Abstract
Background
This study assessed the proportion of risk-stratified Korean patients with dyslipidemia achieving their low-density lipoprotein cholesterol (LDL-C) targets as defined by the European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) (2011) guidelines while receiving lipid-modifying treatments (LMTs).
Methods
In this multicenter, cross-sectional, observational study, we evaluated data from Korean patients aged ≥19 years who were receiving LMTs for ≥3 months and had an LDL-C value within the previous 12 months on the same LMT. Data were collected for demographics, cardiovascular (CV) risk factors, medical history, and healthcare consumption. Patients were risk-stratified according to the ESC Systematic COronary Risk Evaluation (SCORE) chart and LDL-C target achievement rate was assessed.
Results
Guideline-based risk-stratification of the 1,034 patients showed the majority (72.2%) to be in the very high-risk category. Investigators’ assessment of risk was underestimated in 71.6% compared to ESC/EAS guidelines. Overall LDL-C target achievement rate was 44.3%; target achievement was the highest (66.0%) in moderate-risk patients and the lowest (39.0%) in very high-risk patients. Overall 97.1% patients were receiving statin therapy, mostly as a single-agent (89.2%). High-intensity statins and the highest permissible dose of high-intensity statins had been prescribed to only 9.1% and 7.3% patients in the very high-risk group, respectively. Physician satisfaction with patients’ LDL-C levels was the primary reason for non-intensification of statin therapy.
Conclusion
Achievement of target LDL-C level is suboptimal in Korean patients with dyslipidemia, especially in those at very high-risk of CV events. Current practices in LMTs need to be improved based on precise CV risk evaluation posed by dyslipidemia.
Keywords: Dyslipidemias, Cholesterol, LDL, Hydroxymethylglutaryl-CoA reductase inhibitors, Practice guideline, Risk assessment, Korea
INTRODUCTION
Dyslipidemia, characterized by abnormal levels of serum lipids, is a major established risk factor for cardiovascular disease (CVD) [1] and cerebrovascular disease [2] in Korea. At present, these diseases rank as the second- and third-leading cause of mortality in Koreans [3]. According to the results of the Korea National Health and Nutrition Examination Survey (KNHANES), the age-standardized prevalence of dyslipidemia in Korea has risen steadily from 54.0% in 1998 to 59.0% in 2010 [4]. For the period 2005 to 2010, awareness and treatment rates for dyslipidemia in Korea were also found to have gradually increased to 13.7% and 7.4%, respectively [4]. However, these can still be considered as suboptimal.
Various epidemiological studies have shown that low-density lipoprotein (LDL) has negative implications in CVD [5–7]. Korean patients with dyslipidemia have been reported to have a propensity for relatively higher triglycerides (TG) and lower high-density lipoprotein cholesterol (HDL-C) levels. However, in recent years, the incidence of hypercholesterolemia and elevated LDL-cholesterol (LDL-C) levels has been increasing in parallel, without any decrease in hypertriglyceridemia or hypo-HDL-cholesterolemia [8]. Further KNHANES database has shown that approximately 10.0% of Korean adults have LDL-C levels that needed to be pharmacologically lowered, and 19.9% have LDL-C levels that needed therapeutic lifestyle modifications [9]. Consequently, dyslipidemia stands to substantially increase the cardiovascular (CV) risk in the Korean population and needs to be considered as a major health concern in the Korean setting.
Considering the role of LDL-C as a crucial modifiable CV risk factor, various international guidelines recommend lowering of LDL-C levels to minimize the risk of CV events [10–13]. The recently formulated guidelines from the Committee of Clinical Practice of the Korean Society of Lipid and Atherosclerosis for the Management of Dyslipidemia [14] also recommend the reduction of LDL-C levels to target levels or lower as the first goal for management of dyslipidemia. The discovery of statins has revolutionized dyslipidemia treatment and statins are known to substantially reduce LDL-C levels and reduce CV complications and mortality [15–18]. Besides interfering with the biosynthesis of cholesterol, statins also increase receptor-mediated uptake of circulating LDL and its precursors [19]. Despite the establishment of guidelines and proven effectiveness of statins for more than a decade, the achievement of LDL-C and total cholesterol (TC) targets in Koreans is suboptimal with current therapeutic options [20,21]. To address this issue, it is vital to assess existing practice patterns for the treatment of dyslipidemia in real clinical settings. A major limitation to such an undertaking is the paucity of data which is applicable to Korea. A recent study in Korean real-world practice has demonstrated good compliance for at least 18 months in patients who had been started with statins at the fixed doses [22]; however, the study was performed at a single institution and lacks generalizability. Moreover, the indices of metabolic syndrome in Korea have historically been TG and HDL-C [9], hence it is not possible to infer LDL-C levels in the general population from the KNHANES database [23].
The International ChoLesterol Management Practice Study (ICLPS) was an observational study [24] designed to ascertain the proportion of patients with moderate to very high CV risk reaching LDL-C targets as defined by the European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) (2011) guidelines [25] in a real-world setting. In this report, we describe the results from the Korean cohort of the ICLPS. In addition, we also attempt to identify whether the treatment of dyslipidemia in actual practice in Korea differed from that recommended by the guidelines.
METHODS
Study design
Data included in the reported sub-analysis was collected from the Korean cohort of ICLPS—an international, multicenter, cross-sectional, observational study conducted at various locations in Eurasia, Asia, Africa, Middle-East, and South America [24].
Study investigators were primarily those physicians who were representatives in the medical community who managed patients with dyslipidemia and included cardiologists, endocrinologists, gerontologists, internists, and general practitioners. Investigators were selected randomly and independently from a pre-established list of physicians that was meant to ensure appropriate proportion of all specialties managing patients with dyslipidemia.
Patient recruitment and data collection
Patients eligible for inclusion in the study were ≥19 years of age, receiving lipid-modifying treatments (LMTs) for at least 3 months prior to enrollment, and had an LDL-C value measured <12 months prior to enrollment while on the same LMT. Use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors within 6 months prior to enrollment as well as participation in other trials simultaneously were grounds for study exclusion. The present study was approved by the Institutional Review Board of each study site (IRB number: DSMC 2015-06-009, B-1506-304-301, H-1506-102-682, NHIMC 2015-06-008, HYUH 2015-06-010, H-1506-011-030, 2015GR-0156, 15-098, KUGH 2015-06-005, DSMC 2015-06-013, 2015GR-0141, SMC 2015-07-037-001, UUH 2015-08-007, CHOSUN 2015-06-015, 2015-12-151, 4-2015-1029, 2015GR-00326, 16-0002, CNUH 2016-001-022) and was conducted according to the Declaration of Helsinki. All study patients had to provide informed consent. In order to reduce bias, patients were recruited consecutively at each study site over a limited period of time (≤2 weeks). The study involved a single visit during enrollment with no subsequent follow-up.
Data of investigators was collected including age and gender, specialty, years of practice, location, mean number of patients consulted per day (total and with hypercholesterolemia), guidelines adhered to, and definition of statin intolerance used. Data of patients in the Korean cohort was collected between August 2015 and March 2016 and included demographics and socioeconomic profile, physical examination, CV risk factors, dyslipidemia and other medical history, laboratory values, ongoing medications, healthcare consumption (past 12 months), level of CV risk, and target LDL-C value.
Risk stratification of patients was conducted according to the ESC Systematic COronary Risk Evaluation (SCORE) chart [26] and LDL-C target for individual patients was ascertained as defined by ESC/EAS (2011) guidelines (Supplemental Table S1) [25]. Values of both parameters, as defined by the guidelines and as assessed by the investigators, were compared to determine if any gap existed between the guideline and the actual practice. Familial hypercholesterolemia (FH) was assessed/diagnosed using the Dutch Lipid Clinic Network diagnostic criteria [27]. Primary/familial dyslipidemia was defined at least one of the following: (1) FH (heterozygous, homozygous, or unknown), (2) first degree relative with known LDL-C above 95th percentile for age and gender, (3) first degree relative with tendinous xanthomata and/or arcus cornealis. The reason for not prescribing the highest dose of statins was assessed by a questionnaire which was configured to select one of three reasons; physician satisfaction, medically inappropriate, statin intolerance.
Statistical analysis
The population for analysis consisted of all patients who met the inclusion criteria at enrollment in this study. Furthermore, any investigator who recruited at least one patient was included in analysis of physician profile (Supplemental Table S2). Sample size of the international study [24] was calculated in order to ensure a sufficient precision in the assessment of the qualitative data and the study planned to recruit approximately 11,000 patients from around 700 sites in 35 countries.
Descriptive statistics using counts and percentages for categorical variables and mean, median, standard deviation (SD), and range for continuous variables, were used for data analysis. All statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC, USA).
RESULTS
Investigator profile and patient characteristics
Study investigators (n=19) were mostly male (n=16, 84.2%), cardiologists (n=9, 47.4%), or endocrinologists (n=6, 31.6%), and had ≥15 years of clinical experience. All investigators reported following guidelines for management of dyslipidemia and the most common were those by the American College of Cardiology/American Heart Association (ACC/AHA) (n/total number=13/19, 68.4%) and the ESC/EAS (n/total number=6/19, 31.6%). Adherence to domestic [14] and other international guidelines was reported by four (21.6%) and three (15.8%) investigators, respectively. Some investigators reported following multiple guidelines. About half (52.6%), 36.8% and 10.5% of physicians defined patients as “statin-intolerant” when patients were intolerant to one, two, and more than two statins, respectively.
Investigators enrolled 1,069 patients across Korea in between August 2015 and March 2016 and data from 1,034 was eligible for analysis. Study population comprised 54.9% (n=568) males and 45.1% females (n=466) (Table 1). The mean age±SD of study population was 63.3±10.4 years and 49.7% of the patients were in the age range ≥45 to <65 years.
Table 1.
Patient Characteristics, Cardiovascular Risk Factors, and Comorbidities at Enrollment
| Characteristic | Risk category | Total (n=1,034) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Low (n=1) | Moderate (n=47) | High (n=178) | Very high (n=747) | Non-assessablea (n=61) | ||
| Age in years | 30.0 | 56.2±7.9 | 60.9±9.5 | 64.5±10.4 | 62.6±11.0 | 63.3±10.4 |
|
| ||||||
| Gender | ||||||
| Male | 0 | 12 (25.5) | 76 (42.7) | 455 (60.9) | 25 (41.0) | 568 (54.9) |
| Female | 1 (100) | 35 (74.5) | 102 (57.3) | 292 (39.1) | 36 (59.0) | 466 (45.1) |
|
| ||||||
| History of dyslipidemia | 1 (100) | 47 (100) | 167 (93.8) | 536 (72.1) | 45 (73.8) | 796 (77.0) |
|
| ||||||
| Time in years since diagnosis of dyslipidemia | 1.0 | 4.1±3.0 | 5.1±3.6 | 5.0±3.7 | 4.2±2.8 | 4.9±3.6 |
|
| ||||||
| Prevalence of CV risk factors | ||||||
| Hypertensionb | 1 (100) | 22 (46.8) | 100 (56.2) | 569 (76.2) | 38 (62.3) | 730 (70.6) |
| Lack of physical activityc | 1 (100) | 30 (63.8) | 104 (58.4) | 446 (59.7) | 40 (65.6) | 621 (60.1) |
| Diabetesd | 0 | 0 | 140 (78.7) | 377 (50.5) | 0 | 517 (50.0) |
| Regular alcohol consumptione | 0 | 4 (8.5) | 32 (18.0) | 143 (19.1) | 11 (18.0) | 190 (18.4) |
| Familial history of CVDf | 0 | 7 (14.9) | 32 (18.0) | 137 (18.3) | 12 (19.7) | 188 (18.2) |
| Current smokingg | 0 | 1 (2.1) | 19 (10.7) | 116 (15.5) | 8 (13.1) | 144 (13.9) |
|
| ||||||
| CV comorbidities | ||||||
| CAD | 0 | 0 | 0 | 424 (56.8) | 0 | 424 (41.0) |
| ACS/MIh (n=424) | NA | NA | NA | 254 (59.9) | NA | 254 (59.9) |
| PCIh (n=424) | NA | NA | NA | 246 (58.0) | NA | 246 (58.1) |
| CABGh (n=424) | NA | NA | NA | 16 (3.8) | NA | 16 (3.8) |
| Stroke | 0 | 0 | 0 | 140 (18.7) | 0 | 140 (13.5) |
| CKDi | 1 (100.0) | 9 (19.1) | 9 (5.1) | 94 (12.6) | 5 (8.2) | 118 (11.4) |
Values are expressed as mean±standard deviation or number (%).
CV, cardiovascular; CVD, cardiovascular disorder; CAD, coronary artery disease; ACS, acute coronary syndrome; MI, myocardial infarction; NA, not applicable; PCI, percutaneous intervention; CABG, coronary artery bypass graft; CKD, chronic kidney disease.
Patients without a serious pathology classifying them as very high or high cardiovascular risk, and in whom the Systematic COronary Risk Evaluation (SCORE) could not be calculated due to missing data (most commonly baseline low-density lipoprotein cholesterol);
Systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg or a previous history of hypertension;
Patient is not regularly involved in moderate (walking/cycling/gardening) or strenuous exercise (jogging/football/vigorous swimming) for ≥4 hours each week;
Type 1 or 2 diabetes mellitus;
Consumption ≥3 times a week;
Coronary and/or vascular disease <55 years of age in male and <60 years in female first-degree relatives;
Current smokers and individuals who smoked any tobacco in the previous 12 months (including those who have quit smoking within the previous 12 months);
Only assessed in patients with CAD;
GFR <60 mL/min/1.73 m2.
Commonly prevalent CV risk factors were hypertension (n=730, 70.6%), lack of physical activity (n=621, 60.1%), and diabetes (n=517, 50.0%) (Table 1). Patients in the very high-risk category also had a high prevalence of coronary artery disease (CAD; n/total number=424/747; 56.8%). Over half of the very high-risk patients with CAD presented with acute coronary syndrome (ACS)/myocardial infarction (MI) (n=254, 59.9%) or percutaneous coronary intervention (n=246, 58.1%). Elevated consumption of healthcare resources was reported in 22.0% (n=227) of the overall patients and in 25.3% (n/total number=189/747) of the very high-risk patients.
Risk stratification of study patients according to ESC/EAS (2011) guidelines
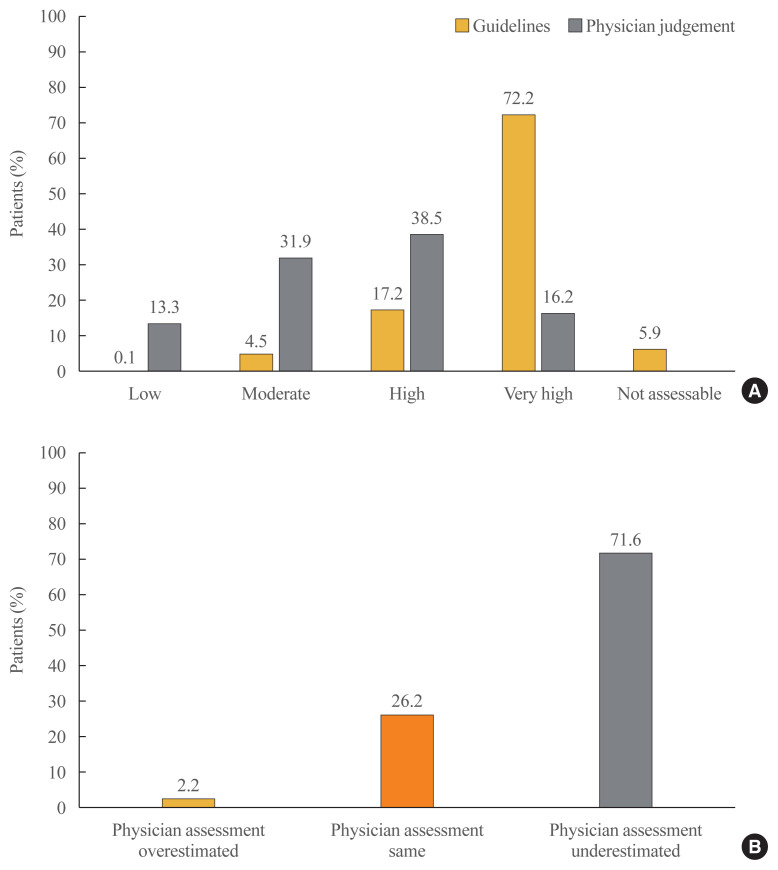
Risk stratification of study patients (n=1,034) according to ESC/EAS (2011) guidelines classified the patients as—747 (72.2%) with very high-risk, 178 (17.2%) with high-risk, 47 (4.5%) with moderate-risk, and one (0.1%) with low-risk. A total of 61 (5.9%) patients were deemed non-assessable for risk according to the guidelines (Fig. 1A). The investigators’ assessment of risk was consistent with guideline-based risk assessment in only 20.1% (n/total number=150/747) very high-risk patients, 46.1% (n/total number=82/178) high-risk patients, and 48.8% (n/total number=23/47) moderate-risk patients (Table 2). Overall, only 26.2% (n/total number=255/973) of physician’s assessment were consistent with the guidelines and in most of the cases (n/total number=697/973, 71.6%), and physician underestimated the risk of patients compared to the guideline (Fig. 1B).
Fig. 1.
(A) Comparison of risk stratification according to European Society of Cardiology (ESC) Systematic COronary Risk Evaluation (SCORE) chart [27] and physician judgement. (B) The matching pattern of physician judgement for risk stratification compared to ESC/European Atherosclerosis Society (EAS) 2011 guidelines. Physician assessment overestimated and underestimated were defined as the physician’s risk assessment was higher and lower than the risk of the patients according to ESC/EAS guideline, respectively.
Table 2.
Risk Assessment According to ESC/EAS 2011 Guidelines versus Risk Assessment by Investigator
| Risk assessed by investigator | Risk category | Total (n=1,034) | ||||
|---|---|---|---|---|---|---|
| Low (n=1) | Moderate (n=47) | High (n=178) | Very high (n=747) | Non-assessablea (n=61) | ||
| Low | 0a | 20 (42.6) | 39 (21.9) | 65 (8.7) | 14 (23.0) | 138 (13.3) |
| Moderate | 1(100) | 23 (48.9)a | 41 (23.0) | 226 (30.3) | 39 (63.9) | 330 (31.9) |
| High | 0 | 4 (8.5) | 82 (46.1)a | 306 (41.0) | 6 (9.8) | 398 (38.5) |
| Very high | 0 | 0 | 16 (9.0) | 150 (20.1)a | 2 (3.3) | 168 (16.2) |
Values are expressed as number (%).
ESC, European Society of Cardiology; EAS, European Atherosclerosis Society.
The investigator’s risk assessment and the guidance-based risk assessment were the same.
Serum LDL-C profile and lipid-modifying treatments prescribed
A total of 796 (77.3%) patients had a history of dyslipidemia for a mean±SD duration of 4.9±3.6 years since diagnosis. Of these patients, 13.3% (n=137) and 47.0% (n=485) were reported to have primary (or familial) and secondary hypercholesterolemia, respectively. At first diagnosis, the mean±SD values for lipid parameters were: LDL-C, 138.2±40.3 mg/dL; TC, 215.9±45.9 mg/dL; HDL-C, 48.0±12.9 mg/dL; and TG, 179.3±123.7 mg/dL. In comparison, mean values for lipid parameters at enrollment were: LDL-C, 83.6±26.3 mg/dL; TC, 154.3±35.3 mg/dL; HDL-C, 50.1±13.6 mg/dL; and TG, 136.2±93.0 mg/dL.
Statins were the most commonly prescribed LMTs, reported in 1,004 (97.1%) patients, mostly as monotherapy (n=922, 89.2%) (Table 3). Of the patients using statins, 7.8% (n=78) of overall patients and 9.1% (n=66) of very high-risk patients were taking high-intensity statins. The maximum permissible dose of high-intensity statins had been prescribed to 7.4% (n=74) of overall patients and 7.3% (n=53) of very high-risk patients. The most common (84.5%) reason for not prescribing highest permissible dose of statins was cited as physician satisfaction with patient’s LDL-C levels from current dosage (Table 3).
Table 3.
Patterns in Prescription of Lipid-Modifying Treatments According to Risk Stratification
| Variable | Risk category | Total (n=1,034) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Low (n=1) | Moderate (n=47) | High (n=178) | Very high (n=747) | Non-assessablea (n=61) | ||
| Lipid-modifying treatment | ||||||
| Statins | 1 (100) | 47 (100) | 169 (94.9) | 729 (97.6) | 58 (95.1) | 1,004 (97.1) |
| Fibrates | 0 | 0 | 10 (5.6) | 27 (3.6) | 4 (6.6) | 41 (4.0) |
| Omega-3 fatty acids | 0 | 2 (4.3) | 8 (4.5) | 29 (3.9) | 1 (1.6) | 40 (3.9) |
| Cholesterol absorption inhibitors | 0 | 6 (12.8) | 6 (3.4) | 19 (2.5) | 0 | 31 (3.0) |
| Others | 0 | 0 | 0 | 6 (0.8) | 0 | 6 (0.6) |
|
| ||||||
| Patients receiving high-intensity statinsb | 0 | 2 (4.3) | 9 (5.3) | 66 (9.1) | 1 (1.7) | 78 (7.8) |
|
| ||||||
| Patients receiving highest permissible dose of statins | 0 | 2 (4.3) | 15 (8.9) | 53 (7.3) | 4 (6.9) | 74 (7.4) |
|
| ||||||
| Reason for not prescribing the highest dose of statins | ||||||
| Assessed number of patients | 1 | 45 | 154 | 664 | 54 | 918 |
| Physician satisfactionc | 0 | 35 (77.8) | 141 (91.6) | 550 (82.8) | 50 (92.6) | 776 (84.5) |
| Medically inappropriated | 1 (100) | 9 (20.0) | 9 (5.8) | 154 (23.2) | 13 (24.1) | 186 (20.3) |
| Statin intolerancee | 0 | 1 (2.2) | 4 (2.6) | 15 (2.3) | 0 | 20 (2.2) |
Values are expressed as number (%).
Patients without a serious pathology classifying them as very high or high cardiovascular risk, and in whom the Systematic COronary Risk Evaluation (SCORE) could not be calculated due to missing data (most commonly baseline low-density lipoprotein cholesterol [LDL-C]);
Atorvastatin 40/80 mg or rosuvastatin 20/40 mg;
Physician determined that the patient’s LDL-C levels were appropriate;
Higher dose not advisable due to patient’s clinical condition;
Patient did not tolerate a higher dose regimen or a higher intensity statin.
LDL-C target achievement
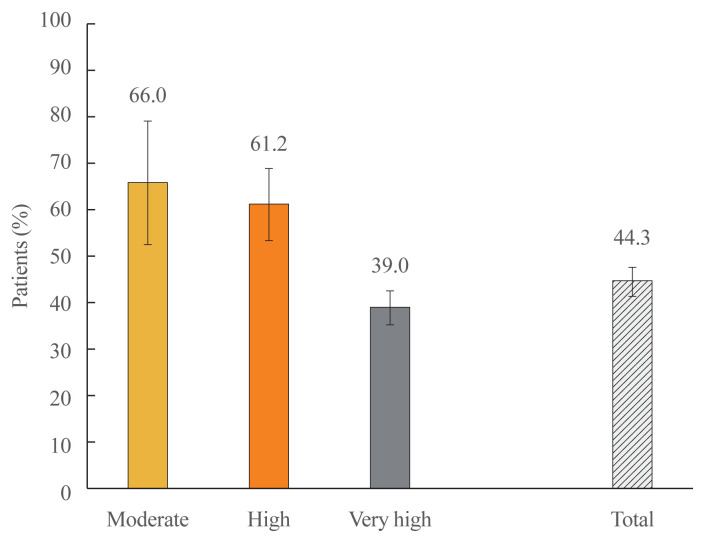
Overall achievement of target LDL-C was reported in 44.3% (n/total number=431/973) of the patients (Fig. 2). Proportions of patients attaining target LDL-C levels in each risk group were −66.0% (n/total number=31/47) in the moderate-risk group; 61.2% (n/total number=109/178) in the high-risk group; and 39.0% (n/total number=291/747) in the very high-risk group. Of the 129 patients who were statin-intolerant, 39.3% (n=55) had achieved their LDL-C target; in contrast to 45.2% (n/total number=376/832) of the remainder of the patients who were statin-tolerant. On the other hand, patients who had experienced ACS/MI in the 12 months preceding study enrollment had a lower rate of target achievement in comparison to those who had no such experience (34.4% vs. 44.8%).
Fig. 2.
Target low-density lipoprotein cholesterol achievement according to cardiovascular risk strata. Error bars represent 95% confidence intervals.
DISCUSSION
Periodic assessment of real-world clinical data on the management of CV risk factors such as dyslipidemia is essential to gauge the effectiveness of disease management guidelines. In our study, aimed at ascertaining target LDL-C achievement in Korean patients with dyslipidemia, risk-stratified according to ESC/EAS (2011) guidelines, a substantial proportion of the study patients were already in the very high-risk category. Overall target LDL-C was achieved in less than half of the patients and this proportion was inversely associated with risk categorization. We also discovered that study patients who were statin-intolerant or had ACS/MI in the preceding 12 months had poorer target achievement rates in comparison to the other patients.
Despite a large volume of clinical data and established guidelines from numerous medical associations, achieving target LDL-C levels in patients with dyslipidemia has been somewhat less than optimal and inconsistent across periods and regions. In one of the first large-scale, multinational studies conducted in almost 10,000 patients, the lipid treatment assessment project 2 (LTAP2), the target achievement rate ranged between 47.0% and 84.0% [28]. Interestingly, this study also enrolled almost 1,000 Korean patients in whom target achievement was reported to be 83.5%, even though 68.0% of the patients were in the high-risk category according to the National Cholesterol Education Program Adult Treatment Panel III guidelines [29]. The Dyslipidemia International Study (DYSIS) which was conducted in 30 countries and analyzed ≥50,000 patients who were risk-stratified according to the ESC/EAS (2011) guidelines, reported a target achievement rate in between 14.3% and 49.5% [30]. In both the LTAP2 and DYSIS investigations, patients in the higher risk strata or with other CV comorbidities had a substantially lower target achievement rate. These findings have also been replicated in the Asian content by the Centralized Pan-Regional Surveys on the Undertreatment of Hypercholesterolaemia (CEPHEUS) Pan-Asian study, which showed LDL-C target achievement in 49.4% of overall patients [31].
Attainment of lipid indices such as LDL-C have been clinically validated to depend on various factors such as disease progression, time to diagnosis and commencement and intensification of treatment regimens. Late detection of dyslipidemia, inertia in timely and appropriate interventions, as well as presence of other CV comorbidities might also contribute to suboptimal reduction in LDL-C levels. As high physicians’ satisfaction (84.5% in overall) with the current LDL-C levels was revealed to be the prime reason for not prescribing high-intensity or highest dose of statins regardless of either accordance to guidelines recommendation or achieving risk-stratified LDL-C target levels in this study, their physicians’ insufficient of awareness of guidelines might be the major factor of non-adherence to guidelines and, consequently, failure in achieving target LDL-C levels [32–34]. In line with this hypothesis, physicians underestimated the risk levels for 71.6% of all patients in this study and target achievement of LDL-C levels was only 44.3%. Moreover, the underestimation and target achievement of LDL-C rates were as high as 79.9% and as low as 39.0% among very high-risk patients, respectively. Since a prerequisite for evidence-based management of dyslipidemia is appropriate risk stratification, the discordance in risk assessment could have a substantial bearing on the outcomes. For example, a recent study [35] showed that only 42.4% of the patients who were recommended for statin therapy were on the recommended statin intensity. In the study, untreated (25.3%) and undertreated (32.3%) patients had significantly higher LDL-C levels than the ones on recommended statin intensity. Interestingly, another recent study revealed that Korean cardiologists tend to be more determined in LMTs and achieve higher goal attainment rate than other Asian cardiologists in the CEPHEUS study [36]. Further investigation would be needed to identify factors influencing physician’s stratification of CV risk and adherence to guidelines’ recommendation for statin intensity especially in Korea.
Given the complex nature of metabolic disease and its relation to CVD, it is practically impossible for a single study to decipher all relevant and multimodal pathways involved. The goal of our study was to answer one key practical question—what is the rate of LDL-C target achievement in Korean patients in real-world settings? Certain aspects of dyslipidemia treatment were beyond the scope of our study; we could not ascertain patient adherence, a factor critical to any chronic therapy. Moreover, no follow-up visits were planned in the study to monitor patients for changes in LDL-C levels. We were also unable to ascertain specific treatments or regimens that were more successful than others in helping patients achieve their LDL-C targets. However, the overall findings of our study not only serve to reiterate the suboptimal attainment of LDL-C reduction in Korean patients presenting CV risk but also highlights the need to revise current practices in management of dyslipidemia in Korea. Further studies will need to be conducted in order to fine tune the existing guidelines for successfully lowering the CV risk posed by dyslipidemia in the Korean population.
Supplementary Information
Footnotes
CONFLICTS OF INTEREST
One of the co-authors, Ji-Hyun Kim, is a current employee at Sanofi-Aventis Korea, but no potential conflict of interest was reported by the author.
This study was supported by Sanofi-Aventis Korea. Medical writing and editorial support in the preparation of this publication was provided by Satyendra Shenoy from Describe Scientific Writing & Communications who was paid for by Sanofi-Aventis Korea and Anahita Gouri and Rohan Mitra from Sanofi-Aventis Korea. The authors individually and collectively are responsible for all content and editorial decisions and received no payment from Sanofi-Aventis Korea directly or indirectly (through a third party) related to the development/presentation of this publication.
AUTHOR CONTRIBUTIONS
Conception or design: J.S.K., K.M.C., K.W.L., S.C.L., J.R.C., S.J.O., J.H.K., S.H.C. Acquisition, analysis, or interpretation of data: Y.S.Y., S.Y.L., J.S.K., K.M.C., K.W.L., S.C.L., J.R.C., S.J.O., J.H.K., S.H.C. Drafting the work or revising: Y.S.Y., S.H.C. Final approval of the manuscript: Y.S.Y., S.Y.L., J.S.K., K.M.C., K.W.L., S.C.L., J.R.C., S.J.O., J.H.K., S.H.C.
REFERENCES
- 1.Kim MH, Kim MK, Choi BY, Shin YJ. Prevalence of the metabolic syndrome and its association with cardiovascular diseases in Korea. J Korean Med Sci. 2004;19:195–201. doi: 10.3346/jkms.2004.19.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung KH, Lee SH, Kim BJ, Yu KH, Hong KS, Lee BC, et al. Secular trends in ischemic stroke characteristics in a rapidly developed country: results from the Korean Stroke Registry Study (secular trends in Korean stroke) Circ Cardiovasc Qual Outcomes. 2012;5:327–34. doi: 10.1161/CIRCOUTCOMES.111.963736. [DOI] [PubMed] [Google Scholar]
- 3.Shin HY, Lee JY, Song J, Lee S, Lee J, Lim B, et al. Cause-of-death statistics in the Republic of Korea, 2014. J Korean Med Assoc. 2016;59:221–32. [Google Scholar]
- 4.Roh E, Ko SH, Kwon HS, Kim NH, Kim JH, Kim CS, et al. Prevalence and management of dyslipidemia in Korea: Korea National Health and Nutrition Examination Survey during 1998 to 2010. Diabetes Metab J. 2013;37:433–49. doi: 10.4093/dmj.2013.37.6.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 6.Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104:1108–13. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 7.Howard BV, Robbins DC, Sievers ML, Lee ET, Rhoades D, Devereux RB, et al. LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: the Strong Heart Study. Arterioscler Thromb Vasc Biol. 2000;20:830–5. doi: 10.1161/01.atv.20.3.830. [DOI] [PubMed] [Google Scholar]
- 8.Kim HC. Epidemiology of dyslipidemia in Korea. J Korean Med Assoc. 2016;59:352–7. [Google Scholar]
- 9.Choi SJ, Park SH, Lee KS, Park HY. The prevalence, awareness and treatment of high low density lipoprotein-cholesterol in Korean adults without coronary heart diseases: the third Korea national health and nutrition examination survey, 2005. Korean Circ J. 2012;42:86–94. doi: 10.4070/kcj.2012.42.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1. full report. J Clin Lipidol. 2015;9:129–69. doi: 10.1016/j.jacl.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Expert Dyslipidemia Panel of the International Atherosclerosis Society Panel members. An International Atherosclerosis Society position paper: global recommendations for the management of dyslipidemia: full report. J Clin Lipidol. 2014;8:29–60. doi: 10.1016/j.jacl.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Committee of Clinical Practice Guideline of the Korean Society of Lipid and Atherosclerosis. Korean Guidelines for the Management of Dyslipidemia. 4th ed. Seoul: Korean Society of Lipid and Atherosclerosis; 2018. 2018. [Internet] [cited 2020 Apr 26]. Available from: http://www.lipid.or.kr/bbs/index.html?code=care&category=&gubun=&page=1&number=903&mode=view&keyfield=&key= [Google Scholar]
- 15.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 16.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 17.Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 18.Cholesterol Treatment Trialists’ (CTT) Collaborators. Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–90. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raghow R. Statins redux: a re-assessment of how statins lower plasma cholesterol. World J Diabetes. 2017;8:230–4. doi: 10.4239/wjd.v8.i6.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang JY, Jung CH, Lee WJ, Park CY, Kim SR, Yoon KH, et al. Low density lipoprotein cholesterol target goal attainment rate and physician perceptions about target goal achievement in Korean patients with diabetes. Diabetes Metab J. 2011;35:628–36. doi: 10.4093/dmj.2011.35.6.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JE, Chiang CE, Munawar M, Pham GK, Sukonthasarn A, Aquino AR, et al. Lipid-lowering treatment in hypercholesterolaemic patients: the CEPHEUS Pan-Asian survey. Eur J Prev Cardiol. 2012;19:781–94. doi: 10.1177/1741826710397100. [DOI] [PubMed] [Google Scholar]
- 22.Khang AR, Song YS, Kim KM, Moon JH, Lim S, Park KS, et al. Comparison of different statin therapy to change low-density lipoprotein cholesterol and high-density lipoprotein cholesterol level in Korean patients with and without diabetes. J Clin Lipidol. 2016;10:528–37. doi: 10.1016/j.jacl.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Kim HC. Knowledge gap regarding low density lipoprotein-cholesterol levels in Koreans. Korean Circ J. 2012;42:80–2. doi: 10.4070/kcj.2012.42.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danchin N, Almahmeed W, Al-Rasadi K, Azuri J, Berrah A, Cuneo CA, et al. Achievement of low-density lipoprotein cholesterol goals in 18 countries outside Western Europe: the International ChoLesterol management Practice Study (ICLPS) Eur J Prev Cardiol. 2018;25:1087–94. doi: 10.1177/2047487318777079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catapano AL, Reiner Z, de Backer G, Graham I, Taskinen MR, Wiklund O, et al. ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Atherosclerosis. 2011;217:3–46. doi: 10.1016/j.atherosclerosis.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 27.WHO Human Genetics Programme. Familial hypercholesterolaemia (FH): report of a second WHO consultation; Geneva. 4 Sep 1998; Geneva: WHO; c2020. [Internet] [cited 2020 Apr 26]. https://apps.who.int/iris/handle/10665/66346. [Google Scholar]
- 28.Waters DD, Brotons C, Chiang CW, Ferrieres J, Foody J, Jukema JW, et al. Lipid treatment assessment project 2: a multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goals. Circulation. 2009;120:28–34. doi: 10.1161/CIRCULATIONAHA.108.838466. [DOI] [PubMed] [Google Scholar]
- 29.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 30.Gitt AK, Lautsch D, Ferrieres J, Kastelein J, Drexel H, Horack M, et al. Low-density lipoprotein cholesterol in a global cohort of 57,885 statin-treated patients. Atherosclerosis. 2016;255:200–9. doi: 10.1016/j.atherosclerosis.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Chiang CE, Ferrieres J, Gotcheva NN, Raal FJ, Shehab A, Sung J, et al. Suboptimal control of lipid levels: results from 29 countries participating in the centralized pan-regional surveys on the undertreatment of hypercholesterolaemia (CEPHEUS) J Atheroscler Thromb. 2016;23:567–87. doi: 10.5551/jat.31179. [DOI] [PubMed] [Google Scholar]
- 32.Reiner Z, Sonicki Z, Tedeschi-Reiner E. Physicians’ perception, knowledge and awareness of cardiovascular risk factors and adherence to prevention guidelines: the PERCRO-DOC survey. Atherosclerosis. 2010;213:598–603. doi: 10.1016/j.atherosclerosis.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Vashitz G, Meyer J, Parmet Y, Henkin Y, Peleg R, Gilutz H. Physician adherence to the dyslipidemia guidelines is as challenging an issue as patient adherence. Fam Pract. 2011;28:524–31. doi: 10.1093/fampra/cmr025. [DOI] [PubMed] [Google Scholar]
- 34.Ding R, Ye P, Zhao S, Zhao D, Yan X, Dong Y, et al. Effect of physician characteristics and knowledge on the quality of dyslipidemia management and LDL-C target goal achievement in China: subgroup analysis of the Dyslipidemia International Study. J Glob Health. 2017;7:020702. doi: 10.7189/jogh.07.020702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navar AM, Wang TY, Li S, Robinson JG, Goldberg AC, Virani S, et al. Lipid management in contemporary community practice: results from the Provider Assessment of Lipid Management (PALM) Registry. Am Heart J. 2017;193:84–92. doi: 10.1016/j.ahj.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sung J, Kim SH, Song HR, Chi MH, Park JE. Lipid-lowering treatment practice patterns in Korea: comparison with the data obtained from the CEPHEUS Pan-Asian study. J Atheroscler Thromb. 2014;21:1219–27. doi: 10.5551/jat.23242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.