Abstract
Ferroptosis is a type of cell death caused by the pathogenic accumulation of lipid hydroperoxides. Pharmacological mechanisms to induce ferroptosis may provide a way to kill cancer cells that are resistant to other forms of cell death like apoptosis. Nonetheless, the proteins that regulate ferroptotic sensitivity in cancer cells remain incompletely understood. Here, we screened a panel of inhibitors of serine hydrolases – an enzyme class important for regulating lipid metabolism – for potentiation of ferroptosis in HT1080 fibrosarcoma cells. We found that DO264, a selective inhibitor of the lyso- and ox-phosphatidylserine (PS) lipase ABHD12, enhances ferroptotic death caused by RSL3, an inhibitor of the lipid peroxidase GPX4. RSL3-induced ferroptosis was also potentiated by genetic disruption of ABHD12. Metabolomic experiments revealed that, in addition to elevated lyso-PS, ABHD12-inactivated cells show higher quantities of arachidonate (C20:4)-containing PS and 2-arachidonoyl glycerol, pointing to potential oxidation-sensitive lipid mediators of ferroptosis regulated by ABHD12.
Ferroptosis is a form of cell death defined by runaway peroxidation of membrane phospholipids.1 This lipid peroxidation is dependent on iron, and ferroptotic cell death can be inhibited by iron chelators.1 Ferroptosis is implicated in several types of pathological cell death, often related to cell and/or organ damage and associated degenerative disorders.2 Ferroptosis has more recently garnered interest as a way to promote cancer cell death independent of the apoptotic pathways that are often defective in transformed cells.3 For instance, drug-resistant, or persister, cancer cells4 and those that have undergone an epithelial-to-mesenchymal transition5 exhibit high susceptibility to ferroptosis.
Ferroptosis can be induced by at least two known pharmacological mechanisms: blockade of the cellular import of cystine by inhibitors of the cystine/glutamate transporter SCL7A11 (or system xc−), such as erastin, or by direct inhibition of the phospholipid hydroperoxide glutathione peroxidase 4 (GPX4).6, 7 The lipid peroxidation step of ferroptosis occurs on phospholipids bearing polyunsaturated fatty acids (PUFAs), such as linoleic (C18:2), arachidonic (C20:4), and adrenic (C22:4) acids,8 and it is either catalyzed by lipoxygenases9 or iron-mediated autoxidation.10 Accordingly, prevention of PUFA incorporation into phospholipids by disruption of enzymes like ACSL411 or LPCAT312 attenuates ferroptosis.
Whether inhibitors of SLC7A11 or GPX4 can induce ferroptosis in cancer cells with sufficient selectivity to avoid general cell or organ toxicity in vivo remains unclear, and it would therefore be of interest to identify other proteins that modulate the ferroptotic potential of cancer cells. Two recent studies used genetic screens of ferroptosis-resistance cancer cells to identify the coenzyme Q oxidoreductase FSP1 (or AIFM2) as a suppressor of ferroptosis in cells that lack dependency on GPX4.13, 14 Here, we considered an alternative, chemical screen to discover potentiators of ferroptosis. Recognizing that the hydrolysis of the sn-1 and sn-2 acyl chains of phospholipids constitutes a principal route for metabolism of these lipids in both unoxidized and oxidized states, and that this reaction is often catalyzed by phospholipase enzymes from the serine hydrolase class15, we reasoned that a screen of serine hydrolase inhibitors may identify enzymes that modulate GPX4-dependent ferroptosis. This screen identified the lyso- and oxidized-phosphatidylserine (PS) lipase ABHD12 as a ferroptosis regulatory enzyme, where pharmacological or genetic disruption of this enzyme enhances GPX4-mediated ferroptosis concurrently with elevating lyso-PS, arachidonoyl-PS, and 2-arachidonoyl glycerol (2-AG).
Serine hydrolases are one of the largest and most diverse enzyme families in Nature, comprising ~1% of all proteins in humans.16 The substrates of serine hydrolases are diverse and include proteins, peptides, and lipids.15 Owing to a shared catalytic mechanism that involves an activated serine nucleophile, serine hydrolases are amenable to functional analysis by the chemical proteomic method activity-based protein profiling (ABPP).17 We and others have used ABPP to generate selective inhibitors for several serine hydrolases, including many that act on lipid substrates, including neutral lipids,18-25 (lyso)phospholipids,26-28 and oxidized phospholipids29. We assembled a set of ~25 serine hydrolase-directed inhibitors for screening in the ferroptosis-sensitive fibrosarcoma cell line HT1080 (see Table S1 for list of serine hydrolase-directed inhibitors). The screen involved pre-treatment for 1 h with individual inhibitors (tested at a concentration that exceeds the EC90 value for the preferred serine hydrolase target) or DMSO control, followed by exposure to a concentration range of the GPX4 inhibitor RSL3 for 24 h, from which EC50 values for ferroptosis induction were calculated. The screen was performed twice and the reported EC50 values reflect average values for the two independent replicate experiments. Ferroptosis-enhancing compounds were defined as those that produced EC50 values for RSL3 that were > 1.5-fold of DMSO control values.
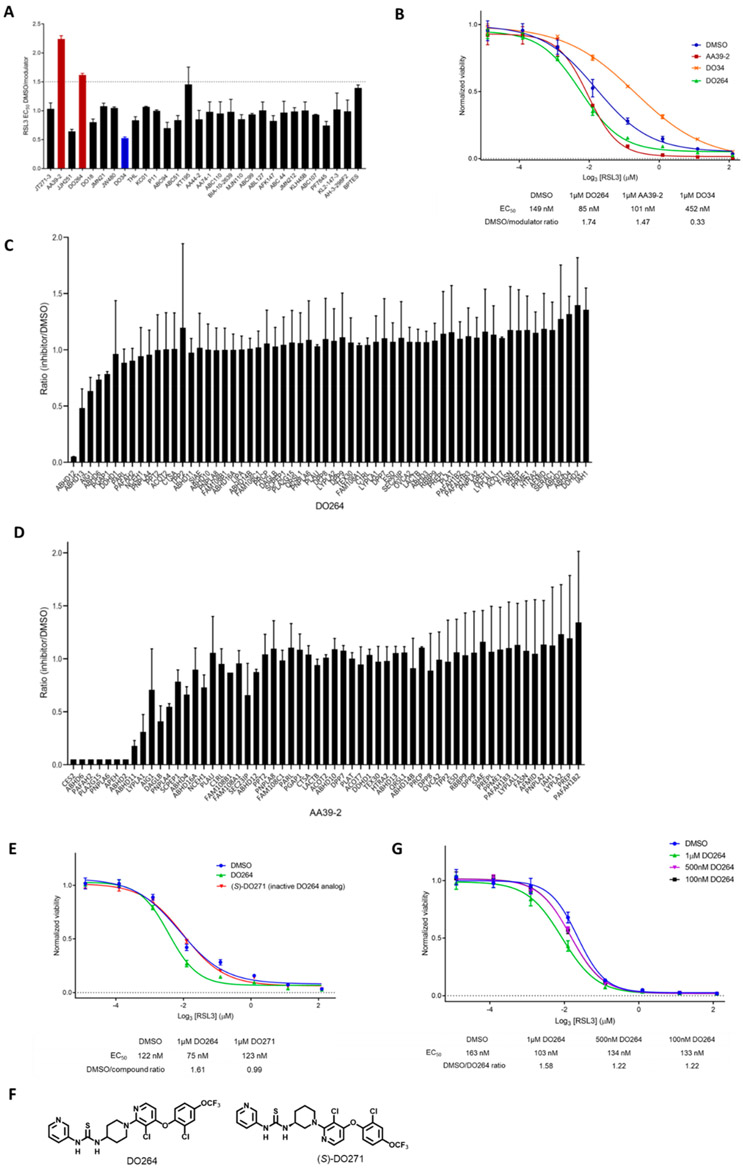
Our screen identified two compounds that substantially enhanced ferroptosis – the ABHD12 inhibitor DO26426 and PAFAH2 inhibitor AA39-229 (Figure 1A). Even though we were primarily interested in identifying mechanisms that potentiated ferroptosis, we also noted that one compound – the DAGLA/DAGLB inhibitor DO3424 – substantially suppressed this process by ~50% (blue bar, Figure 1A). We confirmed the activity of each compound in an independent set of ferroptosis assays (Figure 1B) and proceeded to focus on the ferroptosis-enhancing compounds for further characterization. We first wanted to verify the target profiles of the active compounds, since we have not previously performed ABPP experiments in HT1080 cells. We treated HT1080 cells with DMSO or AA39-2 or DO264 (1 μM each) for 2 h, after which the cells were lysed, and proteomes exposed to the serine hydrolase-directed activity-based probe fluorophosphonate (FP)-biotin30 (10 μM, 1 h) and processed for streptavidin enrichment and quantitative mass spectrometry (MS)-based proteomics. Protein quantitation was performed by isotopic labeling with reductive dimethylation, using heavy and light formaldehyde, as described.31 These MS-based ABPP experiments verified that DO264 selectively inhibited ABHD12 in HT1080 cells (Figure 1C and Table S2; > 90% reductions in ABDH12 activity, with only a single partial cross-reactivity with ABHD13 (~50% inhibition) being observed across 50+ quantified serine hydrolases), but revealed that AA39-2 inhibited several enzymes in addition to PAFAH2 (Figure 1D and Table S2). These data indicate that, while AA39-2 – an electrophilic 1,2,3-triazole urea – may selectively inhibit PAFAH2 at lower concentrations (e.g., < 10 nM in cells),29 the compound is more promiscuous at the screening concentration of 1 μM used in this current study. We therefore chose to mechanistically characterize DO264 due to its selectivity for inhibiting ABHD12.
Figure 1.
A screen of serine hydrolase-directed inhibitors to identify ferroptosis-modulating compounds. (A) Ferroptosis screen. HT1080 cells were treated with individual serine hydrolase inhibitors (all compounds tested at 1 μM, except: DO18 (0.2 μM), THL (10 μM); see Supplementary Table 1 for inhibitor structures and associated serine hydrolase targets) for 1 h before induction of ferroptosis with a concentration range of RSL3 (10 μM with threefold serial dilution, 24 h). Compounds were considered to substantially enhance ferroptosis if they increased the potency of RSL3 (EC50 value) by at least 50% compared to DMSO control. Two compounds met this criteria and are shown in red. Blue marks a compound (DO34) that substantially reduced RSL3-mediated ferroptosis by ~50%. Data represent mean values ± s.d. for two independent experiments. (B) Confirmatory cell viability curves for the hit compounds found to enhance RSL3-dependent ferroptosis from (A). EC50 value shifts for each compound (tested at 1 μM) reported below the curves. Data represent mean values ± s.e.m for four independent experiments. (C, D) Competitive ABPP data for serine hydrolases from HT1080 cells treated with ferroptosis-enhancing compounds DO264 (C) and AA39-2 (D) Cells were treated with each compound at 1 μM for 2 h and then harvested, lysed, treated with the serine hydrolase-directed probe FP-biotin (10 μM), and processed and analyzed following previously described quantitative MS-ABPP protocols31. Ratios represent compound/DMSO (light/heavy), such that low values correspond to inhibition of serine hydrolases. Data represent mean values ± s.d. for median aggregate peptide ratios for each serine hydrolase from two independent experiments.(E) DO264, but not its inactive structural analog (S)-DO271 (1 μM each, 1 h), enhances RSL3-dependent ferroptosis in HT1080 cells. (F) Structures of DO264 and (S)-DO271. (G) DO264 enhances RSL3-dependent ferroptosis in a concentration-dependent manner in HT1080 cells. Data represent mean values ± s.e.m for four independent experiments.
We first verified that (S)-DO271, a structurally related analogue to DO264 that does not inhibit ABHD12,26 did not alter GPX4-dependent ferroptosis (Figure 1E). We also found that DO264, but not (S)-DO271, enhanced ferroptosis in a second cancer cell line, the B cell lymphoma line SU-DHL-5 (Figure S1A). The ferroptosis-potentiating activity of DO264 showed concentration-dependence that generally matched the previously reported in situ potency of the compound for inhibiting ABHD12 (IC50 < 1 μM)26 (Figure 1F and Figure S1B) and was blocked by co-treatment with the ferroptosis inhibitor ferrostatin-1 (Figure S2). DO264 did not potentiate other forms of cell death in HT1080 cells, such as apoptosis induced by staurosporine or necrosis induced by H2O2 (Figure S3), and was not independently cytotoxic in HT1080 or SU-DHL-5 cells at concentrations below 10 μM (Figure S2). These data, taken together, indicate that ABHD12 inhibiton by DO264 specifically potentiates ferroptotic cell death.
To provide further evidence that the ferroptosis-potentiating effects of DO264 occurred through inhibition of ABHD12, we generated ABHD12-knockout (ABHD12-KO) HT1080 cells using CRISPR-Cas9 genome editing. We performed these studies on a population level rather than by selecting clones to avoid potential clonality effects on the basal ferroptotic sensitivity of cells. We confirmed substantial loss of ABHD12 in gene-edited HT1080 cells using a tailored activity-based probe JJH35026 (Figure 2A). The ABHD12-KO cells displayed heightened sensitivity to GPX4-dependent ferroptosis compared to cells treated with control sgRNAs (Figure 2B). The impact of genetic disruption of ABHD12 was lower in magnitude than the effect of DO264 (Figure 2B), which could indicate a residual amount of ABHD12 expression in the ABHD12-KO cell population or that part of the ferroptotic-enhancing effects of DO264 occur through an additional target. Regardless, these data demonstrate that the pharmacological or genetic impairment of ABHD12 enhances the ferroptotic sensitivity of cancer cells.
Figure 2.
Genetic disruption of ABHD12 enhances ferroptosis. (A) Loss of ABHD12 in CRISPR-Cas9-edited HT1080 cells (ABHD12-KO cells), as measured using the ABHD12-directed activity-based probe JJH350 (2 μM, 45 min, 37 °C), following described protocols26. Populations of HT1080 cells were separately generated with three ABHD12-targeting sgRNAs (KO1, KO2, KO3) and two non-targeting control sgRNAs (Ctrl 1, Ctrl 2). (B) Relative RSL3 EC50 values for control (Ctrl) and ABHD12-KO populations treated with DMSO or DO264 (1 μM). Data are normalized to DO264-treated control cells and reflect three independent experiments performed with each control (Ctrl 1, Ctrl 2) and ABHD12-KO (KO1, KO2, KO3) population. **P < 0.01; ***P < 0.001 (two-sided Student’s t-test performed relative to control sgRNA cells treated with DMSO).
ABHD12 has been shown to have at least three metabolic functions in mammals – 1) a lysophospholipase activity that acts on lyso-phosphatidylserine (lysoPS) lipids;32 2) a phospholipase activity that acts on oxidized variants of PUFA PS lipids;33 and 3) a neutral lipase activity that acts on monoacylglycerols (MAGs), including the endocannabinoid 2-arachidonoylglycerol, or 2-AG.34 Genetic or pharmacological blockade of ABHD12 has been shown to increase both the lyso-PS and PUFA (C20:4) PS content of mammalian cells and tissues,26, 32 with the latter change likely reflecting the action of lysophospholipid acyltransferases on elevated lyso-PS. Using targeted lipidomics we found that both DO264-treated cells and ABHD12-KO cells showed substantially elevated 18:0 lysoPS and 18:0/C20:4 PS (Figure 3A, B; Figure S4) The inactive control compound (S)-DO271 did not alter 18:0 lysoPS or 18:0/C20:4 PS lipids (Figure S4). Other phospholipids were largely unchanged in ABHD12-disrupted cells (Table S3), consistent with previous studies pointing to a specialized role for ABHD12 as a lyso-PS lipase.32 Interestingly, exposure to RSL3 further increased 18:0 lyso-PS and dramatically lowered the 18:0/C20:4 PS content of DO264-treated cells (Figure 3C, D). RSL3 had a similar effect on 18:0/C20:4 PS, but did not alter lyso-PS, in DMSO-treated control cells (Figure 3C, D). These data suggest that the heightened PUFA content of PS lipids resulting from ABHD12 blockade can serve as a substrate for lipid peroxidation during ferroptosis, pointing to one potential mechanism for sensitization to this form of cell death. We attempted to identify oxidized PS lipids in DO264/RSL3-treated cells, but were unable to detect these lipids, possibly due to their low abundance and diversified structures.
Figure 3.
Genetic or chemical disruption of ABHD12 elevates lyso-PS and C20:4 PS content of HT1080 cells. (A,B) Quantification of 18:0 lysoPS and 18:0/20:4 PS content of control (Ctrl) or ABHD12-KO HT1080 cells treated with DMSO or DO264 (1 μM, 6 h). Data represent mean values ± s.e.m for three independent experiments for each sgRNA population (two control, three ABHD12-KO). *P < 0.05; **P < 0.01; ***P < 0.001 (two-sided Student’s t-test performed relative to control sgRNA cells treated with DMSO). (C,D) Quantification of 18:0 lysoPS and 18:0/20:4 PS content of ferroptotic HT1080 cells. HT1080 cells were treated with DMSO or DO264 (1 μM) for 1 h, followed by DMSO or RSL3 (2 μM) for 4 h. Data represent mean values ± s.e.m for four independent experiments per group. *P < 0.05; **P < 0.01; ***P < 0.001 (two-sided Student’s t-test performed relative to DMSO control, unless otherwise indicated).
As noted above, ABHD12 can also serve as a 2-AG hydrolase, although this function is primarily performed by MAG lipase (MGLL) in biological systems expressing both enzymes.32 We noticed, however, that MGLL was not detected in our ABPP experiments (Figure 1C, D), indicating that HT1080 cells do not express, or express very low levels, of this enzyme. Consistent with ABHD12 serving as a principal 2-AG lipase in HT1080 cells, ABHD12-KO cells showed substantially lower 2-AG hydrolysis rates compared to control cells (Figure S5). Additionally, both ABHD12-KO and DO264-treated cells displayed heightened 2-AG content compared to control cells (Figure 4A, B). Exposure to RSL3 lowered the 2-AG content of both control and DO264-treated cells (Figure 4C), indicating the potential for 2-AG to undergo peroxidation in ferroptotic cells. Finally, we note that DO34, an inhibitor of the 2-AG biosynthetic enzymes DAGLA and DAGLB, which we found to in our initial screen to protect cells from ferroptosis (Figure 1A, B), substantially lowered the 2-AG content of HT1080 cells (Figure 4D). These data, taken together, indicate that 2-AG and its presumed peroxidation products may contribute to the ferroptotic sensitivity of cancer cells.
Figure 4:
Genetic or chemical disruption of ABHD12 elevates 2-arachidonoylglycerol (2-AG) content of HT1080 cells. (A) Quantification of 2-AG content of HT1080 cells treated with DMSO or DO264 or the inactive control (S)-DO271 (1 μM for each compound, 24 h). Data represent mean values ± s.e.m. for four independent experiments per group. (B) 2-AG content of control (Ctrl) or ABHD12-KO HT1080 cells treated with DMSO or DO264 (1 μM, 24 h). Data represent mean values ± s.e.m for three independent experiments for each sgRNA population (two control, three ABHD12-KO). *P < 0.05; **P < 0.01; ***P < 0.001 (two-sided Student’s t-test performed relative to control sgRNA cells treated with DMSO). (C) Quantification of 2-AG content of ferroptotic HT1080 cells. HT1080 cells were treated with DMSO or DO264 (1 μM, 24 h), followed by DMSO or RSL3 (2 μM, 4 h). Data represent mean values ± s.e.m for three independent experiments per group. *P < 0.05; **P < 0.01; ***P < 0.001 (two-sided Student’s t-test performed relative to DMSO control, unless otherwise indicated). (D) DO34 lowers 2-AG content of HT1080 cells. Quantification of 2-AG content of HT1080 cells treated with DMSO or DO34 (1 μM, 24 h). Data represent mean values ± s.e.m for four independent experiments per group. *P < 0.05; **P < 0.01; ***P < 0.001 (two-sided Student’s t-test performed relative to DMSO control).
Here, we have performed a focused screen of serine hydrolase inhibitors to identify lipid metabolic enzymes that modulate ferroptotic sensitivity of human cancer cells. That the enzymes discovered to either potentiate (ABHD12) or suppress (DAGLA/B) ferroptosis both regulate C20:4 lipids supports the model that ferroptotic potential of cells is coupled to their PUFA content. While past work has emphasized the role of PUFA-containing phosphatidylethanolamine lipids in promoting ferroptosis,8 our data suggest that additional classes of PUFA lipids (e.g. C20:4 PS, C20:4 MAG) may contribute as well. We also cannot exclude that some of the lipid changes caused by ABHD12 (and DAGLA/B) blockade may contribute to ferroptosis through a signaling mechanism that does not involve peroxidation of C20:4 acyl chains. Both lyso-PS and 2-AG, for instance, act on GPCRs, for instance, to regulate diverse cellular processes,35, 36 and it will be important, in the future, to evaluate the potential role of these signaling pathways in ferroptosis.
In considering the potential pathophysiological and translational implications of our findings, we note that loss of ABHD12 in humans leads to an age-dependent polyneuropathy disorder termed PHARC.37 It is therefore interesting to speculate whether ferroptosis in the CNS, where ABHD12 is highly expressed, may contribute to the development of PHARC. ABHD12 is also found at high levels in innate immune cells, and pharmacological or genetic disruption of this enzyme has immunostimulatory effects in vivo.38 Recent work points to an important role for GPX4-dependent lipid peroxidation in macrophage pyroptotic cell death,38 and understanding how ABHD12 and other PUFA-related metabolic enzymes modulate this process in innate immune cells represents an interesting future objective. Finally, we should qualify the extent to which our findings can be interpreted for cancer biology and therapy. First, we have only evaluated the contributions of ABHD12 in two ferroptosis-sensitive human cancer cell lines – HT1080 and SU-DHL-5 – and the magnitude of potentiation of GPX4-induced ferroptosis caused by ABHD12 blockade was relatively modest in these cells (~1.5-fold), precluding assessment of the potential for synergy. Going forward, it will be important to establish a broader understanding of the role that ABHD12 plays in ferroptosis through, for instance, the analysis of additional cancer cell types both in vitro and in vivo. Also, some of the metabolic functions performed by ABHD12 in HT1080 cells, such as the hydrolysis of 2-AG, likely reflects the absence of other enzymes (e.g., MGLL) in this cell type. To the extent that a ferroptosis-relevant lipid species is subject to regulation by different enzymes, it is possible that this context-dependent metabolism could offer a way to tailor ferroptotic outcomes to specific cell types and avoid broader systemic effects that may occur if targeting a central ferroptosis regulator like GPX4. We therefore encourage the continued pursuit of enzymes that can modulate ferroptotic potential in a cell- and/or state-dependent manner.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (CA231991, DA033760) and a fellowship to SGK from the National Cancer Institute (CA228436).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website and includes:
Supporting Figures and Experimental Methods (PDF)
Tables S1-2 (XLSX)
References
- 1.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison III B, Stockwell BR (2012) Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 149, 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD (2017) Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171, 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassannia B, Vandenabeele P, Vanden Berghe T (2019) Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 35, 830–849. [DOI] [PubMed] [Google Scholar]
- 4.Hangauer MJ, Viswanathan VS, Ryan MJ, Bole D, Eaton JK, Matov A, Galeas J, Dhruv HD, Berens ME, Schreiber SL, McCormick F, McManus MT (2017) Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada K, Aguirre AJ, Viswanathan SR, Chattopadhyay S, Tamayo P, Yang WS, Rees MG, Chen S, Boskovic ZV, Javaid S, Huang C, Wu X, Tseng Y-Y, Roider EM, Gao D, Cleary JM, Wolpin BM, Mesirov JP, Haber DA, Engelman JA, Boehm JS, Kotz JD, Hon CS, Chen Y, Hahn WC, Levesque MP, Doench JG, Berens ME, Shamji AF, Clemons PA, Stockwell BR, Schreiber SL (2017) Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seiler A, Schneider M, Förster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Rådmark O, Wurst W, Bornkamm GW, Schweizer U, Conrad M (2008) Glutathione Peroxidase 4 Senses and Translates Oxidative Stress into 12/15-Lipoxygenase Dependent- and AIF-Mediated Cell Death. Cell Metab. 8, 237–248. [DOI] [PubMed] [Google Scholar]
- 7.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR (2014) Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 156, 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kagan VE, Mao G, Qu F, Angeli JPF, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayir H (2016) Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol 13, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR (2016) Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci 113, E4966–E4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah R, Shchepinov MS, Pratt DA (2018) Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent. Sci 4, 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trümbach D, Mao G, Qu F, Bayir H, Füllekrug J, Scheel CH, Wurst W, Schick JA, Kagan VE, Angeli JPF, Conrad M (2016) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol 13, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M, Superti-Furga G, Stockwell BR (2015) Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem. Biol 10, 1604–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, Bassik MC, Nomura DK, Dixon SJ, Olzmann JA (2019) The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Grocin AG, Xavier da Silva TN, Panzilius E, Scheel CH, Mourão A, Buday K, Sato M, Wanninger J, Vignane T, Mohana V, Rehberg M, Flatley A, Schepers A, Kurz A, White D, Sauer M, Sattler M, Tate EW, Schmitz W, Schulze A, O’Donnell V, Proneth B, Popowicz GM, Pratt DA, Angeli JPF, Conrad M (2019) FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698. [DOI] [PubMed] [Google Scholar]
- 15.Long JZ, Cravatt BF (2011) The Metabolic Serine Hydrolases and Their Functions in Mammalian Physiology and Disease. Chem. Rev 111, 6022–6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachovchin DA, Cravatt BF (2012) The pharmacological landscape and therapeutic potential of serine hydrolases. Nat. Rev. Drug Discovery 11, 52–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon GM, Cravatt BF (2010) Activity-based Proteomics of Enzyme Superfamilies: Serine Hydrolases as a Case Study. J. Biol. Chem 285, 11051–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang JW, Cognetta AB, Niphakis MJ, Cravatt BF (2013) Proteome-Wide Reactivity Profiling Identifies Diverse Carbamate Chemotypes Tuned for Serine Hydrolase Inhibition. ACS Chem. Biol 8, 1590–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inloes JM, Hsu K-L, Dix MM, Viader A, Masuda K, Takei T, Wood MR, Cravatt BF (2014) The hereditary spastic paraplegia-related enzyme DDHD2 is a principal brain triglyceride lipase. Proc. Natl. Acad. Sci 111, 14924–14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kornahrens AF, Cognetta AB, Brody DM, Matthews ML, Cravatt BF, Boger DL (2017) Design of Benzoxathiazin-3-one 1,1-Dioxides as a New Class of Irreversible Serine Hydrolase Inhibitors: Discovery of a Uniquely Selective PNPLA4 Inhibitor. J. Am. Chem. Soc 139, 7052–7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson DS, Stiff C, Lazerwith SE, Kesten SR, Fay LK, Morris M, Beidler D, Liimatta MB, Smith SE, Dudley DT, Sadagopan N, Bhattachar SN, Kesten SJ, Nomanbhoy TK, Cravatt BF, Ahn K (2011) Discovery of PF-04457845: A Highly Potent, Orally Bioavailable, and Selective Urea FAAH Inhibitor. ACS Med. Chem. Lett 2, 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niphakis MJ, Cognetta AB, Chang JW, Buczynski MW, Parsons LH, Byrne F, Burston JJ, Chapman V, Cravatt BF (2013) Evaluation of NHS Carbamates as a Potent and Selective Class of Endocannabinoid Hydrolase Inhibitors. ACS Chem. Neurosci 4, 1322–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng H, Kooijman S, van den Nieuwendijk AMCH, Ogasawara D, van der Wel T, van Dalen F, Baggelaar MP, Janssen FJ, van den Berg RJBHN, den Dulk H, Cravatt BF, Overkleeft HS, Rensen PCN, van der Stelt M (2017) Triazole Ureas Act as Diacylglycerol Lipase Inhibitors and Prevent Fasting-Induced Refeeding. J. Med. Chem 60, 428–440. [DOI] [PubMed] [Google Scholar]
- 24.Ogasawara D, Deng H, Viader A, Baggelaar MP, Breman A, den Dulk H, van den Nieuwendijk AMCH, Soethoudt M, van der Wel T, Zhou J, Overkleeft HS, Sanchez-Alavez M, Mori S, Nguyen W, Conti B, Liu X, Chen Y, Liu Q. s., Cravatt BF, van der Stelt M (2016) Rapid and profound rewiring of brain lipid signaling networks by acute diacylglycerol lipase inhibition. Proc. Natl. Acad. Sci 113, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baggelaar MP, den Dulk H, Florea BI, Fazio D, Bernabò N, Raspa M, Janssen APA, Scavizzi F, Barboni B, Overkleeft HS, Maccarrone M, van der Stelt M (2019) ABHD2 Inhibitor Identified by Activity-Based Protein Profiling Reduces Acrosome Reaction. ACS Chem. Biol 14, 2295–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogasawara D, Ichu T-A, Vartabedian VF, Benthuysen J, Jing H, Reed A, Ulanovskaya OA, Hulce JJ, Roberts A, Brown S, Rosen H, Teijaro JR, Cravatt BF (2018) Selective blockade of the lyso-PS lipase ABHD12 stimulates immune responses in vivo. Nat. Chem. Biol 14, 1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamat SS, Camara K, Parsons WH, Chen D-H, Dix MM, Bird TD, Howell AR, Cravatt BF (2015) Immunomodulatory lysophosphatidylserines are regulated by ABHD16A and ABHD12 interplay. Nat. Chem. Biol 11, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cognetta AB, Niphakis MJ, Lee H-C, Martini ML, Hulce JJ,Cravatt BF (2015) Selective N-Hydroxyhydantoin Carbamate Inhibitors of Mammalian Serine Hydrolases. Chem. Biol 22, 928–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adibekian A, Martin BR, Wang C, Hsu K-L, Bachovchin DA, Niessen S, Hoover H, Cravatt BF (2011) Click-generated triazole ureas as ultrapotent in vivo–active serine hydrolase inhibitors. Nat. Chem. Biol 7, 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Patricelli MP, Cravatt BF (1999) Activity-based protein profiling: The serine hydrolases. Proc. Natl. Acad. Sci 96, 14694–14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boersema PJ, Raijmakers R, Lemeer S, Mohammed S, Heck AJR (2009) Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nat. Protoc 4, 484. [DOI] [PubMed] [Google Scholar]
- 32.Blankman JL, Long JZ, Trauger SA, Siuzdak G, Cravatt BF (2013) ABHD12 controls brain lysophosphatidylserine pathways that are deregulated in a murine model of the neurodegenerative disease PHARC. Proc. Natl. Acad. Sci 110, 1500–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelkar DS, Ravikumar G, Mehendale N, Singh S, Joshi A, Sharma AK, Mhetre A, Rajendran A, Chakrapani H, Kamat SS (2019) A chemical–genetic screen identifies ABHD12 as an oxidized-phosphatidylserine lipase. Nat. Chem. Biol 15, 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blankman JL, Simon GM, Cravatt BF (2007) A Comprehensive Profile of Brain Enzymes that Hydrolyze the Endocannabinoid 2-Arachidonoylglycerol. Chem. Biol 14, 1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baggelaar MP, Maccarrone M, van der Stelt M (2018) 2-Arachidonoylglycerol: A signaling lipid with manifold actions in the brain. Prog. Lipid Res 71, 1–17. [DOI] [PubMed] [Google Scholar]
- 36.Makide K, Uwamizu A, Shinjo Y, Ishiguro J, Okutani M, Inoue A, Aoki J (2014) Novel lysophosphoplipid receptors: their structure and function. 55, 1986–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiskerstrand T, H'Mida-Ben Brahim D, Johansson S, M'Zahem A, Haukanes BI, Drouot N, Zimmermann J, Cole AJ, Vedeler C, Bredrup C, Assoum M, Tazir M, Klockgether T, Hamri A, Steen VM, Boman H, Bindoff LA, Koenig M, Knappskog PM (2010) Mutations in ABHD12 Cause the Neurodegenerative Disease PHARC: An Inborn Error of Endocannabinoid Metabolism. Am. J. Hum. Genet 87, 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang R, Zeng L, Zhu S, Xie Y, Liu J, Wen Q, Cao L, Xie M, Ran Q, Kroemer G, Wang H, Billiar TR, Jiang J, Tang D (2018) Lipid Peroxidation Drives Gasdermin D-Mediated Pyroptosis in Lethal Polymicrobial Sepsis. Cell Host Microbe 24, 97–108.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.