Abstract
Individuals show meaningful variability in food choices. Choices are affected by individual differences in sensitivity to food reward and punishment, so understanding correlates of response to food reinforcement can help characterize food choices. Here, we examined behavioral and physiological correlates of individual differences in how individuals learn from food reward and punishment, as measured by performance on an appetitive probabilistic selection task that used sweet and bitter tastes as reinforcement. Sensitivity to food reward, sensitivity to food punishment, and overall learning performance were measured in 89 adults. Multivariate linear regressions were used to test if variables including body mass index (BMI), external eating, emotional eating, behavioral inhibition/behavioral activation scales (BIS/BAS), and perceived sensitivity to reward and punishment (SPQ/SRQ) were associated with measures of learning performance. External eating (β=-.035, p=.019), BIS (β=-.066, p=.004), and SPQ (β=.003, p=.023) were associated with overall learning performance. BMI (β=-.000, p=.012), emotional eating (β=.055, p=.006), and external eating (β=-.062, p=.004) were associated with sensitivity to food reward. No variables were associated with sensitivity to food punishment. In post hoc analyses, the interaction of sex and SPQ was associated with overall performance (β=.005, p=.025), such that the relationship was positive in women only (β=.006, p=0.002). Results support that, controlling for key individual characteristics, BMI and susceptibility to food cues are associated with lower sensitivity to food reward, which may affect future food choices and eating behavior.
Keywords: reinforcement learning, reward, punishment, emotional eating, external eating, behavioral inhibition scale, individual differences
INTRODUCTION
The modern food environment presents adults with many decision-making opportunities, resulting in a host of food choice behaviors. Adult food decisions are influenced through a variety of economical, psychological, and social expectations. Consider a restaurant: diners will choose vastly different menu items based factors including on their expectations about the tastiness of a dish, its healthfulness, or how full the food will make them feel [1,2]. These expectations are generated and maintained through reinforcement learning [3,4], which can be categorized as classical conditioning or instrumental conditioning. Classical conditioning occurs when a previously unknown stimulus (e.g. a logo) is repeatedly paired with a reinforcer (e.g. a sugary, palatable drink) that evokes a response (e.g. salivation) [5]. Over the course of conditioning, the cue becomes associated the response evoked by the reinforcer, referred to as a conditioned response. In eating behavior, classical conditioning describes how environmental stimuli, like food advertisements, can activate psychological and physiological responses that prepare for ingestion, such as food cravings and increased salivation [6,7]. Classically conditioned responses are automatic, and can occur outside conscious awareness. Increased sensitivity to conditioned food cues is associated with increased intake and higher weight gain risk [8,9]. Another type of reinforcement learning is instrumental conditioning. Instrumental conditioning differs from classical conditioning in that reinforcement is associated with a response (or behavior), rather than a cue. Outcomes (e.g. reward or punishment) from the instrumental response shape future actions, promoting behaviors that earn rewards and avoid punishments, thus optimizing behavior [10]. In the context of eating behavior, instrumental conditioning can describe how individuals respond to food outcomes, such as a child following rules to receive candy, or an adult avoiding ordering from the same restaurant after a bad meal [11]. Where classical conditioning is passive, instrumental conditioning is active; individuals must engage in the instrumental response to receive reinforcement. How instrumental behaviors relate to food decisions is a key point of study in ingestive behavior.
Identifying factors related to food conditioning provides insight into how individuals make food choices. Individual differences in how we learn from food reward and punishment shape future food choices, where insensitivity food outcomes can contribute to inflexible behavior [12]. Prior research has identified individual level characteristics associated with classical conditioning and instrumental conditioning, however the majority of studies use secondary reinforcers, like written feedback or monetary gains and losses, to test for characteristics associated with conditioning. In such tasks, physiological characteristics such as body mass index (BMI) and biological sex are related to individual differences in learning [13,14]. Specifically, In an instrumental conditioning task using written feedback, individuals with overweight/obesity showed insensitivity to negative feedback [13]. On a similar learning task, sex was related to performance, where women were more sensitive to positive written feedback and men learned better from negative written feedback [14]. Behavioral characteristics also are associated with an individual’s sensitivity to reinforcement learning from secondary reinforcers. First, working memory, a form of short-term memory that stores and processes information for immediate use [15], is positively associated with performance on a range of secondary reinforcement learning tasks [16]. Measures of perceived sensitivity to reward and punishment are related to individual differences in instrumental conditioning and decision making on tasks using secondary reinforcement. The Behavioral Inhibitory System and Behavioral Approach System (BIS/BAS) scales measure reward motivation (BAS) and punishment avoidance (BIS) [17], and high scores on the BIS have been negatively associated with ability to learn from monetary gains [18]. A similar scale, the Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPQ/SRQ) [19] was associated with performance on a probabilistic learning task, where those with high SRQ scores were better at choosing positive visual outcomes while those with high SPQ were better at avoiding negative visual outcomes [20]. Together, these results demonstrate how individual differences in learning are related to a range of participant characteristics, but these associations may not be the same when learning is assessed using a primary reinforcer. Primary and secondary reinforcement are processed through separable neural pathways [21], and behavioral responses to primary and secondary reinforcement vary [22]. This, it is necessary to test if these associations are specific to secondary reinforcement or if the same relationships emerge on a food-motivated reinforcement learning task.
A small number of tasks have tested for associations between characteristics like obesity and eating constructs and food-motivated reinforcement learning using passive, classical conditioning tasks. First, people who have a high BMI (BMI > 25 kg/m2) show to stronger associative learning when a novel cue is paired with a chocolate milkshake compared to people with a normal body weight [23]. Similarly, external eating which measures an individual’s perceived susceptibility to eat in response to external cues [24], is associated with greater attention towards and preference for cues that predict palatable food receipt [25]. Conversely, dietary restraint, representing an individual's intention to limit their food intake for the means of controlling or losing weight [24], is associated with impaired classical conditioning, where those with high dietary restraint do not form preferences for a novel flavor after it is paired with a food reward [26]. This research provides initial evidence that individual differences in food conditioning exits are related to obesity and eating constructs, but further research is needed to test if the same relationships are observed using an instrumental conditioning task.
Together, these studies provide evidence for a number of associations between physiological and psychological characteristics and reinforcement learning. However, to date, the relationship between individual characteristics and reinforcement learning has not been tested using a food-motivated, instrumental conditioning task. This leaves a gap in our understanding of how participant characteristics can independently or interactively relate to how individuals learn from taste to guide their food choices. In the present study, we assessed the relationship between behavioral and psychological correlates associated with secondary reinforcement learning using a primary reinforcement (taste) paradigm. We used an appetitive probabilistic selection task (PST, based on [27]) that examines instrumental conditioning from food reward (sweet taste) and punishment (bitter taste). We completed multivariate linear regression models to examine whether physiological and/or behavioral variables, including BMI, sex, SPQ/SRQ, BIS/BAS, dietary restraint, emotional eating, external eating, and a measure of working memory (the N-back task [28]), predicted three measures of instrumental conditioning: overall learning performance (ability to choose reward and avoid punishment), sensitivity to food reward (ability to choose reward), and sensitivity to food punishment (ability to avoid punishment). Based on prior associations between physiological/behavioral characteristics and individual differences in learning from primary and secondary reinforcement [13, 16, 18, 20, 24], we hypothesized that BMI would be negatively associated with sensitivity to food punishment, and that external eating would be associated with sensitivity to food reward. Finally, we predicted that N-back accuracy, BIS/BAS, and SPQ/SRQ scores would be positively associated with overall learning performance.
METHODS
2.1. Recruitment
Ninety (n=90) male and female participants were recruited from the Chapel Hill, North Carolina area to complete this cross-sectional imaging study. Eligibility criteria included: 1) aged 18–28 years, 2) body mass index between (BMI) 20.0 kg/m2 and 32.0 kg/m2. Exclusion criteria were: 1) counter-indications of MRI (e.g. metal implants, piercings, pregnancy), 2) current smoking, 3) self-reported current or past diagnoses of an eating disorder, 4) chronic illness or medication requirement that could affect diet, 5) diagnosis of a major psychological condition (bipolar, schizophrenia, major affective disorder), and 6) allergy or intolerance to any study foods. The Institutional Review Board of University of North Carolina at Chapel Hill approved all methods and study participants gave written consent before the start of testing. Study visits took place at the University of North Carolina at Chapel Hill’s Gillings School of Global Public Health and Biomedical Research Imaging Center (BRIC). One participant had missing data, so the final analytic sample was n=89.
2.2. Procedures
All measures were completed in a single study visit, lasting 2.5 hours in duration. To normalize the time since last meal in the sample, participants were instructed fast for 4-hours prior to the visit to mimic a between meal interval. Trained research staff assessed height (to the nearest 0.5 cm) and weight (to the nearest 0.1 kg) measured with a wall-mounted stadiometer and a calibrated. BMI (kg/m2) was calculated using height and weight measurements. Participants completed a N-back task (measurement of working memory) on a computer tablet app, (PsychLab101©, Version 2.0, Neurobehavioral Systems). Participants completed a single, 1-back block and a single, 2-back block of the task with alphabet letter stimuli. Block order was counterbalanced across the sample. Working memory was operationalized as participants’ overall accuracy on the two blocks of the task.
To select which beverages would be used in the modified PST, participants completed a taste test of four sweet beverages with variable amounts of added sugar and four bitter beverages with variable amounts of added quinine. Participants were given a 20mL sample of each beverage to rate pleasantness, desire to consume, sweetness, bitterness and intensity on VAS anchored at −100 and 100. All sweet beverages were sampled, then participants ranked the beverages from most pleasant to least pleasant. The same process was then completed with the bitter beverages. The order within sweet and bitter groups was randomized for each participant. The most pleasantly ranked sweet beverage were selected as the reward stimuli. The bitter beverage ranked as least pleasant was selected as the punishment stimuli. In the case where the lowest ranked bitter beverage was rated as “least pleasant imaginable” (-100 on a visual analog scale [VAS]), the next lowest ranked beverage was used as the punishment stimuli to prevent non-compliance during the scan. The eight beverages were made from a base of water, unsweetened Kool-Aid™ cherry powder, and simple syrup. Additional simple syrup or a quinine solution were added to the beverages to create different levels of sweetness or bitterness. Levels of sweetness and bitterness were selected from a previous study of taste preference [29]. The sweetest beverage contained about 35g of sugar per 300mL, a similar amount as a sugar sweetened beverage (full beverage composition can be found in Supplemental Materials). The beverages were calorically-matched to contain 105 kcal/300mL, using the addition of maltodextrin, a soluble, odorless, and flavorless carbohydrate. The amount of maltodextrin added was not associated with beverage intensity ratings, indicating that maltodextrin didn’t impact the perceived intensity of the beverages.
Following the taste test, participants completed the Behavioral Inhibition System and Behavioral Activation System Questionnaire scales (BIS/BAS; [17]) to assess perceived sensitivity to two general motivational systems, and the Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPQ/SRQ; [19]) to measure perceived sensitivity to general positive and negative reinforcement. The Dutch Eating Behavior Questionnaire (DEBQ; [24]) was used to measure eating constructs, including dietary restraint, external eating, and emotional eating.
2.3. Appetitive Probabilistic Selection Task
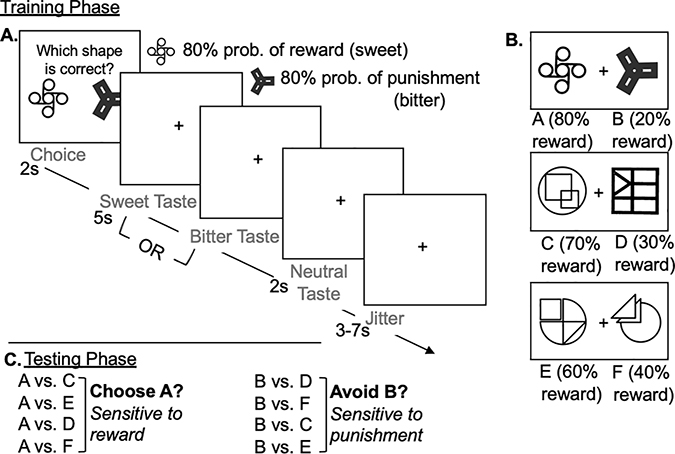
Participants completed a modified version of the Probabilistic Selection Task [27] to measure sensitivity to food reward and punishment. A portion of the task was completed during a functional neuroimaging scan, and imaging data is presented elsewhere [30]. The task was composed of a training during the scan and posttest after the scan. In the training, participants were presented with pairs of novel shapes (similar to logos), and asked to select the “correct” shape to receive a reward. Participants were told that when they choose the "correct” shape, they will receive a sweet taste as a reward, while when they choose the “incorrect” shape, they will receive a bitter taste as a punishment. Participants were instructed to try to choose the “correct” shape on as many trials as possible, but that their feedback was probabilistic, meaning that each shape had a certain probability of being “correct” A visual representation of the task and the reward and punishment frequencies of each shape can be found in Figure 1. In the original version of the task, written feedback was given to provide reinforcement [27]. In the version of the task used here, feedback was given in the form of 3mL of a sweet beverage (food reward) to indicate that participants chose correctly or the same volume of a bitter beverage (food punishment) to indicate that they chose incorrectly. Tastes were delivered for five seconds, followed by a 1 mL rinse of a tasteless solution made to mimic the taste of saliva, delivered over two seconds. The next trial proceeded following a three to seven second jitter. Participants completed 104 training trials over four blocks, each six minutes and 44 seconds in length. Stimuli pairs (AB, CD, or EF) were randomly drawn for each trial. Across the training, each pair was presented on one-third of the trials, representing about 35 exposures to each stimuli pair. Training was completed for a fixed number of trials to accommodate timing constraints associated with neuroimaging and to limit the impact of sensory-specific satiety [31] on results. Following the training, participants completed a posttest outside of the scanner. During the posttest, participants were presented novel pairings of the A, B, C, D, E, and F shapes and asked to select the shape that is more likely to be “correct”. The pairings included one shape from the AB set (A: 80% correct, B: 20% correct), as this set is the most reliable predictor of positive/negative outcome. Participants completed 48 posttest trials. Sensitivity to reward was measured by the percent of trials in which that participant selected the A shape, and sensitivity to punishment was measured by the percent of trials in which that participant avoided the B shape. Overall performance was calculated as the combined percent of trials where participants chose the A shape (highest probability of reward) and avoided the B shape (lowest probability of reward) to capture sensitivity to both types of reinforcement.
Figure 1: Appetitive Probabilistic Selection Task (PST).
A. Visual representation of one trial of the appetitive PST. Boxes represent what is presented to participants. On each trial, participants are first presented with a pair of shapes and instructed to select the shape they think is correct. Based on their selection, they receive either a reward (sweet taste) or punishment (bitter taste). After either taste, they receive a rinse of neutral solution, then there is a jitter of 3–7 seconds between trials. B. The reinforcement probabilities associated with each shape pair. Probabilities shown reflect the likelihood a shape is rewarded with a sweet tasie when selected. For example, shape C is rewarded with a sweet taste 70% of times selected, but punished with a bitter taste 30% of times selected. C. Following training in the scanner, participants’ sensitivity to reward and punishment is tested by presenting the A and B shapes against all other shapes to test if participants have learned to choose the A shape (highest reward likelihood), and avoid the B shape (highest punishment likelihood).
2.4. Data Analysis
One participant’s PST posttest data was lost due to a software error, and they were excluded from regression analyses. The final analytic sample included 89 participants. Scoring and statistical analyses were carried out using the R statistical software package (version 3.5.1, R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics including mean and standard deviation were computed for all variables. To test for differences in variables and outcome variables by sex, Welsh’s independent samples t-tests were employed. To evaluate behavioral and physiological variables associated with posttest performance metrics, we assessed three linear regression models. The nine independent variables in each model were BMI, N-back accuracy, dietary restraint, emotional eating, external eating, BIS, BAS, SPQ, SRQ scores. Sex was included in each model as a control variable, resulting in 10 independent variables in each model. Model 1 regressed variables (BMI, N-back accuracy, dietary restraint, emotional eating, external eating, BIS, BAS, SPQ, SRQ scores and sex) onto overall posttest performance (% choose A and avoid B), model 2 examined posttest sensitivity to reward (% choose A) as the outcome, and model 3’s outcome was posttest sensitivity to reward (% avoid B). To examine the goodness of fit for each model, variance inflation factor (VIF; assesses multicollinearity of independent variables), studentized residuals (tests for possible outliers), and Cook’s distance (checks for influential points [32]) were computed. Additionally, we visually inspected the normality of the residuals, and used the Durbin-Watson Test to check for autocorrelated errors [33]. To address multiple comparisons, the Benjamini-Hochberg procedure was applied to identify significant independent variables using the false-discovery rate adjusted p-FDR < 0.05 [34]. Raw p-values are presented in tables.
2.5. Post Hoc Tests for Interactions with Sex
During data analysis, we identified that five of the independent variables (emotional eating, BIS, BAS, SPQ, SRQ) were statistically significantly different between male and female participants. Thus we probed possible integrations between sex and these independent variables in the statistically significant models predicting overall performance and sensitivity to reward. We ran 5 additional models for each outcome to test for statistically significant interactions between sex and each of the 5 independent variables (e.g. emotional eating*sex interaction). Statistical significance for the post hoc models was considered at an uncorrected threshold of p < 0.05. Likelihood ratio tests were applied to test if the expanded interaction models improved the goodness of fit from the reduced model sans interaction.
RESULTS
3.1. Sample Characteristics and Appetitive Probabilistic Selection Task Performance
Participants in the analytic sample (n=89) were on average young adults (21.5 ± 2.4 years) and healthy weight (24.7 ± 3.2 kg/m2). The majority of participants identified as white and nonHispanic (n=80; 89.9%) and just over half of the participants were women (n=45; 50.6%; Table 1). The mean and standard deviation of the nine independent variables (BMI, N-back accuracy, dietary restraint scores, emotional eating scores, external eating scores, BIS, BAS, SPQ, SRQ) are shown in Table 2, including differences between male and female participants. Male and female participants significantly differed in emotional eating, BAS, BIS, SRQ, and SPQ measures (p’s = 0.007 – 0.04). Outcome variables are also shown in Table 2. During the training, participants completed an average of 96.8 ± 9.1 trials, and the AB pair was shown in 34.4% of trials on average. The average overall posttest accuracy (% choose A and avoid B) was close to chance (51.1 ±7.3%) as was the average sensitivity to reward (% choose A) was 50.9 ± 10.6%, and the average sensitivity to punishment (% avoid B) was 51.2 ± 9.6%.
Table 1:
Participant Characteristics (n=89)
| Characteristic | Count (Frequency) |
|---|---|
| Gender | |
| Female | 45 (50.6%) |
| Male | 44 (49.4%) |
| Race | |
| African American/Black | 4 (4.5%) |
| Asian | 20 (22.5%) |
| White | 55 (61.8%) |
| Middle Eastern | 2 (2.2%) |
| More than 1 race | 5 (5.6%) |
| Other | 3 (3.4%) |
| Ethnicity | |
| Hispanic | 9 (10.1%) |
| Non-Hispanic | 80 (89.9%) |
Table 2:
Descriptive statistics of predictors and outcomes and differences by sex (n=89)
| Variable | Female n=45) | Male (n=44) | Sig. Test (T-value, p) |
|---|---|---|---|
| BMI (kg/m2) | 24.5 (3.41) | 24.9 (2.87) | −0.58, 0.56 |
| N-Back Accuracy (%) | 87.3 (10.1) | 90.6 (6.18) | −1.90, 0.062 |
| Emotional Eating | 2.28 (0.615) | 1.94 (0.694) | 2.44, 0.017* |
| Dietary Restraint | 2.36 (0.808) | 2.12 (0.673) | 1.52, 0.14 |
| External Eating | 3.08 (0.643) | 2.98 (0.621) | 0.78, 0.44 |
| BAS | 2.98 (0.365) | 3.15 (0.378) | −2.07, 0.04* |
| BIS | 3.12 (0.375) | 2.80 (0.469) | 3.54, 0.0007** |
| SRQ | 26.4 (6.52) | 30.2 (6.82) | −2.66, 0.009** |
| SPQ | 29.4 (7.06) | 25.5 (7.64) | 2.46, 0.016* |
| Overall Accuracy (%) | 50.3 (6.17) | 51.9 (8.41) | −1.01,0.32 |
| Sensitivity to Reward (%) | 50.4 (9.82) | 51.5 (11.5) | −.049, 0.63 |
| Sensitivity to Punishment (%) | 50.2 (8.68) | 52.3 (10.5) | −1.01,0.32 |
p < 0.05
p < 0.01
Values are mean (SD). Welch’s T-test used to assess significant differences by sex. BMI = Body Mass Index, BIS = behavioral inhibition scale, BAS = behavioral activation scale, SPQ = sensitivity to punishment questionnaire, SRQ = sensitivity to reward questionnaire
3.2. Multivariate Regression Models to Predict PST Posttest Learning Outcomes
Correlation of the predictor variables ranged from r’s = 0.01 – 0.52 (Supplemental Materials). Despite some high correlations between predictor variables, the variance inflation factor (VIF), which measure multicollinearity for each predictor variable, ranged from 1.20 – 1.98, supporting that there was not collinearity among the predictor variables to warrant variable reduction in the regression analyses.
Of the three multivariate linear models, two were statistically significant: the model to predict overall posttest accuracy (F(10, 78) = 2.35; p = 0.017) and the model to predict sensitivity to reward (F(10, 78) = 2.69; p = 0.007; Table 3). After adjusting for multiple comparisons, three variables were statistically significantly associated with overall posttest accuracy: external eating scores (β = −0.035; p = 0.019), BIS scores (β = −0.066; p = 0.004), and SPQ scores (β = 0.003; p = 0.023). Three variables were statistically significantly associated with sensitivity to reward: body mass index (β= −0.009, p = 0.012), emotional eating scores (β = 0.055, p = 0.006), external eating scores (β = −0.062, p = 0.004), and BIS scores (β = −0.068, p = 0.032). None of the independent variables examined were statistically significantly associated with sensitivity to punishment. Metrics of the goodness of fit for each model, and results of the Benjamini-Hochberg adjustment are provided in Supplemental Materials.
Table 3:
Multivariate linear regression analyses predicting learning performance outcomes from behavioral and physiological variables (n=89)
| Model 1: Linear regression to predict overall accuracy (% choose reward and avoid punishment) | |||||
| β | SE | t-value | p | Model fit | |
| (Intercept) | 0.867 | 0.131 | 6.611 | > 0.001 | Multiple R2 = 0.23 |
| 1. BMI | −0.005 | 0.003 | −1.980 | 0.051 | adjusted R2 = 0.13 |
| 2. N-Back Accuracy | −0.060 | 0.097 | −0.618 | 0.538 | F(10, 78) = 2.36 |
| 3. Dietary Restraint | 0.017 | 0.012 | 1.444 | 0.153 | p = 0.017* |
| 4. Emotional Eating | 0.027 | 0.014 | 2.018 | 0.047 | |
| 5. External Eating | −0.035 | 0.015 | −2.399 | 0.019* | |
| 6. BIS | −0.066 | 0.022 | −2.981 | 0.004* | |
| 7. BAS | −0.011 | 0.026 | −0.416 | 0.679 | |
| 8. SPQ | 0.003 | 0.001 | 2.311 | 0.023* | |
| 9. SRQ | −0.001 | 0.001 | −0.936 | 0.352 | |
| 10. Sex (Male = 1) | 0.027 | 0.017 | 1.625 | 0.108 | |
|
Model 2: Linear regression to predict sensitivity to reward (% choose reward) | |||||
| β | SE | t-value | p | Model fit | |
| (Intercept) | 1.018 | 0.186 | 5.461 | > 0.001 | Multiple R2 = 0.26 |
| 1. BMI | −0.009 | 0.004 | −2.584 | 0.012* | adjusted R2 = 0.16 |
| 2. N-Back Accuracy | −0.100 | 0 138 | −0.724 | 0.471 | F(10, 78) = 2.69 |
| 3. Dietary Restraint | 0.032 | 0.017 | 1.918 | 0.059 | p = 0.007** |
| 4. Emotional Eating | 0.055 | 0.019 | 2.852 | 0.006* | |
| 5. External Eating | −0.062 | 0.021 | −2.937 | 0.004* | |
| 6. BIS | −0.068 | 0.031 | −2.184 | 0.032 | |
| 7. BAS | 0.015 | 0.037 | −0.417 | 0.678 | |
| 8. SPQ | 0.003 | 0.002 | 1.497 | 0.138 | |
| 9. SRQ | −0.001 | 0.002 | −0.613 | 0.542 | |
| 10. Sex (Male = 1) | 0.034 | 0.024 | 1.435 | 0.155 | |
|
Model 3: Linear regression to predict sensitivity to punishment (% avoid punishment) | |||||
| β | SE | t-value | p | Model fit | |
| (Intercept) | 0.716 | 0.187 | 3.831 | > 0.001 | Multiple R2 = 0.08 |
| 1. BMI | −0.001 | 0.004 | −0.201 | 0.841 | adjusted R2 = 0.03 |
| 2. N-Back Accuracy | −0.020 | 0.138 | −0.146 | 0.884 | F(10, 78) = 0.71 |
| 3. Dietary Restraint | 0.002 | 0.017 | 0.113 | 0.91 | p = 0.71 |
| 4. Emotional Eating | 0.000 | 0.019 | −0.012 | 0.99 | |
| 5. External Eating | −0.009 | 0.021 | −0.438 | 0.663 | |
| 6. BIS | −0.063 | 0.031 | −2.005 | 0.048 | |
| 7. BAS | −0.006 | 0.037 | −0.168 | 0.867 | |
| 8. SPQ | 0.003 | 0.002 | 1.750 | 0.084 | |
| 9. SRQ | −0.001 | 0.002 | −0.701 | 0.485 | |
| 10. Sex (Male = 1) | 0.020 | 0.024 | 0.849 | 0.399 | |
p-false discovery rate < 0.05, BMI = Body Mass Index, BIS = behavioral inhibition scale, BAS = behavioral activation scale, SPQ = sensitivity to punishment questionnaire, SRQ = sensitivity to reward questionnaire
3.3. Post Hoc Analyses Testing for Interactions by Sex
We found a statistically significant interaction of SPQ and sex on overall accuracy (Table 4, Figure 2). Holding all other variables constant, in female participants, the relationship between SPQ and overall posttest accuracy was positive (β = 0.006, p = 0.002). However, in male participants, the relationship between SPQ and overall posttest accuracy was not significantly different from zero (β = 0.0008, p = 0.63). Using the Likelihood Ratio Test to test the goodness of fit between the original model to predict overall accuracy and tne model with SPQ-sex interaction, we found that the expanded model better fit the data (χ2(1) = 5.82, p = 0.016). No other significant interactions between sex and the other independent variables examined (emotional eating, BIS, BAS, SRQ) were found to predict overall accuracy (p’s = 0.17 – 0.52). No significant interactions between the independent variables and sex were found to predict sensitivity to reward (p’s = 0.23 – 0.88).
Table 4:
Linear regression to predict posttest overall accuracy and test for a significant interaction of sex and SPQ (n=89)
| Model 4: Linear regression to predict posttest overall accuracy with interaction of SPQ and sex | |||||
|---|---|---|---|---|---|
| β | SE | t-value | p | Model fit | |
| (Intercept) | 0.786 | 0.133 | 5.924 | > 0.001 | Multiple R2 = 0.28 |
| 1. BMI | −0.006 | 0.003 | −2.347 | 0.021* | adjusted R2 = 0.18 |
| 2. N-Back Accuracy | −0.077 | 0.095 | −0.815 | 0.417 | F(11,77) = 2.73 |
| 3. Dietary Restraint | 0.012 | 0.012 | 1.011 | 0.315 | p = 0.005** |
| 4. Emotional Eating | 0.033 | 0.013 | 2.435 | 0.017* | Likelihood Ratio Test (Compared to Model 1) = χ2(1) = 5.82, p = 0.016* |
| 5. External Eating | −0.036 | 0.014 | −2.498 | 0.015* | |
| 6. BIS | −0.056 | 0.022 | −2.562 | 0.012* | |
| 7. BAS | −0.005 | 0.025 | −0.181 | 0.857 | |
| 8. SPQ | 0.006 | 0.002 | 3.281 | 0.002** | |
| 9. SRQ | −0.001 | 0.001 | −1.033 | 0.305 | |
| 10. Sex (Male = 1) | 0.164 | 0.062 | 2.641 | C.010* | |
| 11. SPQ * Sex | −0.005 | 0.002 | −2.282 | 0.025* | |
p < 0.05
p < 0.01
BMI = Body Mass Index, BIS = behavioral inhibition scale, BAS = behavioral activation scale, SPQ = sensitivity to punishment questionnaire, SRQ = sensitivity to reward questionnaire
Figure 2: Interaction of Sex and Sensitivity to Punishment Questionnaire (SPQ) scores on Posttest Overall Accuracy (n=89).
Graph shows the correlation of Sensitivity to Punishment Questionnaire (SPQ) scores with posttest overall accuracy (%) in men (r = −0.11; blue) and women (r = 0.41; red). When tested in a multivariate linear regression model with other predictors (Table 4), female participants had a statistically significant, positive association between SPQ and posttest overall accuracy (β = 0.006, p = 0.002, model R2 = 0.28), while in male participants the relationship was not statistically significant (β = 0.0008, p = 0.63).
DISCUSSION
Individual differences in reinforcement learning are an important predictor of behavioral outcomes, such as food choices and eating habits [35], which in turn impact weight status and overall health. Several studies have established that behavioral and physiological factors are associated with individual differences in classical conditioning from food stimuli [23,25,26] and with reinforcement learning via secondary reinforcers [13,14,16,18,20]. However, few studies have examined how variables related to individual differences in how we learn from taste using a response-dependent, instrumental conditioning task. Here, we tested whether ten variables previously associated with reinforcement learning could predict learning outcomes on an appetitive probabilistic selection task. We found that multivariate linear models statistically significantly predicted two of the three learning outcomes assessed; sensitivity to reward and overall accuracy. BMI, external eating, emotional eating, and BIS scores were associated with overall posttest accuracy and sensitivity to reward. In contrast to our hypothesis, none of variables tested were associated with sensitivity to punishment. Further, when we tested for interactions between emotional eating, BIS, BAS, SPQ, SRQ scores and sex, we found a significant interaction between SPQ and sex to predict overall accuracy on the posttest, such that the relationship was positive in women, but not in men. Compared to the original model to predict overall posttest accuracy, the model containing the interaction of SPQ and sex was a better fit to predict overall accuracy. Because no associations were found with sensitivity to food punishment, individual differences in overall accuracy may be more closely related to sensitivity to food reward.
Similar to other instrumental conditioning paradigms [36–38], the PST task involves a training where participants learn to choose a shape associated with food reward and avoid a shape associated with food punishment. Learning performance was represented by accuracy on the appetitive PST posttest, which measures how sensitivity individuals are to food reward and food punishment outcomes, and relates to the broader construct of probabilistic learning. Probabilistic learning, or learning the likelihood of outcomes in the face of uncertainty, is directly relevant to food choices. Food choices are influenced by reward/punishment expectations [39,40], and often are made in the face of uncertainty about whether or not a food will be palatable [41]. Being able to adapt behavior as more information about reinforcement likelihood is learned should, in theory, help people make better informed choices about what to eat. Successful probabilistic learning can help individuals modify their choices when the motivation value of food changes, such as during sensory-specific satiety [31] or dieting [42]. Thus, learning performance on the appetitive PST has direct implications for how individuals make food choices.
Our analysis found multiple associations between learning performance and individual factors. First, external eating was negatively associated with both overall performance and sensitivity to food reward. While prior research shows that external eating is positively associated with performance on classical conditioning tasks [25] and self-reported reward sensitivity [43,44], we observed the opposite effect. Our results are one of the first to examine how external eating affects sensitivity to food reward using an instrumental conditioning task, so differences between instrumental conditioning and classical conditioning paradigms may explain the divergence of results. Alternatively, the negative association between external eating and task performance may be explained by impaired attentional control seen in those with high external eating [45]. The PST task requires working memory and sustained attention [46], so deficits in attentional control in individuals with high external eating could contribute to lower performance on the task. Second, we found that scores on the behavioral inhibition scale, which measures how individuals respond to expected punishment [17], were negatively related to overall posttest accuracy. In prior research, higher behavioral inhibition scores were associated with disordered eating behaviors [47], suggesting that high punishment avoidance is related to alternations in food motivation. The results presented here suggest similar effects, where high behavioral inhibition scores are associated with decreased sensitivity to food reward and punishment. Despite being highly correlated with scores on the behavioral inhibition scale, scores on the sensitivity to punishment questionnaire were positively associated with overall accuracy. Items on the SPQ assess how much individuals try to avoid experienced punishment [19], while the BIS measures how strongly expectations of punishment induce negative emotions or anxiety [17]. While both measures attempt to assess Gray’s Behavioral Inhibition System [48], the differential emphasis on expectations versus experience may explain why the estimated direction of associations with a behavioral measure of punishment avoidance is not the same.
Emotional eating and body mass index were both associated with sensitivity to food reward. Emotional eating assesses susceptibility to negative affect, and similar to other samples, emotional eating and external eating were positively correlated in our sample [49–51]. However, emotional and external eating had opposite associations with sensitivity to reward. Holding external eating constant, emotional eating was positively related to posttest sensitivity to reward. In other research, emotional eating has been associated with self-reported sensitivity to food reward [44]. Our results support that high emotional eating is also related to increased behavioral sensitivity to food reward. Like external eating, BMI was negatively associated with sensitivity to reward. Many studies show that BMI is associated with changes to performance on various reinforcement learning tasks, but the direction of the estimated effect varies [13,52–55]. While BMI is positively associated with increased food reward motivation in children and adults [54,55], here, high BMI was associated with poorer sensitivity to reward. Notably, when the effect of BMI was assessed in a PST with non-appetitive feedback, BMI did not relate to sensitivity to reward [13], which may be related to differences between food reinforcement and visual feedback on the two PST tasks.
Finally, in a post hoc analysis, we observed a significant interaction between sex and SPQ scores on overall posttest accuracy. In women, SPQ scores significantly predicted posttest accuracy, while in men, SPQ was not associated with accuracy. Unlike the original validation studies [19,56], here, female participants had significantly lower SPQ scores than men. We found that self-reported sensitivity to punishment was positively associated with accuracy in women, while in men, the association was not statistically different from zero. Although sex was not an independent predictor of posttest performance in this study, other applications of the PST task find evidence for differences in performance by sex, such that women were more accurate than men in learning from positive feedback and less sensitive when to negative feedback [14]. Our results suggest that women who report being sensitive to punishment are better able to learn from food reward and punishment, and may be more sensitive to value in making decisions about food.
Punishment was not related to any of the tested variables, though it was very weakly correlated with sensitivity to reward (r = 0.06), suggesting that the likelihood of learning from either type of reinforcer was not related. The variables we examined may be more closely related to sensitivity to food reward than sensitivity to food punishment. Participant’s tendency to learn from only food reward or punishment is seen in other applications of the PST, where individuals demonstrate greater sensitivity to positive or negative feedback, but not both [13,14,27,57]. Our results suggest that learning from reward versus learning from punishment are separable constructs, and that the overall effect of learning may be driven by the valence of reinforcement. While these results identify several factors that make individuals more sensitive to food reward, factors that affect sensitivity to food punishment are important to explore in future research. In real-world choices, sensitivity to both positive and negative outcomes both shape decisions [47,58], so examining both outcomes and overall learning is important for understanding overall eating behavior.
This study has a number of limitations that warrant discussion. First, while the sample here is adequately sized [59] and powered for our analyses, a larger sample may produce models with different associations with learning outcomes and potentially more stable results. Second, to our knowledge, this is the first adaptation of the probabilistic selection task to use a primary reinforcer. However, compared to prior studies, accuracy on the appetitive PST posttest was lower [13,27], which may be related to our adaptation of the task. The appetite PST had fewer training trials thank the original task, and the use of taste reinforcers can introduce effects from adaptive processes like sensory-specific satiety or alliesthesia that could affect hedonic motivation during the training [60–62]. This study did not collect hedonic ratings of the task stimuli following completion of the task training, so we are not able to test for these effects. Third, since sensitivity to punishment was not associated with the variables examined here, it is likely that some unmeasured construct would better explain posttest sensitivity to punishment. An unknown variable may better explain the other learning outcomes assessed as well. Despite this limitation, the models to predict sensitivity to reward and overall accuracy explained 26% and 28% of the variance in performance, respectively, suggesting the variables examined here are useful for understanding individual differences in reinforcement learning. Finally, while this study provides novel information about the combination of variables that relate to food conditioning outcomes, in order to generalize these results, it is critical to test our findings in an independent sample. Future studies are needed to address these constraints. It is plausible that associations may change if a different appetitive instrumental conditioning task is used to assess food-motivated reinforcement learning. A task of particular interest is appetitive Pavlovian-to-Instrumental Transfer (PIT). This task measures the ability of a conditioned cue to modulate previously a conditioned response and measures cue-driven changes in food choices [63]. Individual differences in Pavlovian transfer are associated with cue-reactivity [64], and some studies support that transfer may be increased in individuals with obesity [65], however others show null effects [66]. Exploration of how measures like sex, the BIS/BAS scales, SPQ, and emotional and external eating relate to appetitive PIT would provide unique information, and build on the results presented here.
Conclusions
Using an adapted probabilistic selection task, we examined associations between a range of physiological, psychological and behavioral characteristics and instrumental conditioning via sweet and bitter tastes. We found that learning performance outcomes, specifically overall performance and sensitivity to reward, were associated with individual differences in emotional and external eating behavior, BMI, and trait sensitivity to punishment. Further, we found a statistically significant interaction between sex and self-reported sensitivity to punishment in association with overall posttest performance. Together, our results support that food reinforcement learning may relate to a combination of behavioral and physiological measures, and that in future studies of individual differences in how we learn from taste, it is critical to assess the relationship of multiple constructs in combination to outcomes.
Supplementary Material
HIGHLIGHTS.
We tested individual differences in outcome learning via sweet and bitter tastes
Higher external eating related to poorer learning from sweet and bitter tastes
Higher BMI was associated with lower ability to learn from sweet taste
Sensitivity to punishment related to better ability to learn from taste in women
ACKNOWLEDGEMENTS
The authors would like to thank the participants who provided data for this analysis. Also, the authors would like to thank the Neuropsychology of Ingestive Behavior lab, specifically Katie Gandee, Lia Bauert, Peter Dihn, Ryesa Mansoor, Megan Neff, and Brian Brown for assistance in data collection and study administration. The authors would also like to thank Dianne S. Ward, Jessica R. Cohen, and Dana M. Small for their input on the broader study associated with this manuscript.
FUNDING
This research is supported by The National Institutes of Health (R01DK112317) awarded to KSB and the American Psychological Foundation (Visionary Grant, 2018) awarded to JRS.
Footnotes
DISCLOSURES
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Egger G, Dixon J, Beyond obesity and lifestyle: a review of 21 st century chronic disease determinants., Biomed Res. Int. 2014 (2014) 731685. doi: 10.1155/2014/731685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Serra-Majem L, Bautista-Castaño I, Etiology of obesity: two “key issues” and other emerging factors., Nutr. Hosp. 28 Suppl 5 (2013) 32–43. doi: 10.3305/nh.2013.28.sup5.6916. [DOI] [PubMed] [Google Scholar]
- [3].Davidson TL, Sample CH, Swithers SE, An application of Pavlovian principles to the problems of obesity and cognitive decline., Neurobiol. Learn. Mem. 108 (2014) 172–184. doi: 10.1016/j.nlm.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Johnson AW, Eating beyond metabolic need: how environmental cues influence feeding behavior., Trends Neurosci. 36 (2013) 101–109. doi: 10.1016/j.tins.2013.01.002. [DOI] [PubMed] [Google Scholar]
- [5].Pavlov PI, Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex., Ann. Neurosci. 17 (1927) 136–141. doi: 10.5214/ans.0972-7531.1017309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bouton ME, Learning and behavior: A contemporary synthesis, Sinauer Associates, 2007. [Google Scholar]
- [7].van den Akker K, Schyns G, Jansen A, Learned overeating: applying principles of pavlovian conditioning to explain and treat overeatino, Curr Addict. Rep. 5 (2018) 223–231.. doi: 10.1007/s40429-018-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sobik L, Hutchison K, Craighead L, Cue-elicited craving for food: a fresh approach to the study of binge eating., Appetite. 44 (2005) 253–261. doi: 10.1016/j.appet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- [9].Burger KS, Stice E, Greater striatopallidal adaptive coding during cue-reward learning and food reward habituation predict future weight gain., Neuroimage. 99 (2014) 122–128. doi: 10.1016/j.neuroimage.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rangel A, Camerer C, Montague PR, A framework for studying the neurobiology of value-based decision making., Nat. Rev Neurosci. 9 (2008) 545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Revusky S, Learning as a General Process with an Emphasis on Data from Feeding Experiments, in: Milgram NW. Krames L, Alloway TM (Eds.), Food Aversion Learning, Springer US, Boston, Mfi, 1977. pp. 1–71. doi: 10.1007/978-1-4757-1299-5_1. [DOI] [Google Scholar]
- [12].Yin HH, Knowlton BJ, Addiction and learning in the brain, in: Handbook of Implicit Cognition and Addiction, SAGE Publications, Inc., 2455 Teller Road, Thousand Oaks California 91320 United States, 2006: pp. 167–184. doi: 10.4135/9781412976237.n12. [DOI] [Google Scholar]
- [13].Coppin G, Nolan-Poupart S, Jones-Gotman M, Small DM, Working memory and reward association learning impairments in obesity., Neuropsychologia. 65 (2014) 146–155. doi: 10.1016/j.neuropsychologia.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Evans KL, Hampson E, Sex-dependent effects on tasks assessing reinforcement learning and interference inhibition., Front. Psychol. 6 (2015) 1044. doi: 10.3389/fpsyg.2015.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cowan N, Working Memory Underpins Cognitive Development, Learning, and Education., Educ Psychol Rev. 26 (2014) 197–223. doi: 10.1007/s10648-013-9246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Collins AGE, Albrecht MA, Waltz JA, Gold JM, Frank MJ, Interactions Among Working Memory, Reinforcement Learning, and Effort in Value-Based Choice: A New Paradigm and Selective Deficits in Schizophrenia., Biol. Psychiatry. 82 (2017) 431–439. doi: 10.1016/j.biopsych.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Carver CS, White TL, Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales., J. Pers. Soc. Psychol. 67 (1994) 319–333. doi: 10.1037/0022-3514.67.2.319. [DOI] [Google Scholar]
- [18].Kim SH, Yoon H, Kim H, Hamann S, Individual differences in sensitivity to reward and punishment and neural activity during reward and avoidance learning., Soc. Cogn. Affect. Neurosci. 10 (2015) 1219–1227. doi: 10.1093/scan/nsv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Torrubia R, Ávila C, Moltó J, Caseras X, The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions, Pers. Individ. Dif. 31 (2001) 837–862. doi: 10.1016/S0191-8869(00)00183-5. [DOI] [Google Scholar]
- [20].Aberg KC, Doell KC, Schwartz S, Linking Individual Learning Styles to ApproachAvoidance Motivational Traits and Computational Aspects of Reinforcement Learning., PLoSOne. 11 (2016) e0166675. doi: 10.1371/journal.pone.0166675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Beck SM, Locke HS, Savine AC, Jimura K, Braver TS, Primary and secondary rewards differentially modulate neural activity dynamics during working memory., PLoS One. 5 (2010) e9251. doi: 10.1371/journal.pone.0009251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jensen J, Walter H, Incentive motivational salience and the human brain., Restor Neurol Neurosci. 32 (2014) 141–147. doi: 10.3233/RNN-139006. [DOI] [PubMed] [Google Scholar]
- [23].Meyer MD, Risbrough VB, Liang J, Boutelle KN, Pavlovian conditioning to hedonic food cues in overweight and lean individuals., Appetite. 87 (2015) 56–61. doi: 10.1016/j.appet.2014.12.002. [DOI] [PubMed] [Google Scholar]
- [24].van Strien T, Frijters JER, Bergers GPA, Defares PB, The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior, Int. J. Eat. Disord. 5 (1986) 295–315. doi:. [DOI] [Google Scholar]
- [25].Brignell C, Griffiths T, Bradley BP, Mogg K, Attentional and approach biases for pictorial food cues. Influence of external eatino, Appetite. 52 (2009) 299–306. doi: 10.1016/j.appet.2008.10.007. [DOI] [PubMed] [Google Scholar]
- [26].Brunstrom JM, Downes CR, Higgs S, Effects of dietary restraint on flavour-flavour learning., Appetite. 37 (2001) 197–206. doi: 10.1006/appe.2001.0432. [DOI] [PubMed] [Google Scholar]
- [27].Frank MJ, Seeberger LC, O’reilly RC, By carrot or by stick: cognitive reinforcement learning in parkinsonism., Science. 306 (2004) 1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- [28].Kirchner WK, Age differences in short-term retention of rapidly changing information., J Exp Psychol. 55 (1958) 352–358. doi: 10.1037/h0043688. [DOI] [PubMed] [Google Scholar]
- [29].Charalambous G, Chemistry of foods and beverages: recent developments, Elsevier, 2012. [Google Scholar]
- [30].Sadler JR, Shearrer GE, Acosta NT, Papantonia A, Cohen JR, Small DM, et al. , Network organization during probabilistic learning via taste outcomes, NutriXiv. (2020). https://osf.io/preprints/nutrixiv/3g7h2 (accessed March 23, 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rolls BJ, Rolls ET, Rowe EA, Sweeney K, Sensory specific satiety in man., Physiol. Behav. 27 (1981) 137–142. doi: 10.1016/0031-9384(81)90310-3. [DOI] [PubMed] [Google Scholar]
- [32].Cook RD, Detection of Influential Observation in Linear Regression, Technometrics. 19 (1977) 15. doi: 10.2307/1268249. [DOI] [Google Scholar]
- [33].Durbin J, Watson GS, Testing for serial correlation in least squares regression. II., Biometrika. 38 (1951) 159–178. doi: 10.2307/2332325. [DOI] [PubMed] [Google Scholar]
- [34].Benjamini Y, Hochberg Y, Controlling the false discovery rate: A practical and powerful approach to multiple testing, Journal of the Royal Statistical Society: Series B (Methodological). 57 (1995) 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- [35].Reiss S, Havercamp S, The sensitivity theory of motivation: implications for psychopathology., Behav. Res. Ther. 34 (1996) 621–632. doi: 10.1016/00057967(96)00041-1. [DOI] [PubMed] [Google Scholar]
- [36].O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ, Neural responses during anticipation of a primary taste reward., Neuron. 33 (2002) 815–826. doi: 10.1016/s08966273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- [37].Abraham A, Hermann C, Biases in probabilistic category learning in relation to social anxiety., Front. Psychol. 6 (2015) 1218. doi: 10.3389/fpsyg.2015.01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Holmes NM, Marchand AR, Coutureau E, Pavlovian to instrumental transfer: a neurobehavioural perspective., Neurosci. Biobehav. Rev. 34 (2010) 1277–1295. doi: 10.1016/j.neubiorev.2010.03.007. [DOI] [PubMed] [Google Scholar]
- [39].Hennegan JM, Loxton NJ, Mattar A, Great expectations. Eating expectancies as mediators of reinforcement sensitivity and eating., Appetite. 71 (2013) 81–88. doi: 10.1016/j.appet.2013.07.013. [DOI] [PubMed] [Google Scholar]
- [40].Cardello AV, Consumer expectations and their role in food acceptance, in: MacFie HJH, Thomson DMH (Eds.), Measurement of Food Preferences, Springer US, Boston, MA, 1994: pp. 253–297. doi: 10.1007/978-1-4615-2171-6_10. [DOI] [Google Scholar]
- [41].Anselme P, Güntürkün O, How foraging works: uncertainty magnifies food-seeking motivation., Behav. Brain Sci. (2018) 1–106. doi: 10.1017/S0140525X18000948. [DOI] [PubMed] [Google Scholar]
- [42].Appelhans BM, Neurobehavioral inhibition of reward-driven feeding: implications for dieting and obesity., Obesity (Silver Spring). 17 (2009) 640–647. doi: 10.1038/oby.2008.638. [DOI] [PubMed] [Google Scholar]
- [43].Vandeweghe L, Vervoort L, Verbeken S, Moens E, Braet C, Food Approach and Food Avoidance in Young Children: Relation with Reward Sensitivity and Punishment Sensitivity., Front. Psychol. 7 (2016) 928. doi: 10.3389/fpsyg.2016.00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Loxton NJ, Tipman RJ, Reward sensitivity and food addiction in women., Appetite. 115 (2017) 28–35. doi: 10.1016/j.appet.2016.10.022. [DOI] [PubMed] [Google Scholar]
- [45].Momoi K, Ohara K, Okita Y, Mase T, Miyawaki C, Fujitani T, et al. , Relationship among Eating Behavior, Effortful Control, and Working Memory in Female Young Adults, Health (Irvine, Calif.). 08 (2016) 1187–1194. doi: 10.4236/health.2016.812122. [DOI] [Google Scholar]
- [46].Vartak D, Jeurissen D, Self MW, Roelfsema PR, The influence of attention and reward on the learning of stimulus-response associations., Sci. Rep. 7 (2017) 9036. doi: 10.1038/S41598-017-08200-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Matton A, Goossens L, Braet C, Vervaet M, Punishment and reward sensitivity: are naturally occurring clusters in these traits related to eating and weight problems in adolescents?, Eur Eat Disord Rev. 21 (2013) 184–194. doi: 10.1002/erv.2226. [DOI] [PubMed] [Google Scholar]
- [48].Gray JA, The neuropsychology of emotion and personality, (1987). [Google Scholar]
- [49].Jansen A, Nederkoorn C, Roefs A, Bongers P, Teugels T, Havermans R, The proof of the pudding is in the eating: is the DEBQ-external eating scale a valid measure of external eating?, Int. J. Eat. Disord. 44 (2011) 164–168. doi: 10.1002/eat.20799. [DOI] [PubMed] [Google Scholar]
- [50].Ouwens MA, van Strien T, van Leeuwe JFJ, Possible pathways between depression, emotional and external eating. A structural equation model., Appetite. 53 (2009) 245–248. doi: 10.1016/j.appet.2009.06.001. [DOI] [PubMed] [Google Scholar]
- [51].Van Strien T, Schippers GM, Cox WM, On the relationship between emotional and external eating behavior., Addict. Behav. 20 (1995) 585–594. doi: 10.1016/03064603(95)00018-8. [DOI] [PubMed] [Google Scholar]
- [52].Kube J, Mathar D, Horstmann A, Kotz SA, Villringer A, Neumann J, Altered monetary loss processing and reinforcement-based learning in individuals with obesity., Brain Imaging Behav. 12 (2018) 1431–1449. doi: 10.1007/s11682-017-9786-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Clark EN, Dewey AM, Temple JL, Effects of daily snack food intake on food reinforcement depend on body mass index and energy density., Am. J. Clin. Nutr. 91 (2010) 300–308. doi: 10.3945/ajcn.2009.28632. [DOI] [PubMed] [Google Scholar]
- [54].Epstein LH, Carr KA, Lin H, Fletcher KD, Roemmich JN, Usual energy intake mediates the relationship between food reinforcement and BMI., Obesity (Silver Spring). 20 (2012) 1815–1819. doi: 10.1038/oby.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rollins BY, Loken E, Savage JS, Birch LL, Measurement of food reinforcement in preschool children. Associations with food intake, BMI, and reward sensitivity., Appetite. 72 (2014) 21–27. doi: 10.1016/j.appet.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Caseras X, Avila C, Torrubia R, The measurement of individual differences in behavioural inhibition and behavioural activation systems: a comparison of personality scales, Personality and Individual Differences. 34 (2003) 999–1013. [Google Scholar]
- [57].Simon JR, Howard JH, Howard DV, Adult age differences in learning from positive and negative probabilistic feedback., Neuropsychology. 24 (2010) 534–541. doi: 10.1037/a0018652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Estes WK, Reinforcement in human behavior: reward and punishment influence human actions via informational and cybernetic processes., Am. Sci. 60 (1972) 723–729. [PubMed] [Google Scholar]
- [59].Austin PC, Steyerberg EW, The number of subjects per variable required in linear regression analyses., J. Clin. Epidemiol. 68 (2015) 627–636. doi: 10.1016/j.jclinepi.2014.12.014. [DOI] [PubMed] [Google Scholar]
- [60].Havermans RC, Janssen T, Giesen JCAH, Roefs A, Jansen A, Food liking, food wanting, and sensory-specific satiety., Appetite. 52 (2009) 222–225. doi: 10.1016/j.appet.2008.09.020. [DOI] [PubMed] [Google Scholar]
- [61].Sclafani A, Ackroff K, The relationship between food reward and satiation revisited., Physiol. Behav. 82 (2004) 89–95. doi: 10.1016/j.physbeh.2004.04.045. [DOI] [PubMed] [Google Scholar]
- [62].Cabanac M, Physiological role of pleasure., Science. 173 (1971) 1103–1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- [63].Talmi D, Seymour B, Dayan P, Dolan RJ, Human pavlovian-instrumental transfer., J. Neurosci. 28 (2008) 360–368. doi: 10.1523/JNEUROSCI.4028-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Garofalo S, di Pellegrino G, Individual differences in the influence of task-irrelevant Pavlovian cues on human behavior., Front. Behav. Neurosci. 9 (2015) 163. doi: 10.3389/fnbeh.2015.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lehner R, Balsters JH, Burgler A, Hare TA, Wenderoth N, Food-Predicting Stimuli Differentially Influence Eye Movements and Goal-Directed Behavior in Normal-Weight, Overweight, and Obese Individuals., Front Psychiatry. 8 (2017) 230. doi: 10.3389/fpsyt.2017.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Meemken M-T, Horstmann A, Appetitive Pavlovian-to-lnstrumental Transfer in Participants with Normal-Weight and Obesity., Nutrients. 11 (2019). doi: 10.3390/nu11051037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.