Abstract
BACKGROUND & AIMS:
The safety of different antithrombotic strategies for patients with 1 or more indication for antithrombotic drugs has not been determined. We investigated the risk and time frame for gastrointestinal bleeding (GIB) in patients prescribed different antithrombotic regimens. We proposed that risk would increase over time and with combination regimens, especially among elderly patients.
METHODS:
We performed a retrospective analysis of nationwide claims data from privately insured and Medicare Advantage enrollees who received anticoagulant and/or antiplatelet agents from October 1, 2010, through May 31, 2017. Patients were stratified by their prescriptions (anticoagulant alone, antiplatelet alone, or a combination) and by their primary diagnosis (atrial fibrillation, ischemic heart disease, or venous thromboembolism). The 1-year GIB risk was estimated using parametric time-to-event survival models and expressed as annualized risk and number needed to harm (NNH).
RESULTS:
Our final analysis included 311,211 patients (mean ages, 67 years for monotherapy and 69.8 years for combination antithrombotic therapy). There was no significant difference in the proportion of patients with bleeding after anticoagulant or antiplatelet monotherapy (~3.5%/year). Combination antithrombotic therapy increased GIB risk compared with anticoagulant (NNH, 29) or antiplatelet (NNH, 31) monotherapy, regardless of the patients’ diagnosis or time point analyzed. Advancing age was associated with increasing 1-year probability of GIB. Patients prescribed combination therapy were at the greatest risk for GIB, especially after the age of 75 years (GIB occurred in 10%–17.5% of patients/y).
CONCLUSIONS:
In an analysis of nationwide insurance and Medicare claims data, we found GIB to occur in a higher proportion of patients prescribed combinations of anticoagulant and antiplatelet agents compared with monotherapy. Among all drug exposure categories and cardiovascular conditions, the risk of GIB increased with age, especially among patients older than 75 years.
Keywords: Anticoagulant, Antiplatelet, Ischemic Heart Disease, Venous Thromboembolism, Atrial Fibrillation
Gastrointestinal bleeding (GIB) in cardiac patients is common in an aging and increasingly comorbid population.1,2 Choosing among drug strategies is challenging in the absence of head-to-head safety studies among comparable patients, given the myriad of treatment options, combinations, and increasing patient complexity and comorbidity. Published studies tend to focus on a single cardiovascular risk group when studying adverse events,1,3-7 an approach that does not reflect the clinical reality of patients with more than 1 underlying cardiovascular condition requiring antithrombotic therapy. In addition, there are limited data on GIB risk in the era of direct oral anticoagulants (DOACs) and second-generation antiplatelet agents.3,6,7
We sought to fill this knowledge gap by providing a richer exploration of GI bleeding risk (ie, safety) among patients with multiple comorbid cardiovascular conditions, in whom multiple antithrombotic strategies are indicated.6,8-10 We quantified the risk and time frame of GIB among persons with 1 or more indications (atrial fibrillation, ischemic heart disease, and/or venous thromboembolism) for antithrombotic medications (antiplatelet and/or anticoagulant drugs) prescribed as monotherapy or in combination. We hypothesized that GIB risk would increase over time when combination antithrombotic drug regimens are prescribed and patients age 75 years and older would be at greatest risk.
Methods
Data Source
We evaluated data from the OptumLabs Data Warehouse (Cambridge, MA), which includes claims data for privately insured and Medicare Advantage enrollees in a large private, US health plan.1,5 The database contains longitudinal health information including physician, hospital, and prescription drug services on enrollees of diverse ages, ethnicities, and geographic regions across the United States. Medical claims include International Classification of Diseases, 9th and 10th revisions, Clinical Modification, diagnosis codes and procedure codes, Current Procedural Terminology, version 4, procedure codes, Healthcare Common Procedure Coding System procedure codes, site of service codes, and provider specialty codes. Because this study involved analysis of pre-existing, de-identified data, it was exempt from Institutional Review Board approval.
Patient Identification and Stratification
We identified patients 18 years of age and older with an index prescription of an anticoagulant (vitamin K antagonist or DOAC) and/or an antiplatelet agent (second- or third-generation thienopyridine agent) either as monotherapy or in combination from October 1, 2010, to May 31, 2017. The date of first filled prescription was the index date and was used to assign patients to their exposure strata (anticoagulant, antiplatelet, or anticoagulant and antiplatelet). We excluded patients with evidence of a dispensed prescription in the 12 months before the index date to ensure a new-user cohort. All patients required 12 months of continuous enrollment in the medical and pharmacy plan before the index prescription; this period of time was considered the baseline period for assessment of past medical history. We excluded patients with a current cancer diagnosis who may be at risk for a malignancy-associated GIB. Eligible patients then were stratified by cardiovascular (CV) diagnosis of atrial fibrillation (AF), ischemic heart disease (IHD), and/or venous thromboembolism (VTE) at the time of the index prescription; combinations of these 3 conditions were treated as separate strata. The first occurrence of the diagnostic code of interest during the first 90 days of observation after index prescription determined the cardiovascular condition stratification category.
Exposures and Primary Outcome
Exposure was considered continuous from the index date until the occurrence of an outcome or until censoring because of the end of enrollment (including owing to mortality), switch to another treatment strategy, or treatment termination as defined by the absence of a new prescription by the end of the 30 days after the last identified prescription fill date for the index medication. The last date of follow-up evaluation was May 31, 2017. The primary outcome of interest was a GI bleed as previously defined,1,5,11 expressed as upper GIB, lower GIB, and the composite outcome of total GIB (Supplementary Table 1). Each event was identified using in-patient hospital claims for relevant primary and secondary discharge diagnoses.
Variables of Interest
Baseline demographic characteristics, CHA2DS2-VASC score (ie, CHADS; Congestive heart failure, Hypertension, Age [ ≥ 65 = 1 point, ≥ 75 = 2 points], Diabetes, and Stroke/TIA (2 points). VASc stands for vascular disease (peripheral arterial disease, previous MI, aortic atheroma) and female gender) and concomitant prescribed pharmacologic risk factors (acetylsalicylic acid [ASA], nonsteroidal anti-inflammatory drugs [NSAIDs], selective serotonin reuptake inhibitors, and gastroprotective agents) were assessed as potential confounding variables. Comorbid conditions were identified by administrative codes in the primary or secondary position on any claim during the baseline period and overall comorbidity burden was assessed using the Charlson–Deyo index.
Statistical Analysis
We estimated separate models for each of the antithrombotic drug exposures by CV condition group using a parametric time-to-event survival model with a Weibull distribution.12 This model then was used to predict the GIB risk of each patient averaged over each treatment group at multiple time points within the first 6 months of the prescription and at 1 year. For each prescription strategy, the 1-year risk ratio and 95% CIs were calculated, as was the absolute risk reduction and the number needed to harm (NNH) at 1 year. The analytic data set was created and manipulated using SAS 9.3 (SAS Institute, Inc, Cary, NC) and Stata 15.1 (Stata Corp, College Station, TX).
Sensitivity Analyses
We examined the effect of age and treatment regimen on annualized GIB by including an interaction term in the model. The comparative safety of the antithrombotic prescription was examined in 5 age stratum: 18 to 44, 45 to 54, 55 to 64, 65 to 74, and 75 and older. To address potential underascertainment of over-the-counter (OTC) ASA and proton pump inhibitor (PPI) cohort members were assigned randomly to an increased prevalence of ASA and PPI use (20%, 40%, 60%, and 80%), and the magnitude of effect on the annualized GIB (total, upper, and lower) was calculated.
Results
Baseline Characteristics
We identified 311,211 eligible patients (Figure 1). At index, 164,649 patients were prescribed an anticoagulant agent, 142,433 patients were prescribed a non-ASA/NSAID antiplatelet agent, and 4129 patients were prescribed an anticoagulant concomitantly with a non-ASA/NSAID antiplatelet agent. The mean patient age ranged from 67 years in patients prescribed anticoagulant or antiplatelet monotherapy to 70 years for those prescribed combination therapy with an anticoagulant and concomitant antiplatelet (Table 1). The duration of therapy was shortest for patients prescribed combination antithrombotic therapy (111 days), and longest for patients prescribed antiplatelet monotherapy (291 days). We observed a male sex predominance (67%–70% male), more than 30% were 75 years and older and most were Caucasian (74%). A CHA2DS2-VASC score higher than 4 was noted among 55.4% and 81.3% prescribed anticoagulant or combination antithrombotic therapy, respectively. The Charlson–Deyo comorbidity index suggested the majority had a relatively low, noncardiac comorbidity burden.
Figure 1.
Study flow diagram. GIB, gastrointestinal bleeding.
Table 1.
All Patients
| Full cohort | |||
|---|---|---|---|
| Characteristics | Anticoagulant (N = 164,649) |
Antiplatelets (N = 142,433) |
Anticoagulants & Antiplatelets (N = 4129) |
| Demographics and risk scores | |||
| Age | |||
| Mean (SD) | 67.0 (14.1) | 67.0 (11.7) | 69.8 (11.5) |
| Age Group | |||
| 18-44 | 12,909 (7.84%) | 4838 (3.40%) | 122 (2.95%) |
| 45-54 | 17,665 (10.73%) | 17,032 (11.96%) | 314 (7.60%) |
| 55-64 | 32,268 (19.60%) | 35,515 (24.93%) | 795 (19.25%) |
| 65-74 | 44,416 (26.98%) | 43,874 (30.80%) | 1,280 (31.00%) |
| 75+ | 57,391 (34.86%) | 41,174 (28.91%) | 1,618 (39.19%) |
| Sex | |||
| Female | 80,376 (48.82%) | 56,781 (39.87%) | 1,566 (37.93%) |
| Male | 84,273 (51.18%) | 85,652 (60.13%) | 2,563 (62.07%) |
| Race/Ethnicity | |||
| White | 124,302 (75.50%) | 100,544 (70.59%) | 3,109 (75.30%) |
| Black | 16,288 (9.89%) | 16,124 (11.32%) | 377 (9.13%) |
| Other | 24,059 (14.61%) | 25,765 (18.09%) | 643 (15.57%) |
| Charlson-Deyo score | |||
| 0-1 | 99,315 (60.32%) | 62,989 (44.22%) | 3,344 (80.99%) |
| 2-3 | 33,288 (20.22%) | 39,456 (27.70%) | 308 (7.46%) |
| 4+ | 32,046 (19.46%) | 39,988 (28.07%) | 477 (11.55%) |
| Condition(s) at Treatment Episode Start | |||
| AF | 35,637 (21.64%) | 795 (0.56%) | 60 (1.45%) |
| AF + IHD | 48,308 (29.34%) | 16,482 (11.57%) | 2,346 (56.82%) |
| AF + IHD + VTE | 9,811 (5.96%) | 1,417 (0.99%) | 453 (10.97%) |
| IHD | 14,372 (8.73%) | 118,826 (83.43%) | 658 (15.94%) |
| IHD + VTE | 16,263 (9.88%) | 4,474 (3.14%) | 575 (13.93%) |
| VTE | 35,789 (21.74%) | 399 (0.28%) | 35 (0.85%) |
| VTE + AF | 4,469 (2.71%) | 40 (0.03%) | ~ |
| Previous Treatment Episode | 40,867 (24.82%) | 29,259 (20.54%) | 3,221 (78.01%) |
| Baseline Comorbidities | |||
| Alcoholism | 9,368 (5.69%) | 7,766 (5.45%) | 230 (5.57%) |
| Carotid Revascularization Procedures | 4,275 (2.60%) | 8,058 (5.66%) | 308 (7.46%) |
| Chronic Liver Disease | 11,089 (6.73%) | 9,502 (6.67%) | 282 (6.83%) |
| Chronic Kidney Disease | 22,907 (13.91%) | 19,663 (13.81%) | 857 (20.76%) |
| Chronic Heart Failure | 51,288 (31.15%) | 37,531 (26.35%) | 2,151 (52.09%) |
| Diabetes Treatment | |||
| No Diabetes | 106,627 (64.76%) | 78,970 (55.44%) | 2,045 (49.53%) |
| Diabetes not treated | 28,313 (17.20%) | 25,079 (17.61%) | 929 (22.50%) |
| Diabetes treated with metformin only | 7,982 (4.85%) | 8,794 (6.17%) | 241 (5.84%) |
| Diabetes treated with other non-insulin | 11,040 (6.71%) | 14,145 (9.93%) | 427 (10.34%) |
| medications | |||
| Diabetes treated with insulin | 10,687 (6.49%) | 15,445 (10.84%) | 487 (11.79%) |
| History of GIB | 37,187 (22.59%) | 32,389 (22.74%) | 919 (22.26%) |
| History of Ischemic Heart Disease | 75,065 (45.59%) | 116,024 (81.46%) | 3,592 (86.99%) |
| History of PCI | 11,493 (6.98%) | 76,828 (53.94%) | 2,481 (60.09%) |
| Hypertension | 136,172 (82.70%) | 129,527 (90.94%) | 3,854 (93.34%) |
| Peripheral Arterial Disease | 19,862 (12.06%) | 33,409 (23.46%) | 1,225 (29.67%) |
| Rheumatological Diseases | 12,307 (7.47%) | 9,229 (6.48%) | 330 (7.99%) |
| Sleep Apnea | 22,956 (13.94%) | 15,688 (11.01%) | 608 (14.73%) |
| Smoking | 51,850 (31.49%) | 64,788 (45.49%) | 1,935 (46.86%) |
| Thyroid Disease | 47,278 (28.71%) | 36,950 (25.94%) | 1,155 (27.97%) |
| Valvular Disease | 69,973 (42.50%) | 53,818 (37.78%) | 2,315 (56.07%) |
| Viral Hepatitis | 2,940 (1.79%) | 2,762 (1.94%) | 70 (1.70%) |
| Concomitant drug exposure | |||
| Aspirin and/or NSAID | 22,311 (13.55%) | 22,237 (15.61%) | 516 (12.50%) |
| Anti-hypertensive drugs | 114,977 (69.83%) | 113,978 (80.02%) | 3,716 (90.00%) |
| Anti-arrhythmics | 18,083 (10.98%) | 3,646 (2.56%) | 675 (16.35%) |
| Gastroprotective agents, proton pump inhibitor, H2 blocker | 38,382 (23.31%) | 36,514 (25.64%) | 1,352 (32.74%) |
| Selective serotonin reuptake inhibitors (SSRI) | 20,793 (12.63%) | 17,341 (12.17%) | 532 (12.88%) |
Anticoagulant therapy was the primary strategy for patients with AF alone. However, both AF and IHD patients commonly were prescribed combination anticoagulant and antiplatelet therapy (56.8%). Among patients with AF with IHD and VTE, combination antithrombotic therapy was most prevalent (11.0%), with fewer prescribed anticoagulant monotherapy (6.0%). Patients with IHD predominantly were prescribed antiplatelet therapy (84.4%). Among those patients with IHD and VTE, the addition of an anticoagulant to antiplatelet therapy (13.9%) was more common than anticoagulant monotherapy (9.9%) or antiplatelet monotherapy (3.1%). Patients with VTE or VTE and AF predominantly were prescribed anticoagulant monotherapy, as expected. DOACs were prescribed as the index anticoagulant prescription in 42.7% of patients in this new-user cohort, with the greatest prevalence among patients aged 18 to 64 years (Supplementary Table 2). Among patients receiving DOACs, an apixaban prescription was prescribed to 10.3% of patients aged 18 to 64, to 15.4% of patients aged 65 to 74, and to 17.4% of patients aged 75 years and older (Supplementary Table 3).
Chronic kidney disease was noted in approximately 16% and a past medical history of GIB was present in 22% to 23% of patients. A concomitant prescription of ASA or NSAIDs was noted in 13.6% of patients prescribed an anticoagulant, in 15.6% of patients prescribed antiplatelets, and in 12.5% of patients prescribed combination antithrombotic therapy. Gastroprotective agents, including PPIs and histamine blockers, were prescribed in 23% to 33% of patients whereas prescription of selective serotonin reuptake inhibitors was approximately 12%.
Annual Risk of Gastrointestinal Bleeding, Absolute Risk Reduction, and the Number Needed to Harm
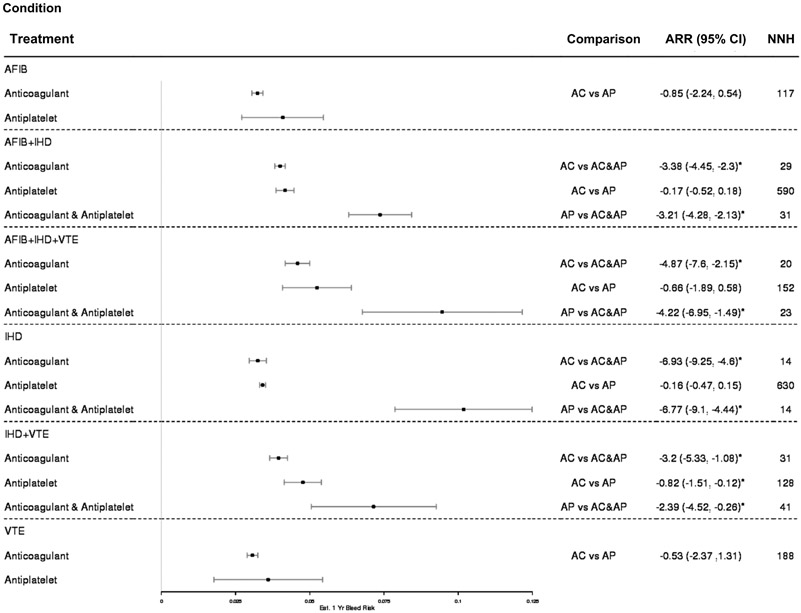
Overall, there were 13,979 GIB events during the period of observation, of which 9929 (3.2%) were upper and 3937 (1.3%) were lower events, and 113 (0.8%) were impossible to classify. Figure 2 shows the 1-year total GIB risk per patient based on prescription and cardiovascular indication, as well as the absolute risk reduction of 2 compared strategies and the subsequent NNH. Among all conditions, the prescription of combination antithrombotic therapy is associated with a higher risk of GIB at 1 year when compared with monotherapy. The same was true at all other time points examined (Supplementary Table 4).
Figure 2.
Annual risk of gastrointestinal (GI) bleeding, absolute risk reduction (ARR), and number needed to harm (NNH). AC, anticoagulant; AF, atrial fibrillation; AP, antiplatelet; IHD, ischemic heart disease; VTE, venous thromboembolism.
In Figure 2, patients with both AF and IHD prescribed combination antithrombotic therapy have a higher 1-year GIB risk at 7.4% (95% CI, 6.3–8.4) than those patients prescribed anticoagulant therapy alone. Only 29 patients with AF + IHD would need to be prescribed combination antithrombotic therapy to incur 1 additional GIB. In this cardiovascular subgroup (ie, AF + IHD) the risk of GIB is similar between anticoagulant monotherapy (4.0%; 95% CI, 3.8–4.2) and antiplatelet monotherapy (4.2; 95% CI, 3.9–4.5), as is the protective benefit of monotherapy over combination antithrombotic therapy. Only 31 patients would need to be prescribed combination antithrombotic therapy as opposed to antiplatelet monotherapy to incur 1 additional clinically significant GIB.
Among patients with concurrent AF, IHD, and VTE the prescription strategy with the lowest GIB risk per patient-year is anticoagulant monotherapy, and the strategy with the greatest GIB risk is combination antithrombotic therapy. As few as 20 patients with AF + IHD + VTE would need to be prescribed combination antithrombotic therapy to result in 1 additional GIB, with its 10% per patient-year risk of GIB. A similar pattern was observed among patients with IHD. If prescribed combination antithrombotic therapy with an anticoagulant and antiplatelet, a patient’s 1-year probability of GIB increased to 10% from 3.5% with anticoagulant or antiplatelet monotherapies.
The prescription of combination antithrombotic therapy was associated with a much higher GIB risk among patients with IHD and VTE. With an estimated 1 year GIB risk per patient of 7.5%, 41 patients with IHD and VTE would need to be prescribed this strategy instead of antiplatelet monotherapy, or 31 patients instead of anticoagulant therapy, to incur 1 additional GIB. Stratification by lower GIB (Supplementary Figure 1) and upper GIB (Supplementary Figure 2) showed a similar pattern across all cardiovascular strata and drug regimens.
Sensitivity Analysis: Impact of Advancing Age on Gastrointestinal Bleeding Risk
Across all cardiovascular subgroups, older age was associated with a greater probability of GI bleeds within 1 year of the index prescription (Figures 3 and 4). The patients’ prescribed concomitant anticoagulants and antiplatelets were at the greatest risk, especially after the age of 75. Among patients with AF (Figure 3), anticoagulant monotherapy strategies were the safest regimens across all age ranges regardless of whether the patient had additional cardiovascular conditions at the time of the index prescription (ie, IHD or IHD and VTE). Among patients older than age 65 years with AF, combination antithrombotic therapy was associated with twice the rate of GIB within 1 year when compared with anticoagulant monotherapy (10%–11% and 5%/y, respectively). The same pattern was seen among the elderly with AF and IHD, and AF with IHD and VTE. In the latter group, combination antithrombotic therapy in patients 75 years and older was associated with more than a 17% probability of GIB per year, as compared with a 9% probability of GIB per year among similar patients prescribed anticoagulant monotherapy (Figure 3). Increased risk with combination antithrombotic therapy and advancing age also was seen among patients with IHD, IHD and VTE, VTE, and VTE and AF (Figure 4).
Figure 3.

Age-stratified analysis. AC, anticoagulant; AF, atrial fibrillation; AP, antiplatelet; GI, gastrointestinal; IHD, ischemic heart disease; VTE, venous thromboembolism.
Figure 4.
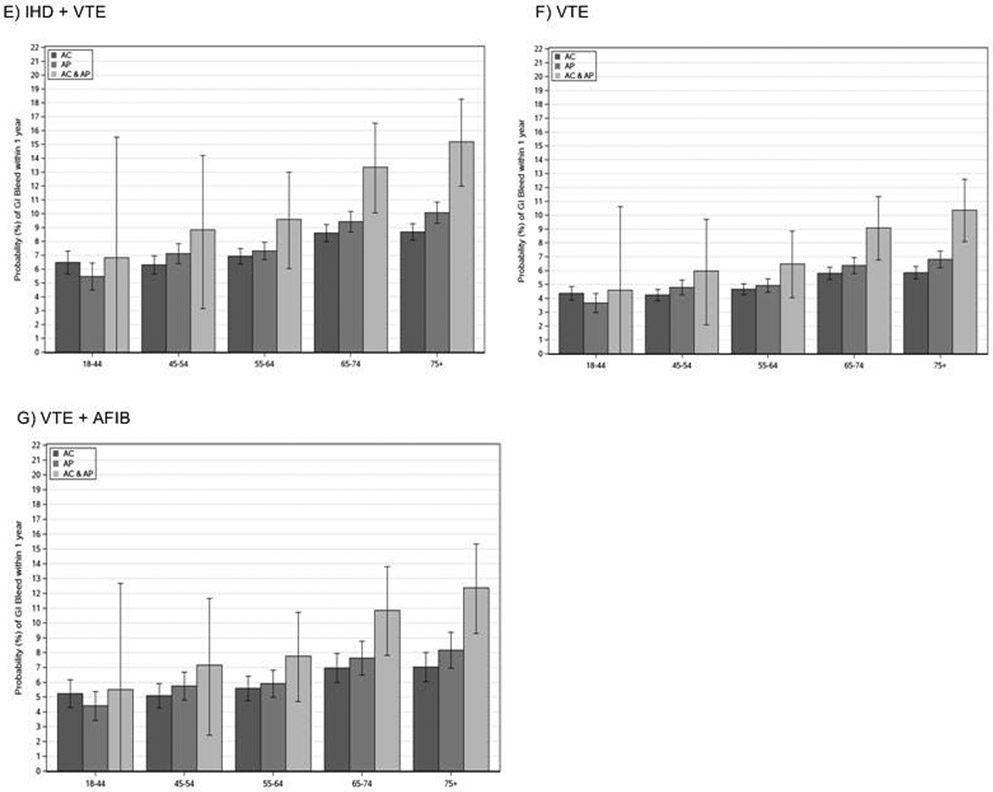
Age-stratified analysis. AC, anticoagulant; AF, atrial fibrillation; AP, antiplatelet; GI, gastrointestinal; IHD, ischemic heart disease; VTE, venous thromboembolism.
Sensitivity Analysis: Impact of Potential Over-the-Counter Acetylsalicylic Acid and Proton Pump Inhibitor Use
OTC drug use is not captured in traditional prescription drug claims data. To explore how risk estimates might change with the addition of OTC ASA and PPI, we conducted exploratory sensitivity analyses. In Supplementary Tables 5 and 6 we highlight the annualized estimates of GIB assuming different clinical thresholds of OTC ASA or OTC PPI use from 20% to 80%. As shown in Supplementary Tables 5 and 6, no significant difference in GIB rates were seen because the prevalence of ASA or PPI use was varied from 20% to 80%. There was also no meaningful difference in location of the GI bleed when OTC drug risk was varied among all CV subgroups prescribed any prescription strategy.
Discussion
The goal of this study was to quantify the safety, as assessed by rates of GIB, of a variety of common antithrombotic regimens among patients with 1 or more cardiovascular conditions. The use of a large national administrative claims database of commercially insured and Medicare Advantage patients during a time period with increasing use of DOACs and second-generation antiplatelet agents permitted a broad exploration of the real-world risk of antithrombotic polypharmacy among American cardiovascular patients. We showed that the use of antithrombotic polypharmacy was associated with a much higher risk of GIB than monotherapies. Our estimates of GIB among cardiovascular patients with 1 or more indication for antiplatelet or anticoagulant therapy show a 2.5% to 10% annual risk per patient depending on the indication for antithrombotic therapy and the choice of monotherapy or combination antithrombotic therapy. These estimates remained stable over multiple time points within the first 6 months of antithrombotic prescription over all cardiovascular patient strata and prescription strategies examined.
Typically, comparative effectiveness and safety studies have been used to estimate the benefits and risks associated with each of the antithrombotic agents.3,7,13,14 However, because we were interested specifically in quantifying the risk of GIB among patients taking various antithrombotic regimens for varying indications, a comparative effectiveness study would not be suitable because it would require limiting our investigation to a homogenous population to ensure accurate assessment of benefit among patients with similar CV conditions and indications for drug therapy. Online calculators/risk prediction tools such as the Stroke Prevention in Atrial Fibrillation Risk Tool already exist to compare the comparative benefits of commonly used medication regimens for the atrial fibrillation population (http://www.sparctool.com/). Similarly, the antiplatelet therapy clinical prediction score was developed to help identify patients with greater expected benefit vs harm from prolonged dual-antiplatelet therapy.15 In this study, we have extended the safety literature by performing a methodologically rigorous study that allows for examination of antithrombotic-related GIB risk as a drug class, regardless of the indication for which these agents are prescribed. The results of this study highlight the importance of careful consideration of risk-benefit before adding antiplatelet and anticoagulant drugs to a patient’s cardiovascular regimen, particularly in patients older than age 75.
In the current study, AF patients had similar bleeding rates whether they were prescribed anticoagulant or antiplatelet therapy. This is consistent with the results from the Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) trial,16 which showed a reduction in the risk of systemic embolism without a significant increase in GIB complications (hazard ratio, 0.86; 95% CI, 0.38–1.90) when apixaban (5 mg/d) was used as compared with aspirin (81–324 mg/d). In the AVERROES trial, a reduced dose of apixaban (2.5 mg/d) was used in the very elderly (age, ≥80 y), in patients with low body weight (≤60 kg), or individuals with impaired renal excretion (serum creatinine level, ≥1.5 mg/dL). Dose reduction among the elderly and in patients with renal impairment, as directed in the Food and Drug Administration drug labeling, has been shown to reduce gastrointestinal and other major bleeding complications significantly.17-19 Both underdosing and overdosing commonly are observed in practice and is associated with suboptimal stroke and bleeding risks.20
The risk-benefit profile of AF patients with concurrent IHD may favor anticoagulant monotherapy, which has been shown to be effective in patients with IHD.14 Our data suggest that combination antithrombotic therapy substantially increases GIB risk when compared with anticoagulant monotherapy (NNH, 29) or antiplatelet monotherapy (NNH, 31). Dual-antiplatelet therapy (ASA + clopidogrel) increased GIB risk without improvement in stroke prevention in the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE W) trial.21 The ACTIVE W trial8 further showed the superiority of oral anticoagulant therapy to dual-antiplatelet therapy (ASA + clopidogrel) in reducing vascular events, with an increase in minor bleeds. Taken together, the results of our study, ACTIVE W trial, and the AVERROES trial suggest that anticoagulant therapy with appropriately dosed anticoagulant may be the most favorable approach for AF patients, with and without IHD, who are at moderate to severe risk of bleeding.
This conclusion also is supported by the 2018 American Heart Association White Paper updating recommendations for antithrombotic therapy in AF patients after percutaneous coronary intervention.22 The American Heart Association authors endorse considering the ischemic/thrombotic and bleeding risk profiles of patient when choosing the duration of a dual-antithrombotic regimen. A double-therapy regimen (anticoagulant plus single thienopyridine antiplatelet agent) should be considered for most patients except for those at high bleeding risk; and triple antithrombotic therapy (anticoagulant plus ASA and thienopyridine agent) should be used only for a limited period of time in very select cases when there is a very high ischemic and low bleeding risk (eg, for 1 month after percutaneous intervention with coronary stent placement). Modification of antithrombotic strategies to minimize bleeding risk is encouraged in susceptible patients, including prescription of clopidogrel as the thienopyridine antiplatelet of choice in most patients, with the use of ticagrelor reserved for patients with a high ischemic/thrombotic and low bleeding risk. These recommendations are consistent with prior clinical data showing high bleeding rates with both prasugrel and ticagrelor; with the most common bleeding location being gastrointestinal.23-25
In these aforementioned studies,23-25 there was an observed absolute increase in major bleeding that is greatest in the elderly (1.2%) when compared with younger patients (0.7%). This study further highlights the risk of antithrombotic GIB among elderly patients, underscoring the necessity of careful consideration of risk when individualizing therapy.1,5 Across all cardiovascular conditions and all antithrombotic regimens, advancing age was associated with the greatest risk of GIB at 1 year (up to 10%/y). Patients aged 75 years and older prescribed dual-antithrombotic therapy experienced a doubling of their probability of GIB (up to 17.5%/y) in some cardiovascular subgroups.
Strengths and Weaknesses
As with any observational study, detected associations may not be causal and treatment choices may be influenced by factors that also influence outcomes. However, this study focused specifically on describing the rates of bleed rather than conducting a comparative effectiveness study. We were not able to capture the rates of over-the-counter medication use (ASA or PPI) in pharmacy claims data. However, the sensitivity analyses performed to better understand the magnitude of effect of underascertainment of OTC ASA and PPI use (Supplementary Table 5) confirm no meaningful differences in the GIB rates (total, upper, and lower) when a wider threshold of clinical exposure is applied, highlighting the robustness of our estimates. This study focused on evaluating outcomes at 1 year and it is possible that treatment effects may differ for different time frames, especially with a longer follow-up period. However, we expect that including 1-year outcomes are meaningful for clinicians as they consider starting these treatments for patients with cardiovascular disease.
Against these limitations, this study also had important strengths worth considering. Outcomes were captured in a large, nationally representative, cohort of patients across all adult age groups and diverse backgrounds. This heterogeneous population provides meaningful information for patients and clinicians with broad applicability. In addition, this study evaluated the risk of GIB in an era with increasing use of DOACs and second-generation antiplatelet agents.
Implications for Health Care Professionals and Patients
There are some key findings from this study for both patients and clinicians. First, although it is known that combination therapy increases the harms associated with these medications, this study quantifies the impact of single and combination therapy over a wide range of ages, and at multiple time points during the initial year of exposure. Just as importantly, this study showed that anticoagulant and antiplatelet monotherapies have similar GIB rates. It has been assumed that antiplatelets may be safer compared with anticoagulants and may be a preferred strategy for patients at moderate-to-high bleeding risks; however, this study shows that these risks may be similar, and in some individuals the use of appropriately dosed anticoagulant monotherapy may be the most favorable approach for AF patients, with and without IHD, who are at moderate to severe risk of bleeding. This is an important consideration as patients and clinicians consider optimal treatment strategies.
Conclusions
In this study, we describe the rates of GIB among various treatment strategies for patients with cardiovascular disease. Combination therapy with both antiplatelets and anticoagulants are associated with significantly higher 30- to 180-day risk of GIB, and 1-year risk of GIB compared with either antiplatelet or anticoagulant monotherapy. The 30- to 180-day and 1-year risks of GIB associated with antiplatelet or anticoagulants are similar. Among all drug exposure categories and cardiovascular conditions, the risk of GIB increases with advancing age. This risk is most pronounced among patients older than 75 years, in whom the risk of GIB doubles when compared with patients who are younger.
Supplementary Material
What You Need to Know.
Background
Antithrombotic drugs are prescribed to patients with atrial fibrillation, ischemic heart disease, and venous thromboembolism. Clinicians tend to underestimate gastrointestinal bleeding risk among complex patients with 1 or more indications for treatment.
Findings
Among cardiovascular subgroups the risk of bleeding was similar (3.5%/y) if 1 agent was prescribed. Among patients age 75 years and older, regardless of the cardiovascular indication, risk increased from 10% to 17.5% per year on combination therapy.
Implications for patient care
We quantify the impact of going from single to combination therapy with risk estimates that are higher than previous studies examining only 1 atrisk patient population. An age of 75 years and older markedly increases risk across all antithrombotic strategies.
Acknowledgments
Funding
This project was supported by grant R01HS025402 (N.S.A.) from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication were solely the responsibility of the authors listed. Jeph Herrin and Jonathan Inselman had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis.
Abbreviations used in this paper:
- ACTIVE
Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events
- AF
atrial fibrillation
- ASA
acetylsalicylic acid
- AVERROES
Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment
- CV
cardiovascular
- DOAC
direct oral anticoagulant
- GIB
gastrointestinal bleeding
- IHD
ischemic heart disease
- NNH
number needed to harm
- NSAID
nonsteroidal anti-inflammatory drug
- OTC
over-the-counter
- PPI
proton pump inhibitor
- VTE
venous thromboembolism
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2019.05.017.
References
- 1.Abraham NS, Noseworthy PA, Yao X, et al. Gastrointestinal safety of direct oral anticoagulants: a large population-based study. Gastroenterology 2017;152:1014–1022.e1. [DOI] [PubMed] [Google Scholar]
- 2.Abraham NS. New clinical paradigms for treating and preventing antiplatelet gastrointestinal bleeding. Curr Opin Gastroenterol 2017;33:467–472. [DOI] [PubMed] [Google Scholar]
- 3.Yao X, Abraham NS, Alexander GC, et al. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noseworthy PA, Yao X, Gersh BJ, et al. Baseline characteristics and event rates among anticoagulated patients with atrial fibrillation in practice and pivotal NOAC trials. Data Brief 2017; 14:563–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ 2015;350:h1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamberts M, Olesen JB, Ruwald MH, et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation 2012;126:1185–1193. [DOI] [PubMed] [Google Scholar]
- 7.Lamberts M, Gislason GH, Olesen JB, et al. Oral anticoagulation and antiplatelets in atrial fibrillation patients after myocardial infarction and coronary intervention. J Am Coll Cardiol 2013; 62:981–989. [DOI] [PubMed] [Google Scholar]
- 8.Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006;367:1903–1912. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502. [DOI] [PubMed] [Google Scholar]
- 10.Steg PG, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 11.Abraham NS, Hartman C, Richardson P, et al. Risk of lower and upper gastrointestinal bleeding, transfusions, and hospitalizations with complex antithrombotic therapy in elderly patients. Circulation 2013;128:1869–1877. [DOI] [PubMed] [Google Scholar]
- 12.Carroll KJ. On the use and utility of the Weibull model in the analysis of survival data. Control Clin Trials 2003; 24:682–701. [DOI] [PubMed] [Google Scholar]
- 13.Noseworthy PA, Yao X, Abraham NS, et al. Direct comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in nonvalvular atrial fibrillation. Chest 2016; 150:1302–1312. [DOI] [PubMed] [Google Scholar]
- 14.Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med 2002;347:969–974. [DOI] [PubMed] [Google Scholar]
- 15.Yeh RW, Secemsky EA, Kereiakes DJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA 2016;315:1735–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–817. [DOI] [PubMed] [Google Scholar]
- 17.Abraham NS, Castillo DL. Novel anticoagulants: bleeding risk and management strategies. Curr Opin Gastroenterol 2013; 29:676–683. [DOI] [PubMed] [Google Scholar]
- 18.Yao X, Tangri N, Gersh BJ, et al. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 2017;70:2621–2632. [DOI] [PubMed] [Google Scholar]
- 19.Del-Carpio Munoz F, Yao X, Abraham NS, et al. Dabigatran versus warfarin in relation to renal function in patients with atrial fibrillation. J Am Coll Cardiol 2016;68:129–131. [DOI] [PubMed] [Google Scholar]
- 20.Yao X, Shah ND, Sangaralingham LR, et al. Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol 2017; 69:2779–2790. [DOI] [PubMed] [Google Scholar]
- 21.The ACTIVE Investigators. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009; 360:2066–2078. [DOI] [PubMed] [Google Scholar]
- 22.Angiolillo DJ, Goodman SG, Bhatt DL, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention - a North American perspective–2018 update. Circulation 2018; 138:527–536. [DOI] [PubMed] [Google Scholar]
- 23.Husted S, James S, Becker RC, et al. Ticagrelor versus clopidogrel in elderly patients with acute coronary syndromes: a substudy from the prospective randomized PLATelet inhibition and patient Outcomes (PLATO) trial. Circ Cardiovasc Qual Outcomes 2012;5:680–688. [DOI] [PubMed] [Google Scholar]
- 24.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 25.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.