Abstract
Photobiomodulation has been shown to improve tissue and cell functions. We evaluated the influence of photobiomodulation, using a B-Cure laser, on: 1) maximal performance, and 2) muscle recovery after resistance exercise. Two separate crossover randomized double-blinded placebo-controlled trials were conducted. Sixty healthy physical education students (28 men, 32 women), aged 20-35, were recruited (30 participants for each trial). Participants performed two interventions for each experiment, with real lasers (GaAlAs, 808 nm) on three quadricep locations in parallel (overall treatment energy of ~150J) or sham (placebo) treatment. In the first experiment muscle total work (TW) and peak torque (PT) were measured by an isokinetic dynamometer in five repetitions of knee extension, and in the second experiment muscle recovery was measured after the induction of muscle fatigue by evaluating TW and PT in five repetitions of knee extension. There were no differences between treatments (real or sham) regarding the TW (F(1,28) = 1.09, p = .31), or PT (F(1,29) = .056, p = .814). In addition, there was no effect of photobiomodulation on muscle recovery as measured by the TW (F(1,27) = .16, p = .69) or PT (F(1,29) = .056, p = .814). Applying photobiomodulation for 10 min immediately before exercise did not improve muscle function or muscle recovery after fatigue.
Key words: fatigue, acute exercise, skeletal muscles, anaerobic power
Introduction
Photobiomodulation (low laser therapy) refers to the use of near-infra-red-light photons at a non-thermal irradiance to alter biological activity. This has been found to accelerate tissue repair (Alves et al., 2014; Woodruff et al., 2004) and regeneration (Gupta et al., 2013), increase angiogenesis (Alves et al., 2014), reduce pain (Chow et al., 2009) and inflammation (de Oliveira et al., 2018), as well as stimulate the formation of new muscle fibers (Rodrigues et al., 2014). The use of photobiomodulation is considered to be safe in the long term. In mice, applying a low-level laser for eight months at higher doses than the optimal for stimulation of bone marrow cells was shown to have no histological effects on various tissues (Tuby et al., 2013).
The basic mechanism of photobiomodulation is thought to be the activation of cellular enzymes. In the mitochondria, activation of cytochrome c oxidase results in the stimulation of cellular cascades (Mantineo et al., 2014; Wong-Riley et al., 2005) and in increased Adenosine triphosphate (ATP) concentration (Oron et al., 2007). Photobiomodulation was found to enhance cytochrome c oxidase expression in intact skeletal muscles of rats (Albuquerque-Pontes et al., 2015). In addition, treatment with a laser increased other mitochondrial enzymes, such as complexes I, II, III and succinate dehydrogenase (Silveira et al., 2009). Furthermore, lactate dehydrogenase (LDH) was found to be inhibited by the treatment (De Marchi et al., 2017). This inhibition may shift metabolism toward aerobic processes. Altogether, it appears that photobiomodulation increases ATP content in cells and accelerates their function.
Given the pivotal role of mitochondria in ATP production in skeletal muscles during exercise, and their ability to absorb light, it seems reasonable to assume that skeletal muscle will benefit from photobiomodulation during exercise. A chronic effect of photobiomodulation combined with resistance exercise was found to be associated with an increased cross-sectional area of the tibialis anterior, with a reduced resting lactate level and decreased muscle glycogen depletion, compared to resistance exercise alone (Patrocinio et al., 2013). Acute effects of photobiomodulation on the contraction of skeletal muscles was shown to delay the onset of exercise fatigue in rats (Leal Junior et al., 2010a).
In humans, the effects of photobiomodulation were evaluated for exercise and sport benefits. In untrained adults, photobiomodulation was applied to quadriceps and gastrocnemius muscles, using a multidiode cluster (650/850nm, energy of 20J per point), 10 min before cardiopulmonary exercise testing (CPET) on an electromagnetic cycle ergometer. The treatment improved CPET performance and increased peak O2 uptake, probably through increased O2 extraction by peripheral muscles (da Silva Alves et al., 2014). Pre-exercise treatment of photobiomodulation with a combination cluster of 12 diodes (905 nm, 875 nm and 670 nm; and energy of 60 J, 80 J and 300 J per muscle group) significantly increased performance of eccentric repetitions of the quadriceps muscle in untrained men compared to the placebo (Antonialli et al., 2014). However, photobiomodulation (808 nm, energy of 56 J per muscle) was found to have no significant effect on delaying muscle fatigue in the biceps brachii muscles of young females (Higashi et al., 2013).
In trained participants, a photobiomodulation cluster probe with 5 laser diodes (810 nm; energy of 60 J per muscle) improved muscle performance in elbow flexion exercise of volleyball players, where subjects repeated flexion/extension at a workload of 75% of their maximal voluntary contraction force until exhaustion. Additionally, the treatment enhanced muscle recovery, mainly when applied prior to exercise (Leal-Junior et al., 2010b).
In male volleyball players, pre-exercise irradiation of the biceps with an LLLT (655 nm, energy of 20 J per muscle) increased endurance for repeated elbow flexion against resistance, and decreased post exercise levels of blood lactate, creatine kinase, and C reactive protein (Leal Junior et al., 2008). Furthermore, this group of researchers showed that photobiomodulation (660 nm/850 nm; energy of 41.7 J per muscle) immediately before exhaustive biceps humeri contractions caused a slight delay in the development of skeletal muscle fatigue (Leal Junior et al., 2009b), and that the mean number of repetitions was significantly higher after real treatment (830 nm, energy of 20 J per muscle) than after the placebo (Leal Junior et al., 2009c).
In physically active young women, treatment with photobiomodulation (780 nm, energy of 23.49 J per muscle) before the induction of a protocol of tibialis anterior muscle fatigue increased muscle torque at the beginning of the exercise, compared to the placebo (dos Santos Maciel et al., 2014).
The positive effects of photobiomodulation on muscle function during and after exercise have the potential to improve exercise performance. However, there are many photobiomodulation devices on the market, each with different characteristics.
In this study we used a small, portable device, which is easy to use independently and can be used at home. This device is already in use by a variety of populations for healing sports injuries and reducing pain. Since many of the device's users are active in competitive sporting events, we hypothesized that the same device would be applicative not only for injuries, but also for enhancing muscle performance. Thus, the aim of this study was to evaluate the influence of photobiomodulation on: 1) maximal muscle performance, and 2) muscle recovery after resistance exercise, in physically active young adults.
Methods
Participants
Sixty healthy physical education students (28 men, 32 women), aged 20-35, were recruited for the study; they participated in two different experiments (30 for each experiment). Participants were excluded if they had sustained musculoskeletal injury to the hips or knees in the previous two months, if they were regularly using pharmacological agents or nutritional supplements, or if they had any physical limitations that could prevent them from performing the tests.
The study was approved by an Ethics Committee. Each participant signed an informed consent form prior to participation in the study.
The sample size that was determined based on the following factors: effect size = 0.25, α = 0.05 power (1-β) = .0.8, r = 0.5 was N = 26. The participants' characteristics are presented in Table 1.
Table 1.
Anthropometric characteristics of study participants.
| Experiment 1 Mean ± SD | Experiment 2 Mean ± SD | |
|---|---|---|
| N (male/female) | 30 (15/15) | 30 (13/17) |
| Age (years) | 28.50 ± 2.94 | 26.57 ± 2.66 |
| Body mass (kg) | 68.10 ± 14.59 | 63.83 ± 8.97 |
| Body height (m) | 1.71 ± 0.09 | 1.69 ± 0.09 |
| BMI | 23.12 ± 2.74 | 22.37 ± 2.37 |
Insert Table 1 here
Photobiomodulation treatment
Treatment consisted of irradiation by three laser/placebo devices in parallel for 10 min, at three sites of the quadriceps femoris (rectus femoris, vastus lateralis, and vastus medialis), as shown in Figure 1. The device was held in direct contact with the skin in the areas with the largest muscle mass, as was felt and marked previously by the technician. The laser characteristics are presented in Table 2.
Figure 1.
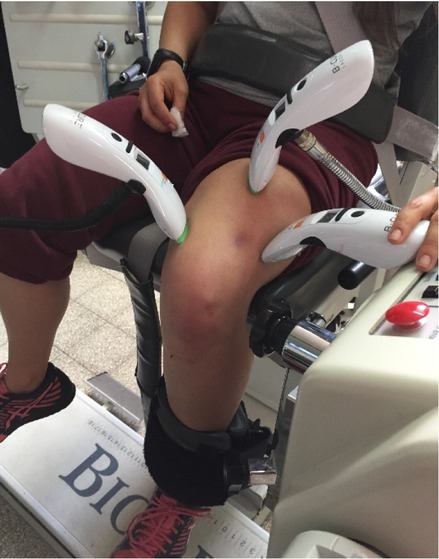
Three parallel lasers placed on the quadriceps femoris during treatment
Table 2.
Laser characteristics –specifications of the B-CURE LASER used in the treatment.
| Parameter | Laser |
|---|---|
| Type of laser | laser diode in solid-state GaAlAs |
| Wavelength | 808 nm |
| Maximum power | 250 mW |
| Laser pulse frequency | 13 kHz |
| Pulse width | 26 μs |
| Avg power | 84.5 mW |
| Dose Rate: | 5.07 J/min (1.13 J/cm2/min) |
| Total energy per treatment | 1.1 J/cm2/min, beam size 1× 4.5 cm2 on 3 quadriceps locations in parallel, overall treatment energy ~150 J |
Procedures
The study protocol designed as a crossover randomized double-blinded placebo-controlled trial included two experiments (maximal strength and muscle recovery). For each experiment participants arrived twice, with at least a one-week interval between the trials. Muscle function during unilateral knee extension movements was measured by a Biodex® system 3 Pro isokinetic dynamometer (Biodex Inc., Shirley, NY, USA). Peak torque (PT) and total work (TW) were measured by N∙m and J units, respectively. Participants were encouraged to put maximum effort in the extension movement in order to evaluate the knee extensors' function.
Resting measurement (measure 1): The session began with measuring the resting heart rate (HR) (using a Polar heart rate monitor – Polar AccurexPlus S610, Polar Electro, Woodbury, NY, USA). In the second (recovery) experiment, we measured blood lactate (Lactate Pro 2, ARKRAY Factory Inc., Japan), which was used as a physiological metabolic marker. The resting measurements were followed by a general physical warm-up.
General warm-up: It consisted of 5 min of cycling on an ergometer (Monark, Ergomedic 894 Ea) with a constant pre-set load of 50 Watts for women and 100 Watts for men, and then dynamic stretching exercises of the lower limbs, including flexion/extension of the hip (4 repetitions of 4 exercises).
Specific isokinetic warm-up of the knee extensor muscles: It consisted of five repetitions of submaximal concentric contractions through a range of motion of the knee 85-180º (180º represents full knee extension), and extension-flexion of the dominant leg’s (jumping leg) knee, at seven descending velocities from 240 to 90º/s. Of these five repetitions, three were performed at moderate intensity and two at high intensity (50%/80% of maximal voluntary contraction [MVC], respectively).
Treatment: Treatment consisted of either photobiomodulation or placebo treatment (crossover) to the quadriceps muscles.
Experiment 1, Maximal Strength:
A schematic representation is presentedin Figure 2.
Figure 2.
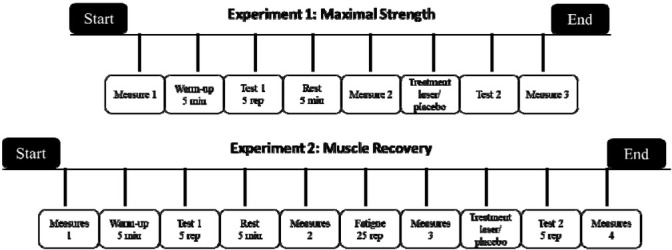
Schematic representation of the recovery experiment time-points (upper panel) and of the maximal strength experiment time-points (lower panel).
Test 1: After the warm-up, which was followed by a 3-min rest interval, participants were tested for maximal strength: five maximal-effort repetitions of isokinetic knee extension at an angular velocity of 60º/s (representing maximal strength in accordance with the isokinetic device manual). This test was followed by a 5-min rest period (and preparation of the laser device). The HR was measured at the end of this assessment (measure 2).
Treatment: After Test 1, participants underwent either photobiomodulation or placebo treatment.
Post-treatment assessment (Test 2): Five min after the termination of the treatment, the pretest treatment was repeated. The HR was measured again at the end of this assessment (measure 3).
In the second session the same procedure was followed, but with a different device (laser or placebo) being used in the treatment phase.
Experiment 2, Muscle Recovery:
A schematic representation is presentedin Figure 2.
Test 1: After the warm-up, which was followed by a 3-min rest interval, participants were tested for maximal strength: they performed five maximal-effort repetitions of isokinetic knee extension at an angular velocity of 90º/s. The test was followed by a 5-min rest period (and preparation of laser equipment). Blood lactate concentration and the HR were measured at the end of this assessment (measure 2).
Fatigue protocol: After the pre-test, participants performed 25 maximal-effort repetitions of knee extension at an angular velocity of 90º/s (representing a fatigue protocol in accordance with the isokinetic device manual). Blood lactate concentration and HR were measured at the end of this assessment (measure 3).
Treatment: After the fatigue protocol, participants underwent either photobiomodulation or a placebo treatment. Laser characteristics are presented in Table 2.
Test 2: One min after the termination of the treatment, a post-test was performed: five maximal-effort repetitions of isokinetic knee extension at an angular velocity of 90º/s. Blood lactate concentration and HR were measured again at the end of this assessment (measure 4).
In the second session, the same procedure was followed, but with a different device (laser or placebo) being used in the treatment phase.
Statistical Analyses
The SPSS program was used to analyze the data. The level of significance was set at .05. TW and PT averaged values were analyzed separately, to compare the two conditions of treatment – with (photobiomodulation) and without (placebo) active treatment, in order to evaluate their effect on maximal strength in Experiment 1, and on muscle recovery in Experiment 2. Analysis of variance with repeated measures (rmANOVA) of 2 x 2 was conducted. In addition, rmAnova of 2 x 4 was conducted to explore the influence of photobiomodulation on the recovery of the muscle in terms of blood lactate; rmAnova of 2 x 3 and 2 x 4 was conducted to determine the influence on the HR in Experiment 1 and in Experiment 2, respectively.
Results
Experiment 1 (Maximal Strength)
The participants’ anthropometric characteristics are shown in Table 1.
No significant differences were found between the laser treatment and the sham treatment with regard to TW (F(1,28) = .008, p = .93, η2 = .000). Additionally, there was no significant difference in work between the two time-points – before and after the treatment (F(1,28) = .097, p = .76, η2 = .003). There was also no significant interaction between the condition and the time-point. (F(1,28) = 1.09, p = .31, η2 = .037) (Figure 3, part A). Similar results were found considering PT. No significant differences in PT were found related to the condition (F(1,29) = .77, p = .39, η2 = .026) or to the time before and after the treatment (F(1,29) = 2.02, p =.166, η2 = .065). There was also no significant interaction between the condition and the time-point (F(1,29) = .056, p = .814, η2 = .002) (Figure 3, part B). There were no significant differences in HR responses related to condition (F(1,29) = .03, p = .88, η2 = .001).
Figure 3.
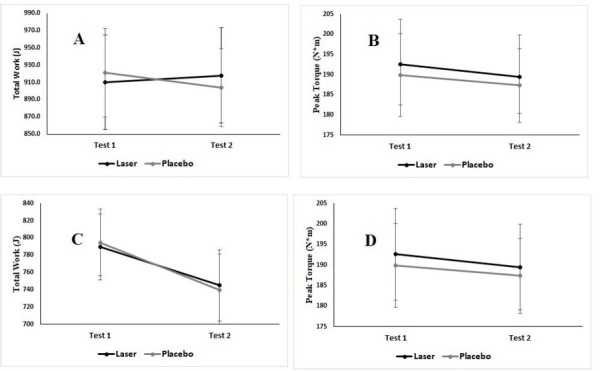
Maximal strength. A: The effect of LLLT (black) or a placebo (gray) on total work (TW) before (Test 1) and after (Test 2) the treatment. B: The effect of LLLT (black) or a placebo (gray) on peak torque (PT) before (Test 1) and after (Test 2) the treatment. Muscle recovery. C: The effect of LLLT (black) or a placebo (gray) on total work (TW) before (Test 1) and after (Test 2) the induction of fatigue and the treatment. D: The effect of LLLT (black) or a placebo (gray) on peak torque (PT) before (Test 1) and after (Test 2) the treatment. Mean and standard error are presented.
Experiment 2 (Muscle Recovery)
There were no significant differences in TW related to the condition (F(1,27) = 0.00, p = .995, η2 = .000). There was a significant decline in TW after applying the fatigue protocol (F(1,27) = 5.66, p < .05, η2 = .173). There was no significant interaction between the condition and the time-point (F(1,27) = .16, p = .69, η2 = .006) (Figure 3, part C). No significant differences in PT related to the condition were observed (F(1,26) = .88, p = .36, η2 = .033). No significant decline was noted in PT after applying the fatigue protocol (F(1,26) = .93, p = .34, η2 = .034). There was no significant interaction between the condition and the time-point (F(1,26) = .01, p = .92, η2 = .000) (Figure 3, part D). No significant differences related to the condition were found in blood lactate concentration (F(1,26) = 0.16, p = .69, η2 = .006). Also there were no significant differences related to HR responses (F(1,27) = .00, p = .997, η2 = .000).
Discussion
The main purpose of the study was to assess the effect of a small, home-use photobiomodulation device on maximal muscle strength and muscle recovery following resistance exercise.
Effect of the treatment on muscle strength
The results of the treatment on muscle strength showed that 10 minutes of photobiomodulation on the quadriceps femoris, before exercise, had no effect on muscle strength compared to the sham treatment. Negative effects were also shown in a previous study in young volleyball players as pre-exercise treatment with LLLT or LEDT (810 nm or 660 nm/850 nm, energy of 12 J or 83.4 J) protocols did not enhance performance in the Wingate tests or reduced post-exercise blood lactate concentration levels (Leal Junior et al., 2009a).
Our results are inconsistent with other findings as de Oliveira et al. (2017) showed that applying photobiomodulation (810 nm, energy of 300 J) before an eccentric protocol of knee extension increased maximal isometric voluntary contraction (MVC). This positive effect may be related to the higher energy delivered in their study. It is possible that the dose of energy in our study may not have been sufficient to induce significant changes in the measured variables, and did not stimulate the tissue enough to exert its effect. A higher amount of energy for the treatment may be achieved by higher doses of irradiation. Indeed, Hemmings et al. (2016) evaluated different doses of photobiomodulation treatment (660 nm, 850 nm and energy of 250 J, 500 J and 1000 J per muscle; data from a meta-analysis of Vanin et al. (2018)) and showed improvement in the number of repetitions after treatment with higher doses of energy. However, all the doses of the treatment did not affect PT values of knee flexion (Hemmings et al., 2016), as in our study. It is possible that the PT value is not sensitive enough to changes compared to the number of repetitions, thus explaining its lack of effect in this study.
Another study evaluated three energy doses on MVC (Vanin et al., 2016). Those authors also showed that laser treatment (810 nm, energy doses of 60 J, 180 J and 300 J per muscle) increased MVC from immediately after exercise to 24 h with a 300 J dose, and from 24 to 96 h with a 60 J dose. However, there were no differences for the 180 J dose, which is the closest dose to the energy delivered in our study (148.5 J). The researchers believe that: "different doses used in their study can lead to different time-windows (which can explain the immediate and delayed responses promoted by different doses) and/or different mechanisms of action" (Vanin et al., 2016).
Effect of Treatment on Muscle Recovery
In the second experiment of the current study, there was no effect of the laser treatment on muscle recovery (as measured by TW, PT, HR, and blood lactate concentrations at several time-points) compared to the sham treatment. The ineffectiveness of the treatment is in accordance with results shown by Higashi et al. (2013), although they used lower energy output (808 nm, energy of 56 J) than in our study.
Other studies have shown positive effects of laser treatment on muscle fatigue. De Souza et al. (2016) showed that participants treated with a laser (808 nm, energy of 25 J per muscle) had small, but significantly lower dynamometric fatigue index scores when compared to controls. Although participants in De Souza et al.'s (2016) study were as young and active as those in our study, and they radiated with less energy, they applied the treatment to a different muscle group (soleus vs. quadriceps). According to these researchers the soleus muscle was more affected by the treatment, as it had more aerobic characteristics compared to the quadriceps.
Leal Junior et al. (2008) showed a positive effect of photobiomodulation on muscle fatigue. They measured the mean number of repetitions and showed that it was increased by the active treatment (650 nm, energy of 20 J per muscle). It is possible that measuring the number of repetitions is more sensitive to treatment than measuring the dynamometer parameters, as it was done in our study.
Another explanation for the lack of positive effects in our study is the time-points of the measures during the procedures. Other studies have shown a lack of an immediate effect of photobiomodulation treatment on the MVC of knee flexion or on recovery. While the effects of a low-level laser on PT were not different immediately after a fatigue protocol, they were improved after 24 hr post induction of fatigue (De Marchi et al., 2017). This result is compatible with our study, as we measured PT immediately after the fatigue protocol.
Another point to be made regarding the findings of the current study is related to the exercise habits of the study participants. Although all participants were physical education students, it is possible that they performed various activities with different intensities, and accordingly their muscle responded to different loads. It is suggested that in a follow-up study, homogeneous groups of participants be examined performing the same types of exercise with unified intensity.
The inconsistency of the effects of photobiomodulation on muscle performance and recovery after fatigue may be the result of differences between various methods, such as the test protocols for measuring muscle performance, fatigue protocols, and/or laser characteristics. In a recently published systemic review and meta-analysis (Vanin et al., 2018), these variables were shown to affect the results of photobiomodulation on muscle performance and recovery from exercise.
This study has two limitations to be acknowledged, and suggestions for future studies. First, the measurement time points should be reconsidered. It might be beneficial to include a follow-up evaluation, in order to monitor delayed effects of the treatments, as in the study by De Marchi (2017). Second, the energy dosage delivered during the current study might not be effective, thus it is further recommended to evaluate treatments with different dosages.
Conclusions
The current photobiomodulation protocol of irradiation that was used (20 min, 808 nm, energy of 150 J) did not show beneficial effects on quadriceps muscle performance or recovery after induction of fatigue, when applied immediately after exercise.
References
- Albuquerque-Pontes GM, Vieira R de P, Tomazoni SS, Caires CO, Nemeth V, Vanin AA, Santos LA, Pinto HD, Marcos RL, Bjordal JM, de Carvalho P de TC, Leal- Junior ECP. Effect of pre-irradiation with different doses, wavelengths, and application intervals of low-level laser therapy on cytochrome c oxidase activity in intact skeletal muscle of rats. Lasers Med Sci. 2015;30:59–66. doi: 10.1007/s10103-014-1616-2. [DOI] [PubMed] [Google Scholar]
- Alves AN, Fernandes KPS, Deana AM, Bussadori SK, Mesquita-Ferrari RA. Effects of low-level laser therapy on skeletal muscle repair. Am J Phys Med Rehabil. 2014;93:1073–1085. doi: 10.1097/PHM.0000000000000158. [DOI] [PubMed] [Google Scholar]
- Antonialli FC, De Marchi T, Tomazoni SS, Vanin AA, dos Santos Grandinetti V, de Paiva PRV, Pinto HD, Miranda EF, de Tarso Camillo de Carvalho P, Leal- Junior ECP. Phototherapy in skeletal muscle performance and recovery after exercise: effect of combination of super-pulsed laser and light-emitting diodes. Lasers Med Sci. 2014;29:1967–1976. doi: 10.1007/s10103-014-1611-7. [DOI] [PubMed] [Google Scholar]
- Aver Vanin A, De Marchi T, Silva Tomazoni S, Tairova O, Leão Casalechi H, de Tarso Camillo de Carvalho P, Bjordal JM, Leal-Junior EC. Pre-exercise infrared low-level laser therapy (810 nm) in skeletal muscle performance and postexercise recovery in humans, what is the optimal dose? A randomized, double-blind, placebo-controlled clinical trial. Photomed Laser Surg. 2016;34:473–482. doi: 10.1089/pho.2015.3992. [DOI] [PubMed] [Google Scholar]
- Chow RT, Johnson MI, Lopes-Martins RAB, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374:1897–908. doi: 10.1016/S0140-6736(09)61522-1. [DOI] [PubMed] [Google Scholar]
- da Silva Alves MA, Pinfildi CE, Neto LN, Lourenço RP, de Azevedo PHSM, Dourado VZ. Acute effects of low-level laser therapy on physiologic and electromyographic responses to the cardiopulmonary exercise testing in healthy untrained adults. Lasers Med Sci. 2014;29:1945–1951. doi: 10.1007/s10103-014-1595-3. [DOI] [PubMed] [Google Scholar]
- de Marchi T, Schmitt VM, Danúbia da Silva Fabro C, da Silva LL, Sene J, Tairova O, Salvador M. Phototherapy for improvement of performance and exercise recovery: Comparison of 3 commercially available devices. J Athl Train. 2017;52:429–438. doi: 10.4085/1062-6050-52.2.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira HA, Antonio EL, Silva FA, de Carvalho Pde TC, Feliciano R, Yoshizaki A, Vieira S de S, de Melo BL, Leal-Junior ECP, Labat R, Bocalini DS, Silva Junior JA, Tucci PJF, Serra AJ. Protective effects of photobiomodulation against resistance exercise-induced muscle damage and inflammation in rats. J Sports Sci. 2018;36:2349–2357. doi: 10.1080/02640414.2018.1457419. [DOI] [PubMed] [Google Scholar]
- de Oliveira AR, Vanin AA, Tomazoni SS, Miranda EF, Albuquerque-Pontes GM, De Marchi T, dos Santos Grandinetti V, de Paiva PRV, Imperatori TBG, de Carvalho Pde TC, Bjordal JM, Leal-Junior ECP. Pre-exercise infrared photobiomodulation therapy (810 nm) in skeletal muscle performance and oostexercise tecovery in humans: What is the optimal power output? Photomed Laser Surg. 2017;35:595–603. doi: 10.1089/pho.2017.4343. [DOI] [PubMed] [Google Scholar]
- de Souza CG, Borges DT, de Brito Macedo L, Brasileiro JS. Low-level laser therapy reduces the fatigue index in the ankle plantar flexors of healthy subjects. Lasers Med Sci. 2016;31:1949–1955. doi: 10.1007/s10103-016-2074-9. [DOI] [PubMed] [Google Scholar]
- dos Santos Maciel T, Muñoz ISS, Nicolau RA, Nogueira DV, Hauck LA, Osório RAL, de Paula Júnior AR. Phototherapy effect on the muscular activity of regular physical activity practitioners. Lasers Med Sci. 2014;29:1145–1152. doi: 10.1007/s10103-013-1481-4. [DOI] [PubMed] [Google Scholar]
- Gupta A, Avci P, Sadasivam M, Chandran R, Parizotto N, Vecchio D, de Melo WCMA, Dai T, Chiang LY, Hamblin MR. Shining light on nanotechnology to help repair and regeneration. Biotechnol Adv. 2013;31:607–631. doi: 10.1016/j.biotechadv.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings TJ, Kendall K, Dobson JL. Identifying dosage effect of LEDT on muscular fatigue in quadriceps. J Strength Cond Res. 2016;31:1. doi: 10.1519/JSC.0000000000001523. [DOI] [PubMed] [Google Scholar]
- Higashi RH, Toma RL, Tucci HT, Pedroni CR, Ferreira PD, Baldini G, Aveiro MC, Borghi-Silva A, de Oliveira AS, Renno ACM. Effects of low-level laser therapy on biceps braquialis muscle fatigue in young women. Photomed Laser Surg. 2013;31:586–594. doi: 10.1089/pho.2012.3388. [DOI] [PubMed] [Google Scholar]
- Leal Junior ECP, Lopes-Martins RÁB, Baroni BM, De Marchi T, Rossi RP, Grosselli D, Generosi RA, de Godoi V, Basso M, Mancalossi JL, Bjordal JM. Comparison between single-diode low-level laser therapy (LLLT) and LED multi-diode (cluster) therapy (LEDT) applications before high-intensity exercise. Photomed Laser Surg. 2009a;27:617–623. doi: 10.1089/pho.2008.2350. [DOI] [PubMed] [Google Scholar]
- Leal Junior ECP, Lopes-Martins RAB, Dalan F, Ferrari M, Sbabo FM, Generosi RA, Baroni BM, Penna SC, Iversen VV, Bjordal JM. Effect of 655-nm low-level laser therapy on exercise-induced skeletal muscle fatigue in humans. Photomed Laser Surg. 2008;26:419–424. doi: 10.1089/pho.2007.2160. [DOI] [PubMed] [Google Scholar]
- Leal Junior ECP, Lopes-Martins RÁB, de Almeida P, Ramos L, Iversen VV, Bjordal JM. Effect of low-level laser therapy (GaAs 904 nm) in skeletal muscle fatigue and biochemical markers of muscle damage in rats. Eur J Appl Physiol. 2010a;108:1083–1088. doi: 10.1007/s00421-009-1321-1. [DOI] [PubMed] [Google Scholar]
- Leal Junior ECP, Lopes-Martins RAB, Frigo L, De Marchi T, Rossi RP, de Godoi V, Tomazoni SS, Silva DP, Basso M, Filho PL, de Valls Corsetti F, Iversen VV, Bjordal JM. Effects of low-level laser therapy (LLLT) in the development of exercise-induced skeletal muscle fatigue and changes in biochemical markers related to postexercise recovery. J Orthop Sports Phys Ther. 2010b;40:524–532. doi: 10.2519/jospt.2010.3294. [DOI] [PubMed] [Google Scholar]
- Leal Junior ECP, Lopes-Martins RAB, Rossi RP, De Marchi T, Baroni BM, de Godoi V, Marcos RL, Ramos L, Bjordal JM. Effect of cluster multi-diode light emitting diode therapy (LEDT) on exercise-induced skeletal muscle fatigue and skeletal muscle recovery in humans. Lasers Surg Med. 2009b;41:572–577. doi: 10.1002/lsm.20810. [DOI] [PubMed] [Google Scholar]
- Leal Junior ECP, Lopes-Martins RAB, Vanin AA, Baroni BM, Grosselli D, De Marchi T, Iversen VV, Bjordal JM. Effect of 830 nm low-level laser therapy in exercise-induced skeletal muscle fatigue in humans. Lasers Med Sci. 2009c;24:425–431. doi: 10.1007/s10103-008-0592-9. [DOI] [PubMed] [Google Scholar]
- Mantineo M, Pinheiro JP, Morgado AM. Low-level laser therapy on skeletal muscle inflammation: evaluation of irradiation parameters. J Biomed Opt. 2014;19:98002. doi: 10.1117/1.JBO.19.9.098002. [DOI] [PubMed] [Google Scholar]
- Oron U, Ilic S, De Taboada L, Streeter J. Ga-As (808 nm) laser irradiation enhances ATP production in human neuronal cells in culture. Photomed Laser Surg. 2007;25:180–182. doi: 10.1089/pho.2007.2064. [DOI] [PubMed] [Google Scholar]
- Patrocinio T, Sardim AC, Assis L, Fernandes KR, Rodrigues N, Renno ACM. Effect of low-level laser therapy (808 nm) in skeletal muscle after resistance exercise training in rats. Photomed Laser Surg. 2013;31:492–498. doi: 10.1089/pho.2013.3540. [DOI] [PubMed] [Google Scholar]
- Rodrigues NC, Brunelli R, Abreu DCC, Fernandes K, Parizotto NA, Renno ACM. Morphological aspects and Cox-2 expression after exposure to 780-nm laser therapy in injured skeletal muscle: an in vivo study. Brazilian J Phys Ther. 2014;18:395–401. doi: 10.1590/bjpt-rbf.2014.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira PCL, Silva LA da, Fraga DB, Freitas TP, Streck EL, Pinho R. Evaluation of mitochondrial respiratory chain activity in muscle healing by low-level laser therapy. J Photochem Photobiol. 2009;95:89–92. doi: 10.1016/j.jphotobiol.2009.01.004. B. [DOI] [PubMed] [Google Scholar]
- Tuby H, Hertzberg E, Maltz L, Oron U. Long-term safety of low-level laser therapy at different power densities and single or multiple applications to the bone marrow in mice. Photomed Laser Surg. 2013;31:269–273. doi: 10.1089/pho.2012.3395. [DOI] [PubMed] [Google Scholar]
- Vanin AA, Verhagen E, Barboza SD, Costa LOP, Leal-Junior ECP. Photobiomodulation therapy for the improvement of muscular performance and reduction of muscular fatigue associated with exercise in healthy people: a systematic review and meta-analysis. Lasers Med Sci. 2018;33:181–214. doi: 10.1007/s10103-017-2368-6. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- Woodruff LD, Bounkeo JM, Brannon WM, Dawes KS, Barham CD, Waddell DL, Enwemeka CS. The efficacy of laser therapy in wound repair: a meta-analysis of the literature. Photomed Laser Surg. 2004;22:241–247. doi: 10.1089/1549541041438623. [DOI] [PubMed] [Google Scholar]


