Abstract
Background
Adenovirus type 5 (Ad5) is widely used as a vehicle for vaccine delivery in the treatment of infectious disease and cancer. However, the efficacy of Ad5 vectors has been limited in humans because exposure to Ad5 infections results in most adults having neutralizing antibodies against Ad5. To overcome this limitation, the hexon epitope present in the fifth hypervariable region of Ad5 was modified.
Methods
To evaluate the ability of Ad5 vectors encoding the HIV env protein to induce Ag-specific immune responses in the face of pre-existing anti-Ad5 immunity, mice were administrated intramuscularly with the Ad-Luc vector, and then vaccinated with parental or hexon-modified Ad5 vectors (Ad-HisHIV, Ad-END/AAAHIV or Ad-HIV) at week 8. HIV-specific cell-mediated immune responses were detected through a combination of tetramer assays and intracellular cytokine staining from weeks 8–23.
Results
The hexon-modified Ad vector was able to escape from anti-Ad5 neutralizing antibody, and mice with the modified vector generated significantly lower individual neutralizing antibody than those immunized with the parental vector. Furthermore, mice with pre-existing anti-Ad immunity immunized with the modified vector generated significantly stronger cell-mediated anti-env responses than those immunized with the parental vector.
Conclusions
These data demonstrate that Ad5 vector with hexon modification reduce their sensitivity to pre-existing anti-Ad immunity and improve their clinical utility.
Keywords: HIV, neutralizing antibody, vaccine, virus vector
Introduction
HIV infection continues to increase worldwide, emphasizing the importance of developing an effective vaccine against this retrovirus. Many strategies are being pursued to achieve this goal, including the production of HIV subunit peptide vaccines [1], DNA vaccines [2], recombinant virus-vector vaccines (vaccinia virus [3], adenovirus (Ad) [4,5], rabies virus [6], flavivirus [7], Sendai virus [8], Venezuelan equine encephalitis virus [9] and adeno-associated virus [10–12]), and bacterial vector vaccines [13–15]. Many of these strategies show promising results in animal models.
For example, studies indicate that Ad5-based vaccines can be very effective immunogens, since many cells express the Coxsackievirus and Ad receptors recognized as targets by these vectors [16]. In addition, the efficacy and safety of Ad5 based vaccines have been established in clinical trials involving patients with HIV [5,17] and cancer [18,19]. Despite their promise, several issues must be resolved to enable the widespread use of Ad5-based vaccines. In particular, pre-existing immunity against Ad5 is present in 50–90% of normal human adults [20], and these neutralizing antibodies reduce vaccine efficacy. Only by studying animals in which immunity against Ad5 has been induced can the effect of such a response be adequately modelled.
The Ad5 mainly contains three structural proteins, hexon, penton and fiber. The hexon protein is the most abundant of these, with each Ad5 containing 240 copies of the trimeric hexon molecule (accounting for 63% of the total protein mass [21]). Antibodies against the hexon protein dominate the neutralizing response elicited when humans are infected by Ad [22–26]. The hexon protein is composed of seven short hypervariable regions (HVRs), each of which can elicit a serotype-specific immune response [27,28]. Efforts to alter the hexon protein of Ad5 vectors (i.e. to circumvent the problems raised by pre-existing immunity) have been complicated because hexon proteins interact extensively with other capsid proteins. These interactions are critical to hexon and capsid stability, thereby reducing the type and nature of mutational changes that can be introduced. For example, chimeric viruses in which the entire Ad5 hexon was replaced with those from Ad1, Ad2, Ad6 or Ad12 [22,29] yielded vectors that were structurally unstable, thereby decreasing viral yield [30].
The present study examined the impact of modifying the major neutralizing epitope present in HVR5 on the immunogenicity of an Ad5 vector expressing the HIV env gene in a relevant murine model of pre-existing anti-Ad immunity.
Materials and methods
Recombinant vectors
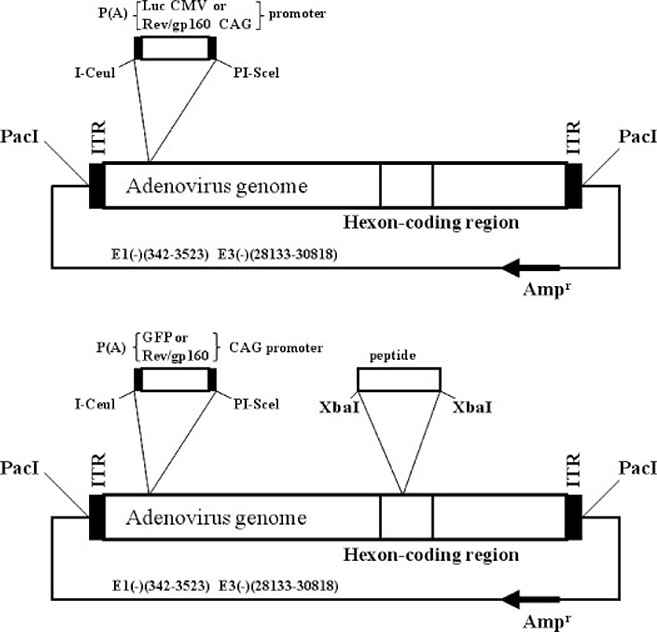
The pAdHM4 plasmid which contains the full-length Ad5 genome with an E1/E3 region deletion (Figure 1, upper panel) was used to construct the control replication-deficient Ad5 vectors [31]. A 5.2-kbp fragment containing a CAG promoter-HIVIIIBrev/gp160-poly(A) [32] or cytomegalovirus (CMV) promoter-luciferase gene-poly(A) was subcloned into the E1 region of the pAdHM4 plasmid at the I-CeuI/PI-SceI site to produce an HIV env gene-expressing Ad5 (Ad-HIV) or luciferase-expressing Ad5 (Ad-Luc), respectively. To construct the HVR5-modified Ad5 vector, the pAdHM62 plasmid (Figure 1, bottom panel) containing the full-length Ad5 genome with E1/E3 and hexon HVR5 deletions was used [33]. pAdHM62 con-tains a unique XbaI site in the hexon HVR5-coding region and I-CeuI/PI-SceI sites in the E1 deletion region. Oligonucleotides and an XbaI linker were used to synthesize the following fragments: HIV-V3 (RGPGRAFVTI), HIV-V3s (LGSRGPGRAFVTILGS), histidine acid residues (SSLGSHHHHHHLGSPR) and END/AAA (TTAATAGAGANLAP). The fragments were inserted into the XbaI site of the hexon region, and the CAG promoter-HIVIIIB rev/gp160-poly(A) cassette, CMV promotoer-luciferase-poly(A) or CAG promoter-green fluorescent protein (GFP)-poly(A) cassette was inserted into the I-CeuI/PI-SceI sites of pAdHM62 to generate the corresponding Ad-producing plasmids. These plasmids were transfected into low-passage human embryonic kidney (HEK293) cells (Micro-bix Biosystems, Inc., Toronto, Ontario, Canada) by using FuGENE 6 Transfection Reagent (Roche Diagnostics, Indianapolis, IN, USA). The recombinant viruses thus produced were propagated in HEK293 cells and purified twice over a CsCl gradient as previously described [34]. The viral titer was measured on the basis of the optical density at 260 nm (OD260) using formula: 1 OD260 = 1012 virus particles (vp)/ml [35]. In addition, the number of viral plaque-forming units (pfu) was determined using tissue culture infectious doses (TCID50).
Figure 1.
Schematic representation of Ad vectors. The plasmid pAdHM4 contains the full-length of the Ad5 genome with an E1/E3 deletion. Plasmid pAdHM62 was derived from plasmid pAdHM4 and the HVR5 of hexon was replaced with a unit restriction enzyme site, XbaI
Western blotting
The human embryonic kidney cell line (HEK293) and human lung carcinoma cell line (A549) were used for western blotting. Replication-deficient Ad vectors were able to replicate in HEK293 but not A549 cells. The HEK293 and A549 cells were infected with Ads at 0.01 and 5 pfu/cell, respectively, for 24 h. The cells were washed in phosphate-buffered saline (PBS) and lysed with 0.1 M Tris-HCl (pH 7.8) and 0.125%Nonidet P-40. The cell lysates were mixed with an equal volume of 2 × sodium dodecyl sulfate (SDS) buffer (125 mM Tris-HCl at pH 6.8, 4% SDS, 20% glycerol, 0.01% bromophenol blue, and 10% β-mercaptoethanol) and boiled for 10 min. The cell lysates were then loaded on an 8% polyacrylamide gel and transferred to a Hybond ECL nitrocellulose membrane (Amersham Pharmacia Biotech, Bucks, UK). The HIV gp160, Ad hexon and cellular β-actin proteins were detected using a mouse anti-HIV gp120 monoclonal antibody (mAb)(Hybridoma 902; AIDS Research and Reference Reagent Program, National Institute of Health, Bethesda, MD, USA), polyclonal mouse anti-Ad5, and rabbit anti-human β-actin mAb (Sigma, St Louis, MO, USA), respectively. Affinity-purified horseradish peroxidase (HRP)-labelled anti-mouse immunoglobulin (Ig)G (ICN Pharmaceuticals, Inc., OH, USA) or HRP-labelled anti-rabbit IgG (ICN Pharmaceuticals, Inc.) were used as the secondary antibody. The protein intensity was detected using the ECL Plus Western Blotting Detection System (Amersham Pharmacia Biotech).
Analysis of Ad vector genome with restriction
HEK293 cells at 90% confluency in a 24-well plate were infected with Ad vectors at 0.001 pfu per cell for 48 h. The cells were harvested and washed twice with PBS, then treated with 40 μg of proteinase K (Qiagen GmbH, Hilden, Germany), 50 mM of Tris-HCl (pH 7.5), 10 mM of EDTA (pH 7.5), and 100 mM of NaCl for 1.5 h at 50 °C, followed by phenol/chloroform extraction. Total sample was suspended in TE containing 20 μg/ml of RNase and digested with XhoI. The sample was extracted with phenol/chloroform again followed by 0.7% agarose gel electrophoresis.
Virus stability
Ad viral vectors were passaged in HEK293 cells for 15 generations (0.01 pfu per cell). Two days after infection, the HEK293 cells from each generation were washed in PBS and lysed with 0.1 M Tris-HCl (pH 7.8) and 0.125% Nonidet P-40, followed by phenol/chloroform extraction. The HIV gp160 region was amplified from DNA using the primer pair: sense 5′-GTGGAGGGGAATTTTTC-3′, antisense 5′-ATAGTGCTTCCTGCTGCT-3′ to obtain a 700-bp fragment. The polymerase chain reaction (PCR) product was analysed by 1% agarose gel.
Animal immunization
Female BALB/c mice (aged 6–8 weeks; H-2Dd) were purchased from Japan SLC, Inc. (Shizuoka, Shizuoka-ken, Japan). The mice were injected intramuscularly (i.m.) with 5 × 108 pfu of Ad-Luc to induce anti-Ad neutralizing antibodies. The mice were immunized i.m. with 108 pfu of Ad-HIV, Ad-HisHIV or Ad-END/AAAHIV at week 8 after the initial Ad-Luc injection. The study was approved by the Animal Administer Community of Yokohama City University.
Enzyme-linked immunosorbent assay
The serum anti-Ad5 or HIV env antibody titer were determined by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates (Nunc, Roskilde, Denmark) were coated with 100 μl of 1 μg/ml heat-inactivated wild-type Ad5 virus protein or 10 μg/ml HIV env V3 region epitope peptide (NNTRKRIRQRGPGRAFVTIGKIGN) overnight at 4 °C. The wells were then blocked for 2 h at 37 °C with PBS containing 1% bovine serum albumin and 0.05% Tween-20. After washing three times with 0.05% Tween-20-PBS, diluted mouse serum was applied, and the plates were incubated for 2 h at 37 °C. The plate was washed three times with PBS containing 0.05% Tween-20 and incu-bated with a 1 : 2000 dilution of peroxidase-conjugated rabbit anti-mouse antibody for 2 h at 37 °C. The anti-bodies were washed again three times and Ad5 or HIV env-specific antibodies were detected by adding a sub-strate solution (OPT tablet in 0.1 M citric acid, pH 5.6; 1 μl/ml 30% H2O2). The substrate reaction was termi-nated by adding 1 M H2SO4, and The absorbance was determined at 450 nm by using microplate reader (Bio-Rad, Hercules, CA, USA).
Neutralization assay
Neutralization assay was performed using a luciferase-based assay [36]. In brief, 5 × 105 pfu of Ad-Luc virus, Ad-HisLuc virus or Ad-END/AAALuc virus was incubated with an equal volume of series diluted serum from the mice administered with 5 × 108 pfu of Ad-Luc, Ad-HisLuc or Ad-END/AAA post 8 weeks or PBS at 37 °C for 2 h. Subsequently, the mixture was incubated with 104 A549 human lung carcinoma cells/well (50 pfu per cell) in a 96-well plate at 37 °C for 24 h. The luciferase activity of the cells was measured with a Bright-Glo Luciferase Reagent System (Promega, Madison, WI, USA). Percentage of neutralization was calculated using the formula: (luciferase activity of PBS incubated with Ad-Luc – luciferase activity of immune sera incubated with Ad-Luc)/luciferase activity of PBS incubated with Ad-Luc × 100. Neutralizing activity by each vectors were compared at the serum dilution fold with the greatest significant difference (320-fold serum dilution).
Tetramer assay
The H-2Dd/p 18 tetramer (RGPGRAFVTI) was synthesized by the NIH Tetramer Core Facility (Atlanta, GA, USA) and labelled with phycoerythrin (PE) [11,37,38]. In brief, 100 μl of heparin-preserved whole mouse blood was stained with 0.5 μg fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD8 antibody and 0.05 μg of tetramer reagent at room temperature for 30 min. The cells were fixed with 100 μl of OptiLyse B-Lysing solution (Beckman Coulter, Marseille Cedex, France) for 10 min. Erythrocytes were lysed by adding 1 ml of H2O for 10 min at room temperature, and then washing twice with PBS. The samples were analysed by fluorescence-activated cell sorting.
Intercellular cytokine staining assay
Interferon (IFN)-γ secreting CD8+ T cells were detected as recommended (Cytofix/CytoPerm Plus kit; Pharmingen, San Diego, CA, USA). In brief, erythrocytes in 200 μl of heparin-preserved whole mouse blood were lysed with BD Pharm Lyse Lysing buffer (BD Biosciences, San Diego, CA, USA). The cells were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS) and 10 μg/ml of HIV V3 peptide (RGPGRAFVTI) for 24 h at 37 °C. Two hours before the end of incubation, 1 μg/ml of GolgiPlug was added. The cells were washed with staining buffer (3% FCS and 0.1% sodium azide in PBS), stained with PE-conjugated anti-mouse CD8 antibody at 4 °C for 30 min, followed by fixing in Cytofix/Cytoperm solution at 4 °C for 20 min and finally washed with Perm/Wash solution. Next, the cells were stained with FITC-conjugated anti-mouse IFN-γ antibody at 4 °C for 30 min, and then subjected to flow cytometric analysis. PBS injected mouse peripheral blood mononuclear cells were used as a control.
Challenge study with recombinant vaccinia virus
Virus challenge experiments were performed as previously described [32]. Vaccinated mice were challenged intraperitoneally with 108 pfu of the recombinant vaccinia virus vPE16 (provided by the AIDS Research and Reference Reagent Program; NIH, Rockville, MD, USA) expressing HIV-1 gp160 and β-galactosidase [32]. Three days after challenge, the mice were killed, and their total ovaries were sonicated. The amount of vPE16 virus in the ovaries was determined by incubating ten-fold serial dilutions in 96-well plates containing CV1 cells. Infected cells were detected by staining with crystal violet and plaques were counted at each dilution. As an alternative method, β-galactosidase expression levels were monitored using the Beta-Glo Assay System (Promega). We calculated vPE16 titer (pfu) and β-galactosidase activity in the total ovary.
Results
Viability and stability of hexon-modified Ad vectors
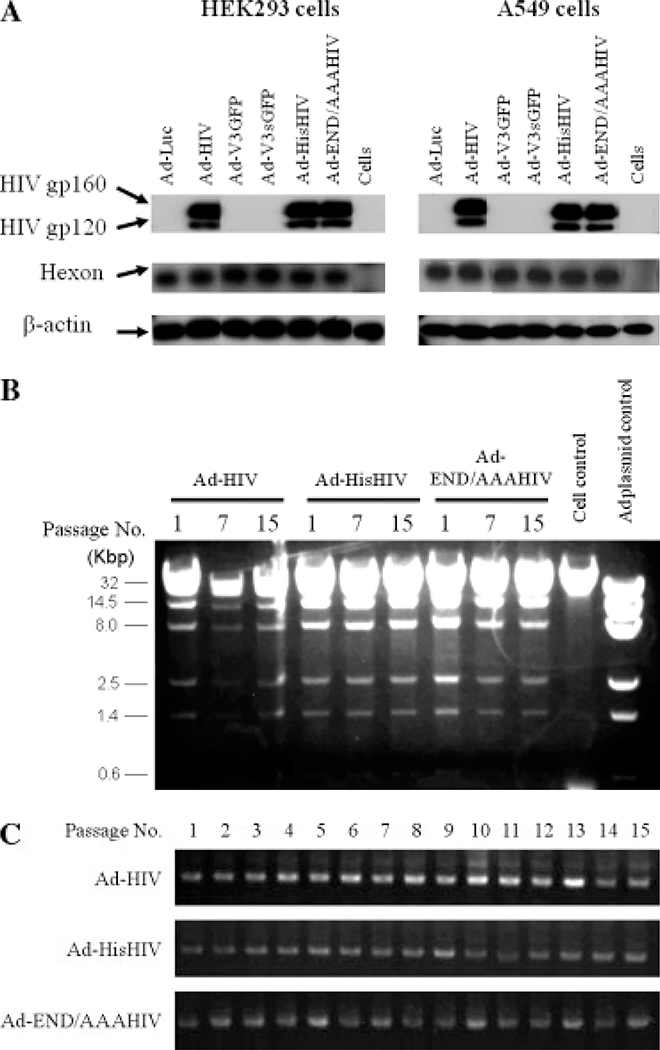
Several hexon HVR5 region modified Ad vectors were prepared that expressed either the env gene of HIV or a reporter gene in the E1 region (Figure 1 and Table 1). The infectivity, protein expression, and replication of these Vectors were analysed in vitro.HEK293 and A549 cell lines were infected with either the parental or hexon-modified Ad vectors. As shown in Figure 2A, similar amounts of hexon protein and HIV gp160 were produced after infection with parental (Ad-HIV) and hexon-modified Ad vectors, indicating that the infection and replication abilities of both vector preparations was similar.
Table 1.
Recombinant Ad vectors used in the present study
| Origin vector | Vector name | Insert in hexon | Sequence in hexon HVR5 region | Gene in E1 region | vp produced per cell | vp/pfu ratio |
|---|---|---|---|---|---|---|
| pAdHM4 | Ad-Luc | Naive | 269TTEATAGNGDNLTP282 | Luciferase | 5000 | 125 |
| pAdHM4 | Ad-HIV | Naive | 269TTEATAGNGDNLTP282 | HIV rev/gp160 | 6500 | 141 |
| pAdHM62 | Ad-V3GFP | HIV-V3 loop epitope | RGPGRAFVTI | GFP | 4850 | 234 |
| pAdHM62 | Ad-V3sGFP | HIV-V3 loop epitope with spacer | LGSRGPGRAFVTILGS | GFP | 4400 | 112 |
| pAdHM62 | Ad-HisHIV | His | LGSHHHHHHLGS | HIV rev/gp160 | 5500 | 117 |
| pAdHM62 | Ad-HisLuc | His | LGSHHHHHHLGS | Luciferase | 6000 | 126 |
| pAdHM62 | Ad-END/AAA HIV | With three amino acid mutants | 269TTAATAGAGANLTP282 | HIV rev/gp160 | 6000 | 110 |
| pAdHM62 | Ad-END/AAALuc | With three amino acid mutants | 269TTAATAGAGANLTP282 | Luciferease | 4950 | 129 |
LGSxxxxxxxxLGS is a spacer for the inserted peptide. The viral titer was expressed as viral particles (vp, measured by OD260) and plaque forming units (pfu, measured by TCID50).
Figure 2.
Analysis of virus expression and stability. (A) HEK293 cells (0.01 pfu per cell) and A549 cells (5 pfu per cell) were infected with Ad vectors for 24 h, and the expression of HIV env gp160 protein and hexon protein was detected using western blotting. (B) The total DNA sample from Ad-infected HEK293 cells was digested with XhoI, subjected to 0.7% agarose gel electrophoresis. (C) The transgene stability of the Ad vectors through 15 serial passages. PCR reactions were performed in crude lysates from passages 1–15, and the resulting products analysed using 1% agarose gel electrophoresis
To explore the stability of the Ad genome and their ability to express the encoded gene product, the Ad-HIV, Ad-HisHIV and Ad-END/AAAHIV vectors were passaged in HEK293 cells for 15 generations. Ad genome was stabled in each passage (Figure 2B). Persistence of the HIV gene was detected after each passage, indicating that the HIV gene was not lost during the replication and that the hexon-modified virions were stable (Figure 2C).
Comparison of the neutralizing activity against Ad5 or individual vector by the modified-Ad vector
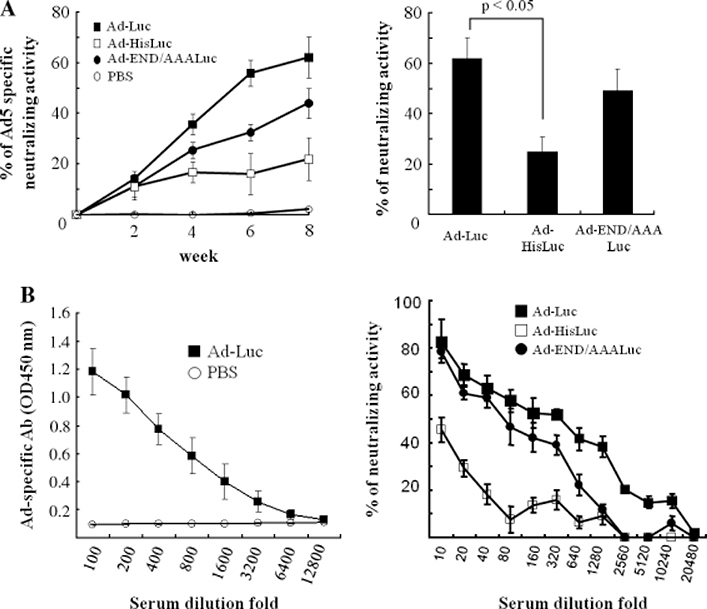
To evaluate the neutralizing activity of sera by modified-Ad vector, mice were administrated i.m. with the Ad-Luc, Ad-HisLuc or Ad-END/AAALuc vector, and neutralizing activity against Ad5 was measured from 0–8 weeks (Figure 3A, left panel). The Ad-HisLuc vector generated the lowest Ad5-specific neutralizing activity, which was significantly lower than that generated by Ad-Luc at weeks 6 and 8 (p < 0.01), and by Ad-END/AAALuc vector at week 8 (p < 0.05). The neutralizing activity elicited by the modified Ad vectors against their individual vector was monitored using 8-week sera post vector-administration (Figure 3A, right panel). The individual neutralizing activity of Ad-HisLuc immunized mice was significantly lower than that of Ad-Luc immunized mice (p < 0.01).
Figure 3.
Anti-Ad antibody titer and neutralizing assay. (A) Mice (eight per group) were immunized i.m. with 5 × 108 pfu of Ad-Luc, Ad-HisLuc, Ad-END/AAALuc or PBS. Ad5-specific serum antibody levels were measured by a neutralizing assay in A549 cells (left panel). The serum neutralizing activity against their individual vectors was measured by a neutralizing assay in A549 cells (right panel). Neutralizing activity by each vectors were compared at 320-fold serum dilution. (B) Mice (eight per group) were immunized i.m. with 5 × 108 pfu of Ad-Luc or PBS. Eight weeks later, Ad-specific serum antibody levels were measured by ELISA (left panel). The neutralizing activity against Ad vectors of sera derived from Ad-Luc-treated mice at week 8 was measured by a neutralizing assay in A549 cells (right panel)
Developing mice with pre-existing immunity to Ad5
To prepare mice with pre-existing anti-Ad5 antibodies, the Ad-Luc vector was injected i.m. into mice. Administration of Ad-Luc induced anti-Ad5 antibody (Figure 3A, left panel). To explore the neutralizing ability of the anti-Ad5 antibodies to Ad vectors in vitro, we performed a neutralizing assay using the pooled sera in A549 cells. As shown in Figure 3B (right panel), the neutralizing ability of the anti-Ad5 antibodies to Ad-HisLuc was significantly lower than that to Ad-Luc and Ad-END/AAALuc by 320-fold serum dilution. These results demonstrate that Ad-HisLuc can partially escape neutralization of anti-Ad5 antibody.
Comparison of the adaptive immune response induced by various Ad5 vectors
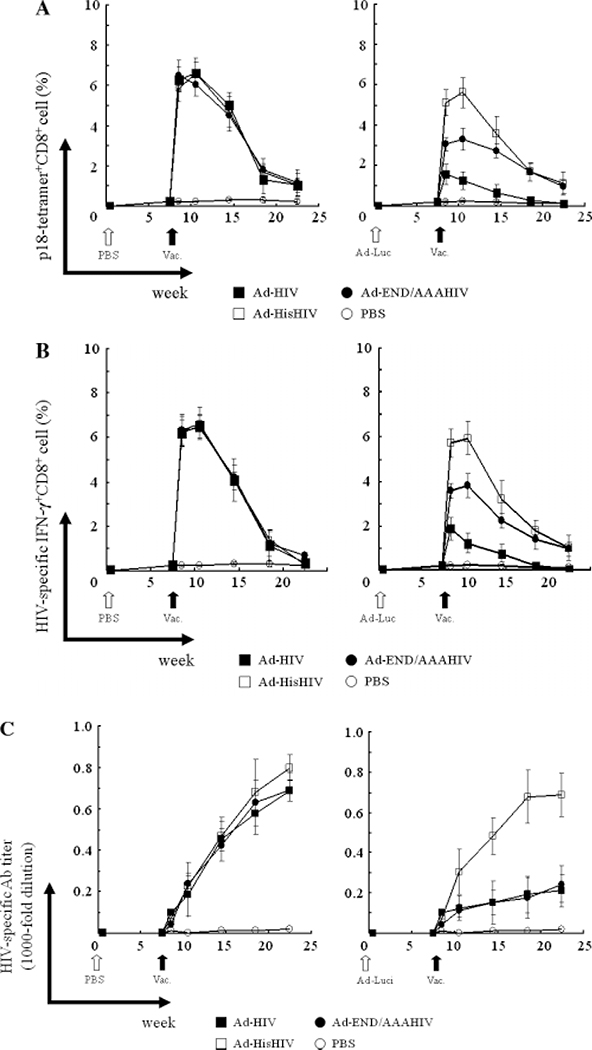
To evaluate the ability of Ad5 vectors encoding the HIV env protein to induce Ag-specific immune responses in the face of pre-existing anti-Ad5 immunity, mice were administrated i.m. with the Ad-Luc vector and measured anti-Ad5 antibody titer (Figure 3B), and then vaccinated with parental or hexon-modified Ad5 vectors (Ad-HisHIV, Ad-END/AAAHIV or Ad-HIV) at week 8. There was no significant difference of anti-Ad5 antibody titer between each group (Ad-HisHIV, Ad-END/AAAHIV and Ad-HIV) at week 8 (data not shown). HIV-specific cell-mediated immune responses were detected through a combination of tetramer assays and intracellular cytokine staining (ICCS) from weeks 8–23. Administration of the Ad-HIV, Ad-HisHIV and Ad-END/AAAHIV vectors stimulated similar numbers of tetramer specific CD8 T cells in control mice (animals without pre-existing anti-Ad immunity, Figure 4A, left panel; p > 0.05 among the groups immunized with Ad-HisHIV, Ad-END/AAAHIV and Ad-HIV from weeks 8–23). The peak response was observed 2 weeks after immunization, and gradually decreased.
Figure 4.
HIV-specific cell-mediated immunity induced by a single vaccination. All studies were conducted in eight mice per group without (left panel) or with (right panel) pre-existing anti-Ad5 neutralizing antibodies (induced by vaccination 8 weeks earlier with 5 × 108 pfu of Ad-Luc). HIV-specific cell-mediated immunity after a single vaccination was measured (A) using a tetramer binding assay and (B) using an ICCS assay. (C) HIV-specific IgG serum antibody levels were measured by ELISA
By contrast, the magnitude of the tetramer response elicited by these vectors varied markedly in mice with pre-existing immunity to Ad5 (Figure 4A, right panel). The maximal frequency of HIV-specific CD8 T cells induced by Ad-HisHIV was significantly greater than that induced by Ad-END/AAAHIV at weeks 9, 11 and 15 (p < 0.05), which was greater than that elicited by the conventional Ad-HIV vector at weeks 9, 11, 15 and 19 (p < 0.05). The magnitude and duration of the CD8 T cell response induced by the Ad-HisHIV vector was similar in mice with or without pre-existing immunity, but was significantly diminished in animals previously exposed to Ad5 immunized with the other vectors (Figure 4A). The same response pattern was observed in measurements of HIV-specific IFN-γ secreting CD8 T cells (Figure 4B). The Ad-HisHIV vector induced strong responses in mice unrelated to previous Ad5 exposure, whereas significantly weaker and shorter responses were induced when the other vectors were administered to mice with pre-existing immunity. These results indicate that a hexon-modified Ad5 vector can escape the neutralizing immune response induced by prior exposure to native Ad5.
The production of env-specific antibodies was monitored in vaccinated mice. The IgG anti-env response of control mice increased after each vaccination, and achieved similar magnitudes with each Ad5 vector (Figure 4C, left panel). By contrast, only the Ad-HisHIV vector elicited a strong IgG anti-env response in mice with pre-existing anti-Ad5 immunity (Ad-HisHIV versus Ad-END/AAA and Ad-HIV, p < 0.05 from weeks 11–23). Significantly lower initial antibody responses were observed when mice with pre-existing immunity were vaccinated with either the Ad-HIV or Ad-END/AAA vectors (non-significant difference between the two groups at all time points, p > 0.05), which were significantly higher than PBS-administered group at weeks 11, 15, 19 and 23. The response to administration of these vectors was also blunted (Figure 4C, right panel).
Comparison of the protective immunity induced by various Ad5 vectors
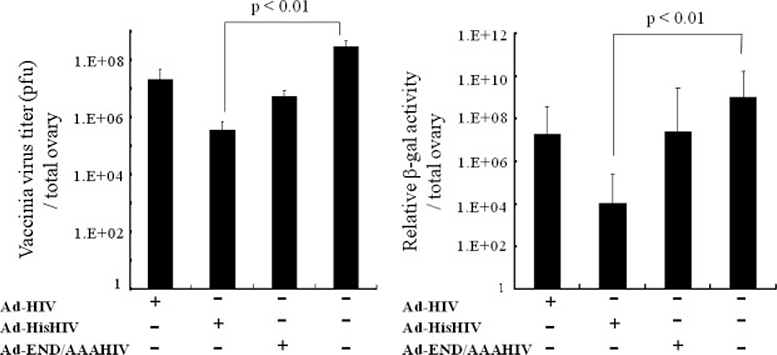
To determine whether the differences in adaptive immune responses observed above correlated with differences in protective immunity, mice with pre-existing Ad antibodies were vaccinated with each env-expressing vector and challenged 15 weeks after the final immunization with an HIV env and β-galactosidase expressing vaccinia virus [32]. The viral load of Ad-HisHIV immunized mice was >100-fold lower than that of unimmunized mice (p < 0.01, Figure 5). By comparison, the viral loads of mice immunized with the Ad-HIV or Ad-END/AAAHIV vectors were not significantly lower than those of control mice.
Figure 5.
Challenge with recombinant vaccinia virus. Protection against challenge with HIV gp160-expressing vaccinia virus (vPE16) was examined 15 weeks after the immunization. Three days after challenge, vaccinia virus titers were examined in mouse ovaries using a plaque assay (left panel) or β-galactosidase assay (right panel)
Discussion
The present study examined the effect of modifying the HVR5 region of the Ad5 hexon on the vector’s ability to avoid neutralization by pre-existing anti-Ad5 immunity. Initial studies established that the hexon-modified Ad vector was stable and could replicate normally in HEK293 cells as well as their parental Ad vector. The hexon-modified Ad vector was able to escape from anti-Ad5 neutralizing antibody, and mice with the modified vector generated significantly lower individual neutralizing antibody than those immunized with the parental vector. Importantly, this vector induced significantly stronger T and B cell responses in mice with pre-existing anti-Ad immunity than did the parental vector.
Research on Ad5-based vaccines targeting HIV and other pathogens has advanced to the stage of large-scale clinical trials [39]. Further development is hampered by evidence that most adults have been exposed to the naturally-circulating strain of this cold virus, and thus mount a strong anti-Ad immune response that can compromise the efficacy of conventional Ad5 vectors [40–42]. The antibody response against Ad5 can be directed against all elements of the capsid, including the fiber, penton base and hexon proteins [25,43]. However, the dominant neutralizing antibody typically target the hexon [20], which contains seven hypervariable regions. HVR5 is situated in the outer layer of the viral capsid, and is a major target of the neutralizing antibody response that is naturally elicited in humans infected by this cold virus [27,44].
The hexon protein interacts with many other capsid proteins. Thus, the magnitude and nature of changes that the hexon protein can tolerate while remaining structurally and functionally active may be limited. To evade the pre-existing immunity against Ad5 found in a majority of adult humans, the Ad5 hexon has been replaced with hexons from Ad1, Ad2, Ad6 and Ad12 [22,23,29,45]. In the present study, several control epitopes (10–16 amino acid residues) were inserted into HVR5. Compared to the parental vector, the stability of these hexon-modified vectors was not impaired (Figure 2A). However, a 24-amino acid residue of the B cell-epitope inserted in the HRV5 region resulted in a failure to rescue the virus (data not shown). While, the virus can be harvested when the short B cell-epitope (16-amino acid residues) was inserted in the HRV5 region. These results are contrast with those obtained by McConnell et al. [46], who introduced a 36-amino acid insert into the HRV5 region and found that the Ad virion still can be available. These differences may reflect their use of very long inserts or inserts encoding sequences that alter the secondary structure within the hexon core, thereby affecting hexon folding and virus stability.
The present studies were conducted using vectors in which the HVR5 of Ad5 was replaced with His6 (Ad-His) or modified by three point mutations (Ad-END/AAA). First, to evaluate the usefulness of modified Ad5 vector, we measured the neutralizing activity of sera by a modified-Ad vector. Ad-HisLuc vector elicited the lowest Ad5-specific neutralizing activity, which was significantly lower than that elicited by Ad-Luc and Ad-END/AAA, and the elicited individual neutralizing antibody was lower than parental Ad vector (Figure 3A). These results indicated that Ad-His vector generated much low anti-vector antibody. Further, experiments are required using human sera containing high anti-wild type Ad neutralizing antibody. Second, a mouse model in which immunity against Ad5 was induced by pre-immunization with a different construct was used to assess whether these modified vectors retained their immunogenicity despite the presence of anti-Ad5 antibodies. Significantly stronger cellular immune responses were induced by vaccinating these mice with Ad-HisHIV versus the parental Ad-HIV vector (Figure 4). The Ad-END/AAA vector also induced a stronger response than the unmodified vector, although this response was significantly lower than that elicited by Ad-HisHIV. This finding suggests that a major change in the immunogenic epitope present in HVR5 is required to avoid pre-existing neutralizing antibodies, and that such a change was more completely achieved by the Ad-HisHIV than by Ad-END/AAA vector. On the other hand, this finding suggests that not only the LGS-HHHHHH-LGS sequence, but also other peptides (but not all) can serve a similar function because we found that when the HVR5 was replaced with a B-cell epitope, a higher magnitude of humoral immunity against the B-cell epitope and very low levels of cell-mediated immunity against transgene were elicited [47]. In a challenge study with recombinant vaccinia virus, the viral load of Ad-HisHIV immunized mice was significantly lower than other Ad vectors (105 pfu in Ad-HisHIV immunized mice versus 107 pfu in Ad-HIV immunized mice) in the mouse model (Figure 5). However, the model using vaccinia virus vPE16 challenge does not comprise a perfect animal model. The real protective efficacy of the vaccine should be confirmed in a monkey model employing a SIVmac239 virus challenge. These results suggest that the Ad-HisHIV vaccine can escape from neutralizing antibodies, even in mice with pre-existing neutralizing antibodies against the vector, and, thus, it has high HIV-specific immunogenicity.
A recent large-scale clinical study involving an Ad5-vectored HIV vaccine was suspended because vaccination did not reduce the incidence of the HIV-infection or HIV viral load after infection compared to control group [39]. That vaccine contained three different Ad5 vectors each expressing the HIV gag, pol or nef gene, and the trial participants received three injections of the three vectors. To explore whether a boost from a homological vector can enhance transgene-specific immune responses, Ad-Luc pre-administrated mice were primed with parental or hexon-modified Ad5 (Ad-HisHIV, Ad-END/AAAHIV or Ad-HIV) at week 8, and boosted at weeks 10 and 14 (data not shown). We found that the first boost slightly increased cell-mediated immune responses detected by both tetramer assay and ICCS, but not the second boost (data not shown). A recent study using Ad26 vector prime and an Ad5 vector boost regimen generated better protective immunity than a single vector prime/boost [48]. On the other hand, we found that priming/boosting with different vectors elicited a higher immune response than that with homological vector [49]. These results demonstrate that multiple vaccination with a homological vector may not be an ideal regimen, even when using hexon-modified Ad5 vector.
Previous studies suggest that introducing peptides with spacers into the hexon region may further improve vector immunogenicity [33,44]. To examine this possibility, the immunogenicity of two hexon-modified Ad vectors containing GFP with (Ad-V3sGFP) or without (Ad-V3GFP) a spacer was compared in a mouse model. The Ad-V3sGFP vector induced significantly stronger cell-mediated responses than Ad-V3GFP (data not shown). This finding suggests that spacer inclusion impacts on epitope presentation. The magnitude of the cell-mediated immune response against the V3 epitope located in the hexon was considerably lower than the response against the rev/env gene located in the E1 region (data not shown). This result may be attributable to the HIV gene in the E1 region being driven by a strong promoter, resulting in each virion containing a large copy number of the HIV gene.
In summary, the present study describes a novel Ad-based HIV vaccine containing an HVR5 region modification. The B and T cell responses induced by this vector in normal mice were equivalent to that of the parental vector. By evading the neutralizing effects of antibodies directed against the hexon protein, the immunogenicity of this novel vector far exceeded that of the parental vector in mice with pre-existing immunity to Ad. These results suggest that hexon-modified Ad5 vectors represent a promising strategy for further development of Ad-based vaccines for use in humans.
Acknowledgements
We thank NIH Tetramer Core Facility (Atlanta, GA, USA) for the tetramer. This work was partially supported by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan; a Grant for the Strategic Research Project of Yokohama City University, Japan; a Research Foundation for the Advanced Medical Research Center and a Grant from the Japanese National Institute of Biomedical Innovation (No. 05-1).
References
- 1.Bukawa H, Sekigawa K, Hamajima K, et al. Neutralization of HIV-1 by secretory IgA induced by oral immunization with a new macromolecular multicomponent peptide vaccine candidate. Nat Med 1995; 1: 681–685. [DOI] [PubMed] [Google Scholar]
- 2.Barouch DH, Santra S, Schmitz JE, et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 2000; 290: 486–492. [DOI] [PubMed] [Google Scholar]
- 3.Robinson HL, Montefiori DC, Johnson RP, et al. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat Med 1999; 5: 526–534. [DOI] [PubMed] [Google Scholar]
- 4.Patterson LJ, Malkevitch N, Venzon D, et al. Protection against mucosal simian immunodeficiency virus SIV (mac251) challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J Virol 2004; 78: 2212–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiver JW, Fu TM, Chen L, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 2002; 415: 331–335. [DOI] [PubMed] [Google Scholar]
- 6.Schnell MJ, Foley HD, Siler CA, McGettigan JP, Dietzschold B, Pomerantz RJ. Recombinant rabies virus as potential live-viral vaccines for HIV-1. Proc Natl Acad Sci USA 2000; 97: 3544–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandl CW, Aberle JH, Aberle SW, Holzmann H, Allison SL, Heinz FX. In vitro-synthesized infectious RNA as an attenuated live vaccine in a flavivirus model. Nat Med 1998; 4: 1438–1440. [DOI] [PubMed] [Google Scholar]
- 8.Matano T, Kobayashi M, Igarashi H, et al. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J Exp Med 2004; 199: 1709–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caley IJ, Betts MR, Davis NL, Swanstrom R, Frelinger JA, Johnston RE. Venezuelan equine encephalitis virus vectors expressing HIV-1 proteins: vector design strategies for improved vaccine efficacy. Vaccine 1999; 17: 3124–3135. [DOI] [PubMed] [Google Scholar]
- 10.Xin KQ, Mizukami H, Urabe M, et al. Induction of robust immune responses against human immunodeficiency virus is supported by the inherent tropism of adeno-associated virus type 5 for dendritic cells. J Virol 2006; 80: 11 899–11 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xin KQ, Ooki T, Mizukami H, et al. Oral administration of recombinant adeno-associated virus elicits human immunodeficiency virus-specific immune responses. Hum Gene Ther 2002; 13: 1571–1581. [DOI] [PubMed] [Google Scholar]
- 12.Xin KQ, Urabe M, Yang J, et al. A novel recombinant adeno-associated virus vaccine induces a long-term humoral immune response to human immunodeficiency virus. Hum Gene Ther 2001; 12: 1047–1061. [DOI] [PubMed] [Google Scholar]
- 13.Aldovini A, Young RA. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature 1991; 351: 479–482. [DOI] [PubMed] [Google Scholar]
- 14.Someya K, Cecilia D, Ami Y, et al. Vaccination of rhesus macaques with recombinant Mycobacterium bovis Bacillus Calmette–Guerin Env V3 elicits neutralizing antibody-mediated protection against simian-human immunodeficiency virus with a homologous but not a heterologous V3 motif. J Virol 2005; 79: 1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xin KQ, Hoshino Y, Toda Y, et al. Immunogenicity and protective efficacy of orally administered recombinant Lactococcus lactis expressing surface-bound HIV Env. Blood 2003; 102: 223–228. [DOI] [PubMed] [Google Scholar]
- 16.Roelvink PW, Lizonova A, Lee JG, et al. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol 1998; 72: 7909–7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan NJ, Sanchez A, Rollin PE, et al. Development of a preventive vaccine for Ebola virus infection in primates. Nature 2000; 408: 605–609. [DOI] [PubMed] [Google Scholar]
- 18.Jiao Y, Ge CM, Meng QH, et al. Adenovirus-mediated expression of Tob1 sensitizes breast cancer cells to ionizing radiation. Acta Pharmacol Sin 2007; 28: 1628–1636. [DOI] [PubMed] [Google Scholar]
- 19.Wohlfahrt ME, Beard BC, Lieber A, et al. A capsid-modified, conditionally replicating oncolytic adenovirus vector expressing TRAIL Leads to enhanced cancer cell killing in human glioblastoma models. Cancer Res 2007; 67: 8783–8790. [DOI] [PubMed] [Google Scholar]
- 20.Sumida SM, Truitt DM, Lemckert AA, et al. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol 2005; 174: 7179–7185. [DOI] [PubMed] [Google Scholar]
- 21.van Oostrum J, Burnett RM. Molecular composition of the adenovirus type 2 virion. J Virol 1985; 56: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gall JG, Crystal RG, Falck-Pedersen E. Construction and characterization of hexon-chimeric adenoviruses: specification of adenovirus serotype. J Virol 1998; 72: 10 260–10 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy S, Shirley PS, McClelland A, Kaleko M. Circumvention of immunity to the adenovirus major coat protein hexon. J Virol 1998; 72: 6875–6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rux JJ, Burnett RM. Adenovirus structure. Hum Gene Ther 2004; 15: 1167–1176. [DOI] [PubMed] [Google Scholar]
- 25.Wohlfart C Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J Virol 1988; 62: 2321–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu H, Dmitriev I, Kashentseva E, et al. Construction and characterization of adenovirus serotype 5 packaged by serotype 3 hexon. J Virol 2002; 76: 12775–12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crawford-Miksza L, Schnurr DP. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J Virol 1996; 70: 1836–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rux JJ, Burnett RM. Type-specific epitope locations revealed by X-ray crystallographic study of adenovirus type 5 hexon. Mol Ther 2000; 1: 18–30. [DOI] [PubMed] [Google Scholar]
- 29.Youil R, Toner TJ, Su Q, et al. Hexon gene switch strategy for the generation of chimeric recombinant adenovirus. Hum Gene Ther 2002; 13: 311–320. [DOI] [PubMed] [Google Scholar]
- 30.Roberts DM, Nanda A, Havenga MJ, et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing antivector immunity. Nature 2006; 441: 239–243. [DOI] [PubMed] [Google Scholar]
- 31.Maruyama K, Akiyama Y, Nara-Ashizawa N, et al. Adenovirus-mediated MUC1 gene transduction into human blood-derived dendritic cells. J Immunother 2001; 24: 345–353. [DOI] [PubMed] [Google Scholar]
- 32.Jounai N, Okuda K, Kojima Y, et al. Contribution of the rev gene to the immunogenicity of DNA vaccines targeting the envelope glycoprotein of HIV. J Gene Med 2003; 5: 609–617. [DOI] [PubMed] [Google Scholar]
- 33.Kurachi S, Koizumi N, Sakurai F, et al. Characterization of capsid-modified adenovirus vectors containing heterologous peptides in the fiber knob, protein IX, or hexon. Gene Ther 2007; 14: 266–274. [DOI] [PubMed] [Google Scholar]
- 34.Lieber A, He CY, Kirillova I, Kay MA. Recombinant adenoviruses with large deletions generated by Cre-mediated excision exhibit different biological properties compared with first-generation vectors in vitro and in vivo. J Virol 1996; 70: 8944–8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ugai H, Yamasaki T, Hirose M, et al. Purification of infectious adenovirus in two hours by ultracentrifugation and tangential flow filtration. Biochem Biophys Res Commun 2005; 331: 1053–1060. [DOI] [PubMed] [Google Scholar]
- 36.Sprangers MC, Lakhai W, Koudstaal W, et al. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J Clin Microbiol 2003; 41: 5046–5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altman JD, Moss PA, Goulder PJ, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science 1996; 274: 94–96. [PubMed] [Google Scholar]
- 38.Villacres MC, Zuo J, Bergmann CC. Maintenance of CD8(+) T-cell memory following infection with recombinant sindbis and vaccinia viruses. Virology 2000; 270: 54–64. [DOI] [PubMed] [Google Scholar]
- 39.Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med 2008; 205: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnett BG, Crews CJ, Douglas JT. Targeted adenoviral vectors. Biochim Biophys Acta 2002; 1575: 1–14. [DOI] [PubMed] [Google Scholar]
- 41.Nemerow GR. Adenoviral vectors – new insights. Trends Microbiol 2000; 8: 391–394. [DOI] [PubMed] [Google Scholar]
- 42.Vorburger SA, Hunt KK. Adenoviral gene therapy. Oncologist 2002; 7: 46–59. [DOI] [PubMed] [Google Scholar]
- 43.Gahery-Segard H, Farace F, Godfrin D, et al. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J Virol 1998; 72: 2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu H, Han T, Belousova N, et al. Identification of sites in adenovirus hexon for foreign peptide incorporation. J Virol 2005; 79: 3382–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roy S, Clawson DS, Calcedo R, et al. Use of chimeric adenoviral vectors to assess capsid neutralization determinants. Virology 2005; 333: 207–214. [DOI] [PubMed] [Google Scholar]
- 46.McConnell MJ, Danthinne X, Imperiale MJ. Characterization of a permissive epitope insertion site in adenovirus hexon. J Virol 2006; 80: 5361–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ura T, Yoshida A, Xin KQ, et al. Designed recombinant adenovirus type 5 vector induced envelope-specific CD8(+) cytotoxic T lymphocytes and cross-reactive neutralizing antibodies against human immunodeficiency virus type 1. J Gene Med 2009; 11: 139–149. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, O’Brien KL, Lynch DM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 2009; 457: 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimada M, Wang HB, Kondo A, et al. Effect of therapeutic immunization using Ad5/35 and MVA vectors on SIV infection of rhesus monkeys undergoing antiretroviral therapy. Gene Ther 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]