Abstract
The transcription factors Prdm1 (Blimp1) and Vsx2 (Chx10) work downstream of Otx2 to regulate photoreceptor and bipolar cell fates in the developing retina. Mice that lack Vsx2 fail to form bipolar cells while Prdm1 mutants form excess bipolars at the direct expense of photoreceptors. Excess bipolars in Prdm1 mutants appear to derive from rods, suggesting that photoreceptor fate remains mutable for some time after cells become specified. Here we tested whether bipolar cell fate is also plastic during development. To do this, we created a system to conditionally misexpress Prdm1 at different stages of bipolar cell development. We found that Prdm1 blocks bipolar cell formation if expressed before the fate choice decision occurred. When we misexpressed Prdm1 just after the decision to become a bipolar cell was made, some cells were reprogrammed into photoreceptors. In contrast, Prdm1 misexpression in mature bipolar cells did not affect cell fate. We also provide evidence that sustained misexpression of Prdm1 was selectively toxic to photoreceptors. Our data show that bipolar fate is malleable, but only for a short temporal window following fate specification. Prdm1 and Vsx2 act by stabilizing photoreceptor and bipolar fates in developing OTX2+ cells of the retina.
Keywords: Retinal Development, Bipolar cells, Photoreceptors, VSX2, PRDM1
INTRODUCTION
During development, a population of retinal progenitor cells gives rise to all six major classes of neurons within the eye: rod and cone photoreceptors, ganglion, amacrine, bipolar, and horizontal cells, as well as Müller glia (Turner and Cepko, 1987; Turner et al., 1990). Retinal progenitors permanently exit the cell cycle (their birthdate) and generate these cell fates in a stereotyped overlapping fashion from approximately embryonic (E) day 11.5 to postnatal (P) day 7 in mice (Carter-Dawson and LaVail, 1979; Rapaport et al., 2004; Sidman, 1961; Young, 1985). These progenitors have more than one fate option they can select from at nearly any given time in retinal development. Nonetheless, it is unclear how these cells choose their fate and subsequently make that decision permanent.
Mouse retinal progenitors in the postnatal period choose between four fates: amacrine cells, Müller glia, bipolar cells, and rod photoreceptors (Turner and Cepko, 1987; Young, 1985). Of these, rods and bipolars are the most abundant and both types express the key transcription factor Otx2 (Beby and Lamonerie, 2013; Fossat et al., 2007; Nishida et al., 2003; Shekhar et al., 2016). Otx2 is required for the formation of photoreceptors and bipolar cells (Nishida et al., 2003; Sato et al., 2007). Its expression is activated in the final cell cycle and precedes the decision to adopt photoreceptor versus bipolar cell fates (Muranishi et al., 2011). Otx2 directly regulates two downstream transcription factors, Vsx2 (Chx10) and Prdm1 (Blimp1), through defined enhancer sequences (Brzezinski et al., 2013; Kim et al., 2008; Mills et al., 2017; Wang et al., 2014). When Prdm1 is knocked out in the developing retina, there is a severe reduction in the number of photoreceptors that form (Brzezinski et al., 2010; Brzezinski et al., 2013; Katoh et al., 2010). In these mutants, there is a 1:1 fate-shift within OTX2+ cells such that bipolars are increased at the expense of photoreceptors. Overexpression of Prdm1 in early development suppresses bipolar cell formation (Brzezinski et al., 2010; Katoh et al., 2010). No bipolar cells are formed when Vsx2 is mutated (Burmeister et al., 1996; Green et al., 2003; Phillips et al., 2014). In contrast, when Vsx2 is overexpressed it inhibits photoreceptor gene expression and increases bipolar cell formation at the expense of rods (Dorval et al., 2006; Livne-Bar et al., 2006). These data suggest that there is a mutually inhibitory gene regulatory network within OTX2+ cells where Prdm1 represses bipolar fate and Vsx2 blocks photoreceptor formation. Correspondingly, loss of Prdm1 results in precocious and excess expression of Vsx2 in the retina (Brzezinski et al., 2010; Brzezinski et al., 2013; Katoh et al., 2010). We observed that bipolar cells in Prdm1 mutants could be derived from cells that make rod-specific markers (Brzezinski et al., 2013). This suggests that photoreceptor fate is transiently plastic after it has been selected and is subsequently stabilized or else superseded by the bipolar cell program. In Vsx2 mutant retinas, non-functional Vsx2 mRNA remains and was found in developing photoreceptors (Livne-Bar et al., 2006). This raises the possibility that OTX2+ cells destined for bipolar fate can have their identity superseded by the photoreceptor program. Here, we asked whether and when bipolar fate can be superseded in developing OTX2+ cells.
Since Prdm1 can block bipolar cell formation, we misexpressed PRDM1 at different stages of retinal development to determine whether bipolar fate is plastic. To do this, we first created a mouse line that allowed us to conditionally drive Prdm1 expression via CRE-mediated recombination. We then drove constitutive PRDM1 expression using three distinct sources of CRE during unique timeframes in development: (1) before bipolar fate choice is made, (2) just after bipolar fate selection, and (3) in mature bipolar cells. Prdm1 had different effects at each of these developmental stages. It blocked bipolar formation if present before the choice, partially converted bipolars into photoreceptors just after bipolar specification, and had no effect on the fate of mature bipolar cells. Surprisingly, we also observed that long-term misexpression of Prdm1 appeared to be toxic only to photoreceptors. Taken together, our data show that bipolar cells are transiently plastic after their fate is selected. Nonetheless, shortly after fate selection bipolar cells become stabilized and refractory to the effects of PRDM1. A major role of the interplay between Prdm1 and Vsx2 in OTX2+ cells is to stabilize fate choices made during retinal development.
RESULTS
Photoreceptor and bipolar cell formation requires the transcription factor Otx2 (Nishida et al., 2003; Sato et al., 2007). OTX2 directly activates two downstream transcription factors, Prdm1 and Vsx2, which have been hypothesized to play a cross-repressive role to set the balance of photoreceptors and bipolar cells formed (Brzezinski et al., 2010; Brzezinski and Reh, 2015; Brzezinski et al., 2013; Katoh et al., 2010; Kim et al., 2008; Mills et al., 2017; Wang et al., 2014). If so, constitutive expression of PRDM1 in OTX2+ cells should block bipolar genesis and force these cells to adopt photoreceptor fate. Since Prdm1 is not permanently expressed in photoreceptors, there may only be a transient period when Prdm1 can affect fate choice. To test how Prdm1 affects cell fate, we built systems to misexpress Prdm1 in a spatially and temporally controlled fashion. To drive Prdm1 expression, we first constructed a CRE-inducible Prdm1 mouse line. We replaced the red fluorescent protein sequence (tdTomato) in the Ai9 targeting vector (Madisen et al., 2010) with the cDNA for mouse Prdm1. This targeting vector was used to make knock-in mice, resulting in the insertion of a Lox-stop-Lox Prdm1 allele into the ROSA26 locus on chromosome 6 (Fig 1A). Throughout, we refer to these ROSA-Prdm1 knock-in animals as PRDM1 mice. As detailed below, we permanently misexpressed Prdm1 in different spatial and temporal patterns by combining these PRDM1 mice with multiple CRE expression systems.
Figure 1. Constitutive PRDM1 expression driven by PAX6-CRE does not prevent progenitor VSX2 expression.
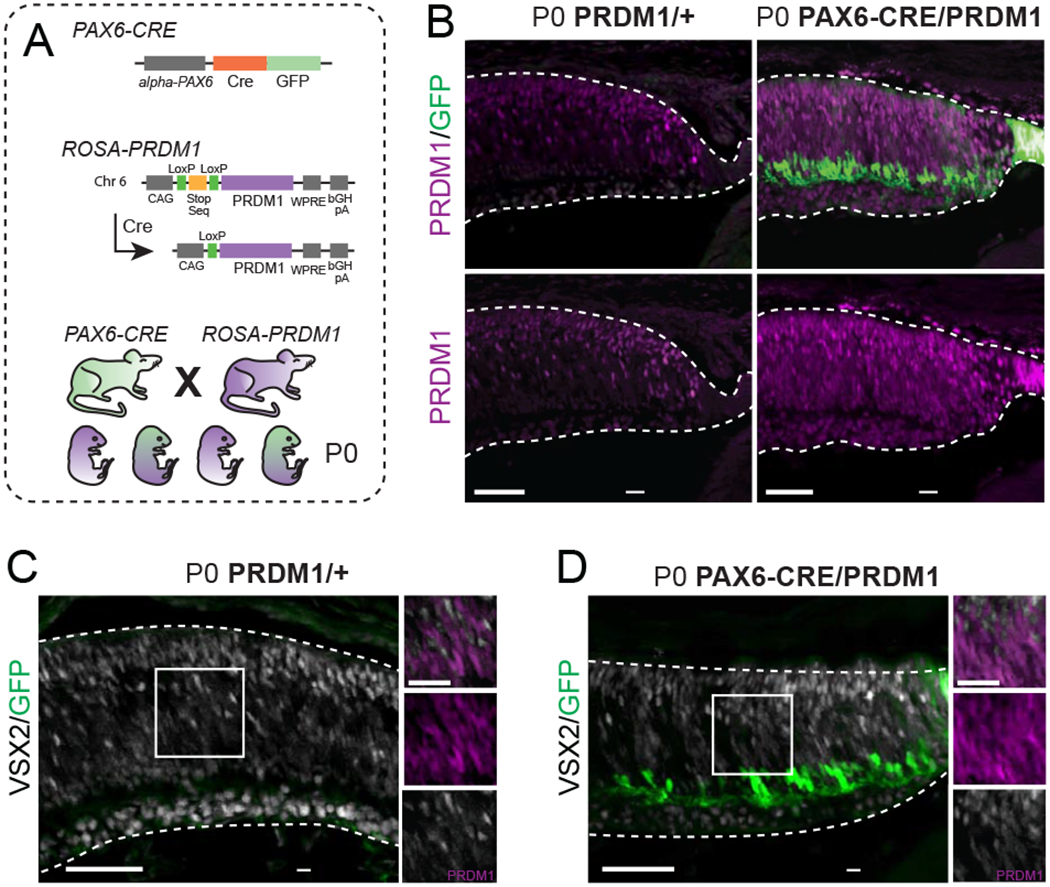
A) Schematic of transgenic mice utilized. To make the PRDM1 strain, the ROSA26 locus on chromosome 6 was targeted to insert the Prdm1 cDNA downstream of a CAG enhancer and a LoxP-stop-LoxP sequence. Further downstream is a woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) and a bovine growth hormone polyadenylation (bGH pA) site. Introduction of CRE removes the stop sequence and allows for permanent PRDM1 expression. B) Representative immunohistochemistry of PRDM1/+ control compared to PAX6-CRE/PRDM1 retinas stained for Prdm1 (purple) and GFP (green). Note the ectopic expression of Prdm1 in the RPE and ciliary body of PAX6-CRE/PRDM1 eyes (arrowheads). C-D) P0 PRDM1/+ control compared to PAX6-CRE/PRDM1 retinas stained for VSX2 (white), GFP (green), and PRDM1 (purple, inset-only). Arrowheads mark PRDM1+/VSX2+ cells. There is VSX2 background staining in the ganglion cell layer in both conditions. bars=50μm, inset bars=25μm.
Constitutive Prdm1 expression alters cell fates within the retina
To test that our misexpression system worked and that Prdm1 can induce cell fate changes, we crossed PRDM1 mice with αPAX6-Cre-IRES-GFP mice (PAX6-CRE) (Fig 1A) (Marquardt et al., 2001). The PAX6-CRE line drives CRE and GFP expression in nearly all retinal progenitors of the peripheral retina starting before the onset of neurogenesis and persisting in a subset of amacrine cells across the whole mature retina (Lefebvre et al., 2008; Marquardt et al., 2001). This transgene also drives expression in the developing ciliary structure of the eye that is immediately adjacent to the retina and within a small number of peripheral retinal pigmented epithelial (RPE) cells. We collected PAX6-CRE/PRDM1 experimental and PRDM1/+ control mice at P0. Immunostaining for PRDM1 showed a dramatic increase in the number and distribution of labeled cells in the peripheral retinas of PAX6-CRE/PRDM1 eyes compared to controls (Fig 1B). This included PRDM1+ cells in the RPE and ciliary body, which were never observed in controls (Fig 1B). Thus, the PRDM1 mice operate as expected, leading to robust PRDM1 misexpression following CRE-mediated recombination in the eye.
Misexpressing PRDM1 throughout the periphery had no observable effect on retinal architecture when examined at P0 (Fig 1B–D). Despite the increase in PRDM1 expression in experimental mice, immunostaining retinal progenitors in the P0 peripheral retina with VSX2 showed no changes in gross morphological pattern or overlap with PRDM1+ cells compared to control (Burmeister et al., 1996; Green et al., 2003; Livne-Bar et al., 2006; Sigulinsky et al., 2015) (Fig 1C–D). This is consistent with a role for PRDM1 in controlling cell fate choice within OTX2+ cells, rather than proliferative progenitors.
To examine cell fate choice, we next collected mice at P7, when bipolar cells are readily detectable in the retina (Fig 2A). PAX6-CRE/PRDM1 mice had morphologically similar peripheral retinas compared to CRE-negative controls (Fig 2B–C). We stained P7 sections with multiple markers for bipolar cells. These include: (1) OTX2, which marks newly postmitotic rods and bipolars at higher intensity than mature photoreceptors, (2) VSX2, which marks bipolars intensely and progenitors weakly, (3) ISLET 1/2, which marks cone ON and rod bipolar cells, and (4) Secretagogin (SCGN), which marks a subset of ON and OFF cone bipolars (Burmeister et al., 1996; Elshatory et al., 2007a; Elshatory et al., 2007b; Fossat et al., 2007; Nishida et al., 2003; Puthussery et al., 2010; Shekhar et al., 2016). PAX6-CRE/PRDM1 mice had fewer intensely labeled VSX2+ and OTX2+ cells (Fig 2B–E). The overall number of OTX2+ cells was slightly, but not significantly, reduced (Fig 2F). We observed mosaic patterns of VSX2+ bipolar cell reduction (Fig 2D) and continuous stretches of near complete bipolar cell loss (Fig 2E). When we quantified bright VSX2 cells, which generally represent bipolar-specific expression, we observed a significant decrease in our test condition compared to control (Fig 2D–E, G, p<0.001). This was consistent with a reduction in bipolar cells. We found that SCGN+ cone bipolar cells were reduced in experimental retinas compared to control (Fig 2D–E, H, p<0.001). ISLET1/2+ bipolar cells also showed a severe loss of labeling in PAX6-CRE/PRDM1 retinas compared to control (Fig 2B–C, I, p<0.001). Indeed, all areas that lacked intense OTX2 or VSX2 staining also lacked SCGN or ISLET1/2 staining (Fig 2B–E, H–I). In contrast, we saw no differences in the number of amacrine and ganglion cells that were also labeled by ISLET1/2 between conditions (Fig 2B–C, J, p=0.355) (Elshatory et al., 2007a; Elshatory et al., 2007b). Our results show that misexpressing Prdm1 before the onset of bipolar cell formation can prevent their genesis. This aligns with previous findings where overexpression of Prdm1 in postnatal retinal progenitors suppressed bipolar cell formation (Brzezinski et al., 2010; Katoh et al., 2010). Moreover, our results show that Prdm1 misexpression does not significantly interfere with other cell fate choices that occur in retinal development.
Figure 2. Constitutive PRDM1 expression driven by PAX6-CRE prevents bipolar cell formation at P7.
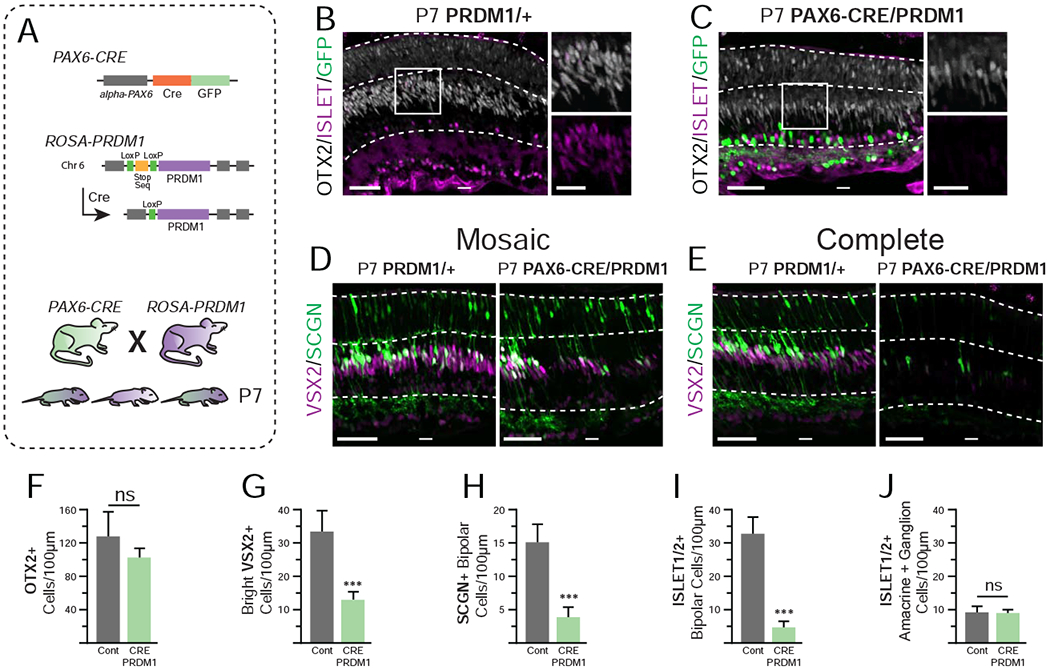
A) Schematic of transgenic mice utilized. B-C) P7 PRDM1/+ control compared to PAX6-CRE/PRDM1 retinas stained for ISLET1/2+ (purple), OTX2 (white), and GFP (green). Stain of VSX2 (purple) and SCGN (green) with D) mosaic loss and E) complete loss of bipolars in the peripheral retina. Note that SCGN marks some photoreceptors at this stage. F) The total number of OTX2+ cells (bright and faint) is not significantly decreased. G-I) Quantification of bipolar cell markers. There are fewer G) bright VSX2+ cells, H) SCGN+ bipolars, and I) ISLET1/2+ bipolars in in PAX6-CRE/PRDM1 retinas compared to controls. J) There is no difference in the number of ISLET1/2+ amacrines or ganglion cells between conditions. A total of 4,260 cells were quantified from 43 images. Statistics calculated based on number of mice (N), Cont N=4, PAX6-CRE/PRDM1 N=5. Error bars=standard deviation. ns=not significant, ***p<0.001. bars=50μm, inset bars=25μm, INL=Inner Nuclear Layer, ONL=Outer Nuclear Layer.
We next tested whether Prdm1 could affect fate choice in OTX2+ cells that have already decided to become bipolar cells. To do this, we first obtained VSX2-CRE mice from Jackson Labs (Nickerson et al., 2011). This line uses sequences upstream of Vsx2 to drive CRE in a bipolar cell-specific fashion starting shortly after they become specified in the early postnatal period (Nickerson et al., 2011). We then crossed the VSX2-CRE mice with our PRDM1 animals to test whether Prdm1 misexpression at the onset of bipolar fate specification could alter fate choice (Fig S2A). However, we consistently had trouble breeding these mice. When litters were obtained, they were small (3-5 pups) and no VSX2-CRE/PRDM1 double-transgenic mice were seen in dozens of crosses. Since Prdm1 promotes the germ cell lineage at the expense of somatic development (Magnusdottir et al., 2013; Ohinata et al., 2005; Sybirna et al., 2019), we suspected that CRE expression might be occurring early in development and preventing embryogenesis. To test this, we crossed the VSX2-CRE mice with ROSA-RFP (RFP) reporter mice (Madisen et al., 2010) (Fig S2). When mature VSX2-CRE/RFP retinas were stained for CRE, the pattern of expression was limited to bipolar cells as expected (Fig S2D). However, these mice had visibly red skin and their entire eye was RFP+, including the lens and surrounding ocular tissues (Fig S2D). When the VSX2-CRE was maternally derived, all pups (even those that were VSX2-CRE-negative) ubiquitously expressed RFP (Fig S2E). When VSX2-CRE was paternally inherited, VSX2-Cre/RFP pups had mosaic, yet global, RFP expression (Fig S2F). We concluded that VSX2-CRE mice have CRE activity in retinal bipolar cells, but also at very early stages of development. When crossed with PRDM1 mice, the VSX2-CRE allele will drive widespread Prdm1 expression. Based on Prdm1’s early role in development, this is likely incompatible with embryogenesis. We propose that such early PRDM1 misexpression explains why we never recovered any VSX2-CRE/PRDM1 mice and necessitated a different approach to misexpress Prdm1 in bipolar cells. To do this, we designed viral and plasmid-electroporation strategies to deliver CRE recombinase to mature and developing bipolar cells, respectively.
AAV driven PRDM1 does not alter bipolar fate choice and appears toxic to mature photoreceptors
To ascertain if PRDM1 expression in mature bipolar cells is sufficient to cause a fate-shift to photoreceptors we created adeno-associated viruses (AAV) that drive CRE-recombinase under the control of an ON bipolar cell-specific PCP2 enhancer (PCP2-CRE-AAV) (de Leeuw et al., 2014; Scalabrino et al., 2015). This AAV serotype 2 virus contains a modified capsid that allows for the robust transduction of the inner and outer retina upon intravitreal delivery (see Methods) (Kay et al., 2013). PRDM1 mice were crossed with RFP mice to generate trans-heterozygous experimental (RFP/PRDM1) and control (RFP/+) mice. PCP2-CRE-AAV was injected into the vitreous of P28 eyes and the mice were raised until P60 (Fig 3A). This allowed us to track cells that expressed the bipolar-specific CRE because they permanently express RFP. We then compared RFP/+ controls to RFP/PRDM1 mice by immunostaining for RFP. Cell fates were assessed by morphology and location within the retina. In addition to RFP+ bipolar cells, we also observed RFP+ ganglion cells, amacrines, Müller glia, and photoreceptors (Fig 3B). We quantified cells and found that both control mice and their RFP/PRDM1 littermates had an equal number of amacrines and ganglion cells, which were proximal to the site of the intravitreal injections (Fig 3D, data not shown). This suggested that amacrine and ganglion cell labeling was the result of transient off-target CRE expression, as previously noted with similar viruses (Scalabrino et al., 2015). Thus, we excluded amacrine and ganglion cells from further calculations. While off-target expression accounts for RFP labeling of photoreceptors and Müller glia in controls, we quantified them to determine whether any cell fate changes occurred upon PRDM1 misexpression. When summed, the number of RFP+ bipolars and photoreceptors were similar between conditions (Fig 3C). If PRDM1 reprogrammed bipolar cell fate, we expected to see an increase in RFP+ photoreceptors at the expense of bipolars. However, when we specifically examined RFP+ cells, we observed fewer photoreceptors and more bipolar cells in our Prdm1 misexpression condition compared to our controls, exactly the opposite of our hypothesis (Fig 3E–G). Indeed, there were almost no RFP+ photoreceptors found in the RFP/PRDM1 condition (Fig 3E, S3).
Figure 3. Overexpression of PRDM1 in mature retinas driven by PCP2-CRE-AAV.
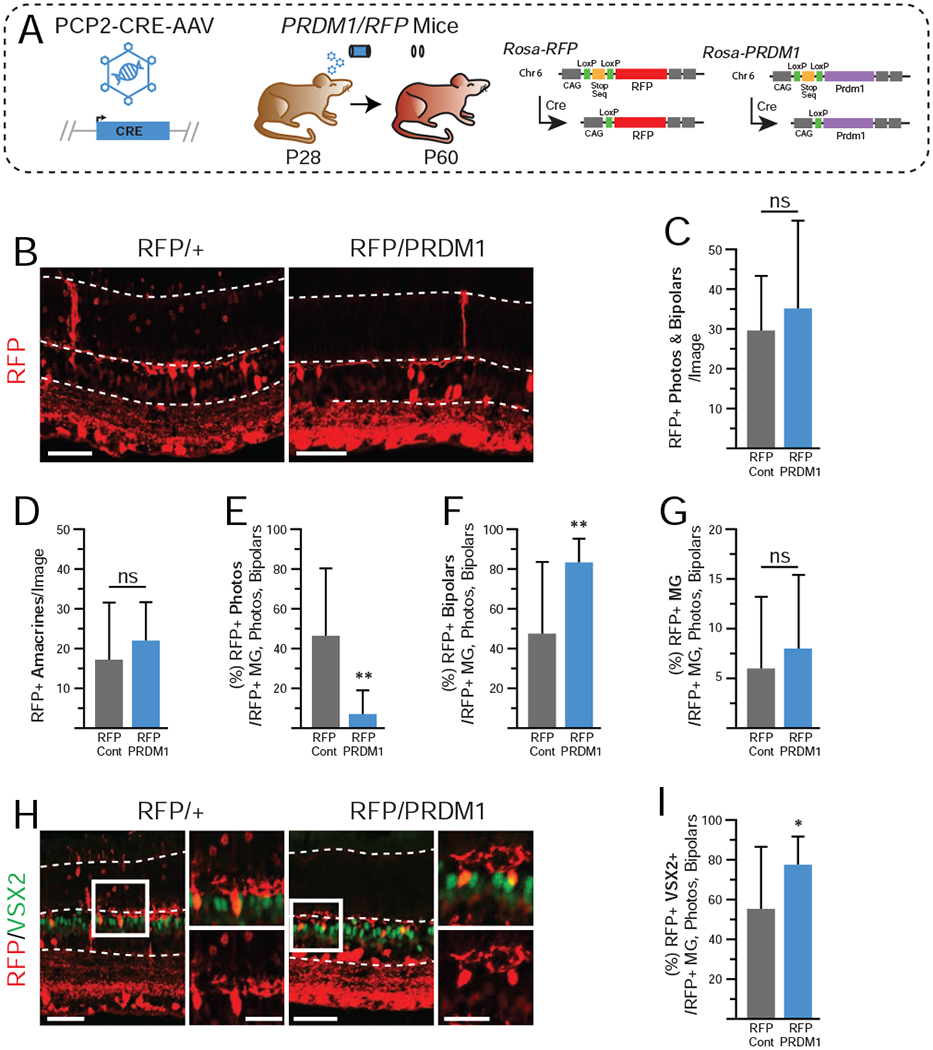
A)Schematic of experimental design. ROSA-RFP and PRDM1 mice are as described in Figure 1. B) Representative immunohistochemistry of transduced RFP/+ controls compared to RFP/PRDM1 littermates. Arrowheads mark bipolar cells and asterisks mark photoreceptors.Note that the RFP labeling intensity of photoreceptors is less than other cell types in the retina (see also Figure S3). C-D) There is no difference in the total number of RFP+ bipolars and photoreceptors when summed, or in the total number of amacrines and ganglion cells. Amacrines and ganglion cells were excluded from the rest of the calculations. Transduced RFP/PRDM1 mice have E) a significantly lower percentage of RFP+ cells that are photoreceptors, F) an increased percentage that are bipolar cells, G) and no change in the percentage that are Muller glia compared to controls. H) There is no observable difference in VSX2 intensity (green) in RFP+ bipolars and I) there is a significantly higher fraction of RFP+ cells that co-express VSX2+ in RFP/PRDM1 mice. A total of 1,951 cells (excluding the ganglion cell layer) were quantified from 36 images. Statistics calculated based on number of eyes (N), Cont (RFP/+) N=6, RFP/PRDM1 N=11. Error bars=standard deviation. ns=not significant, *p<0.05, **p<0.01. bars=50μm, inset bars=25μm, INL=Inner Nuclear Layer, ONL=Outer Nuclear Layer.
We next tested whether PRDM1 misexpression repressed VSX2 expression in RFP+ bipolar cells. VSX2 stains revealed that significantly more RFP+ cells in RFP/PRDM1 mice were VSX2+ compared to controls (Fig 3H–I). However, we did not observe diminished VSX2 staining intensity compared to neurons in the control condition or in RFP-negative bipolar cells within the same image. We concluded that constitutive PRDM1 expression cannot alter the fate of mature bipolar cells, does not affect VSX2 expression, and may be toxic to mature photoreceptors.
Overexpression of PRDM1 in nascent bipolar cells causes a fate-shift to rods
In the absence of Prdm1, VSX2 is precociously upregulated in the retina (Brzezinski et al., 2010; Brzezinski et al., 2013; Katoh et al., 2010). Early overexpression of Prdm1 reduces VSX2 and bipolar cell formation (Fig 2) (Brzezinski et al., 2010; Katoh et al., 2010). Nonetheless, long-term misexpression of Prdm1 in adult bipolar cells does not repress VSX2 expression or change cell fate (Fig 3). We hypothesized that there is a critical period in OTX2+ cells where PRDM1 can suppress VSX2 and promote photoreceptor fate at the expense of bipolar cell development.
To test this, we designed a plasmid containing a 164bp bipolar-specific Vsx2 enhancer (Kim et al., 2008) driving CRE-recombinase expression that we called VE-CRE (Fig 4A). Previous work showed that this enhancer is sufficient to drive bipolar-specific expression (Kim et al., 2008) and our recent findings (Goodson, Park, Brzezinski et al, in press) suggest it is necessary for VSX2 expression and bipolar cell formation. We electroporated VE-CRE into the retina of P0 RFP/+ controls and RFP/PRDM1 littermates and raised the pups to P7 (Fig 4A). RFP+ cells were quantified based on morphology and location within the retina. As expected, the majority of RFP+ cells were bipolars (Fig 4B, D). However, about 12% of RFP+ cells were photoreceptors, suggesting that some VE-expressing cells are poised between bipolar and photoreceptor fates (Fig 4C). We also observed a small number of amacrines and Müller glia in control electroporations, which likely reflects off-target CRE activity (Fig 4B, E, H). VE-CRE electroporations in RFP/PRDM1 mice resulted in RFP+ cells that were three times more likely to be photoreceptors compared to control, which came at the direct expense of RFP+ bipolar cells (Fig 4B–D). We stained for VSX2 and found that the loss of RFP+ bipolar cells perfectly matched the loss of RFP+/VSX2+ cells (Fig 4E–F). The presence of VSX2 in morphologically identified bipolar cells suggested that they were accurately quantified and had repressed photoreceptor fate despite the misexpression of PRDM1. Photoreceptors did not co-express VSX2 in either condition. Thus, overexpression of PRDM1 in VSX2+ nascent bipolars was sufficient to force a fate change in some (~25%), but not all of the cells. Upon quantification, we observed no differences in the percentage of RFP+ cells that were amacrines or Müller glia between conditions (Fig 4G, data not shown). We also stained for OTX2 and the pan-amacrine marker PAX6 (de Melo et al., 2003) and found no differences in either between conditions (Fig 4H–J). This suggests that Prdm1 misexpression only alters fate choice within OTX2+ cells.
Figure 4. Overexpression of PRDM1 in developing retinas driven by VE-CRE at P7.
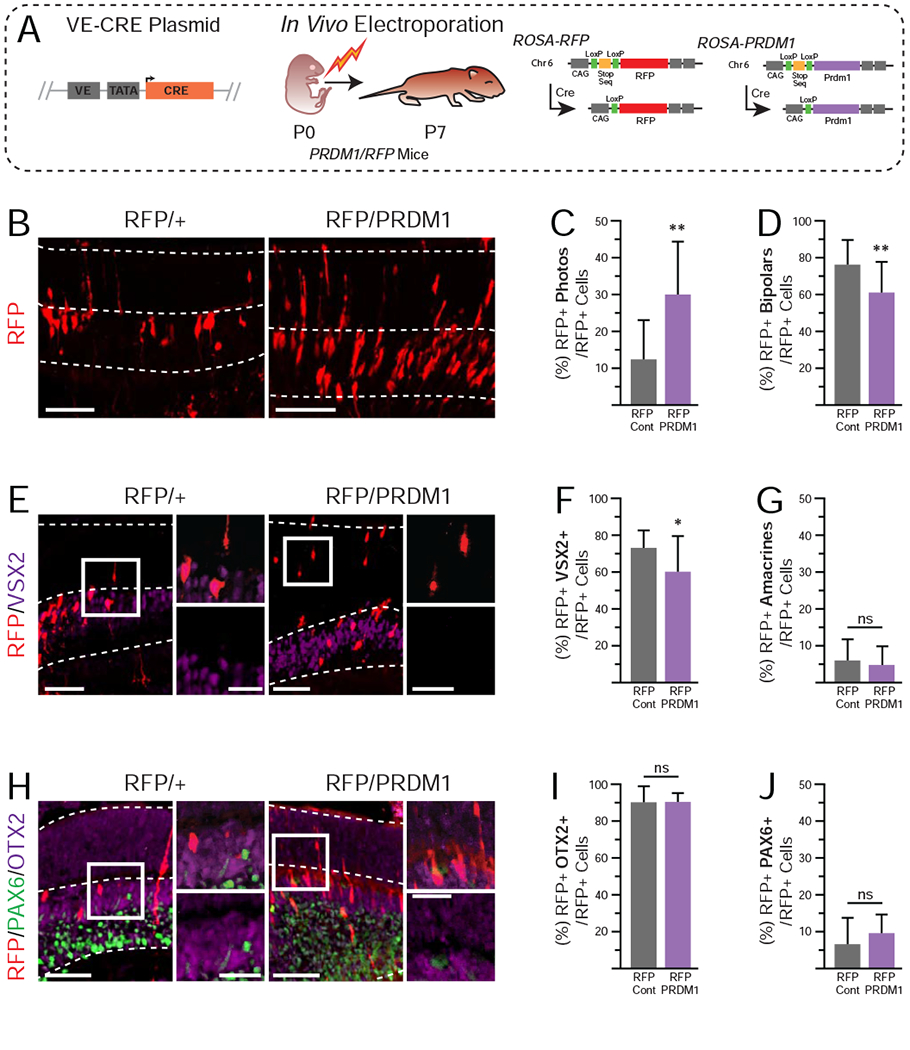
A)Schematic of experimental design. Mice are as described in Figure 1. B) Representative immunohistochemistry of electroporated RFP/+ controls compared to RFP/PRDM1 littermates. Arrowheads mark bipolar cells and asterisks mark photoreceptors. Electroporated RFP/PRDM1 pups have C) a significantly higher percentage of RFP+ cells that are photoreceptors, D) a significantly lower percentage that are bipolar cells, and G) no change in the percentage that are amacrines compared to controls. E) Stains of electroporated cells (red) and VSX2 (purple). F) Quantification showing a significant reduction in the percentage of RFP+ cells that co-express VSX2+ in RFP/PRDM1 mice. There is no apparent change in the intensity of VSX2 staining in bipolar cells from control or RFP/PRDM1 mice. H) Stains for electroporated cells (red) that express PAX6 (green) or OTX2 (purple). I-J) There is no difference in the expression patterns of PAX6 and OTX2. A total of 1,057 cells were quantified from 38 images. Statistics calculated based on number of mice (N), RFP/+ N=6, RFP/PRDM1 N=3. Error bars=standard deviation. ns=not significant, ***p<0.001. bars=50μm, inset bars=25μm, INL=Inner Nuclear Layer, ONL=Outer Nuclear Layer.
Constitutive PRDM1 expression appears toxic to mature photoreceptors
PRDM1 is expressed by OTX2+ cells throughout retinal development, but is downregulated in the early postnatal period and becomes undetectable by immunohistochemistry between P6 to P10 (Brzezinski et al., 2010). Although PRDM1 is important for photoreceptor formation, it is not appreciably expressed by mature rods or cones. When Prdm1 is turned on earlier in developing bipolars, it can cause a fate-shift to photoreceptor identity (Fig 4). However, our PCP2-CRE-AAV experiment suggests that expressing Prdm1 in mature photoreceptors is toxic (Fig 3). This suggested that constitutive PRDM1 expression would eventually kill photoreceptors that were formed from nascent bipolar cells.
To test this, we electroporated P0 RFP/+ control pups and RFP/PRDM1 littermates with the VE-CRE plasmid and raised them to P28 when the retina is mature. We assessed the fate of RFP+ cells by morphology and immunohistochemistry (Fig 5A). Unlike at P7, we did not observe differences in the percentage of RFP+ cells that became photoreceptors and bipolars between control and RFP/PRDM1 conditions (Fig 5B–D). The percentage of electroporated cells that were photoreceptors at P28 in the RFP/PRDM1 condition matched controls at both P7 and P28 (~10%). This argues that the increase in photoreceptors caused by Prdm1 misexpression was lost by P28. While it is likely that photoreceptor cell death is the cause, the sparse label nature of our approach and the narrow time frame of cell death markers prevented us from directly measuring cell death. Additionally, there was large variation in the total number of electroporated cells per eye that prevented us from determining if there were significantly fewer cells in the RFP/PRDM1 condition at P7 and P28. There were no differences in the percentage of RFP+ cells that were amacrines or Müller glia at P28 (Fig 5G, data not shown). There were also no differences in the number of RFP+ cells that co-expressed VSX2+, OTX2+, or PAX6+ between conditions (Fig 5E–J). Taken together with the PCP2-CRE-AAV findings, we infer that sustained Prdm1 expression in photoreceptors is toxic. In contrast, Prdm1 misexpression in other cell types does not appear to affect their survival.
Figure 5. Overexpression of PRDM1 in developing retinas driven by VE-CRE at P28.
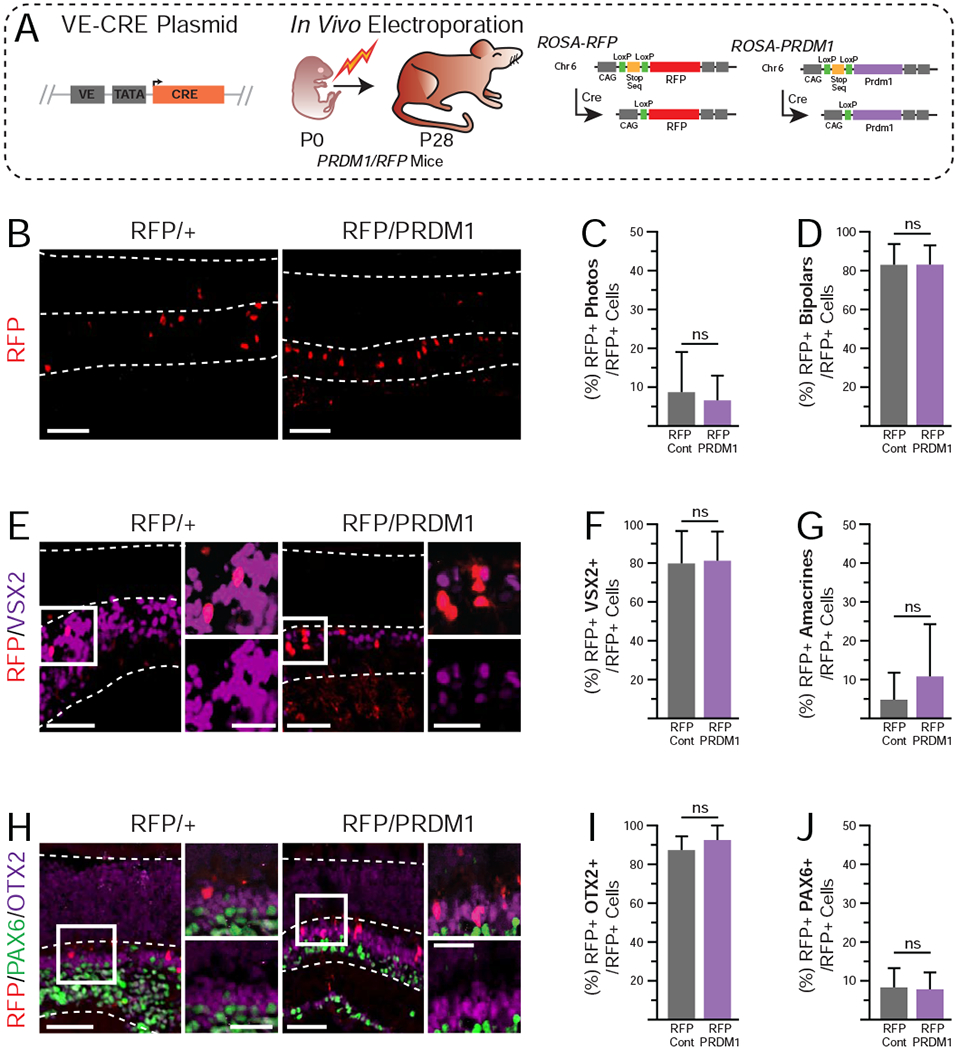
A)Schematic of experimental design. Mice are as described in Figure 1. B) Representative immunohistochemistry of electroporated RFP/+ controls compared to RFP/PRDM1 littermates. Arrowheads mark bipolar cells and asterisks mark photoreceptors. Note that the RFP labeling intensity of photoreceptors is less than other cell types in the retina. Electroporated RFP/PRDM1 pups have no differences in C) the percentage of RFP+ cells that are photoreceptors, D) bipolar cells, or G) amacrines compared to controls. E) Stains of electroporated cells (red) and VSX2 (purple). F) Quantification showing no change in the percentage of RFP+ cells that co-express VSX2. H) Stains for electroporated cells (red) that express PAX6 (green) or OTX2 (purple). I-J) there is no difference in the expression patterns of PAX6 and OTX2. A total of 826 cells were quantified from 41 images. Statistics calculated based on number of mice (N), RFP/+ N=6, RFP/PRDM1 N=4. Error bars=standard deviation. ns=not significant, bars=50μm, inset bars=25μm, INL=Inner Nuclear Layer, ONL=Outer Nuclear Layer.
DISCUSSION
During late retinogenesis, OTX2+ cells decide between rod photoreceptor and bipolar fates. This is driven in part by the actions of OTX2’s downstream targets, Vsx2 and Prdm1 (Fig 6D). The loss of Prdm1 from the retina results in a fate-shift to VSX2+ bipolar cells, even after cells have started to show photoreceptor-like morphology and markers (Brzezinski et al., 2013). This suggests that there is some window wherein developing OTX2+ cells have selected a specific fate but remain susceptible to signals driving an alternative identity. Here, we tested whether OTX2+ cells can change their identity at different stages of maturity (Fig 6A–C). Our findings show that a subset of specified bipolar interneurons can be shifted to photoreceptor fates by PRDM1 (Fig 4, 6B). However, there is a narrow temporal limit to this fate conversion, and mature bipolars do not change their fate in response to PRDM1 (Fig 3, 6C). While Prdm1 is critical for photoreceptor development, we provide evidence that if PRDM1 is constitutively expressed in mature photoreceptors it will cause their death (Fig 3, 5, 6C).
Figure 6. Summary of findings.
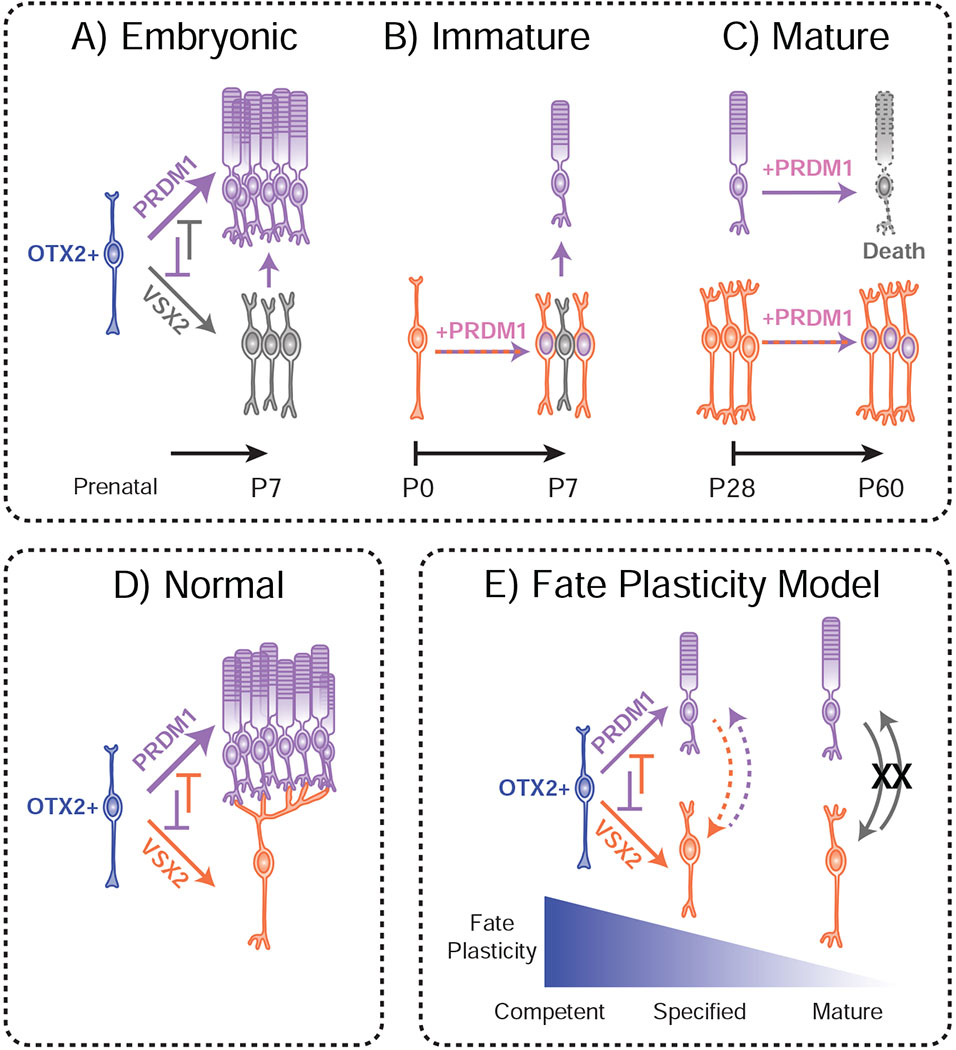
A) Broadly turning on PRDM1 in embryonic retinas prevents bipolar cell formation, ostensibly resulting in the increased formation of rods. B) Misexpressing PRDM1 in nascent bipolar cells causes some to convert into rods by P7. C) Activating PRDM1 in a mature retina has no apparent effect on bipolar cell fate, but is likely toxic to rods. These PRDM1-expressing bipolars still make VSX2, however, it is possible that these bipolar cells have abnormal gene expression patterns despite their normal morphology. D) During normal development, OTX2+ cells express PRDM1 and VSX2. These transcription factors interact to control the decision between rod photoreceptor and bipolar cell interneuron fates. This is done, in part, by stabilizing fate decisions. E) OTX2+ cells are initially competent to form rods and bipolars. Rod and bipolar fates are plastic even after fate is specified, but this plasticity is rapidly lost as the cells mature.
Bipolar cell fate choice is plastic over a narrow window of development
We used Prdm1 misexpression to ask whether bipolar cell fate can be superseded at three different stages of retinal development. Using PAX6-CRE mice, we were able to drive Prdm1 misexpression before the decision to become a bipolar cell is normally made. As predicted from prior work, this early Prdm1 misexpression was highly effective at preventing bipolar cell formation (Fig 1–2, 6A) (Brzezinski et al., 2010; Katoh et al., 2010). Next, we used an electroporation approach to permanently activate Prdm1 in newly specified bipolar cells. Misexpression at this stage blocked a portion of bipolar cell formation while increasing photoreceptors (Fig 4). Thus, bipolar fate can be superseded by Prdm1 around the time of specification, although at a modest efficacy compared to early misexpression (Fig 6A–B). This moderate efficiency could reflect a technical limit of our experiment based on the time it takes for VE-CRE to recombine the ROSA-PRDM1 allele and upregulate Prdm1 expression. This would be especially true if the time window for changing fates is brief. The bipolar fate plasticity is similar to what we saw in Prdm1 conditional knock-out retinas, where a substantial number of rod photoreceptors appear to directly transition into bipolar cells in the early postnatal period (Brzezinski et al., 2013). Lastly, we activated Prdm1 expression in mature bipolar cells using an AAV strategy. This had no discernable effect on bipolar cell fate stability (Fig 3, 6C). Taken together, our results suggest that bipolar cell fate is malleable. Nonetheless, plasticity is limited to a narrow time period around the bipolar fate specification event (Fig 6).
Bipolar cell plasticity is similar to what has been observed in rods. Knocking out the rod-instructive factor Nrl prevents rod formation and massively increases cone genesis if done early in development (Mears et al., 2001; Montana et al., 2013). Removing Nrl shortly after birth incompletely converts rods into cones, while targeting Nrl in the adult retina has a more modest effect that is limited to changes in rod- and cone-specific gene expression (Montana et al., 2013; Yu et al., 2017; Zhu et al., 2017). These data argue that OTX2+ neurons are transiently plastic and progressively alter their epigenetic states to “lock-in” their identities (Montana et al., 2013) (Fig 6E). The ability to reprogram bipolar cell identity around the time of fate specification appears more modest than what occurs to photoreceptors in Nrl or Prdm1 knockouts. This raises the possibility that some cell types, such as photoreceptors, take longer to “lock-in” their identities. This may correlate with the highly specialized chromatin architecture that slowly forms in rods over several weeks in postnatal mice (Solovei et al., 2009). In this model, Prdm1 may inhibit bipolar fate while rods develop their “locked-in” stabilized state. This could explain why PRDM1 is only active for a relatively short period during photoreceptor development.
Our previous loss-of-function data and current PRDM1 gain-of-function experiments show that cell fate choice is plastic in developing OTX2+ cells (Fig 6E) (Brzezinski et al., 2013). While this fate plasticity appears to be short-lived in newly specified bipolar cells, it is unclear how long nascent photoreceptors remain plastic. As PRDM1 expression is low in photoreceptors by P7, it is unlikely that plasticity extends beyond the first postnatal week (Fig 6E). Since fate plasticity is only widespread in genetically perturbed conditions, it is likely that Prdm1 and VSX2 robustly stabilize fate choices in OTX2+ cells. How PRDM1 and VSX2 interact with OTX2 and other factors to select and maintain cell fate decisions remains to be determined.
PRDM1 acts in a context-specific fashion in OTX2+ cells
OTX2 directly activates the expression of Prdm1 and Vsx2 through essential retina-specific enhancer elements (Brzezinski et al., 2013; Kim et al., 2008; Mills et al., 2017; Wang et al., 2014) (Goodson, Park, Brzezinski et al, in press). Gain- and loss-of-function experiments argue that the balance of photoreceptors and bipolar cells is controlled by direct cross-repression between Prdm1 and Vsx2 (Brzezinski et al., 2010; Brzezinski et al., 2013; Dorval et al., 2006; Katoh et al., 2010; Livne-Bar et al., 2006). Overexpression of Prdm1 early in development blocked the intense VSX2 labeling that is characteristic of bipolar cells (Fig 2). However, permanently misexpressing Prdm1 at later times did not block VSX2 expression in morphologically identified bipolar cells (Figs 3–5). There are several potential explanations for this observation. As mentioned above, one possibility is that mature bipolar cells become “locked-in” and can no longer respond to PRDM1. This could be through changes in the epigenetic state that prevents PRDM1 from silencing Vsx2 in mature bipolar cells. This could also occur if there are unique enhancers that maintain VSX2 expression in mature bipolar cells that are not regulated by PRDM1. We observed that misexpression of Prdm1 in early development did not block VSX2 expression in progenitor cells (Fig 1C–D), which would be expected to severely limit retinal progenitor proliferation (Burmeister et al., 1996; Green et al., 2003; Livne-Bar et al., 2006; Sigulinsky et al., 2015). This argues that PRDM1 regulates Vsx2 expression in a context-specific fashion. This could occur if PRDM1 has no regulatory effect on Vsx2 enhancers that control progenitor-specific expression, while binding the bipolar-specific enhancer and suppressing its activity. However, the 164bp bipolar-specific Vsx2 enhancer sequence (Kim et al., 2008) lacks a high-confidence PRDM1 binding site, raising the possibility that PRDM1 silences Vsx2 by binding at a different site (Katoh et al., 2010) or by acting indirectly on Vsx2 expression. We observed that most retinal cell types were unaffected by Prdm1 misexpression during development or in adults (Fig 1–5). This further argues that Prdm1 acts in a context-specific fashion, only affecting fate in cells that are competent to become bipolar cells. Other cells are either unaffected by PRDM1 activity or “locked-in” and refractory to its influence. Additional studies examining PRDM1 and VSX2 binding sites in the developing retina are needed to understand the mechanisms these two factors use to control photoreceptor and bipolar fate choice.
PRDM1 toxicity in mature photoreceptors
In the retina, Prdm1 is transiently expressed in OTX2+ cells. Expression does not persist into mature photoreceptors (Brzezinski et al., 2010). There are several other systems where transient PRDM1 expression affects fate determination, such as primordial germ cell genesis in mice as well as slow twitch muscle and neural crest development in zebrafish (Baxendale et al., 2004; Bikoff et al., 2009; Hernandez-Lagunas et al., 2005; Liew et al., 2008; Magnusdottir et al., 2013; Ohinata et al., 2005; Powell et al., 2013). We presumed that Prdm1 expression was transient because it was no longer needed in mature photoreceptors. However, we were surprised to see that sustained Prdm1 expression appeared to kill photoreceptors. This was in contrast to other retinal cell types, including mature bipolar cells, which seemed unaffected by Prdm1 misexpression. Thus, the apparent toxicity of PRDM1 was not simply due to its overexpression, but rather because of a specific effect on photoreceptors (Fig 6C). We did not observe acute toxicity with Prdm1 overexpression at P0 or P7. This suggests that toxicity occurs relatively late, during or after the process of maturation. It is unclear why sustained PRDM1 expression is toxic only in photoreceptors. One possibility is that photoreceptors are particularly sensitive to the misexpression of proteins in general. For example, even modest overexpression of Rhodopsin can be toxic to rods (Tan et al., 2001). PRDM1 may thus act in a non-specific way to impart toxicity. Another possibility is that PRDM1 inhibits both bipolar formation and photoreceptor maturation. The inability to mature may then cause photoreceptor cell death. Another reason may be that PRDM1 expression is incompatible with the formation or maintenance of the specialized rod nuclear architecture that forms in the postnatal period (Solovei et al., 2009). Due to chromatin accessibility differences between photoreceptors versus other retinal cell types, PRDM1 may have the ability to regulate deleterious gene expression networks only in photoreceptors. Lastly, alterations in the dosage of key transcription factors may destabilize gene regulatory networks required for photoreceptor function and survival. In support of this possibility, a recent report showed that overexpressing OTX2 in photoreceptors caused mild toxicity within rods (Yamamoto et al., 2020). Taken together, these data suggest that careful control of transcription factor expression timing and levels are essential for rod survival. The mechanisms that underlie PRDM1-induced photoreceptor toxicity remain to be uncovered.
METHODS
Mice
All animals were utilized in accordance with procedures approved by the University of Colorado Anschutz Medical Campus IACUC. ROSA-RFP mice (B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J) (Strain #007914) (Madisen et al., 2010) and VSX2-CRE mice (129S1.Cg-Tg(Vsx2-cre)2690Chow/J) (Strain #026200) (Nickerson et al., 2011) were obtained from Jackson Labs (Bar Harbor, ME, USA). The αPax6-Cre-IRES-GFP mice (Marquardt et al., 2001) were a gift from Dr. Ruth Ashery-Padan (Tel Aviv University, Israel). All pups were housed with their parent and sibling following electroporation until collected. Animals older than P28 were separated by sex and housed in groups of 3-5 mice until tissues were collected. Mice were euthanized by CO2 asphyxiation or decapitation.
ROSA-PRDM1 Mouse construction
To build the ROSA-PRDM1 (PRDM1) mouse line, we first obtained the Ai9 targeting vector from Dr. Hongkui Zeng (Allen Institute, Seattle, WA, USA) (Madisen et al., 2010). This construct is designed to insert a CAG enhancer/promoter, LoxP-STOP-LoxP sequence, and a tdTomato cassette into the ROSA26 locus on chromosome 6 (Madisen et al., 2010). We cut the Ai9 vector with FseI to remove the tdTomato sequence and re-ligated the plasmid. We PCR-amplified the mouse Prdm1 cDNA sequence and used In-Fusion cloning (Takara Bio, Mountain View, CA, USA) to insert it into the remaining FseI site in the modified Ai9 vector. This drives the expression of an 823 amino acid PRDM1 protein (NCBI: NP_031574.2). The targeting vector was validated by Sanger sequencing and used to target C57BL/6 embryonic stem cells with the assistance of the University of Colorado Bioengineering Core facility. After selection for neomycin resistance, several properly targeted clones were obtained and two were karyotyped. One normal karyotyped embryonic stem cell line was then used to generate chimeras. From this, one chimera was used to establish the ROSA-PRDM1 line (Fig S1). RFP and PRDM1 mice were genotyping using primers for both wildtype and mutant genes, including the forward primer 5’-CTCTGCTGCCTCCTGGCTTCT and the reverse primer 5’-CGAGGCGGATCACAAGCAATA for wildtype or 5’-TCAATGGGCGGGGGTCGTT for mutants. Primers specific for PRDM1 mice included 5’-GCTGATCCGGAACCCTTAAT and 5’-GATCCCAGTCTCTGCCAGTC (Fig S1). PCR was performed using 35 cycles of 94°C for 25”, 61°C for 30”, and 72°C for 30”. Cre PCR genotyping was performed at 59°C annealing as previously described (Brzezinski et al., 2010).
CRE-AAV Production
Adeno-Associated Virus (AAV) vector preparations were produced by the 2-plasmid, co-transfection method (Zolotukhin et al., 1999; Zolotukhin et al., 2002). Briefly, approximately 1 X 109 HEK 293 cells was cultured in one Cell Stack (Corning Inc., Corning, NY, USA) in Dulbecco’s Modified Eagle’s Medium (Hyclone Laboratories, Logan, UT, USA), supplemented with 5% fetal bovine serum and antibiotics. A CaPO4 transfection precipitation was done by mixing a 1:1 molar ratio of recombinant (r) AAV vector plasmid DNA and serotype-specific rep-cap helper plasmid DNA. For the virus payload, we used a 1.65kb DNA sequence upstream of the human PCP2 gene (also known as Ple155) to drive bipolar cell-specific expression of Cre recombinase (de Leeuw et al., 2014; Scalabrino et al., 2015). The capsid was engineered to generate AAV2 vectors with the following substitutions: Y272F, Y444F, Y500F, Y730F, and T491V (AAV2, quad Y-F + T471V) (Kay et al., 2013). After transfection, the cells grew at 37°C, 7% CO2, for 60 hours and were then harvested and lysed by three freeze/thaw cycles. The crude lysate was clarified by centrifugation and the resulting vector-containing supernatant was divided among four discontinuous iodixanol step gradients. The gradients were centrifuged at 350,000g for 1 hour, and 5mL of the 60–40% step interface was removed from each gradient and combined. This iodixanol fraction was further purified and concentrated by column chromatography on a 5-mL HiTrap Q Sepharose (anion exchange) column using a Pharmacia AKTA FPLC system (GE Healthcare Life Sciences, Pittsburgh, PA, USA). The vector was eluted from the column using 215mM NaCl, pH 8.0, and the rAAV peak collected. The vector-containing fraction was then concentrated, and buffer exchanged in Alcon BSS with 0.014% Tween 20, using a Biomax 100K concentrator (Millipore, Billerica, MA, USA). The vector was titered for DNase-resistant vector genomes by real-time PCR relative to a standard. The purity of the vector was validated using three standard assays. First by silver-stained SDS-PAGE, the three AAV capsid proteins are the only visible protein bands in an acceptable prep. The second assay screened for bioburden by adding 10μL of the final product to 15mL of non-selective LB and monitoring for 5 days. Lastly, the sample was assayed for Endotoxin using an Endosafe-PTS test system (Charles River, Durham, NC, USA). Passing criteria was ≤5 EU/mL.
AAV Injections
In Vivo CRE-AAV injections were performed on P28 mice utilizing common intraocular injection methods. In short, the concentrated CRE-AAV stock (7.28 X 1013 vector genomes per mL) was diluted 1:10 in sterile nuclease-free water. Mice were anesthetized utilizing 2% isoflurane mixed with oxygen and were kept under anesthesia utilizing a nose cone giving a continuous 2% isoflurane and oxygen mixture. Under a dissecting microscope the mice were held steady and a 31G needle was used to create a perforation of the sclera nasally near the junction with the cornea. A Hamilton syringe (7653-01, Hamilton Company, Reno, NV) was inserted into the vitreous and 0.5μL of diluted AAV solution was injected. The mouse was allowed to recover on a heat block before being returned to housing.
VE-CRE Plasmid Construction
To create the VE-CRE plasmid, we modified the pMIN-nGFP plasmid used previously to track enhancer activity in the retina (Mills et al., 2017; Wilken et al., 2015). This vector has a TATA box minimal promoter followed by a nuclear-localized GFP cassette and a polyadenylation sequence. We inserted a P2A self-cleavage peptide sequence and a Cre cassette (based on NCBI: AAS78497) in frame downstream of GFP using In Fusion cloning (Takara). Next, we PCR amplified the 164bp Vsx2 bipolar-specific enhancer (Kim et al., 2008) from C57BL/6J genomic DNA. This was inserted into the EcoRI and KpnI sites just upstream of the TATA box in the plasmid using In Fusion cloning. The resulting VE-CRE plasmid was validated by Sanger sequencing.
Electroporation
In Vivo electroporation’s were performed on P0 pups following previously described methods (de Melo and Blackshaw, 2011). All electroporated DNA was delivered at a concentration of 2μg/μL. Mice were cryoanesthetized for approximately five minutes until all response to external stimulation ceased. Under a dissecting microscope and light, the mice were placed on a frozen ice pack and held steady while a 31G needle was used to create an opening along the eye-crease. The lid was then opened and a second 31G needle was utilized to create a perforation of the sclera nasally near the junction with the cornea. A Hamilton syringe was inserted into the opening and pressed medially back against the central anterior portion of the retina. 0.5μL of 2μg/μL DNA in sterile H2O was then injected between the retina and underlying membrane and the syringe removed. The mouse’s head was placed between a tweezertrode electrode (BTX, Holliston, MA) and electroporated with 5 80V square wave pulses for 50ms with a 950ms delay between each pulse using a Bio-Rad Gene Pulser Xcell (BioRad, Hercules, CA, USA). Neosporin was placed on the surface of the eyelid to prevent infection. The mouse was brought back up to normal body temperature on a heat pad and returned to the mother.
Retina collection and Immunohistochemistry
Under a dissecting scope, a 31G needle was used to create 3-4 perforations in the sclera near the junction of the cornea ensuring fluid access to the vitreous, the eyes were then placed in 2% paraformaldehyde for 2-4 hours, followed by cryoprotection at 4°C with an increasing concentration series (10-30%) of sucrose solutions in PBS. Eyes were stored in 30% sucrose overnight and flash frozen in OCT (Sakura Finetech, Torrance, CA, USA). A shorter fixation time of 30 minutes was utilized with P0 eyes to improve Prdm1 immunostaining. Eyes were cryosectioned at 12μm and transferred to Shandon Colorfrost Plus microscope slides (ThermoFisher Scientific, Waltham, MA, USA). Slides were stored in a −20°C freezer until immunohistochemistry was performed.
Immunohistochemistry procedures were conducted as previously described (Brzezinski et al., 2010; Brzezinski et al., 2012; Goodson et al., 2018; Mills et al., 2017; Park et al., 2017). Slides were washed in PBS, blocked for two hours in 5% milk block (the supernatant of a solution of 5% powdered milk, 0.5% Triton X100, 0.2% NaN3, in PBS), and placed in primary antibody in 5% milk block overnight at room temperature. The next day slides were washed with PBS. Secondary antibodies in 5% milk block were applied and the slides left one to two hours in the dark. Slides were washed in PBS and covered with Fluoromount-G (eBioscience, San Diego, CA, USA) and a glass coverslip.
The following primary antibodies were used in the given concentrations: mouse anti-CRE (1:250; MAB3120, Millipore); mouse anti-GFP (1:1000; ab13970, Abcam, Cambridge, MA, USA); mouse anti-ISLET1/2 (1:200, 39.4D5, Developmental Studies Hybridoma Bank, Iowa City, IA USA), goat anti-OTX2 (1:250; AF1979, Bio-Techne Corporation, Minneapolis, MN, USA); rabbit anti-PAX6 (1:500; PRB-278P, Covance, Princeton, NJ, USA); chicken anti-RFP (1:100; 600-901-379, Rockland Antibodies & Assays); mouse anti-RFP (1:1000; ab65856, Abcam); Rabbit anti-Secretagogin (1:2500, RD181120100; Biovendor, Ashville, NC, USA); and sheep anti-VSX2 (1:400; X1179P, Exalpha Biologicals, Shirley, MA, USA)
Imaging and Cell Counts
Slides were imaged following immunohistochemical staining with a 20x objective on a Nikon C2 laser scanning confocal scope (Melville, NY, USA). Retinas were imaged at multiple 1.5 μm thick steps and 4-6 images were collected per section (as a Z-stack) and then compressed to a single layer utilizing a maximum intensity stack compression in ImageJ (Schneider 2012). All images were minimally processed in Adobe Photoshop (San Diego, CA, USA), and cell counts were conducting utilizing manual counting.
Images were taken from the eyes of both male and female mice. PCP2-CRE-AAV images were taken in both central and peripheral retina, but no differences were observed between these regions. Images from PAX6-CRE mice were taken in the peripheral retina. Due to the nature of in vivo retina electroporations, most sections covered central or mid-peripheral retina, however, some mice had electroporated cells that extended to the periphery. In the case of electroporations that required a subretinal injection, counts were only conducted on sections of relatively intact retina such that disrupted sections were excluded from quantification. We also excluded images with less than 10 labeled cells. Retinas were imaged in 2-6 locations and the counts pooled to calculate the mean and standard deviation.
For all plots the error bars represent standard deviation. One-tailed unpaired t-tests with the assumption of heteroscedasticity were used for statistics. The degrees of freedom were based on the number (N) of eyes in AAV experiments and the number of animals examined when electroporations or PAX6-CRE mice were involved. Throughout, p<0.05 was considered significant, and was calculated utilizing GraphPad Prism 8 (GraphPad Software, La Jolla California, USA).
Supplementary Material
Supplemental Figure 1. ROSA-PRDM1 transgene. Schematic of the targeted ROSA26 locus on mouse chromosome (chr) 6 approximately to scale in base pairs (bp). The transgene includes a CAG enhancer/promoter sequence followed by a Flp recombination site (Frt) and a LoxP flanked STOP cassette. The stop cassette contains numerous stop codons in each reading frame and three poly adenylation (pA) sequences. Upon CRE-mediated recombination, the STOP sequence is deleted and the Prdm1 cDNA becomes permanently expressed. Downstream of Prdm1 is a woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) and a bovine growth hormone (bGH) pA sequence. Next, there is a phosphoglycerate kinase (PGK) promoter driving the expression of a neomycin resistance (NeoR) cassette. It is followed by a PGK pA sequence. An additional Frt site is upstream of the NeoR cassette to allow for Flp-mediated deletion of the LoxP-STOP-LoxP Prdm1 portion of the transgene. There are also AttB and AttP sequences flanking the NeoR cassette that allow for PhiC31-mediated deletion of the NeoR cassette. For genotyping, primers 1 and 2 are used to detect ROSA-PRDM1 and ROSA-RFP transgenes. Primers 3 and 4 detect only the ROSA-PRDM1 transgene. Primers 1 and 5, which target ROSA26 genomic DNA, are used to detect wildtype (untargeted) alleles.
Supplemental Figure 2. Early CRE expression in VSX2-CRE mice leads to a broad recombination pattern in adult mice. A) Schematic of experimental design. The VSX2-CRE mice are designed to drive bipolar-specific CRE expression (see Nickerson et al, 2011). ROSA-RFP and PRDM1 mice are as described in Figure 1. B-F) Adult mice stained for CRE (green), RFP (red) and OTX2 (purple). B). No CRE or RFP staining is present in RFP/+ mice. C) VSX2-CRE retinas have CRE expression in OTX2+ bipolar cells but lack RFP staining. The green vascular staining is non-specific background. D) The VSX2-CRE/RFP mice have bipolar CRE expression, but pan-retinal RFP expression. E) An RFP/+ mouse, without VSX2-CRE or CRE staining, has global RFP expression when the mother carried a VSX2-CRE allele. F) Paternally inherited VSX2-CRE mice also show global, yet mosaic RFP expression. Bars=50μm, INL=Inner Nuclear Layer, ONL=Outer Nuclear Layer.
Supplemental Figure 3. Increased exposure of virally infected cells to show photoreceptor labeling. Levels of the red channel (RFP) are increased to reveal photoreceptors. Panel A corresponds to Figure 3B and panel B corresponds to Figure 3H. All increases in brightness were linear and matched across images to preserve relative exposure between conditions.
HIGHLIGHTS.
PRDM1 misexpression can block bipolar cell development
The presence of PRDM1 in new bipolar cells can convert them into photoreceptors
Mature bipolar cells do not respond to PRDM1 misexpression
Constitutive PRDM1 expression appears toxic to mature photoreceptors
ACKNOWLEDGEMENTS
The authors thank Michael Kaufman and Grace Randazzo for technical support and for critically reading the manuscript. The authors thank Hongkui Zeng and Ruth Ashery-Padan for key plasmid and mouse reagents and the University of Colorado Denver Bioengineering Core Facility for assistance generating the ROSA-PRDM1 knock-in mice.
FUNDING
Work was supported in part by the National Institutes of Health R01-EY024272 (JAB), T32-NS099042 (NBG) and by NIH/NCATS Colorado CTSA Grant Number TL1 TR002533 (NBG). Work was partially supported by a Challenge Grant to the CU Department of Ophthalmology from Research to Prevent Blindness, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baxendale S, Davison C, Muxworthy C, Wolff C, Ingham PW, Roy S, 2004. The B-cell maturation factor Blimp-1 specifies vertebrate slow-twitch muscle fiber identity in response to Hedgehog signaling. Nat Genet 36, 88–93. [DOI] [PubMed] [Google Scholar]
- Beby F, Lamonerie T, 2013. The homeobox gene Otx2 in development and disease. Exp Eye Res 111, 9–16. [DOI] [PubMed] [Google Scholar]
- Bikoff EK, Morgan MA, Robertson EJ, 2009. An expanding job description for Blimp-1/Prdm1. Current opinion in genetics & development 19, 379–385. [DOI] [PubMed] [Google Scholar]
- Brzezinski JA, Lamba DA, Reh TA, 2010. Blimp1 controls photoreceptor versus bipolar cell fate choice during retinal development. Development 137, 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski JA, Prasov L, Glaser T, 2012. Math5 defines the ganglion cell competence state in a subpopulation of retinal progenitor cells exiting the cell cycle. Developmental biology 365, 395–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski JA, Reh TA, 2015. Photoreceptor cell fate specification in vertebrates. Development 142, 3263–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski JA, Uoon Park K, Reh TA, 2013. Blimp1 (Prdm1) prevents re-specification of photoreceptors into retinal bipolar cells by restricting competence. Developmental biology 384, 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister M, Novak J, Liang MY, Basu S, Ploder L, Hawes NL, Vidgen D, Hoover F, Goldman D, Kalnins VI, Roderick TH, Taylor BA, Hankin MH, McInnes RR, 1996. Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat Genet 12, 376–384. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM, 1979. Rods and cones in the mouse retina. II. Autoradiographic analysis of cell generation using tritiated thymidine. J Comp Neurol 188, 263–272. [DOI] [PubMed] [Google Scholar]
- de Leeuw CN, Dyka FM, Boye SL, Laprise S, Zhou M, Chou AY, Borretta L, McInerny SC, Banks KG, Portales-Casamar E, Swanson MI, D’Souza CA, Boye SE, Jones SJ, Holt RA, Goldowitz D, Hauswirth WW, Wasserman WW, Simpson EM, 2014. Targeted CNS Delivery Using Human MiniPromoters and Demonstrated Compatibility with Adeno-Associated Viral Vectors. Mol Ther Methods Clin Dev 1, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo J, Blackshaw S, 2011. In vivo electroporation of developing mouse retina. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo J, Qiu X, Du G, Cristante L, Eisenstat DD, 2003. Dlx1, Dlx2, Pax6, Brn3b, and Chx10 homeobox gene expression defines the retinal ganglion and inner nuclear layers of the developing and adult mouse retina. J Comp Neurol 461, 187–204. [DOI] [PubMed] [Google Scholar]
- Dorval KM, Bobechko BP, Fujieda H, Chen S, Zack DJ, Bremner R, 2006. CHX10 targets a subset of photoreceptor genes. J Biol Chem 281, 744–751. [DOI] [PubMed] [Google Scholar]
- Elshatory Y, Deng M, Xie X, Gan L, 2007a. Expression of the LIM-homeodomain protein Isl1 in the developing and mature mouse retina. J Comp Neurol 503, 182–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshatory Y, Everhart D, Deng M, Xie X, Barlow RB, Gan L, 2007b. Islet-1 Controls the Differentiation of Retinal Bipolar and Cholinergic Amacrine Cells. J. Neurosci. 27, 12707–12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossat N, Le Greneur C, Beby F, Vincent S, Godement P, Chatelain G, Lamonerie T, 2007. A new GFP-tagged line reveals unexpected Otx2 protein localization in retinal photoreceptors. BMC Dev Biol 7, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson NB, Nahreini J, Randazzo G, Uruena A, Johnson JE, Brzezinski J.A.t., 2018. Prdm13 is required for Ebf3+ amacrine cell formation in the retina. Developmental biology 434, 149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green ES, Stubbs JL, Levine EM, 2003. Genetic rescue of cell number in a mouse model of microphthalmia: interactions between Chx10 and G1-phase cell cycle regulators. Development 130, 539–552. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lagunas L, Choi IF, Kaji T, Simpson P, Hershey C, Zhou Y, Zon L, Mercola M, Artinger KB, 2005. Zebrafish narrowminded disrupts the transcription factor Prdm1 and is required for neural crest and sensory neuron specification. Developmental biology 278, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Omori Y, Onishi A, Sato S, Kondo M, Furukawa T, 2010. Blimp1 suppresses Chx10 expression in differentiating retinal photoreceptor precursors to ensure proper photoreceptor development. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 6515–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay CN, Ryals RC, Aslanidi GV, Min SH, Ruan Q, Sun J, Dyka FM, Kasuga D, Ayala AE, Van Vliet K, Agbandje-McKenna M, Hauswirth WW, Boye SL, Boye SE, 2013. Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors. PLoS One 8, e62097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Matsuda T, Cepko CL, 2008. A core paired-type and POU homeodomain-containing transcription factor program drives retinal bipolar cell gene expression. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 7748–7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre JL, Zhang Y, Meister M, Wang X, Sanes JR, 2008. gamma-Protocadherins regulate neuronal survival but are dispensable for circuit formation in retina. Development 135, 4141–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew HP, Choksi SP, Wong KN, Roy S, 2008. Specification of vertebrate slow-twitch muscle fiber fate by the transcriptional regulator Blimp1. Developmental biology 324, 226–235. [DOI] [PubMed] [Google Scholar]
- Livne-Bar I, Pacal M, Cheung MC, Hankin M, Trogadis J, Chen D, Dorval KM, Bremner R, 2006. Chx10 is required to block photoreceptor differentiation but is dispensable for progenitor proliferation in the postnatal retina. Proc Natl Acad Sci U S A 103, 4988–4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H, 2010. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13, 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusdottir E, Dietmann S, Murakami K, Gunesdogan U, Tang F, Bao S, Diamanti E, Lao K, Gottgens B, Azim Surani M, 2013. A tripartite transcription factor network regulates primordial germ cell specification in mice. Nat Cell Biol 15, 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P, 2001. Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105, 43–55. [DOI] [PubMed] [Google Scholar]
- Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, Sieving PA, Swaroop A, 2001. Nrl is required for rod photoreceptor development. Nat Genet 29, 447–452. [DOI] [PubMed] [Google Scholar]
- Mills TS, Eliseeva T, Bersie SM, Randazzo G, Nahreini J, Park KU, Brzezinski J.A.t., 2017. Combinatorial regulation of a Blimp1 (Prdm1) enhancer in the mouse retina. PLoS One 12, e0176905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana CL, Kolesnikov AV, Shen SQ, Myers CA, Kefalov VJ, Corbo JC, 2013. Reprogramming of adult rod photoreceptors prevents retinal degeneration. Proc Natl Acad Sci U S A 110, 1732–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranishi Y, Terada K, Inoue T, Katoh K, Tsujii T, Sanuki R, Kurokawa D, Aizawa S, Tamaki Y, Furukawa T, 2011. An essential role for RAX homeoprotein and NOTCH-HES signaling in Otx2 expression in embryonic retinal photoreceptor cell fate determination. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 16792–16807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson PE, Ronellenfitch K, McEwan J, Kim H, McInnes RR, Chow RL, 2011. A transgenic mouse line expressing cre recombinase in undifferentiated postmitotic mouse retinal bipolar cell precursors. PLoS One 6, e27145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida A, Furukawa A, Koike C, Tano Y, Aizawa S, Matsuo I, Furukawa T, 2003. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci 6, 1255–1263. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Payer B, O’Carroll D, Ancelin K, Ono Y, Sano M, Barton SC, Obukhanych T, Nussenzweig M, Tarakhovsky A, Saitou M, Surani MA, 2005. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 436, 207–213. [DOI] [PubMed] [Google Scholar]
- Park KU, Randazzo G, Jones KL, Brzezinski J.A.t., 2017. Gsg1, Trnp1, and Tmem215 Mark Subpopulations of Bipolar Interneurons in the Mouse Retina. Invest Ophthalmol Vis Sci 58, 1137–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MJ, Perez ET, Martin JM, Reshel ST, Wallace KA, Capowski EE, Singh R, Wright LS, Clark EM, Barney PM, Stewart R, Dickerson SJ, Miller MJ, Percin EF, Thomson JA, Gamm DM, 2014. Modeling human retinal development with patient-specific induced pluripotent stem cells reveals multiple roles for visual system homeobox 2. Stem Cells 32, 1480–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DR, Hernandez-Lagunas L, LaMonica K, Artinger KB, 2013. Prdm1a directly activates foxd3 and tfap2a during zebrafish neural crest specification. Development 140, 3445–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthussery T, Gayet-Primo J, Taylor WR, 2010. Localization of the calcium-binding protein secretagogin in cone bipolar cells of the mammalian retina. J Comp Neurol 518, 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport DH, Wong LL, Wood ED, Yasumura D, LaVail MM, 2004. Timing and topography of cell genesis in the rat retina. J Comp Neurol 474, 304–324. [DOI] [PubMed] [Google Scholar]
- Sato S, Inoue T, Terada K, Matsuo I, Aizawa S, Tano Y, Fujikado T, Furukawa T, 2007. Dkk3-Cre BAC transgenic mouse line: a tool for highly efficient gene deletion in retinal progenitor cells. Genesis 45, 502–507. [DOI] [PubMed] [Google Scholar]
- Scalabrino ML, Boye SL, Fransen KM, Noel JM, Dyka FM, Min SH, Ruan Q, De Leeuw CN, Simpson EM, Gregg RG, McCall MA, Peachey NS, Boye SE, 2015. Intravitreal delivery of a novel AAV vector targets ON bipolar cells and restores visual function in a mouse model of complete congenital stationary night blindness. Hum Mol Genet 24, 6229–6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar K, Lapan SW, Whitney IE, Tran NM, Macosko EZ, Kowalczyk M, Adiconis X, Levin JZ, Nemesh J, Goldman M, McCarroll SA, Cepko CL, Regev A, Sanes JR, 2016. Comprehensive Classification of Retinal Bipolar Neurons by Single-Cell Transcriptomics. Cell 166, 1308–1323 e1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman RL, 1961. Histogenesis of mouse retina studied with thymidine H3, in: Smelser GK (Ed.), Structure of the Eye. Academic Press, New York, pp. 487–506. [Google Scholar]
- Sigulinsky CL, German ML, Leung AM, Clark AM, Yun S, Levine EM, 2015. Genetic chimeras reveal the autonomy requirements for Vsx2 in embryonic retinal progenitor cells. Neural Dev 10, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovei I, Kreysing M, Lanctot C, Kosem S, Peichl L, Cremer T, Guck J, Joffe B, 2009. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell 137, 356–368. [DOI] [PubMed] [Google Scholar]
- Sybirna A, Wong FCK, Surani MA, 2019. Genetic basis for primordial germ cells specification in mouse and human: Conserved and divergent roles of PRDM and SOX transcription factors. Curr Top Dev Biol 135, 35–89. [DOI] [PubMed] [Google Scholar]
- Tan E, Wang Q, Quiambao AB, Xu X, Qtaishat NM, Peachey NS, Lem J, Fliesler SJ, Pepperberg DR, Naash MI, Al-Ubaidi MR, 2001. The relationship between opsin overexpression and photoreceptor degeneration. Invest Ophthalmol Vis Sci 42, 589–600. [PubMed] [Google Scholar]
- Turner DL, Cepko CL, 1987. A common progenitor for neurons and glia persists in rat retina late in development. Nature 328, 131–136. [DOI] [PubMed] [Google Scholar]
- Turner DL, Snyder EY, Cepko CL, 1990. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron 4, 833–845. [DOI] [PubMed] [Google Scholar]
- Wang S, Sengel C, Emerson MM, Cepko CL, 2014. A gene regulatory network controls the binary fate decision of rod and bipolar cells in the vertebrate retina. Developmental cell 30, 513–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilken MS, Brzezinski JA, La Torre A, Siebenthall K, Thurman R, Sabo P, Sandstrom RS, Vierstra J, Canfield TK, Hansen RS, Bender MA, Stamatoyannopoulos J, Reh TA, 2015. DNase I hypersensitivity analysis of the mouse brain and retina identifies region-specific regulatory elements. Epigenetics Chromatin 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kon T, Omori Y, Furukawa T, 2020. Functional and Evolutionary Diversification of Otx2 and Crx in Vertebrate Retinal Photoreceptor and Bipolar Cell Development. Cell Rep 30, 658–671 e655. [DOI] [PubMed] [Google Scholar]
- Young RW, 1985. Cell differentiation in the retina of the mouse. Anat Rec 212, 199–205. [DOI] [PubMed] [Google Scholar]
- Yu W, Mookherjee S, Chaitankar V, Hiriyanna S, Kim JW, Brooks M, Ataeijannati Y, Sun X, Dong L, Li T, Swaroop A, Wu Z, 2017. Nrl knockdown by AAV-delivered CRISPR/Cas9 prevents retinal degeneration in mice. Nat Commun 8, 14716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Ming C, Fu X, Duan Y, Hoang DA, Rutgard J, Zhang R, Wang W, Hou R, Zhang D, Zhang E, Zhang C, Hao X, Xiong W, Zhang K, 2017. Gene and mutation independent therapy via CRISPR-Cas9 mediated cellular reprogramming in rod photoreceptors. Cell Res 27, 830–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyczka N, 1999. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther 6, 973–985. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ Jr., Chiodo VA, Phillipsberg T, Muzyczka N, Hauswirth WW, Flotte TR, Byrne BJ, Snyder RO, 2002. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 28, 158–167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. ROSA-PRDM1 transgene. Schematic of the targeted ROSA26 locus on mouse chromosome (chr) 6 approximately to scale in base pairs (bp). The transgene includes a CAG enhancer/promoter sequence followed by a Flp recombination site (Frt) and a LoxP flanked STOP cassette. The stop cassette contains numerous stop codons in each reading frame and three poly adenylation (pA) sequences. Upon CRE-mediated recombination, the STOP sequence is deleted and the Prdm1 cDNA becomes permanently expressed. Downstream of Prdm1 is a woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) and a bovine growth hormone (bGH) pA sequence. Next, there is a phosphoglycerate kinase (PGK) promoter driving the expression of a neomycin resistance (NeoR) cassette. It is followed by a PGK pA sequence. An additional Frt site is upstream of the NeoR cassette to allow for Flp-mediated deletion of the LoxP-STOP-LoxP Prdm1 portion of the transgene. There are also AttB and AttP sequences flanking the NeoR cassette that allow for PhiC31-mediated deletion of the NeoR cassette. For genotyping, primers 1 and 2 are used to detect ROSA-PRDM1 and ROSA-RFP transgenes. Primers 3 and 4 detect only the ROSA-PRDM1 transgene. Primers 1 and 5, which target ROSA26 genomic DNA, are used to detect wildtype (untargeted) alleles.
Supplemental Figure 2. Early CRE expression in VSX2-CRE mice leads to a broad recombination pattern in adult mice. A) Schematic of experimental design. The VSX2-CRE mice are designed to drive bipolar-specific CRE expression (see Nickerson et al, 2011). ROSA-RFP and PRDM1 mice are as described in Figure 1. B-F) Adult mice stained for CRE (green), RFP (red) and OTX2 (purple). B). No CRE or RFP staining is present in RFP/+ mice. C) VSX2-CRE retinas have CRE expression in OTX2+ bipolar cells but lack RFP staining. The green vascular staining is non-specific background. D) The VSX2-CRE/RFP mice have bipolar CRE expression, but pan-retinal RFP expression. E) An RFP/+ mouse, without VSX2-CRE or CRE staining, has global RFP expression when the mother carried a VSX2-CRE allele. F) Paternally inherited VSX2-CRE mice also show global, yet mosaic RFP expression. Bars=50μm, INL=Inner Nuclear Layer, ONL=Outer Nuclear Layer.
Supplemental Figure 3. Increased exposure of virally infected cells to show photoreceptor labeling. Levels of the red channel (RFP) are increased to reveal photoreceptors. Panel A corresponds to Figure 3B and panel B corresponds to Figure 3H. All increases in brightness were linear and matched across images to preserve relative exposure between conditions.


