in stroke. Free Radic. Biol. Med, 2003, 35(12), 1619–1631. [DOI] [PubMed] [Google Scholar]
- [43].Fujii H; Sato-Akaba H; Kawanishi K; Hirata H Mapping of redox status in a brain-disease mouse model by three-dimensional EPR imaging. Magn. Reson. Med, 2011, 65(1), 295–303. [DOI] [PubMed] [Google Scholar]
- [44].Yamato M; Shiba T; Yamada K; Watanabe T; Utsumi H Separable detection of lipophilic- and hydrophilic-phase free radicals from the ESR spectrum of nitroxyl radical in transient MCAO mice. Free Radic. Res, 2009, 43(9), 844–851. [DOI] [PubMed] [Google Scholar]
- [45].Sano T; Umeda F; Hashimoto T; Nawata H; Utsumi H, Oxidative stress measurement by in vivo electron spin resonance spectroscopy in rats with streptozotocin-induced diabetes. Diabetologia, 1998, 41(11), 1355–1360. [DOI] [PubMed] [Google Scholar]
- [46].Ojha N; Roy S; He G; Biswas S; Velayutham M; Khanna S; Kuppusamy P; Zweier JL; Sen CK, Assessment of wound-site redox environment and the significance of Rac2 in cutaneous healing. Free Radic. Biol. Med, 2008, 44(4), 682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Herrling T; Jung K; Fuchs J Measurements of UV-generated free radicals/reactive oxygen species (ROS) in skin. Spectrochim. Acta A Mol. Biomol. Spectrosc, 2006, 63(4), 840–845. [DOI] [PubMed] [Google Scholar]
- [48].Herrling T; Fuchs J; Rehberg J; Groth N UV-induced free radicals in the skin detected by ESR spectroscopy and imaging using nitroxides. Free Radic. Biol. Med, 2003, 35(1), 59–67. [DOI] [PubMed] [Google Scholar]
- [49].Miura Y; Anzai K; Urano S; Ozawa T, In vivo electron paramagnetic resonance studies on oxidative stress caused by X-irradiation in whole mice. Free Radic. Biol. Med, 1997, 23 (4), 533–540. [DOI] [PubMed] [Google Scholar]
- [50].Quintanilha AT; Packer L Surface localization of sites of reduction of nitroxide spin-labeled molecules in mitochondria. Proc .Natl. Acad. Sci. USA 1977, 74(2), 570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chapman DA; Killian GJ; Gelerinter E; Jarrett MT, Reduction of the spin-label TEMPONE by ubiquinol in the electron transport chain of intact rabbit spermatozoa. Biol. Reprod, 1985, 32(4), 884–893. [DOI] [PubMed] [Google Scholar]
- [52].Chen K; Glockner JF; Morse PD 2nd; Swartz HM Effects of oxygen on the metabolism of nitroxide spin labels in cells. Biochemistry, 1989, 28(6), 2496–501. [DOI] [PubMed] [Google Scholar]
- [53].Samuni Y; Gamson J; Samuni A; Yamada K; Russo A; Krishna MC; Mitchell JB Factors influencing nitroxide reduction and cytotoxicity in vitro. Antioxid. Redox. Signal, 2004, 6(3), 587–595. [DOI] [PubMed] [Google Scholar]
- [54].Boveris A; Oshino N; Chance B, The cellular production of hydrogen peroxide. Biochem. J, 1972, 128 (3), 617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Grek CL; Tew KD, Redox metabolism and malignancy. Curr Opin Pharmacol 2010, 10 (4), 362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Turrens JF; Alexandre A; Lehninger AL, Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch. Biochem. Biophys, 1985, 237 (2), 408–14. [DOI] [PubMed] [Google Scholar]
- [57].Finkelstein E; Rosen GM; Rauckman EJ Superoxide-dependent reduction of nitroxides by thiols. Biochimica et Biophysica Acta, 1984, 802, 90–98. [Google Scholar]
- [58].Yamada KI; Kuppusamy P; English S; Yoo J; Irie A; Subramanian S; Mitchell JB; Krishna MC Feasibility and assessment of non-invasive in vivo redox status using electron paramagnetic resonance imaging. Acta Radiol, 2002, 43(4), 433–440. [DOI] [PubMed] [Google Scholar]
- [59].Miura Y; Hamada A; Utsumi H, In vivo ESR studies of antioxidant activity on free radical reaction in living mice under oxidative stress. Free Radic. Res, 1995, 22 (3), 209–214. [DOI] [PubMed] [Google Scholar]
- [60].Rui B; Shen T; Zhou H; Liu J; Chen J; Pan X; Liu H; Wu J; Zheng H; Shi Y A systematic investigation of Escherichia coli central carbon metabolism in response to superoxide stress. BMC Syst. Biol, 2010, 4, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Brumaghim JL; Li Y; Henle E; Linn S Effects of hydrogen peroxide upon nicotinamide nucleotide metabolism in Escherichia coli: changes in enzyme levels and nicotinamide nucleotide pools and studies of the oxidation of NAD(P)H by Fe(III). J. Biol. Chem, 2003, 278(43), 42495–42504. [DOI] [PubMed] [Google Scholar]
- [62].Singh R; Mailloux RJ; Puiseux-Dao S; Appanna VD Oxidative stress evokes a metabolic adaptation that favors increased NADPH synthesis and decreased NADH production in Pseudomonas fluorescens. J. Bacteriol 2007, 189(18), 6665–6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ben-Yoseph O; Boxer PA; Ross BD Assessment of the role of the glutathione and pentose phosphate pathways in the protection of primary cerebrocortical cultures from oxidative stress. J. Neurochem, 1996, 66(6), 2329–2337. [DOI] [PubMed] [Google Scholar]
- [64].Gessner T; Vaughan LA; Beehler BC; Bartels CJ; Baker RM Elevated pentose cycle and glucuronyltransferase in daunorubicin-resistant P388 cells. Cancer Res, 1990, 50(13), 3921–3927. [PubMed] [Google Scholar]
- [65].Guitton J; Servanin S; Francina A Hexose monophosphate shunt activities in human erythrocytes during oxidative damage induced by hydrogen peroxide. Arch. Toxicol, 2003, 77(7), 410–417. [DOI] [PubMed] [Google Scholar]
- [66].H Hyodo F; Chuang KH; Goloshevsky AG; Sulima A; Griffiths GL; Mitchell JB; Koretsky AP; Krishna MC Brain redox imaging using blood-brain barrier-permeable nitroxide MRI contrast agent. J. Cereb. Blood Flow Metab, 2008, 28 (6), 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Nordsmark M; Bentzen SM; Rudat V; Brizel D; Lartigau E; Stadler P; Becker A; Adam M; Molls M; Dunst J; Terris DJ; Overgaard J Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother. Oncol 2005, 77(1), 18–24. [DOI] [PubMed] [Google Scholar]
- [68].Brizel DM; Dodge RK; Clough RW; Dewhirst MW Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol, 1999, 53 (2), 113–117. [DOI] [PubMed] [Google Scholar]
- [69].Hockel M; Knoop C; Schlenger K; Vorndran B; Baussmann E; Mitze M; Knapstein PG; Vaupel P Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother. Oncol 1993, 26 (1), 45–50. [DOI] [PubMed] [Google Scholar]
- [70].Gatenby RA; Kessler HB; Rosenblum JS; Coia LR; Moldofsky PJ; Hartz WH; Broder GJ Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int. J. Radiat. Oncol. Biol. Phys, 1988, 14 (5), 831–838. [DOI] [PubMed] [Google Scholar]
- [71].Swartz HM; Chen K; Pals M; Sentjurc M; Morse PD 2nd, Hypoxia-sensitive NMR contrast agents. Magn. Reson. Med, 1986, 3 (1), 169–174. [DOI] [PubMed] [Google Scholar]
- [72].Iannone A; Tomasi A; Vannini V; Swartz HM Metabolism of nitroxide spin labels in subcellular fraction of rat liver. I. Reduction by microsomes. Biochim. Biophys. Acta, 1990, 1034(3), 285–289. [DOI] [PubMed] [Google Scholar]
- [73].Matsumoto S; Hyodo F; Subramanian S; Devasahayam N; Munasinghe J; Hyodo E; Gadisetti C; Cook JA; Mitchell JB; Krishna MC Low-field paramagnetic resonance imaging of tumor oxygenation and glycolytic activity in mice. J. Clin. Invest, 2008, 118 (5), 1965–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ilangovan G; Li H; Zweier JL; Krishna MC; Mitchell JB; Kuppusamy P, In vivo measurement of regional oxygenation and imaging of redox status in RIF-1 murine tumor: effect of carbogen-breathing. Magn. Reson. Med 2002, 48 (4), 723–730. [DOI] [PubMed] [Google Scholar]
- [75].Ilangovan G; Li H; Zweier JL; Kuppusamy P In vivo measurement of tumor redox environment using EPR spectroscopy. Mol. Cell Biochem, 2002, 234–235 (1–2), 393–398. [PubMed] [Google Scholar]
- [76].Takeshita K; Kawaguchi K; Fujii-Aikawa K; Ueno M; Okazaki S; Ono M; Krishna MC; Kuppusamy P; Ozawa T; Ikota N Heterogeneity of regional redox status and relation of the redox status to oxygenation in a tumor model, evaluated using electron paramagnetic resonance imaging. Cancer Res, 2010, 70 (10), 4133–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Yasui H; Matsumoto S; Devasahayam N; Munasinghe JP; Choudhuri R; Saito K; Subramanian S; Mitchell JB; Krishna MC Low-field magnetic resonance imaging to visualize chronic and cycling hypoxia in tumor-bearing mice. Cancer Res, 2010, 70 (16), 6427–6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].De Jaeger K; Kavanagh MC; Hill RP Relationship of hypoxia to metastatic ability in rodent tumours. Br. J. Cancer, 2001, 84(9), 1280–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Walshe J; Serewko-Auret MM; Teakle N; Cameron S; Minto K; Smith L; Burcham PC; Russell T; Strutton G; Griffin A; Chu FF; Esworthy S; Reeve V; Saunders NA Inactivation of glutathione peroxidase activity contributes to UV-induced squamous cell carcinoma formation. Cancer Res, 2007, 67(10), 4751–4758. [DOI] [PubMed] [Google Scholar]
- [80].Chen HH; Kuo MT Role of glutathione in the regulation of Cisplatin resistance in cancer chemotherapy. Met .Based Drugs, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hamilton TC; Winker MA; Louie KG; Batist G; Behrens BC; Tsuruo T; Grotzinger KR; McKoy WM; Young RC; Ozols RF, Augmentation of adriamycin, melphalan, and cisplatin cytotoxicity in drug-resistant and -sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochem. Pharmacol 1985, 34(14), 2583–2586. [DOI] [PubMed] [Google Scholar]
- [82].Bratasz A; Selvendiran K; Wasowicz T; Bobko A; Khramtsov VV; Ignarro LJ; Kuppusamy P NCX-4040, a nitric oxide-releasing aspirin, sensitizes drug-resistant human ovarian xenograft tumors to cisplatin by depletion of cellular thiols. J. Transl. Med, 2008, 6, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kramer RA; Zakher J; Kim G Role of the glutathione redox cycle in acquired and de novo multidrug resistance. Science, 1988, 241 (4866), 694–697. [DOI] [PubMed] [Google Scholar]
- [84].Grogan TM; Fenoglio-Prieser C; Zeheb R; Bellamy W; Frutiger Y; Vela E; Stemmerman G; Macdonald J; Richter L; Gallegos A; Powis G Thioredoxin, a putative oncogene product, is overexpressed in gastric carcinoma and associated with increased proliferation and increased cell survival. Hum. Pathol, 2000, 31 (4), 475–481. [DOI] [PubMed] [Google Scholar]
- [85].O’Brien ML; Tew KD, Glutathione and related enzymes in multidrug resistance. Eur. J. Cancer, 1996, 32A(6), 967–978. [DOI] [PubMed] [Google Scholar]
- [86].Ghibelli L; Coppola S; Rotilio G; Lafavia E; Maresca V; Ciriolo MR, Non-oxidative loss of glutathione in apoptosis via GSH extrusion. Biochem. Biophys. Res. Commun, 1995, 216(1), 313–320. [DOI] [PubMed] [Google Scholar]
- [87].Ghibelli L; Fanelli C; Rotilio G; Lafavia E; Coppola S; Colussi C; Civitareale P; Ciriolo MR Rescue of cells from apoptosis by inhibition of active GSH extrusion. FASEB J, 1998, 12(6), 479–486. [DOI] [PubMed] [Google Scholar]
- [88].Ushio-Fukai M, Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovas. Res, 2006, 71 (2), 226–235. [DOI] [PubMed] [Google Scholar]
- [89].Chen KY; McLaughlin MG Differences in the reduction kinetics of incorporated spin labels in undifferentiated and differentiated mouse neuroblastoma cells. Biochim. Biophys. Acta, 1985, 845(2), 189–195. [DOI] [PubMed] [Google Scholar]
- [90].Demizu Y; Sasaki R; Trachootham D; Pelicano H; Colacino JA; Liu J; Huang P, Alterations of cellular redox state during NNK-induced malignant transformation and resistance to radiation. Antioxid. Redox Signal, 2008, 10(5), 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Terpstra M; Henry PG; Gruetter R, Measurement of reduced glutathione (GSH) in human brain using LCModel analysis of difference-edited spectra. Magn. Reson. Med, 2003, 50 (1), 19–23. [DOI] [PubMed] [Google Scholar]
- [92].Roshchupkina GI; Bobko AA; Bratasz A; Reznikov VA; Kuppusamy P; Khramtsov VV, In vivo EPR measurement of glutathione in tumor-bearing mice using improved disulfide biradical probe. Free Radic. Biol. Med, 2008, 45(3), 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kuppusamy P; Li H; Ilangovan G; Cardounel AJ; Zweier JL; Yamada K; Krishna MC; Mitchell JB, Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res 2002, 62 (1), 307–312. [PubMed] [Google Scholar]
- [94].Takeshita K; Hamada A; Utsumi H Mechanisms related to reduction of radical in mouse lung using an L-band ESR spectrometer. Free Radic. Biol. Med, 1999, 26(7–8), 951–960. [DOI] [PubMed] [Google Scholar]
- [95].Mitchell JB; Russo A, The role of glutathione in radiation and drug induced cytotoxicity. Br. J. Cancer Suppl, 1987, 8, 96–104. [PMC free article] [PubMed] [Google Scholar]
- [96].Rosen GM; Rauchman E Formation and reduction of a nitroxide radical by liver microsomes. Biochem. Pharmacol, 1977, 26 (7), 675–668. [DOI] [PubMed] [Google Scholar]
- [97].Stier A; Reitz I Radical production in amine oxidation by liver microsomes. Xenobiotica, 1971, 1 (4), 499–500. [DOI] [PubMed] [Google Scholar]
- [98].Iannone A; Bini A; Swartz HM; Tomasi A; Vannini V Metabolism in rat liver microsomes of the nitroxide spin probe tempol. Biochem. Pharmacol, 1989, 38(16), 2581–2586. [DOI] [PubMed] [Google Scholar]
- [99].Utsumi H; Shimakura A; Kashiwagi M; Hamada A Localization of the active center of nitroxide radical reduction in rat liver microsomes: its relation to cytochrome P-450 and membrane fluidity. J. Biochem, 1989, 105(2), 239–244. [DOI] [PubMed] [Google Scholar]
- [100].Morgan WA; Kaler B; Bach PH The role of reactive oxygen species in adriamycin and menadione-induced glomerular toxicity. Toxicol. Lett, 1998, 94(3), 209–215. [DOI] [PubMed] [Google Scholar]
- [101].Yeh GC; Occhipinti SJ; Cowan KH; Chabner BA; Myers CE Adriamycin resistance in human tumor cells associated with marked alteration in the regulation of the hexose monophosphate shunt and its response to oxidant stress. Cancer Res, 1987, 47(22), 5994–5999. [PubMed] [Google Scholar]
- [102].Branca M; Denurra T; Turrini F Reduction of nitroxide free radical by normal and G6PD deficient red blood cells. Free Radic. Biol. Med, 1988, 5 (1), 7–11. [DOI] [PubMed] [Google Scholar]
- [103].Belkin S; Mehlhorn RJ; Hideg K; Hankovsky O; Packer L Reduction and destruction rates of nitroxide spin probes. Arch. Biochem. Biophys, 1987, 256 (1), 232–243. [DOI] [PubMed] [Google Scholar]
- [104].Keana JF; Pou S; Rosen GM Nitroxides as potential contrast enhancing agents for MRI application: influence of structure on the rate of reduction by rat hepatocytes, whole liver homogenate, subcellular fractions, and ascorbate. Magn. Reson. Med, 1987, 5(6), 525–536. [DOI] [PubMed] [Google Scholar]
- [105].Vissers MC; Bozonet SM; Pearson JF; Braithwaite LJ Dietary ascorbate intake affects steady state tissue concentrations in vitamin C-deficient mice: tissue deficiency after suboptimal intake and superior bioavailability from a food source (kiwifruit). Am. J. Clin. Nutr, 2010, 93(2), 292–301. [DOI] [PubMed] [Google Scholar]
- [106].Ki MR; Lee HR; Park JK; Hong IH; Han SY; You SY; Lee EM; Kim AY; Lee SS; Jeong KS, Ascorbate promotes carbon tetrachloride-induced hepatic injury in senescence marker protein 30-deficient mice by enhancing inflammation. J. Nutr. Biochem, 2010. (E-Pub Ahead of Print) [DOI] [PubMed] [Google Scholar]
- [107].Gallez B; Bacic G; Goda F; Jiang J; O’Hara JA; Dunn JF; Swartz HM Use of nitroxides for assessing perfusion, oxygenation, and viability of tissues: in vivo EPR and MRI studies. Magn. Reson. Med, 1996, 35(1), 97–106. [DOI] [PubMed] [Google Scholar]
- [108].Takeshita K; Utsumi H; Hamada A ESR measurement of radical clearance in lung of whole mouse. Biochem. Biophys. Res. Commun, 1991, 177(2), 874–880. [DOI] [PubMed] [Google Scholar]
- [109].Citrin D; Cotrim AP; Hyodo F; Baum BJ; Krishna MC; Mitchell JB Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist., 2010, 15(4), 360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kouvaris JR; Kouloulias VE; Vlahos LJ Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist., 2007, 12(6), 738–747. [DOI] [PubMed] [Google Scholar]
- [111].Rades D; Fehlauer F; Bajrovic A; Mahlmann B; Richter E; Alberti W Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother. Oncol, 2004, 70 (3), 261–264. [DOI] [PubMed] [Google Scholar]
- [112].Iannone A; Hu HP; Tomasi A; Vannini V; Swartz HM, Metabolism of aqueous soluble nitroxides in hepatocytes: effects of cell integrity, oxygen, and structure of nitroxides. Biochim. Biophys. Acta, 1989, 991(1), 90–96. [DOI] [PubMed] [Google Scholar]


