Abstract
Background
Vitamin D deficiency or insufficiency, has been associated with atopy and lack of asthma control. Our objective was to investigate associations between variants in genes of vitamin D pathway with serum levels of 25-hydroxyvitamin D (25(OH)D), atopy, asthma and asthma severity in teenagers from Northeast Brazil.
Methods
This is a cross sectional study nested in a cohort population of asthma. 25(OH)D was quantified from 968 of 11–17 years old individuals by ELISA. Asthma diagnosis was obtained by using the ISAAC Phase III questionnaire. Specific IgE was determined by ImmunoCAP; genotyping was performed using the 2.5 HumanOmni Biochip from Illumina. Statistical analyses were performed in PLINK 1.07 and SPSS 22.1.
Results
After quality control, 104 Single Nucleotides Variants (SNVs) in vitamin D pathway genes, typed in 792 individuals, were included in the analysis. The allele A of rs10875694 on VDR was positively associated with atopy (OR = 1.35; 95% CI 1.01–1.81). The allele C of rs9279 on VDR, was negatively associated with asthma risk (OR = 0.66; 95% CI 0.45–0.97), vitamin D insufficiency (OR = 0.78; 95% CI 0.70–0.96) and higher VDR expression. Two variants in VDR were associated with asthma severity, the allele A of rs2189480 (OR = 0.34; 95% CI 0.13–0.89) and the allele G of rs4328262 (OR = 3.18; 95% CI 1.09–9.28). The combination of variants in CYP2R1 and CYP24A1 (GAC, to rs10500804, rs12794714 and rs3886163, respectively) was negatively associated with vitamin D production (β = − 1.24; 95% CI − 2.42 to − 0.06).
Conclusions
Genetic variants in the vitamin D pathway affect vitamin D serum levels and, thus, atopy and asthma.
Keywords: Vitamin D, CYP2R1, VDR, CYP24A1, IgE, 25(OH)D, SNVs, Asthma, Atopy
Background
Asthma affects more than 339 million people worldwide and it is estimated leading to the death of almost 400,000 people by year [1, 2]. This disease is characterized by a chronic inflammation of lower airways that include complex pathophysiological mechanisms involving several pro-inflammatory cells and molecules, including different cytokine profiles that can change according to environmental and genetic factors [3, 4]. Asthma immunopathological processes leads to reversible airflow obstruction, increased mucus secretion and airway remodelling. Allergic (or atopic) asthma is characterized by the presence of ILC2 and T helper 2 (Th2) response that covers the production of cytokines such as, interleukin (IL)-4, IL-5 and IL-13 which all together orchestrate the migration of eosinophils, mast cells activation and Immunoglobulin E (IgE) production [5, 6]. Subjects with asthma may be atopic or nonatopic. Those with Type 2 inflammation, likewise can also be nonatopic [7]. Atopy is an inherited predisposition to produce IgE in response to exposure to allergens, such as house dust mites, pollen, fungi and food proteins. Atopic individuals can present dermatitis, rhinitis, asthma or can be asymptomatic [8]. In addition to that, all the other asthma phenotypes that do not include sIgE production (specific Immunoglobulin E) are classified as non-atopic [3]. However, asthma heterogeneity involve many different immunological mechanisms and there are likely overlaps among them [9]. Previous studies from our group in the same population of the current study have shown that 24,5% of asthma cases were attributed to atopy [10] and that IFN-γ could be an important biomarker of non-atopic asthma in this population [11]. The main risk factors for this asthma phenotype include poverty and dirt conditions [12]. These findings suggest that asthma in Latin America could differ from Europe and other developed countries [13].
Beyond its role in mineral bone regulation, vitamin D also has a key role in immune regulation [14]. Vitamin D is the general term for a group of secosteroid metabolites whose active form is 1α-25-dihydroxyvitamin D (1,25(OH)D) [15]. This hormone is involved in the regulation of several immune cells, such as lymphocyte, macrophage, monocyte and eosinophils [14] and immune biomarkers, such as CD86/80, FOXP3, MHC, cytokines and IgE [14, 16–18]. The regulation of immune system occurs with vitamin D receptor (VDR) binding on elements responsive to vitamin D (VDRE) on many target genes of immune cells, determining their transcription or silencing [19]. Thus, this molecule has been shown to be a protective factor to diverse immunopathologies such as diabetes type I, multiple sclerosis, psoriasis, allergies and asthma [15, 20–22].
Studies have shown that low serum levels of vitamin D are associated with asthma risk and reduced forced expiratory capacity in one second (FEV1) as well as forced vital capacity (FVC) [23–25]. Moreover, some studies assert that supplementation of asthmatic children with vitamin D resulted in an improvement of pulmonary function, prevention of asthma exacerbation and reduction of IgE sensitization [26–29], although there are controversial findings in the literature [30–33]. Otherwise, maternal intake of vitamin D during pregnancy has been correlated with lower asthma diagnostic in offsprings [34].
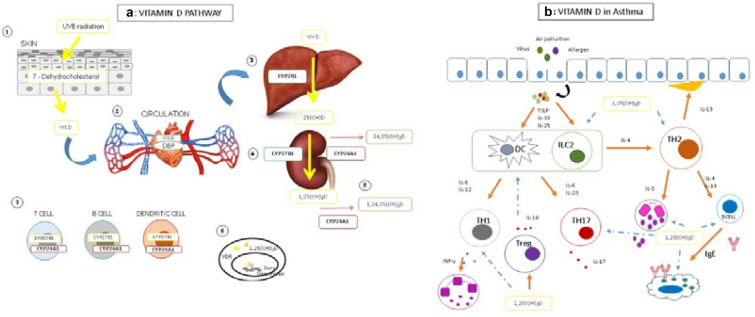
The synthesis of active vitamin D includes reactions that occur in three different tissues [35]. The initial production occurs in the skin, by conversion of 7-dehydrocholesterol following UV irradiation to Vitamin D3. Vitamin D3 is transported in blood circulation by DPB (Vitamin D Protein Binding) and in the liver it is metabolized to 25 hydroxyvitamin D3 (25(OH)D) by CYP2R1; in the kidney it undergoes other hydroxylation, by CYP27B1, leading to the active form 1,25(OH)2D. It is worth highlighting that immune cells also present CYP27B1 [14]. The 1,25(OH)2D binds to the VDR (Vitamin D receptor), a nuclear receptor that regulates target gene transcription. The vitamin D levels are regulated by a feedback mechanism over CYP24A1 that hydroxylates 1,25(OH)2D and/or 25(OH)D in position 24, generating an inactive metabolite [15]. Nevertheless, there are observations about the function of the “inactive” metabolite 24,25 dihydroxyvitamin D, in bone metabolism [36]. Figure 1 shows vitamin D pathway and vitamin D possible effect in asthma immunopathology.
Fig. 1.
Vitamin D pathway and immunological activity.a Vitamin D pathway. 1—Vitamin D starting your synthesis in skin by UVB radiation; 7-dehydrol cholesterol is converted to pre vitamin D3 by UVB radiation. 2—A subsequent thermal isomerization form vitamin D3, that reaches bloodstream and binds to DBP being transported to the tissues. 3—In the liver it is metabolized to 25-hydroxyvitamin D3 by CYP2R1, 4—following another hydroxylation occurs in kidney by CYP27B1 to form 1,25 dihydroxyvitamin D3. The vitamin D levels are regulated by a feedback mechanism over CYP24A1 that hydroxylates 1,25(OH)2D and/or 25(OH)D in position 24, generating an inactive metabolite. 5—Immune cells express CYP27B1 and CYP24A1, being able to regulate vitamin D paracrine metabolism. 6—1,25(OH)2D binds to VDR and regulates gene expression. b Vitamin D in asthma. Asthma can be triggered by many factors such virus, allergens and air pollution; allergens frequently lead epithelial cells to release cytokines IL-25, IL-33 and TSLP, that lead to a Th2 maturation, including ILC2 activation. Th2 cells release Il-4, IL-5 and IL-13 that lead to mast cells and eosinophils recruitment and IgE production that leads to mast cell degranulation and amplification of inflammation. IL-13 leads to increase mucus production in epithelium. The inflammatory cell infiltration leads smooth muscle thickening and contraction; reduction of airway lumen culminates to induce asthma symptoms. A non Th2 asthma profile, can be trigged by virus and air Pollutants, and lead to a Th1 or Th17 response, of neutrophilic profile. T reg cells reduce Th1, Th2 and Th17 response controlling inflammation. 1,25(OH)D can inhibit ILC2, eosinophilic and mast cell proliferation; Inhibits IL-4 release and IgE synthesis. 1.25(OH)D induces Treg response. Orange arrow—activation. Blue arrow—inhibition. TSLP thymic stromal lymphoprotein
Genetic studies have contributed to identifying the molecular pathways that affects asthma [37, 38]. A recent review showing a survey of 10 years of genome-wide studies of asthma, highlighted 28 main genes involved in asthma with reproducible data. Some of these genes are involved in immune function and were related with asthma or other allergies, including TSLP, TNFSF4, CD247, GATA-3, RORA, TLR1, IL6R and IL2RB [39]. Once vitamin D is an important modulator of immune system response and VDR is a map to chromosome 12q, near a region linked to asthma, variants in genes from vitamin D pathway can affect asthma and atopy. Variants in and vitamin D pathway gene (VDR, CYP2R1, CYP24A1, CYP27B1, DBP) are highlighted in immunopathologies including allergies, particularly in asthma [40–48].
Preliminary observation of ours indicate insufficient vitamin D levels were found in 60% of adolescents of the city of Salvador, in the Northeast of Brazil of tropical climate with an average yearly temperature of 27 °C and estimated radiation of 5.9 kWh/m2 [49]. Similar observations were reported in a population of South-eastern Brazil [50]. To explore this paradox, we hypothesized that genetic variants in vitamin D pathway genes may affect 25(OH)D levels and then may affect atopy and asthma in the Brazilian population.
Materials and methods
Population and study design
This work was conducted in the SCAALA Cohort (Social Change Asthma and Allergy in Latin America, Salvador, Bahia, Brazil) [51]. The study design has been described previously [52, 53]. Briefly, the original study population was composed by 1445 children, living in 24 deprived areas from the city of Salvador, Northeast Brazil, enrolled in the study to evaluate the impact of a sanitation programme on diarrhoea occurrence over the period from 1997 to 2003, when the participants were 0–3 years old [54]. The first survey on risk factors for wheezing and atopy was conducted in 2005, the second in 2007 and the third in 2013 [10, 52, 55]. The information for the present study were obtained in 2013 when data from 1206 participants were collected. At this time, the population aged 11 to 19 years old. Seven hundred and ninety-two individuals with complete data of interest were included in the current analysis. In addition, the 25(OH)D levels and specific IgE for aeroallergens (sIgE) were quantified, and vitamin D pathway genes were typed for variants. Asthma diagnosis was obtained using the International Study of Asthma and Allergy in Childhood phase III questionnaire (ISAAC PHASE III) adapted to Portuguese, answered by parents or legal guardians of each participant, or the participant himself if 18 years or older.
Ethical approval was obtained through the Ethical Committee for Health Research of the Institute of Public Health of the Federal University of Bahia, Brazil (Num. 120.616). Written informed consent was obtained from the legal guardian of each individual if they were under 18 years, and themselves if they were older than 18 years old.
Asthma definition
Asthma was defined as wheezing in the last 12 months, plus at least one of the following: (1) history of asthma ever, (2) 4 or more wheezing episodes in the last 12 months, (3) wheezing with exercise in the last 12 months, and (4) sleep disorder due to wheezing in the last 12 months [55]. All other individuals were classified as non-asthmatics.
The asthma severity was also obtained by ISAAC questionnaire as previously described [55]. Severe asthma, from the epidemiological stand point, was defined as individuals having at least one of the following symptoms in the last 12 months: (1) ≥ 12 wheezing episodes, (2) wheezing and breathlessness resulting in difficulty in speaking, and (3) > 1 day of disturbed sleep/week due to asthma. The other cases were considered as mild/moderate asthma.
Atopy definition
Previous study in our population, have indicated that the prevalence of allergen-specific IgE (sIgE) for the studied aeroallergens was greater than the skin prick test (SPT) positivity, and the frequency of SPT positivity between those without sIgE was very low [56]. For this reason, atopy was defined as the presence of at least one positive test for a relevant aeroallergen with sIgE ≥ 0.70 kU/L. The sIgE was determined by ImmunoCap using caps to Blomia tropicalis, Dermatophagoides pteronyssinus, Blatela germanica, and Periplaneta americana from Phadia (AB, Uppsala Sweden).
25-hydroxy vitamin D serum levels quantification
An inhibitory enzyme immunosorbent assay (IDS OCTEIA EIA, IDS Bolton, UK) was used to quantify serum levels of 25(OH)D. This is a diagnostic method recognized by the Vitamin D External Quality Assurance Survey (DEQAS). The lower detection limit was 2 ng/mL. Intra-assay and inter-assay coefficients of variation for concentrations between 15.6 and 52.8 ng/mL were < 5.9% and < 6.6%, respectively.
There is no consensus so far for vitamin D deficiency or insufficiency categorization, some authors define deficiency as < 12 ng/mL and insufficiency < 20 ng/mL [57], and others deficiency < 20 ng/mL and insufficiency < 30 ng/mL [58]. We use vitamin D classification as follows: (1) deficient (< 20 ng/mL); (2) insufficient (≥ 20–30 ng/mL) and (3) sufficient (≥ 30 ng/mL). To carry out logistic regression analyses, we used dichotomous variables to define 25(OH)D serum levels using two different cut-offs, first 20 ng/mL(deficiency), second 30 ng/mL (Deficiency + Insufficiency) [43, 58, 59].
Genotyping and quality control
DNA was extracted from peripheral blood using a commercial kit (Gentra Purgene Blood Kit (Qiagen, Gemantown, ML USA). Genotyping tests were developed using Illumina Human Omni 2.5 BeadChip (San Diego, CA, USA). Five genes in the vitamin D pathway were used in this study, VDR, CYP2R1, CYP27B1, CYP24A1 and CG/DBP. The VDR genetic information was extracted from 48,235,320 to 48,298,814 (Location: NC_000012.12) position at chromosome 12; CYP2R1 information was extracted from 1,489,951 to 14,913,874 (location: NC_000011.10) position at chromosome 11; CYP24A1 information was extracted from 52,769,985 to 52,790,516 (location: NC 000,020.11) position at chromosome 20; CYP27B1 information was extracted from 58,156,117 to 58,160,976 (location: NC 000012.12) position at chromosome 12 and DBP information was extracted from 49,133,817 to 49,140,639 (location: NC000019.10) position at chromosome 19. Quality control was carried out in PLINK version 1.07. SNVs were excluded if MAF (minor allele frequency) was less than 1%, imbalance of Hardy–Weinberg equilibrium with P value less than 10−4 and percentage of missing loci more than 1%.
In silico analysis
To analyse genetic expression, an online browser of the Genotype Tissue Expression Project (GTEx) was used (http://www.gtexportal.org). This project established a database which contains tissue gene expression according to the genetic variation. We examined whether genotypes of two VDR SNV’s, rs9729 and rs731236, were associated with differential expression of VDR receptor in whole blood.
Statistical analysis
The statistical analysis for genetics associations between polymorphisms in vitamin D pathway (VDR, CYP2R1, CYP24A1, CYP27B1 and DBP) and asthma, atopy and Vitamin D were performed using PLINK 1.07. Logistic regression was done to estimate ORs and 95% confidence intervals to categorical variables. Linear regression was made to estimate Beta and 95% confidence intervals to continuous variables. For such analyses, we used covariates (sex, age, and individual ancestry estimated although 269 informative markers identified to principal component analyses compound two variables PC1 and PC2) [37]. The additive genetic model was applied in these analyses and adaptive permutations were employed to the multivariate analysis. To evaluate the combined effect of SNV’s on CYP24A1 and CYP2R1 in 25(OH)D serum levels, and VDR SNV’s effect in atopy, asthma and 25(OH)D serum levels, we performed genetic risk score analysis using SNPstats platform (http://www.snpstats.net/start.htm).
The LD plot was done using the Haploview software. The statistical analysis for serum 25(OH)D and SNV’s rs12794714, rs10500804 and rs3886163 were performed using the GRAPHPAD Prisma 7 software (GraphPad Software, San Diego, CA, USA), using Kruskal–Wallis and Dunn’s post-test. We considered as significant associations, those with P-values ≤ 0.05.
Results
Description of population
We assessed 942 individuals with blood sample, of these 821 were genotyped and 792 remained in the analyses after the genetics quality control tests. The descriptive data of the studied population are shown in Table 1. The asthmatics individuals correspond to 63 (7.9%) and atopic 364 (45.9%). Four hundred and fifteen (52.4%) were males while 377 (47.6%) were female. Males were significantly more atopic than females (p < 0.001), and younger age [11–14] was significantly more frequent among asthmatics (p = 0,032). About vitamin D status, 165 (20.8%) were deficient, 322 (40.7%) were insufficient and 305 (38.5%) had sufficient levels. No association between vitamin D and atopy or asthma was observed. However, considering the whole population studied (942), vitamin D deficiency was associated with atopy and insufficiency was associated with asthma only among females (data on submission).
Table 1.
Characteristic of 792 studied subjects
| Asthma n/N (%) | *p value | Atopy n/N (%) | *p value | |
|---|---|---|---|---|
| Variables | 63/792 (7.9) | 364/792 (45.9) | ||
| Gender | ||||
| Males | 30/415 (7.2) | 0.434 | 216/415 (52.0) | < 0.001 |
| Female | 33/377 (8.8) | 148/377 (39.3) | ||
| Age | ||||
| 11–14 | 46/478 (9.8) | 0.032 | 218/478 (45.6) | 0.927 |
| 15–19 | 17/314 (5.4) | 146/314 (46.5) | ||
| Vitamin D (ng/mL) | ||||
| m ± SDa | 27.95 ± 8.98/27.33 ± 9.60 | 0.935 | 26.98 ± 10.12/27.92 ± 9.01 | 0.156 |
| Vitamin D levels | ||||
| Sufficient | 25/305 (8.2) | 0.893 | 131/305 (43.0) | 0.188 |
| Insufficiency/deficiency | 38/487 (7.9) | 233/487 (47.8) | ||
| Vitamin D levels | ||||
| Sufficiency/insufficiency | 52/627 (8.3) | 0.627 | 277/627 (44.2) | 0.054 |
| Deficient | 11/165 (6.7) | 87/166 (52.7) | ||
* Mean Whitney test; Numbers in italics are statically significant
am ± SD median and standard deviation
Description of genetic data
After quality control for SNVs and individuals, 104 SNVs in vitamin D pathway genes [DBP [2]; CYP2R1 [4]; CYP24A1 [37]; CYP27B1 [1] and VDR [59] ] were included in this study in 792 studied individuals. We have identified 20 genetic variants associated with at least one of the studied outcomes (Additional file 1: Table S1).
VDR SNVs are associated with atopy, asthma and asthma severity
Table 2 summarizes the significant associations between VDR SNVs and the outcomes, atopy, asthma or asthma severity. Regarding atopy, 792 individuals were included, 364 cases and 428 controls. Allele (A) of SNV rs10875694 located in an intronic region of the gene was more frequent on atopic individuals and positively associated with atopy OR = 1.35 (95% CI 1.01–1.81; p-value = 0.043). In the analysis for asthma, 63 cases were included (all asthma cases of the study) and 729 controls. The allele C of variant rs9279, a 3-UTR-prime, was associated with lower risk of asthma OR = 0.66 (95% CI 0.45–0.97; p-value = 0.033). Regarding asthma severity 38 severe asthma cases and 25 mild/moderate asthma controls were included. The variants in VDR showed to be associated were the rs2189480 and the rs4328262, both placed on the intronic region. The first variant was negatively associated (OR = 0.34; 95% CI 0.13–0.89; p-value = 0.029) and the second one was positively (OR = 3.18; 95% CI 1.09–9.28; p-value = 0.034) associated with severe asthma.
Table 2.
Significant associations between SNVs in VDR gene with atopy, asthma symptoms and asthma severity
| Phenotype | SNVs | Alele | Model | ORa | CI 95% | p valueb |
|---|---|---|---|---|---|---|
| Atopyc | ||||||
| VDR | rs10875694 | A | ADD | 1.35 | 1.01–1.81 | 0.043 |
| Asthma symptoms | ||||||
| VDR | rs9729 | C | ADD | 0.66 | 0.45–0.97 | 0.033 |
| Severe asthma | ||||||
| VDR | rs2189480 | A | ADD | 0.34 | 0.13–0.89 | 0.029 |
| rs4328262 | G | ADD | 3.18 | 1.09–9.28 | 0.034 | |
aUsing logistic regression adjusted by sex, age, and individual genetic ancestry
bPermutational analysis
csIgE ≥ 0.70 to common aeroallergens; ADD additive analysis model
SNVs on Vitamin D pathway (CYP2R1, CYP24A1 and VDR) were associated with vitamin D levels
Table 3 shows the significant associations on SNVs of vitamin D pathway genes with different classifications of vitamin D levels in serum. Using logistic regression, we found negative associations for vitamin D insufficiency and VDR gene for 6 SNV’s (rs7967152, rs9729, rs739837, rs11168287, rs7963776 and rs4237855) and in CYP24A1 for 2 SNVs (rs4809960 and rs2245153), which means that the presence of above variants reduce the possibility of a certain individual to be insufficient for 25(OH)D serum levels. While positive associations were found for 3 SNVs in VDR (rs59128934, rs7965274 and rs2853564), 2 SNVs in CYP24A1 (rs56229249 and rs34043203) and 2 in CYP2R1 (rs12794714 and rs10500804), indicating that the presence of such variations increase risk to vitamin D insufficiency. One SNV (rs59128934) on VDR and another (rs3886163) on CYP24A1 were associated with increased risk to Vitamin D deficiency. When we assessed continuous levels of serum 25(OH)D, two SNVs in CYP2R1 (rs12794714 and rs105008804) and one in CYP24A1 were associated with a lower levels of 25(OH)D in serum.
Table 3.
Significant associations between SNVs in VDR, CYP2R1, CYP24A1, vitamin D status and 25(OH)D serum levels
| Phenotype | SNVs | Alele | Model | ORa | CI 95% | P value |
|---|---|---|---|---|---|---|
| 25(OH)D serum levels | ||||||
| Alele | Model | Bc | CI 95% | P value | ||
| CYP2R1 | ||||||
| rs12794714 | A | ADD | − 1.38 | − 2.40 to 0.35 | 0.009 | |
| rs10500804 | G | ADD | − 1.37 | − 2.40 to 0.35 | 0.009 | |
| CYP24A1 | ||||||
| rs3886163 | T | ADD | − 1.48 | − 2.77 to 0.18 | 0.026 | |
| Vitamin D defficiency | ||||||
| VDR | ||||||
| rs59128934 | G | ADD | 1.78 | 1.12 to 2.83 | 0.014 | |
| CYP24A1 | ||||||
| rs3886163 | T | ADD | 1.44 | 1.05 to 1.99 | 0.025 | |
| Vitamin D insufficiency | ||||||
| VDR | ||||||
| rs7967152 | A | ADD | 0.77 | 0.62 to 0.95 | 0.013 | |
| rs9729 | C | ADD | 0.78 | 0.70 to 0.96 | 0.017 | |
| rs739837 | G | ADD | 0.78 | 0.63 to 0.96 | 0.019 | |
| rs11168287 | G | ADD | 0.78 | 0.63 to 0.97 | 0.028 | |
| rs7963776 | G | ADD | 0.79 | 0.64 to 0.98 | 0.029 | |
| rs4237855 | G | ADD | 0.79 | 0.63 to 0.99 | 0.038 | |
| rs59128934 | G | ADD | 2.07 | 1.28 to 3.34 | 0.002 | |
| rs7965274 | T | ADD | 1.31 | 1.01 to 1.70 | 0.044 | |
| rs2853564 | C | ADD | 1.30 | 1.00 to 1.70 | 0.049 | |
| CYP2R1 | ||||||
| rs12794714 | A | ADD | 1.41 | 1.11 to 1.79 | 0.005 | |
| rs10500804 | G | ADD | 1.40 | 1.11 to 1.77 | 0.006 | |
| CYP24A1 | ||||||
| rs4809960 | C | ADD | 0.69 | 0.53 to 0.91 | 0.008 | |
| rs2245153 | C | ADD | 0.79 | 0.63 to 0.99 | 0.042 | |
| rs56229249 | G | ADD | 1.42 | 1.04 to 1.94 | 0.028 | |
| rs34043203 | A | ADD | 1.49 | 1.00 to 2.22 | 0.049 | |
ADD additive analysis model
aUsing logistic regression adjusted by sex, age and individual genetic ancestry
bPermutational analysis
cLinear regression B coefficient adjusted by sex, age, helminth infection and individual genetic ancestry
The variant in VDR rs59128934 (allele G) was associated with risk to vitamin D insufficiency (OR 2.07; 95% CI 1.28–3.34; p = 0.002) as well as to deficiency (OR 1.78; 95% CI 1.12–2.83; p = 0.014), when compared to controls, ≥ 30 ng/mL and ≥ 20 ng/mL, respectively. The variant rs9729 (allele C), was negatively associated with asthma, and also negatively associated with insufficiency of vitamin D (OR 0.78; 95% CI 0.70–0.96; p = 0.017), which means that the carrier of this variant had a lower possibility to be asthmatic and had insufficient levels of 25(OH)D. The genotypic frequency of the studied SNVs by outcomes are shown in Additional file 1: Table S2.
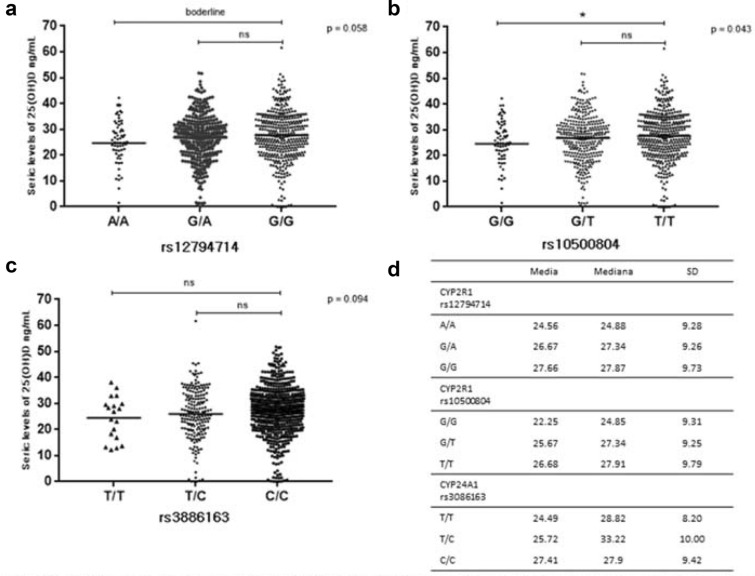
The SNVs rs12794714 (allele A) and rs10500804 (allele G) on CYP2R1 and rs3886163 (allele T) on CYP24A1 were associated with low levels of vitamin D. To better view the effect of genetic variants on vitamin D levels, we have represented in Fig. 2 the 25(OH)D serum levels by the genotype of the SNVs above mentioned. Carriers of (G) allele of rs10500804 variant on CYP2R1 have lower 25(OH)D serum levels (Fig. 2b; p < 0.05). The other two variants presented had no statistical significance with 25(OH)D by using Kruskal–Wallis test (Fig. 2a, d).
Fig. 2.
25OH(D) seric levels by genotypes on SNVs in CYP2R1 and CYP24A1. The a, b and c graphic shows the distribution of 25OH(D) seric levels according genotypes to related SNV’s, horizontal bars represent mean values. p values refers Kruskal–Wallis test and superior bar refer Dunn post test. D table represent measures of central tendency and dispersion to each SNV genotype. p = Kruskal–Wallis analysis. ns non-significant. *p < 0.05 Dunn post test
Genetic risk score using CYP variants influence 25(OH)D serum levels
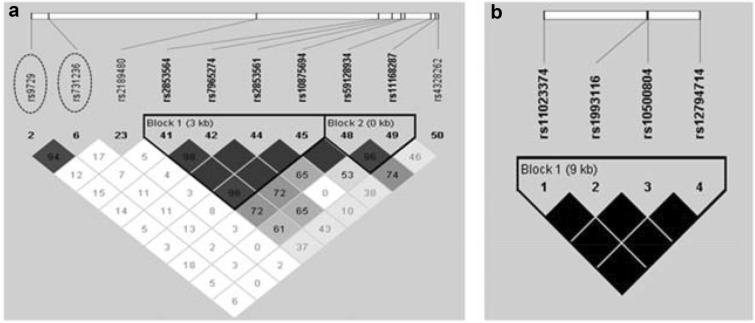
To understand the combined effect of variants on CYP2R1 and CYP24A1 we performed a genetic risk score analysis using SNPStats web version (Table 4). Together, both polymorphic alleles on CYP2R1 (G;A, to rs10500804, rs12794714, respectively) were associated with decreased 25(OH)D serum level (B = − 1.24; CI 95% − 2.42 to − 0.06; p-value = 0.040). The association increased when we added the polymorphic allele on CYP24A1 (T to rs3886163) in the analysis (B = − 3.29; CI 95% − 6.19 to − 0.39; p-value = 0.027). The CYP2R1 variants are in complete linkage disequilibrium (Fig. 3).
Table 4.
Genetic risk score analysis between SNVs in CYP2R1, CYP24A1 and 25(OH)D serum levels
| CYP2R1 | CYP24A1 | 25(OH)D serum concentration | ||||
|---|---|---|---|---|---|---|
| rs10500804 | rs12794714 | rs3886163 | Frequency | β (CI 95%)a | *p value | |
| 1 | T | G | C | 0.6016 | 00 | – |
| 2 | T | G | T | 0.1042 | − 1.41 (− 3.25 to 0.42) | 0.130 |
| 3 | G | A | C | 0.2513 | − 1.24 (− 2.42 to − 0.06) | 0.040 |
| 4 | G | A | T | 0.0422 | − 3.29 (− 6.19 to − 0.39) | 0.027 |
aLinear regression B coefficient adjusted by sex, age and individual genetic ancestry
*Snpstat p value
In italic statistically significant association, in italic borderline association
Fig. 3.
Linkage disequilibrium (r2) in VDR gene on SCAALA population. The LD plot was generates by Haploview program using PLINK 1.07 data set. The top horizontal bar illustrates the SNV’s location on physical scale. The squares colour illustrates the strength of pairwise r2 values scale, where black indicate perfect LD (r2 = 1), grey indicate imperfect LD (1 > r2 < 0) and white indicate equilibrium (r2 = 0). LD value is also indicate inside each square. In a VDR, the rs9729 and rs731236 are in high LD. In b CYP2R1, rs10500804 and rs12794714 are in perfect LD
Variants in VDR increase VDR gene expression
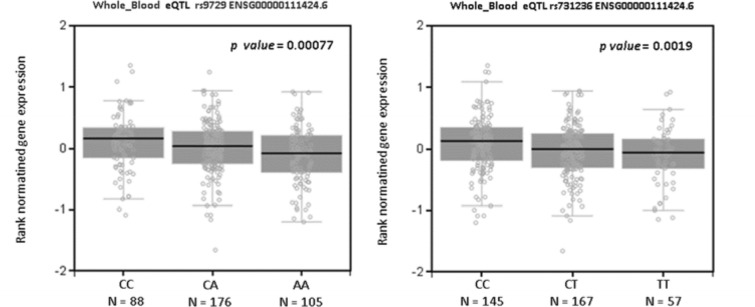
To better understand how such variants affect vitamin D activity we checked 25(OH)D distribution by genotypes (data not shown) and checked how it affects VDR expression using the online platform GTEx. As can be seen in Fig. 4, the C allele of rs9729 increased VDR expression (p-value = 0.0007). This variant (rs9729) is placed in a 3-prime-UTR region and is in high linkage disequilibrium (LD) with other SNV in VDR, such as rs731236 (Fig. 3) that is placed on exon 9 a synonymous polymorphism. Therefore, we analysed the variant rs731236 in GTEx and we had similar results to rs9729, the C allele increases VDR expression, GTEx p-value = 0.0019 (Fig. 4).
Fig. 4.
VDR expression on whole bloond to rs9729 and rs731236. The NES is similar to both variants, their reduce VDR expression. NES normatined gene expression
Discussion
Genetic studies have helped to understand the pathologic pathways of complex diseases such as asthma and other allergic diseases determined by a complex interaction of a variety of genes and environmental factors [39, 60]. There is solid evidence that Vitamin D plays an important biological role in the immune system and affects immune-mediated diseases [61]. However, its role in asthma and allergies is controversial. While most studies have shown protection others have shown no effect [62–64]. Several studies have found single-nucleotide variants in genes of vitamin D pathway associated with asthma and atopy [47, 65, 66].
Vitamin D production is known to be affected by sun exposure [67]. In this way, countries with a higher solar incidence should present population with higher levels of 25(OH)D. Nevertheless, vitamin D insufficiency or deficiency is frequently reported in populations from sunny countries [68, 69]. Therefore, genetic factors could be involved in vitamin D production on such populations.
There are significant reports about SNV’s in vitamin pathway associated with asthma or atopy [48]. The most reported VDR SNV’s associated with asthma are, rs1544410 (BsmI), rs7975232 (ApaI), rs731236 (TaqI) and rs2228570 (FokI) in several populations [42, 48] include Brazilian [43]. In the present study the selected SNV’s was part of a commercial chip contained 2.5 million SNVs (Illumina Human Omni 2.5 BeadChip), that of above mentioned contain only BsmI and TaqI SNV’s, however in our population BsmI is out of HWE (p = 0.01), being excluded of analysis and TaqI was not directly associated with any outcome.
The present study has been carried out in a population from a city of tropical climate, located in Northeast Brazil, with the average yearly temperature of 27 °C (IBGE), and an estimated average of radiation of 5.9 kWh/m2 [49]. However, we found that at least 60% of the studied subjects had insufficient levels of 25(OH)D(Submitted paper).
In our present study, the C allele of variant rs9729 on VDR showed to be negatively associated with asthma and vitamin D insufficiency. This variant was previously reported in a study about allergen sensitization using haplotype analysis [70–72]. The variant rs9729 was poorly explored in asthma and atopy contexts. It is located on 3-UTR-prime, thus it can regulate RNAm stability and VDR expression or translation [73]. GTEx in silico gene expression analysis showed that allele C increases VDR expression. In our population, this variant is in high linkage disequilibrium (r2 = 1) with a synonymous variant widely described in asthma studies, the SNV rs731236 (TaqI). The T allele of rs731236 was associated with risk to atopy and asthma [74]. While in Irland, the C allele of rs731236 was associated with risk to uncontrolled asthma [75], 2018).
In the present work, two VDR variants were associated with severe asthma, the rs2189480 (allele A) as a protective factor and rs4328262 (allele G) as a risk factor. To our knowledge, it is the first study that reports these associations. In a previous GWAS work, conducted in a paediatrics population elsewhere, these variants were not associated with asthma [76]. The variant rs2189480 allele T was described as a risk factor to melanoma and C allele with protection to Type 2 diabetes [77]. We hypothetized that rs2189480 affect regulatory T cell function, once T help regulate inflammatory activity on asthma [65] and possibility tumour proliferation [66], thus explained opposite association found. Functional study of this variant in T cell was requested to elucidate this question.
The variant rs4328262 (allele G) was described in association with breast cancer reduced risk in North America [78], European and East Asian women [79]. Also, it was described in association with increased visceral adipose tissue [80]. However, again it is the first report of this association on asthma. The functional effect on VDR is not clear yet in the presence of this variant.
Also on VDR, we also found the A allele of rs10875694 is associated with risk to atopy. This is also the first study that describes this association. However, Reimers and collaborators (2015) have described that carriers of A allele presented slightly lower levels of 25(OH)D than T carriers [81]. At this point, we did not observe a difference in vitamin D production according to different alleles of this variant. This variant is placed in an intronic region, however, no functional data analysed here using both RegulomeDB and GTEx, did not shown a possible functional impact of this polymorphism on VDR activity. Although one could consider that being an intronic SNVs they could modify messenger RNA (RNAm) stability and also play a role in translational efficiency [73, 82]. Thus, we can suppose that this genetic variant may affect VDR expression, and VDR can, in its turn, control the expression of other genes involved in the development of atopy.
We found three SNV’s on two important enzymes of vitamin D metabolic pathway [rs10500804 and rs12794714 (CYP2R1) and rs3886163 (CYP24A1)] associated with decreased serum level of vitamin D. Additionally the genetic risk score analysis showed that in the presence of the three alleles all together, the effect in decreasing vitamin D serum levels is even stronger.
CYP2R1 is the principal 25-hydroxylase enzyme of the vitamin D pathway. Mutations on this enzyme that inhibits hydroxylase activity can result in 25(OH)D deficiency [83]. The SNV’s [rs10500804 (G) and rs12794714 (A)] on this gene were associated with lower 25(OH)D concentrations in Arabic population; the homozygote carriers of this SNV in South Asian presented lower levels but there was not statistically significant association in comparison with T and G carrier respectively. [84]. Also, these variants were associated with lower 25(OH)D levels after supplementation with vitamin D in USA [85]. In this way, such variants can affect CYP2R1, reducing its metabolic activities. Further studies are needed in order to address if carriers of these variants may have any benefit of vitamin D intake having a deficiency in vitamin D levels due to CYP2R1 enzymatic alterations.
CYP24A1 is the principal catabolic enzyme in vitamin D pathway; knockout mice to this enzyme are not able to reduce vitamin D levels and its loss causes idiopathic infancy hypercalcemia [86, 87]. Genetic polymorphisms in this enzyme affect vitamin D metabolism, and are associated with cancer and coronary atherosclerosis risks [88]. In a previous study about evaluating the pulmonary function, the allele T of the variant rs3886163 on CYP24A1 was associated with better forced expiratory volume in 1 s (FEV1), although no association was found with vitamin D serum levels [89]. It was also associated with a lower coronary artery calcification [88]. In the present study, its association with lower 25(OH)D levels suggests that the T allele of rs3886163 possibly increases enzymatic activity. In North American population the variant allele (T) was associated with better pulmonary function and in European it was associated with less calcification in lungs, thus, suggesting that such SNV increases vitamin D levels, once higher levels of vitamin D cause less artery calcification [90] and increased pulmonary function [91]. The opposite results should be explored, once CYP24A1 affects 1,25(OH)2D and 25(OH)D levels, and metabolites formed by this enzyme are ten times more frequent than 1,25(OH)2D; these metabolites (1,24,25(OH) 3D or 24,25(OH)2D) can have some non-related activity in immunologic cells that could help to understand the vitamin D role in a series of immunological related-diseases. In addition, more functional studies should be performed to understand the impact of this SNV in CYP24A1 activity and in 25(OH)D levels in blood.
Regarding vitamin insufficiency, one SNV in VDR (rs59128934 allele G), and one SNV in CYP24A1 (rs3886163 allele T), were associated with higher risk. The rs59128934 is placed in an intronic region on VDR gene, and from our knowledge there was no report about this SNV in any previous study, once more this is the first report of any association with this variant. Here (in this study), although no association was found for rs59128934 with asthma, we hypothesized that since this SNV was able to decrease vitamin D levels, that could lead to asthma. Unfortunately, we were unable to capture this association probably due to the number of asthma cases with detectable levels of vitamin D. The rs3886163 T allele was reportedly associated with better FEV1 in American Africans, but their effect on vitamin D levels was not demonstrated [89].
Six variants in VDR gene were associated reducing the risk of insufficiency, rs7967152 (A), rs9729(C), rs739837(G), rs11168287(G), rs7963776(G), rs4237855(G). While three other variants were associated with increased risk of insufficiency rs59128934(G), rs7965274(T) and rs2853564(C). There are few studies showing a relationship between VDR polymorphism and 25(OH)D levels. The VDR regulates vitamin D metabolism, the binding of 1,25(OH)2D in VDR, promoting reduced CYP2R1 and CYP27B1 gene transcription, and promoting CYP24A1 expression leading reduction of vitamin D levels. It’s a negative feedback mechanism to avoid excessive levels of the active metabolite [92]. Its important to highlight that rs739837 C allele creates a binding site to miRNA-34b in VDR, decreasing their expression [93].
The SNV’s in CYP2R1 rs10500804 and rs12794714 correlated with a lower levels of vitamin D, were also associated with risk to vitamin D insufficiency. The variant rs3886163 in CYP24A1 correlated with reduction of vitamin D levels also were associated with deficiency. This finding deserves attention once these variants related to lower serum 25(OH)D at the time to be a risk factor to individual health.
In this work we we had found associations between variants in VDR gene and different categories vitamin D levels in blood, however no previous study was found in literature describing such associations before
A limitation of this study was that we did not measure the 1,25(OH)2D levels, due to a lack of funding to perform it in all our sample. But there is a plan to do at some moment in the future. This result would allow us to better understand the complete vitamin D metabolism down to its active metabolite.
Conclusions
In conclusion, we have demonstrated that variants in genes from the vitamin D pathway may affect vitamin D levels and can lead to asthma and atopy. In our population, the vitamin D insufficiency can be due to genetic variations in genes of enzymes that directly influence vitamin D levels. Further studies are necessary, including strategies looking at markers of vitamin D pathway such as PTH and 1,25(OH)2D to better understand the impact of genetic variants on the metabolism of vitamin D, including as interaction analysis to understand its role in asthma and atopy.
Supplementary information
Additional file 1: Table S1. Genetic variants in vitamin pathway associated with atopy or asthma, or 25 (OH) D serum levels. Table S2. Genotypic Frequency of associates SNV’s in vitamin D pathway by outcomes.
Acknowledgements
The authors want to thank the Brazilian agencies (FAPESB, CNPq, CAPES) that supported this work and all the individuals and parents or tutors who have agreed to participate in this work. This study was conducted through the Social Change of Asthma and Allergy in Latin America (SCAALA) Programme and funded by the Wellcome Trust (grant no. 072405/Z/03/Z).
Author contributions
All authors have actively participated of the analysis and approve the version that being submitted. CAF, NMA-N, MLB and AAC designed the orginal work. AAG wrote the first draft. AAG, FAS, EMMA were involved in data collection and laboratorial analysis. AAG, GNOC, RSC, MBRS performed the data analysis. All authors read and approved the final manuscript.
Funding
This work was supported by Fundação de Amparo á Pesquisa do Estado da Bahia (FAPESB), Grant No. SUS010/2014, Edital FAPESB 008/2014 PRONEX Grant No. 8665/2014, CNPq Grant No. 477036/2012-0 and Cordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) – Finance code 001.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Ethical approval was obtained through the Ethical Committee for Medical Research of the Institute of Public Health of the Federal University of Bahia, Brazil, clearance no. 120.616. Written informed consent was obtained from the legal guardian of each individual if they were under 18 years, and themselves if they were older than 18 years old.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alana Alcântara Galvão, Email: alana_alcantara@hotmail.com.
Flávia de Araújo Sena, Email: fasena_@hotmail.com.
Emília Maria Medeiros de Andrade Belitardo, Email: emilia_mandrade@hotmail.com.
Maria Borges Rabelo de Santana, Email: mariabrs12@hotmail.com.
Gustavo Nunes de Oliveira Costa, Email: gustavokosta@gmail.com.
Álvaro Augusto Cruz, Email: cruz.proar@gmail.com.
Maurício Lima Barreto, Email: mauricio@ufba.br.
Ryan dos Santos Costa, Email: ryanscosta@yahoo.com.br.
Neuza Maria Alcantara-Neves, Email: neuzalcantara@gmail.com.
Camila Alexandrina Figueiredo, Email: cavfigueiredo@gmail.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13223-020-00460-y.
References
- 1.GBD. Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD. Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368(9537):804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 4.Morales E, Duffy D. Genetics and gene-environment interactions in childhood and adult onset asthma. Front Pediatr. 2019;7:499. doi: 10.3389/fped.2019.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 6.McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433–445. doi: 10.1164/rccm.201810-1944CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson D, Humbert M, Buhl R, Cruz AA, Inoue H, Korom S, et al. Revisiting Type 2-high and Type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy. 2017;47(2):161–175. doi: 10.1111/cea.12880. [DOI] [PubMed] [Google Scholar]
- 8.Thomsen SF. Epidemiology and natural history of atopic diseases. Eur Clin Respir J. 2015;2:24642. doi: 10.3402/ecrj.v2.24642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai M, Oppenheimer J. Elucidating asthma phenotypes and endotypes: progress towards personalized medicine. Ann Allergy Asthma Immunol. 2016;116(5):394–401. doi: 10.1016/j.anai.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 10.da Cunha S, Barreto ML, Fiaccone RL, Cooper PJ, Alcantara-Neves NM, Simões SM, et al. Asthma cases in childhood attributed to atopy in tropical area in Brazil. Rev Panam Salud Publica. 2010;28(6):405–411. doi: 10.1590/s1020-49892010001200001. [DOI] [PubMed] [Google Scholar]
- 11.Figueiredo CA, Rodrigues LC, Alcantara-Neves NM, Cooper PJ, Amorim LD, Silva NB, et al. Does IFN-γ play a role on the pathogenesis of non-atopic asthma in Latin America children? Allergy Asthma Clin Immunol. 2012;8(1):18. doi: 10.1186/1710-1492-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper PJ, Rodrigues LC, Barreto ML. Influence of poverty and infection on asthma in Latin America. Curr Opin Allergy Clin Immunol. 2012;12(2):171–178. doi: 10.1097/ACI.0b013e3283510967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong Neto HJ, Rosário NA, Solé D, Group LAI Asthma and rhinitis in south america: how different they are from other parts of the world. Allergy Asthma Immunol Res. 2012;4(2):62–67. doi: 10.4168/aair.2012.4.2.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. 2011;134(2):123–139. doi: 10.1111/j.1365-2567.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, Bergamini A. 1Alpha,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. 2009;45(3):190–197. doi: 10.1016/j.cyto.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Urry Z, Chambers ES, Xystrakis E, Dimeloe S, Richards DF, Gabryšová L, et al. The role of 1α,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3 + and IL-10 + CD4 + T cells. Eur J Immunol. 2012;42(10):2697–2708. doi: 10.1002/eji.201242370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heine G, Anton K, Henz BM, Worm M. 1alpha,25-dihydroxyvitamin D3 inhibits anti-CD40 plus IL-4-mediated IgE production in vitro. Eur J Immunol. 2002;32(12):3395–3404. doi: 10.1002/1521-4141(200212)32:12<3395::AID-IMMU3395>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Wöbke TK, Sorg BL, Steinhilber D. Vitamin D in inflammatory diseases. Front Physiol. 2014;5:244. doi: 10.3389/fphys.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 21.Correale J, Ysrraelit MC, Gaitán MI. Gender differences in 1,25 dihydroxyvitamin D3 immunomodulatory effects in multiple sclerosis patients and healthy subjects. J Immunol. 2010;185(8):4948–4958. doi: 10.4049/jimmunol.1000588. [DOI] [PubMed] [Google Scholar]
- 22.Illescas-Montes R, Melguizo-Rodríguez L, Ruiz C, Costela-Ruiz VJ. Vitamin D and autoimmune diseases. Life Sci. 2019;233:116744. doi: 10.1016/j.lfs.2019.116744. [DOI] [PubMed] [Google Scholar]
- 23.Man L, Zhang Z, Zhang M, Zhang Y, Li J, Zheng N, et al. Association between vitamin D deficiency and insufficiency and the risk of childhood asthma: evidence from a meta-analysis. Int J Clin Exp Med. 2015;8(4):5699–5706. [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Dong YQ, Yin J, Yao J, Shen J, Sheng GJ, et al. Meta-analysis of vitamin D and lung function in patients with asthma. Respir Res. 2019;20(1):161. doi: 10.1186/s12931-019-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozturk Thomas G, Tutar E, Tokuc G, Oktem S. 25-hydroxy Vitamin D levels in pediatric asthma patients and its link with asthma severity. Cureus. 2019;11(3):e4302. doi: 10.7759/cureus.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riverin BD, Maguire JL, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLoS ONE. 2015;10(8):e0136841. doi: 10.1371/journal.pone.0136841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lilani MZHA, Elahi RT, Ahmed A, Alam MM, Batool SS. Vitamin D supplementation: an innovative way to prevent asthma exacerbation in developing countries? J Taibah Univ Med Sci. 2019;14(1):99–100. doi: 10.1016/j.jtumed.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorisdottir B, Gunnarsdottir I, Vidarsdottir AG, Sigurdardottir S, Birgisdottir BE, Thorsdottir I. Infant Feeding, Vitamin D and IgE Sensitization to Food Allergens at 6 Years in a Longitudinal Icelandic Cohort. Nutrients. 2019;11:7. doi: 10.3390/nu11071690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang YH, Sim HB, Moon SY, Lee WJ, Lee SJ, Jin M, et al. House dust mite sensitization is inversely associated with plasma 25-hydroxyvitamin D3 levels in patients with severe atopic dermatitis. Ann Dermatol. 2017;29(4):400–406. doi: 10.5021/ad.2017.29.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall SC, Agrawal DK. Vitamin D and bronchial asthma: an overview of data from the past 5 years. Clin Ther. 2017;39(5):917–929. doi: 10.1016/j.clinthera.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo J, Liu D, Liu CT. Can Vitamin D supplementation in addition to asthma controllers improve clinical outcomes in patients with asthma?: a meta-analysis. Medicine (Baltimore). 2015;94(50):e2185. doi: 10.1097/MD.0000000000002185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malliaraki N, Lakiotaki K, Vamvoukaki R, Notas G, Tsamardinos I, Kampa M, et al. Translating vitamin D transcriptomics to clinical evidence: analysis of data in asthma and chronic obstructive pulmonary disease, followed by clinical data meta-analysis. J Steroid Biochem Mol Biol. 2019;197:105505. doi: 10.1016/j.jsbmb.2019.105505. [DOI] [PubMed] [Google Scholar]
- 33.Swangtrakul N, Manuyakorn W, Mahachoklertwattana P, Kiewngam P, Sasisakulporn C, Jotikasthirapa W, et al. Effect of vitamin D on lung function assessed by forced oscillation technique in asthmatic children with vitamin D deficiency: a randomized double-blind placebo-controlled trial. Asian Pac J Allergy Immunol. 2019 doi: 10.12932/AP-010519-0553. [DOI] [PubMed] [Google Scholar]
- 34.Allan KM, Prabhu N, Craig LC, McNeill G, Kirby B, McLay J, et al. Maternal vitamin D and E intakes during pregnancy are associated with asthma in children. Eur Respir J. 2015;45(4):1027–1036. doi: 10.1183/09031936.00102214. [DOI] [PubMed] [Google Scholar]
- 35.Schuster I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim Biophys Acta. 2011;1814(1):186–199. doi: 10.1016/j.bbapap.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz Z, Dean DD, Walton JK, Brooks BP, Boyan BD. Treatment of resting zone chondrocytes with 24,25-dihydroxyvitamin D3 [24,25-(OH)2D3] induces differentiation into a 1,25-(OH)2D3-responsive phenotype characteristic of growth zone chondrocytes. Endocrinology. 1995;136(2):402–411. doi: 10.1210/endo.136.2.7530645. [DOI] [PubMed] [Google Scholar]
- 37.Costa GN, Dudbridge F, Fiaccone RL, da Silva TM, Conceição JS, Strina A, et al. A genome-wide association study of asthma symptoms in Latin American children. BMC Genet. 2015;16:141. doi: 10.1186/s12863-015-0296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez-Pacheco N, Pino-Yanes M, Flores C. Genomic predictors of asthma phenotypes and treatment response. Front Pediatr. 2019;7:6. doi: 10.3389/fped.2019.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vicente CT, Revez JA, Ferreira MAR. Lessons from ten years of genome-wide association studies of asthma. Clin Transl Immunol. 2017;6(12):e165. doi: 10.1038/cti.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wjst M, Altmüller J, Faus-Kessler T, Braig C, Bahnweg M, André E. Asthma families show transmission disequilibrium of gene variants in the vitamin D metabolism and signalling pathway. Respir Res. 2006;7:60. doi: 10.1186/1465-9921-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raby BA, Lazarus R, Silverman EK, Lake S, Lange C, Wjst M, et al. Association of vitamin D receptor gene polymorphisms with childhood and adult asthma. Am J Respir Crit Care Med. 2004;170(10):1057–1065. doi: 10.1164/rccm.200404-447OC. [DOI] [PubMed] [Google Scholar]
- 42.Makoui MH, Imani D, Motallebnezhad M, Azimi M, Razi B. Vitamin D receptor gene polymorphism and susceptibility to asthma: meta-analysis based on 17 case-control studies. Ann Allergy Asthma Immunol. 2020;124(1):57–69. doi: 10.1016/j.anai.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 43.Simões TM, da Silva R, Bianco B, Fonseca FLA, Solé D, Sarni R. Vitamin D Levels, Frequency of Vitamin D receptor gene polymorphisms, and associations with overweight and asthma in Brazilian school children. Ann Nutr Metab. 2019;75(4):238–245. doi: 10.1159/000504872. [DOI] [PubMed] [Google Scholar]
- 44.Nasiri-Kalmarzi R, Abdi M, Hosseini J, Tavana S, Mokarizadeh A, Rahbari R. Association of vitamin D genetic pathway with asthma susceptibility in the Kurdish population. J Clin Lab Anal. 2019;31:e23039. doi: 10.1002/jcla.23039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amo G, Martí M, García-Menaya JM, Cordobés C, Cornejo-García JA, Blanca-López N, et al. Identification of novel biomarkers for drug hypersensitivity after sequencing of the promoter area in 16 genes of the vitamin d pathway and the high-affinity IgE receptor. Front Genet. 2019;10:582. doi: 10.3389/fgene.2019.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bossé Y, Lemire M, Poon AH, Daley D, He JQ, Sandford A, et al. Asthma and genes encoding components of the vitamin D pathway. Respir Res. 2009;10:98. doi: 10.1186/1465-9921-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vimaleswaran KS, Cavadino A, Hyppönen E. Evidence for a genetic interaction in allergy-related responsiveness to vitamin D deficiency. Allergy. 2012;67(8):1033–1040. doi: 10.1111/j.1398-9995.2012.02856.x. [DOI] [PubMed] [Google Scholar]
- 48.Morales E, Sanchez-Solis M, Garcia-Marcos L. Vitamin D Metabolism Genes in Asthma and Atopy. Mini Rev Med Chem. 2015;15(11):913–926. doi: 10.2174/1389557515666150519105944. [DOI] [PubMed] [Google Scholar]
- 49.Lima FJL. Previsão de irradiação solar no Nordeste do Brasil empregando o modelo WRF ajustado por Redes Neurais Artificiais (RNAs). São José dos Campos, SP: Instituto Nacional de Pesquisas Espaciais (INPE); 2015.
- 50.Peçanha MB, Freitas RB, Moreira TR, Silva LS, Oliveira LL, Cardoso SA. Prevalence of vitamin D deficiency and its relationship with factors associated with recurrent wheezing. J Bras Pneumol. 2019;45(1):e20170431. doi: 10.1590/1806-3713/e20170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barreto ML, Cunha SS, Alcântara-Neves N, Carvalho LP, Cruz AA, Stein RT, et al. Risk factors and immunological pathways for asthma and other allergic diseases in children: background and methodology of a longitudinal study in a large urban center in Northeastern Brazil (Salvador-SCAALA study) BMC Pulm Med. 2006;6:15. doi: 10.1186/1471-2466-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barreto ML, Cunha SS, Fiaccone R, Esquivel R, Amorim LD, Alvim S, et al. Poverty, dirt, infections and non-atopic wheezing in children from a Brazilian urban center. Respir Res. 2010;11:167. doi: 10.1186/1465-9921-11-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Figueiredo CA, Amorim LD, Alcantara-Neves NM, Matos SM, Cooper PJ, Rodrigues LC, et al. Environmental conditions, immunologic phenotypes, atopy, and asthma: new evidence of how the hygiene hypothesis operates in Latin America. J Allergy Clin Immunol. 2013;131(4):1064–1068. doi: 10.1016/j.jaci.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barreto ML, Genser B, Strina A, Teixeira MG, Assis AM, Rego RF, et al. Effect of city-wide sanitation programme on reduction in rate of childhood diarrhoea in northeast Brazil: assessment by two cohort studies. Lancet. 2007;370(9599):1622–1628. doi: 10.1016/S0140-6736(07)61638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Magalhães Simões S, da Cunha SS, Cruz Á, Dias KC, Alcântara-Neves NM, Amorim LD, et al. A community study of factors related to poorly controlled asthma among Brazilian urban children. PLoS ONE. 2012;7(5):e37050. doi: 10.1371/journal.pone.0037050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mendonça LR, Veiga RV, Dattoli VC, Figueiredo CA, Fiaccone R, Santos J, et al. Toxocara seropositivity, atopy and wheezing in children living in poor neighbourhoods in urban Latin American. PLoS Negl Trop Dis. 2012;6(11):e1886. doi: 10.1371/journal.pntd.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manson JE, Brannon PM, Rosen CJ, Taylor CL. Vitamin D deficiency—is there really a pandemic? N Engl J Med. 2016;375(19):1817–1820. doi: 10.1056/NEJMp1608005. [DOI] [PubMed] [Google Scholar]
- 58.Ferreira CES, Maeda SS, Batista MC, Lazaretti-Castro M, Vasconcellos LS, Madeira M, et al. Consensus – reference ranges of vitamin D [25(OH)D] from the Brazilian medical societies Brazilian Society of Clinical Pathology/Laboratory Medicine (SBPC/ML) and Brazilian Society of Endocrinology and Metabolism (SBEM) Jornal Brasileiro de Patologia e Medicina Laboratorial. 2017;53:377–381. [Google Scholar]
- 59.Han YY, Forno E, Celedón JC. Vitamin D Insufficiency and Asthma in a US Nationwide Study. J Allergy Clin Immunol Pract. 2017;5(3):790–796. doi: 10.1016/j.jaip.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewis CM. Genetic association studies: design, analysis and interpretation. Brief Bioinform. 2002;3(2):146–153. doi: 10.1093/bib/3.2.146. [DOI] [PubMed] [Google Scholar]
- 61.Heine G, Tabeling C, Hartmann B, González Calera CR, Kühl AA, Lindner J, et al. 25-hydroxvitamin D3 promotes the long-term effect of specific immunotherapy in a murine allergy model. J Immunol. 2014;193(3):1017–1023. doi: 10.4049/jimmunol.1301656. [DOI] [PubMed] [Google Scholar]
- 62.Gupta A, Sjoukes A, Richards D, Banya W, Hawrylowicz C, Bush A, et al. Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma. Am J Respir Crit Care Med. 2011;184(12):1342–1349. doi: 10.1164/rccm.201107-1239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mirzakhani H, Al-Garawi A, Weiss ST, Litonjua AA. Vitamin D and the development of allergic disease: how important is it? Clin Exp Allergy. 2015;45(1):114–125. doi: 10.1111/cea.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brehm JM. Vitamin D and asthma-life after VIDA? Curr Allergy Asthma Rep. 2014;14(9):461. doi: 10.1007/s11882-014-0461-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han JC, Du J, Zhang YJ, Qi GB, Li HB, Yu XL. Vitamin D receptor polymorphisms may contribute to asthma risk. J Asthma. 2016;53(8):790–800. doi: 10.3109/02770903.2016.1158267. [DOI] [PubMed] [Google Scholar]
- 66.Wang M, Liu M, Wang C, Xiao Y, An T, Zou M, et al. Association between vitamin D status and asthma control: a meta-analysis of randomized trials. Respir Med. 2019;150:85–94. doi: 10.1016/j.rmed.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 67.Huldschinsky K. Heilung von rachitis durch kunstliche hohensonne. Germany: Dtsch. Med. Wochenschr; 1919. pp. 712–713. [Google Scholar]
- 68.Bandeira F, Griz L, Dreyer P, Eufrazino C, Bandeira C, Freese E. Vitamin D deficiency: a global perspective. Arq Bras Endocrinol Metabol. 2006;50(4):640–646. doi: 10.1590/s0004-27302006000400009. [DOI] [PubMed] [Google Scholar]
- 69.Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev. 2008;66(10 Suppl 2):S153–S164. doi: 10.1111/j.1753-4887.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- 70.Correa-Rodríguez M, Schmidt Rio-Valle J, González-Jiménez E, Rueda-Medina B. A Cross-Sectional Study of the Association of VDR Gene, Calcium Intake, and Heel Ultrasound Measures in Early Adulthood. Calcif Tissue Int. 2016;98(3):226–234. doi: 10.1007/s00223-015-0086-2. [DOI] [PubMed] [Google Scholar]
- 71.Ramos-Lopez E, Jansen T, Ivaskevicius V, Kahles H, Klepzig C, Oldenburg J, et al. Protection from type 1 diabetes by vitamin D receptor haplotypes. Ann N Y Acad Sci. 2006;1079:327–334. doi: 10.1196/annals.1375.050. [DOI] [PubMed] [Google Scholar]
- 72.Tian HQ, Chen XY, Lu Y, Lu WM, Wang ML, Zhao HL, et al. Association of VDR and CYP2R1 Polymorphisms with Mite-Sensitized Persistent Allergic Rhinitis in a Chinese Population. PLoS ONE. 2015;10(7):e0133162. doi: 10.1371/journal.pone.0133162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steri M, Idda ML, Whalen MB, Orrù V. Genetic variants in mRNA untranslated regions. Wiley Interdiscip Rev RNA. 2018;9(4):e1474. doi: 10.1002/wrna.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poon AH, Laprise C, Lemire M, Montpetit A, Sinnett D, Schurr E, et al. Association of vitamin D receptor genetic variants with susceptibility to asthma and atopy. Am J Respir Crit Care Med. 2004;170(9):967–973. doi: 10.1164/rccm.200403-412OC. [DOI] [PubMed] [Google Scholar]
- 75.Hutchinson K, Kerley CP, Faul J, Greally P, Coghlan D, Louw M, et al. Vitamin D receptor variants and uncontrolled asthma. Eur Ann Allergy Clin Immunol. 2018;50(3):108–116. doi: 10.23822/EurAnnACI.1764-1489.46. [DOI] [PubMed] [Google Scholar]
- 76.Michel S, Liang L, Depner M, Klopp N, Ruether A, Kumar A, et al. Unifying candidate gene and GWAS Approaches in Asthma. PLoS ONE. 2010;5(11):e13894. doi: 10.1371/journal.pone.0013894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ogbah Z, Visa L, Badenas C, Ríos J, Puig-Butille JA, Bonifaci N, et al. Serum 25-hydroxyvitamin D3 levels and vitamin D receptor variants in melanoma patients from the Mediterranean area of Barcelona. BMC Med Genet. 2013;14:26. doi: 10.1186/1471-2350-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O’Brien KM, Sandler DP, Kinyamu HK, Taylor JA, Weinberg CR. Single-Nucleotide Polymorphisms in Vitamin D-Related Genes May Modify Vitamin D-Breast Cancer Associations. Cancer Epidemiol Biomarkers Prev. 2017;26(12):1761–1771. doi: 10.1158/1055-9965.EPI-17-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi J, Grundy A, Richardson H, Burstyn I, Schuetz JM, Lohrisch CA, et al. Genetic variation in vitamin D-related genes and risk of breast cancer among women of European and East Asian descent. Tumour Biol. 2016;37(5):6379–6387. doi: 10.1007/s13277-015-4417-8. [DOI] [PubMed] [Google Scholar]
- 80.Khan RJ, Riestra P, Gebreab SY, Wilson JG, Gaye A, Xu R, et al. Vitamin D Receptor Gene Polymorphisms Are Associated with Abdominal Visceral Adipose Tissue Volume and Serum Adipokine Concentrations but Not with Body Mass Index or Waist Circumference in African Americans: the Jackson Heart Study. J Nutr. 2016;146(8):1476–1482. doi: 10.3945/jn.116.229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reimers LL, Crew KD, Bradshaw PT, Santella RM, Steck SE, Sirosh I, et al. Vitamin D-related gene polymorphisms, plasma 25-hydroxy vitamin D, and breast cancer risk. Cancer Causes Control. 2015;26(2):187–203. doi: 10.1007/s10552-014-0497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shastry BS. SNPs: impact on gene function and phenotype. Methods Mol Biol. 2009;578:3–22. doi: 10.1007/978-1-60327-411-1_1. [DOI] [PubMed] [Google Scholar]
- 83.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA. 2004;101(20):7711–7715. doi: 10.1073/pnas.0402490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Elkum N, Alkayal F, Noronha F, Ali MM, Melhem M, Al-Arouj M, et al. Vitamin D insufficiency in Arabs and South Asians positively associates with polymorphisms in GC and CYP2R1 genes. PLoS ONE. 2014;9(11):e113102. doi: 10.1371/journal.pone.0113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muindi JR, Adjei AA, Wu ZR, Olson I, Huang H, Groman A, et al. Serum vitamin D metabolites in colorectal cancer patients receiving cholecalciferol supplementation: correlation with polymorphisms in the vitamin D genes. Horm Cancer. 2013;4(4):242–250. doi: 10.1007/s12672-013-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Masuda S, Byford V, Arabian A, Sakai Y, Demay MB, St-Arnaud R, et al. Altered pharmacokinetics of 1alpha,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 in the blood and tissues of the 25-hydroxyvitamin D-24-hydroxylase (Cyp24a1) null mouse. Endocrinology. 2005;146(2):825–834. doi: 10.1210/en.2004-1116. [DOI] [PubMed] [Google Scholar]
- 87.Ji HF, Shen L. CYP24A1 mutations in idiopathic infantile hypercalcemia. N Engl J Med. 2011;365(18):1741. doi: 10.1056/NEJMc1110226. [DOI] [PubMed] [Google Scholar]
- 88.Shen H, Bielak LF, Ferguson JF, Streeten EA, Yerges-Armstrong LM, Liu J, et al. Association of the vitamin D metabolism gene CYP24A1 with coronary artery calcification. Arterioscler Thromb Vasc Biol. 2010;30(12):2648–2654. doi: 10.1161/ATVBAHA.110.211805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reardon BJ. THE ROLE OF VITAMIN D IN PULMONARY FUNCTION AND LUNG GENE EXPRESSION. Ithaca: Faculty of the Graduate Schoolof Cornell University; 2012. [Google Scholar]
- 90.de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009;20(8):1805–1812. doi: 10.1681/ASN.2008111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li F, Peng M, Jiang L, Sun Q, Zhang K, Lian F, et al. Vitamin D deficiency is associated with decreased lung function in Chinese adults with asthma. Respiration. 2011;81(6):469–475. doi: 10.1159/000322008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dusso AS. Kidney disease and vitamin D levels: 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and VDR activation. Kidney Int Suppl. 2011;1(4):136–141. doi: 10.1038/kisup.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ji X, Mao J, Zhou S. Rs739837 Polymorphism in MiR-885-3p Binding Site Within 3′-Untranslated Region of Vitamin D Receptor is Associated with a Decreased Risk of Pressure Ulcers. Cell Physiol Biochem. 2017;44(6):2129–2137. doi: 10.1159/000485952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Genetic variants in vitamin pathway associated with atopy or asthma, or 25 (OH) D serum levels. Table S2. Genotypic Frequency of associates SNV’s in vitamin D pathway by outcomes.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.