Abstract
Poly-(ADP-ribose) polymerase 1 and 2 (PARP1 and PARP2), upon binding damaged DNA, become activated to add long chains of poly-(ADP-ribose) (PAR) to themselves and other nuclear proteins. This activation is an essential part of the DNA damage response. The PAR modifications recruit the DNA repair machinery to sites of DNA damage and result in base excision and single-strand break repair, homologous recombination, nucleotide excision repair, and alternative non-homologous end-joining. More recently, both PARP1 and PARP2 have been shown to bind to or be activated by RNA, a property that could interfere with the function of PARP1 and PARP2 in the response to DNA damage or lead to necrosis by depletion of cellular NAD+. We have quantitatively evaluated the in vitro binding of a variety of RNAs to PARP1 and PARP2 and queried the ability of these RNAs to switch on enzymatic activity. We find that while both proteins bind RNAs without specificity toward sequence or structure, their interaction with RNA does not lead to auto-PARylation. Thus, although PARP1 and PARP2 are promiscuous with respect to activation by DNA, they both demonstrate exquisite selectivity against activation by RNA.
Keywords: Poly-(ADP-ribose) polymerase, RNA, DNA repair, enzyme activity
Graphical Abstract
Human poly-(ADP-ribose) polymerase 1 and 2 (PARP1, UniProtKB P09874; PARP2, UniProtKB Q9UGN5) are essential components of the DNA damage response pathway1–4. Both proteins are members of the large family of diphtheria toxin-like ADP-ribosyltransferases and both are enzymatically activated upon binding to a diverse collection of DNA lesions. When active, they use NAD+ to polymerize long chains of poly-(ADP-ribose) (PAR) onto themselves and other nuclear acceptor proteins such as histones. These PAR chains recruit many DNA repair proteins that contain PAR-binding motifs5. Knock-out experiments in mice show that PARP1 directly activates base excision repair, homologous recombination, nucleotide excision repair, and alternative non-homologous end-joining6, whereas PARP2 appears to be important in single-strand break repair and homologous recombination7,8. PARP1 is a validated target for cancer therapy, with olaparib, niraparib, and rucaparib in clinical use for treatment of ovarian and/or breast cancer in BRCA1/2 negative patients9. Hundreds of Phase II and Phase III clinical trials for inhibitors of PARPs are currently ongoing to treat breast and ovarian cancer, non-small cell lung cancer, prostate cancer, and glioblastoma, either as monotherapy or in combination with chemo- or radiotherapy.
Both PARP1 and PARP2 are primarily associated with the acute response to DNA damage that leads to the large increase in PARylation activity4, triggered by the conformational changes that result in enzymatic activation10. Essentially all types of DNA lesions activate PARP1, including single stranded DNA, single strand breaks of double-stranded DNA with or without 5’-phosphorylation, blunt ended or overhanging double strand breaks, hairpins, and cruciforms11–16. PARP2 shows more selectivity than PARP1, with a preference for DNA breaks containing a 5’-phosphate17,18. Although the catalytic domains and mechanisms of activation by DNA-binding appear to be conserved between PARP1 and PARP210, the two proteins have very different DNA-binding domains (Fig. 1). PARP1 has three Zn-fingers and a WGR-domain, while PARP2 only has an unstructured N-terminal domain and a WGR-domain.
Figure 1:
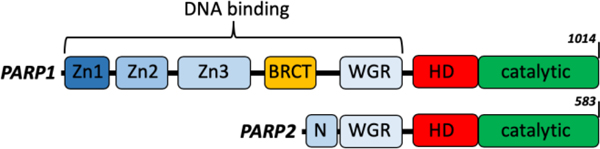
Domain organization of PARP1 and PARP2 showing the differences in DNA-binding domains and the conserved catalytic domains.
PARP1, PARP2, and PARylation activity are involved in other cellular processes such as processing of Okazaki fragments19, rRNA processing20, and formation of ribonucleoprotein stress granules21. In some of these other roles, RNA (both double strand and single strand, with various secondary structures) has been shown to bind and/or activate PARP1 and/or PARP216,22,23. PARP1 might play a role in RNA biogenesis through its interaction with GC-rich regions of RNA24. Most recently, the role of PARP1 in RNA biogenesis has been further elucidated by showing that small nucleolar RNAs (snoRNAs) bind and activate PARP1, leading to the association between PARP1 and the rRNA processing factor DDX2125. In contrast, RNA/DNA hybrids appear to be disfavored for binding to PARP126.
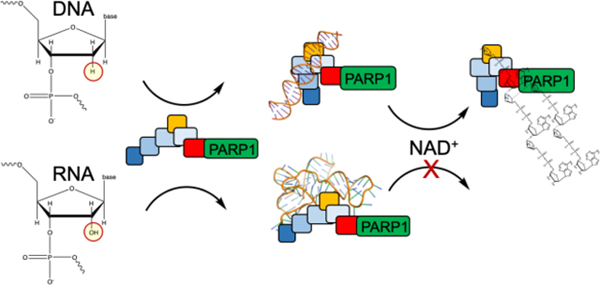
Given that the concentrations of nuclear RNA and DNA are both extremely high, we were interested in testing the generality and specificity of RNA binding and RNA-triggered activation of both PARP1 and PARP2. Additionally, given that activation of PARP1/2 is primarily associated with binding to damaged DNA, we wanted to investigate the generality of activation by RNA, as spurious activation of PARP1/2 could lead to the improper recruitment of the DNA repair machinery to RNA and/or depletion of NAD+ resulting in cell death27.
To test this, we prepared 18 different RNAs representing a wide diversity in terms of length and secondary structure, including some that have been previously shown to interact with PARP1 or PARP2 (Table 1, Fig. S1, Table S1). Each of these RNAs (except the 19mer, which was prepared by chemical synthesis) was prepared by in vitro transcription. This strategy has the advantage that DNA templates used for transcription are significantly longer than the product RNAs, facilitating the purification of the RNAs by gel electrophoresis. For binding assays, RNAs were labeled at the 3’-end with a fluorescein isothiocyanate (FITC)-tag. The integrity of both labeled and unlabeled RNAs was verified by gel electrophoresis (Fig. S2 and S3).
Table 1. Binding constants (KDs) and percent activation (%act) for 18 different RNAs with PARP1 and PARP2.
All values, except those for experiments performed only once, are shown with standard deviations and number of replicates. For KDs, values are derived from fitting to the tight-binding equation as described in Supporting Information. For %act, values (at 1 μM RNA) are derived by comparison with the amount of activity seen by saturating concentrations of DNA (200 nM) as described in the Supporting Information. For PARP1, %act is shown for untreated RNA, RNA treated with RNase, and RNA treated with DNase. For PARP2, the %act is shown for untreated RNA only.
| PARP1 | PARP2 | ||||||
|---|---|---|---|---|---|---|---|
| name | Length (nt) | KD (nM) | %act | %act DNase | %act RNase | KD (nM) | %act |
| p18mer (DNA) | 18 | 13 ± 8 n=11 | 90 ± 13 n=3 | n.d. | n.d. | 59 ± 15 n=10 | 99 ± 11 n=3 |
| 19mer | 19 | 700 ± 230 n=7 | 0 ± 4 n=4 | −3 ± 3 n=3 | 1 ± 7 n=3 | 55 ± 15 n=4 | 2 ± 1 n=3 |
| Kcons1 | 25 | 480 ± 190 n=3 | −7 | n.d. | n.d. | 19 ± 6 n=4 | −4 |
| Kcons3 | 25 | 470 ± 250 n=4 | −5 ± 3 n=3 | −2 ± 1 n=2 | 0 ± 1 n=2 | 24 ± 6 n=4 | 3 ± 2 n=2 |
| Rab7 | 28 | 252 ± 64 n=4 | −5 | n.d. | n.d. | 16 ± 9 n=4 | 4 |
| env8 | 35 | 440 ± 140 n=4 | −6 ± 2 n=3 | −6 ± 3 n=2 | −4 ± 5 n=2 | 27 ± 9 n=6 | −3 ± 2 n=2 |
| B3 CtoU | 41 | 890 ± 150 n=4 | −5 | n.d. | n.d. | 89 ± 17 n=6 | −5 |
| gas5 | 43 | 350 ± 34 n=4 | −4 ± 4 n=4 | −4 ± 3 n=3 | −4 ± 3 n=3 | 40 ± 8 n=4 | −6 ± 5 n=2 |
| h12h13 | 82 | 122 ± 59 n=4 | 10 | n.d. | n.d. | 28 ± 6 n=4 | −1 |
| Arep4&5 | 85 | 34 ± 14 n=4 | 12 ± 5 n=4 | −2 ± 5 n=3 | 10 ± 7 n=3 | 8 ± 3 n=4 | −1 ± 4 n=2 |
| ID205 | 86 | 35 ± 8 n=3 | 12 ± 4 n=3 | −1 ± 1 n=2 | 10 ± 4 n=2 | 12 ± 2 n=4 | 2 |
| tRNALeu | 87 | 159 ± 36 n=6 | 6 ± 2 n=3 | −5 ± 3 n=2 | 1 ± 5 n=2 | 37 ± 7 n=4 | −2 ± 3 n=2 |
| ID411 | 109 | 51 ± 25 n=4 | 25 | n.d. | n.d. | 25 ± 11 n=4 | 2 |
| ID302 | 111 | 27 ± 14 n=3 | 28 ± 7 n=6 | −2 ± 3 n=5 | 14 ± 5 n=3 | 13 ± 1 n=4 | 0 ± 4 n=2 |
| ID509 | 114 | 36 ± 22 n=4 | 7 | n.d. | n.d. | 12 ± 2 n=4 | 2 |
| sno15 | 134 | 14 ± 11 n=3 | 68 ± 15 n=6 | −1 ± 4 n=3 | 48 ± 10 n=3 | 21 ± 6 n=4 | −1 ± 3 n=5 |
| 45s | 146 | 13 ± 5 n=6 | 18 ± 3 n=4 | −1 ± 4 n=3 | 25 ± 19 n=3 | 17 ± 1 n=4 | 5 ± 4 n=3 |
| c5 | 166 | 14 ± 4 n=6 | 14 ± 4 n=2 | −1 | 5 | 22 ± 2 n=4 | 2 |
| sno74 | 202 | 16 ± 7 n=4 | 51 ± 12 n=6 | −1 ± 3 n=3 | 37 ± 4 n=3 | 19 ± 3 n=6 | 1 ± 3 n=4 |
Binding of this set of RNAs to PARP1 and/or PARP2, was monitored through the change in polarization of the fluorescently labeled RNAs upon titrating protein. As a control we included the well-characterized p18mer DNA28, which yielded KDs of 13 and 59 nM for PARP1 and PARP2, respectively (Fig. 2A, Table 1). In agreement with previous reports, the 19mer bound weakly (700 nM)24, and sno74 bound tightly (16 nM)25 to PARP1 (Fig. 2A, Table 1). Comparing all 18 RNAs, the longer the RNA the tighter it bound to PARP1, with a linear correlation between the ln(KD) and length of RNA (Table 1, Fig. 2B). These results suggest non-specific binding of RNA with respect to sequence and structure. The most notable outlier to the linear correlation, sno74 RNA, in Fig. 2B is readily understood. This RNA is sufficiently long (202 nts, molecular weight 65 kDa) that it exceeds the size of the DNA-binding domains of PARP1(10 – 15 kDa) and thus does not bind more tightly than the shorter c5 and 45S RNAs. The increase in binding affinity with length suggests that a single DNA-binding domain in PARP1 (e.g. Zn1, Zn2, Zn3, WGR) engages a short RNA whereas multiple domains, potentially, far removed, are involved in binding a long RNA.
Figure 2:
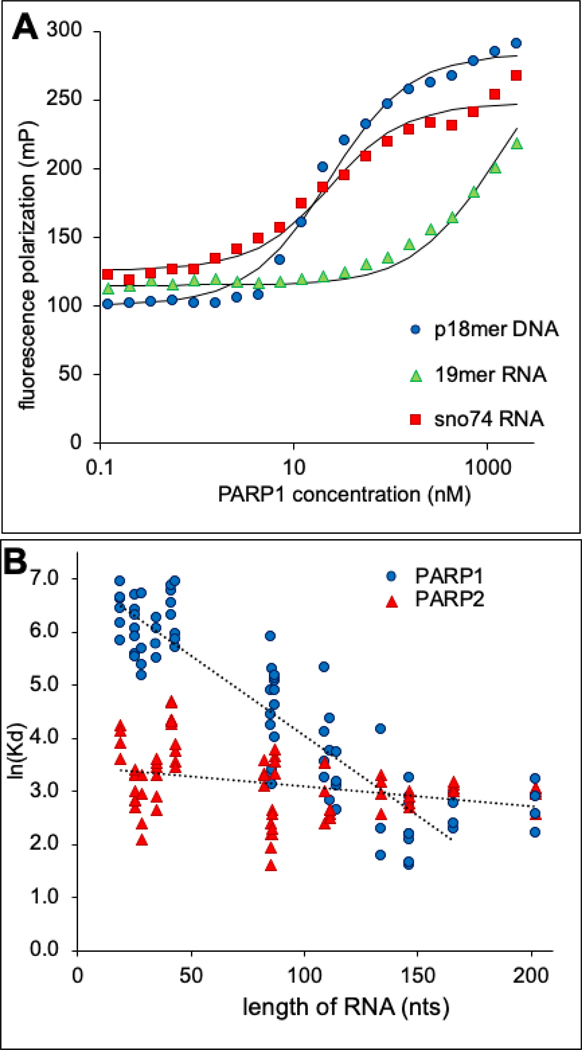
A) Representative binding curves for p18mer DNA, 19mer RNA, and sno74 RNA to PARP1 as determined by fluorescence polarization. The KD values derived from these and replicate determinations are listed in Table 1. B) A plot comparing the KDs (from Table 1) with the length of the RNA demonstrating a strong length-dependence in affinity for PARP1 (linear fit R2 = 0.88) but not for PARP2 (R2 = 0.17).
In contrast, PARP2 bound to all RNAs with an affinity around 20 nM, with only a slightly weaker affinity observed for RNAs shorter than 50 nts (Fig. 2B, Table 1). These results suggest that PARP2 also binds RNA non-specifically with respect to sequence and structure, but that PARP2 has a smaller RNA-binding region most likely encompassing the small (76 residues) and highly cationic (pI = 11.4) N-terminal domain (Fig. 1).
We next tested the 18 different RNAs in an activation assay wherein we monitored the incorporation of 32P-ADPR from 32P-NAD+ into protein using an acid precipitation/filtration assay. The advantages of this assay compared to smear29,30 or other gel-based assays17 are high sensitivity, reproducibility and throughput, allowing us to perform assays under conditions of linearity with respect to product formation (Fig. S4). Additionally, this assay does not rely on a modified NAD+ (e.g. biotinylated-NAD+)31, which may have unanticipated consequences for the activity or specificity of PARP1 or PARP2. As it is possible that the fluorescent tag in the RNA could disrupt a potentially productive interaction with PARP1 or PARP2, we utilized untagged RNAs. We emphasize that adherence to good enzymological practices is a prerequisite to interpreting the potentially different levels of activation between DNA and RNA.
While none of the 18 RNAs tested at saturating concentrations (1 μM) activated PARP2 significantly, some of the RNA samples (at 1 μM) did activate PARP1 to levels almost comparable to the p18mer DNA (at 200 nM) (Table 1). There was no obvious pattern with respect to sequence, length, predicted secondary structure, or affinity as to which RNAs triggered PARylation. We therefore tested the possibility that the activating RNA preparations were contaminated with DNA by treating them with DNase, or as a control, RNase, and then repeating the activation experiments. Treatment with RNase, but not DNase, eliminated most of the nucleic acid material seen following denaturing gel electrophoresis (Fig. S5). Dramatically, DNase treatment reduced all of the apparent RNA-dependent activity of PARP1 to background levels (Fig. 3, Table 1) suggesting that contaminating DNA is responsible for the observed activation. In contrast, RNase treatment led to only a small reduction in apparent activation, supporting the conclusion that the observed activation was caused by DNA contamination (Table 1). We estimate that the contaminating DNA comprises at most 3% of the amount of RNA, a level that would generally be undetectable by gel electrophoresis. Of note, control p18mer DNA treated with DNase retained some activity (19%, Table 1), despite using a rigorous digestion protocol. This incomplete loss of DNA-dependent activation is not surprising given that DNase digests of DNA yield primarily 2mers, 4mers, and 8mers32, the latter being sufficiently long to be a robust activator of PARP133.
Figure 3:
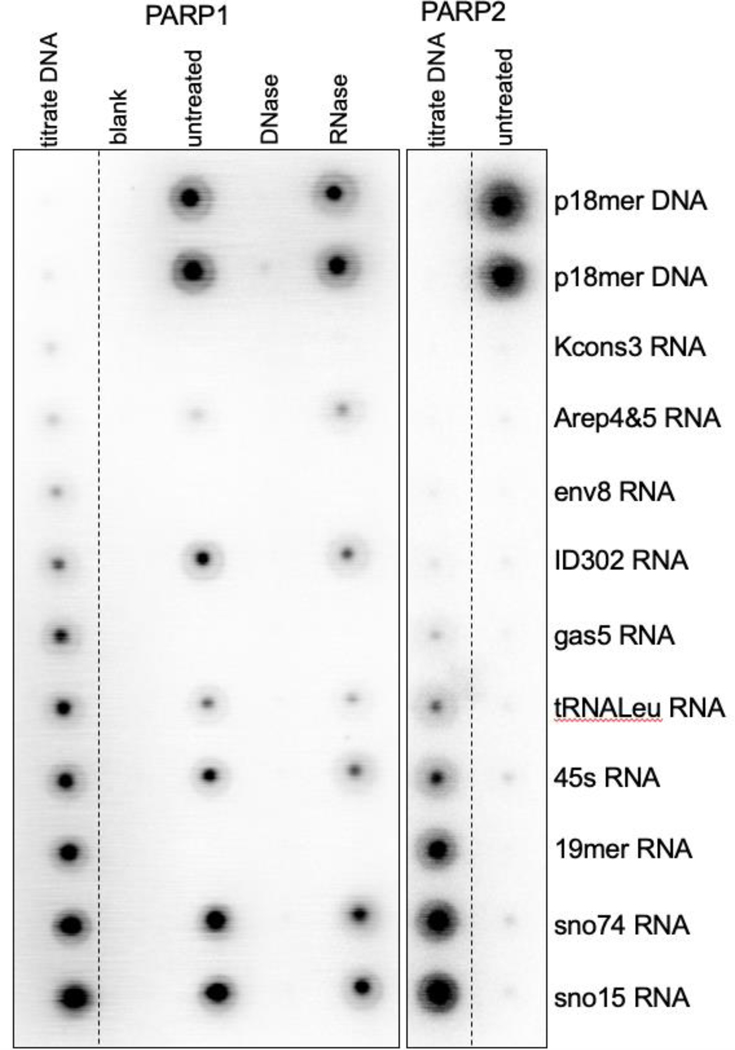
Representative phosphoimager scan of 10 different RNAs (and p18mer DNA control), as indicated on the right, tested for activation of PARylation by monitoring the incorporation of 32P-ADP from 32P-NAD+ for PARP1 (left) and PARP2 (right). The columns labeled titrate DNA contain serial dilutions (1:2) of p18mer DNA (0 – 200 nM). The columns labeled untreated, DNase, RNase contain the RNA (or DNA) samples, as indicated on the right (at 1 μM), after no additional treatment, DNase treatment, or RNase treatment.
Our results demonstrate how difficult it is to completely eliminate DNA contamination in RNA samples prepared by in vitro transcription, and they provide a reasonable explanation for the activation of PARP1 by RNA observed previously22,23,25. Also, our finding that contaminating DNA in the RNA preparations is the source of PARP1 activation is consistent with the lack of activation of PARP2 by any RNA. PARP2 requires 5’-phosphorylation of its DNA and commercially synthesized DNAs do not typically have a 5’-phosphate. Because of the high affinity of PARP1 and PARP2 for DNA and typical PARP assay conditions (15 – 60 min), contamination of DNA in RNA (and protein) samples is a particularly treacherous problem that can lead to misleading results.
In conclusion, we have shown that whereas RNA binds to both PARP1 and PARP2 with a variety of affinities, it does so in a mode that is distinct from DNA as it does not lead to activation of either PARP1 or PARP2. Given that PARP1 is activated by a wide variety of DNA structures (from single stranded DNA to blunt-ended breaks to G-quadruplexes), such discrimination against activation by RNA is surprising, especially since PARP1 is capable of binding tightly to RNA. DNA-mediated activation of PARP1 involves a complex series of structural changes wherein DNA-protein contacts in the Zn1, Zn3, and WGR domains mediates the opening of the HD-domain and access of NAD+ to the active site10. Clearly, the binding of RNA does not trigger analogous conformational changes that lead to activation. Our findings also highlight that DNA contamination is prevalent in RNA samples made by commonly accepted practices, and that it is very difficult to remove to levels that will not trigger activation of PARP1.
Supplementary Material
Acknowledgments
Funding Sources
No competing financial interests have been declared. Funding was provided by the National Cancer Institute R01 CA218255 (to KL), by the Howard Hughes Medical Institute (to KL).
ABBREVIATIONS
- PARP1
Poly-(ADP-ribose) polymerase 1
- PARP2
Poly-(ADP-ribose) polymerase 2
- PAR
poly-(ADP-ribose)
- FITC
fluorescein isothiocyanate
Footnotes
ASSOCIATED CONTENT
Supporting Information. Detailed Methods and Supplementary Figures are available as a combined PDF are available free of charge on the ACS Publications website. A table of the sequences of all the RNAs is available as a TXT file (Table S1).
REFERENCES
- (1).Morales J, Li L, Fattah FJ, Dong Y, Bey E.a, Patel M, Gao J, and Boothman D. a. (2014) Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit. Rev. Eukaryot. Gene Expr 24, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Crawford K, Bonfiglio JJ, Mikoč A, Matic I, and Ahel I (2018) Specificity of reversible ADP-ribosylation and regulation of cellular processes. Crit. Rev. Biochem. Mol. Biol 53, 64–82. [DOI] [PubMed] [Google Scholar]
- (3).Gibson BA, and Kraus WL (2012) New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat. Rev. Mol. Cell Biol 13, 411–424. [DOI] [PubMed] [Google Scholar]
- (4).Beck C, Robert I, Reina-San-Martin B, Schreiber V, and Dantzer F (2014) Poly(ADP-ribose) polymerases in double-strand break repair: Focus on PARP1, PARP2 and PARP3. Exp. Cell Res 329, 18–25. [DOI] [PubMed] [Google Scholar]
- (5).Rack JGM, Perina D, and Ahel I (2016) Macrodomains: Structure, Function, Evolution, and Catalytic Activities. Annu. Rev. Biochem 85, 431–454. [DOI] [PubMed] [Google Scholar]
- (6).de Murcia JM, Niedergang C, Trucco C, Ricoul M, Dutrillaux B, Mark M, Oliver FJ, Masson M, Dierich A, LeMeur M, Walztinger C, Chambon P, and de Murcia G (1997) Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc. Natl. Acad. Sci. U. S. A 94, 7303–7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Schreiber V, Amé JC, Dollé P, Schultz I, Rinaldi B, Fraulob V, Ménissier-de Murcia J, and De Murcia G (2002) Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J. Biol. Chem 277, 23028–23036. [DOI] [PubMed] [Google Scholar]
- (8).Ame JC, Rolli V, Schreiber V, Niedergang C, Apiou F, Decker P, Muller S, Hoger T, de Murcia JM, and de Murcia G (1999) PARP-2, A Novel Mammalian DNA Damage-dependent Poly(ADP-ribose) Polymerase. J Biol Chem 274, 17860–17868. [DOI] [PubMed] [Google Scholar]
- (9).Franzese E, Centonze S, Diana A, Carlino F, Guerrera LP, Napoli M Di Vita, De F, Pignata S, Ciardiello F, and Orditura M (2019) PARP inhibitors in ovarian cancer. Cancer Treat. Rev 73, 1–9. [DOI] [PubMed] [Google Scholar]
- (10).Eustermann S, Wu WF, Langelier MF, Yang JC, Easton LE, Riccio AA, Pascal JM, and Neuhaus D (2015) Structural Basis of Detection and Signaling of DNA Single-Strand Breaks by Human PARP-1. Mol. Cell 60, 742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Langelier MF, Planck JL, Roy S, and Pascal JM (2011) Crystal structures of poly(ADP-ribose) polymerase-1 (PARP-1) zinc fingers bound to DNA: Structural and functional insights into DNA-dependent PARP-1 activity. J. Biol. Chem 286, 10690–10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Eustermann S, Videler H, Yang JC, Cole PT, Gruszka D, Veprintsev D, and Neuhaus D (2011) The DNA-binding domain of human PARP-1 interacts with DNA single-strand breaks as a monomer through its second zinc finger. J. Mol. Biol 407, 149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Lonskaya I, Potaman VN, Shlyakhtenko LS, Oussatcheva EA, Lyubchenko YL, and Soldatenkov VA (2005) Regulation of poly(ADP-ribose) polymerase-1 by DNA structure-specific binding. J. Biol. Chem 280, 17076–17083. [DOI] [PubMed] [Google Scholar]
- (14).Pion E, Bombarda E, Stiegler P, Ullmann GM, Mély Y, De Murcia G, and Gérard D (2003) Poly(ADP-ribose) Polymerase-1 Dimerizes at a 5’- Recessed DNA End in Vitro: A Fluorescence Study. Biochemistry 42, 12409–12417. [DOI] [PubMed] [Google Scholar]
- (15).Silva ID, Pelletier D, Lagueux J, Amours DD, Chaudhry MA, Weinfeld M, Lees-miller SP, and Poirier GG (1999) Relative affinities of poly ( ADP-ribose ) polymerase and DNA-dependent protein kinase for DNA strand interruptions. Biochim. Biophys. Acta 1430, 3–10. [DOI] [PubMed] [Google Scholar]
- (16).Chen Q, Kassab MA, Dantzer F, and Yu X (2018) PARP2 mediates branched poly ADP-ribosylation in response to DNA damage. Nat. Commun 9, 3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Langelier M-F, Riccio AA, and Pascal JM (2014) PARP-2 and PARP-3 are selectively activated by 5′ phosphorylated DNA breaks through an allosteric regulatory mechanism shared with PARP-1. Nucleic Acids Res. 42, 7762–7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Obaji E, Haikarainen T, and Lehtiö L (2016) Characterization of the DNA dependent activation of human ARTD2/PARP2. Sci. Rep 6, 34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Hanzlikova H, Kalasova I, Demin AA, Pennicott LE, Cihlarova Z, and Caldecott KW (2018) The Importance of Poly(ADP-Ribose) Polymerase as a Sensor of Unligated Okazaki Fragments during DNA Replication. Mol. Cell 71, 319–331.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Meder VS, Boeglin M, de Murcia G, and Schreiber V (2005) PARP-1 and PARP-2 interact with nucleophosmin/B23 and accumulate in transcriptionally active nucleoli. J. Cell Sci 118, 211–222. [DOI] [PubMed] [Google Scholar]
- (21).Duan Y, Du A, Gu J, Duan G, Wang C, Gui X, Ma Z, Qian B, Deng X, Zhang K, Sun L, Tian K, Zhang Y, Jiang H, Liu C, and Fang Y (2019) PARylation regulates stress granule dynamics, phase separation, and neurotoxicity of disease-related RNA-binding proteins. Cell Res. 29, 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Huambachano O, Herrera F, Rancourt A, and Satoh MS (2011) Double-stranded DNA binding domain of poly(ADP-ribose) polymerase-1 and molecular insight into the regulation of its activity. J. Biol. Chem 286, 7149–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Léger K, Bär D, Savić N, Santoro R, and Hottiger MO (2014) ARTD2 activity is stimulated by RNA. Nucleic Acids Res. 42, 5072–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Melikishvili M, Chariker JH, Rouchka EC, and Fondufe-Mittendorf YN (2017) Transcriptome-wide identification of the RNA-binding landscape of the chromatin-associated protein PARP1 reveals functions in RNA biogenesis. Cell Discov. 3, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Kim D-S, Camacho CV, Nagari A, Malladi VS, Challa S, and Kraus WL (2019) Activation of PARP-1 by snoRNAs Controls Ribosome Biogenesis and Cell Growth via the RNA Helicase DDX21. Mol. Cell 75, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Fox J, Hafner M, Ravazian N, Zhu Z, Wang IX, Cheung VG, Grunseich C, and Burdick J (2018) Human proteins that interact with RNA/DNA hybrids. Genome Res. 28, 1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, and Swanson RA (2010) NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J. Neurosci 30, 2967–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Rudolph J, Mahadevan J, Dyer PN, and Luger K (2018) Poly(ADP-ribose) polymerase 1 Searches DNA via a “Monkey Bar” Mechanism. Elife 7, e37818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Wacker DA, Ruhl DD, Balagamwala EH, Hope KM, Zhang T, and Kraus WL (2007) The DNA binding and catalytic domains of poly(ADP-ribose) polymerase 1 cooperate in the regulation of chromatin structure and transcription. Mol Cell Biol 27, 7475–7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Karch KR, Langelier M-F, Pascal JM, and Garcia BA (2017) The nucleosomal surface is the main target of histone ADP-ribosylation in response to DNA damage. Mol. Biosyst 13, 2660–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Brown JA, and Marala RB (2002) Development of a high-throughput screening-amenable assay for human poly(ADP-ribose) polymerase inhibitors. J. Pharmacol. Toxicol. Methods 47, 137–141. [DOI] [PubMed] [Google Scholar]
- (32).Pedrini AM, and Grossman L (1983) Purification and Characterization of DNase VIII. J. Biol. Chem 258, 1536–1543. [PubMed] [Google Scholar]
- (33).Altmeyer M, Messner S, Hassa PO, Fey M, and Hottiger MO (2009) Molecular mechanism of poly(ADP-ribosyl)ation by PARP1 and identification of lysine residues as ADP-ribose acceptor sites. Nucleic Acids Res. 37, 3723–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


