Abstract
INTRODUCTION:
Gastrointestinal symptoms in irritable bowel syndrome (IBS) have been correlated with psychological factors using retrospective symptom assessment. However, real-time symptom assessment might reveal the interplay between abdominal and affective symptoms more reliably in a longitudinal perspective. The aim was to evaluate the association between stress and abdominal pain, using the Experience Sampling Method (ESM) as a real-time, repeated measurement method.
METHODS:
Thirty-seven patients with IBS (26 women; mean age 36.7 years) and 36 healthy controls (HC; 24 women; mean age 31.1 years) completed an electronic ESM during 7 consecutive days. Abdominal pain and stress were scored on an 11-point Numeric Rating Scale at a maximum of 10 random moments each day.
RESULTS:
Abdominal pain scores were 2.21 points higher in patients with IBS compared with those in HC (P < 0.001), whereas stress levels did not differ significantly (B: 0.250, P = 0.406). In IBS, a 1-point increase in stress was associated with, on average, 0.10 points increase in abdominal pain (P = 0.017). In HC, this was only 0.02 (P = 0.002). Stress levels at t = −1 were not a significant predictor for abdominal pain at t = 0 in both groups, and vice versa.
DISCUSSION:
Our results demonstrate a positive association between real-time stress and abdominal pain scores and indicate a difference in response to stress and not a difference in experienced stress per se. Furthermore, an in-the-moment rather than a longitudinal association is suggested. This study underlines the importance of considering the individual flow of daily life and supports the use of real-time measurement when interpreting potential influencers of abdominal symptoms in IBS.
INTRODUCTION
Irritable bowel syndrome (IBS) is a disorder of gut–brain interaction, in which psychological factors play a role in both symptom origination and perpetuation (1). Associations between anxiety and depression and IBS have extensively been studied, but daily stress and life hassles have also been associated with gastrointestinal (GI) symptoms in IBS (2,3). However, whether those psychological features precede or follow the occurrence of GI symptoms remains to be elucidated (4,5). Furthermore, it is likely that the order of occurrence of these symptoms and the magnitude of the association between both differ between subjects. Further unraveling this association by considering the heterogeneity between patients with IBS could give insight in individual interactions between psychological and GI symptoms, leading the way to a more personalized approach of IBS.
Most previous studies on this issue are either cross-sectional studies that concurrently assessed both psychological and GI symptoms for between-subject analyses or longitudinal studies that reported within-subject analyses based on repeated assessments on daily or weekly basis (6–8). Although the latter methodology offers a better opportunity to analyze the relationship between both symptoms over time, it does not take into account the individual daily life flow of symptom interactions.
IBS has been described to present as a relapsing-remitting disorder, with symptoms varying over time, where highly fluctuating symptom patterns are often even reported within 1 day (9,10). This underlines the importance of considering this daily life symptom variability when evaluating associations with possible triggering or concomitant factors, such as stress. In addition, as is true for the assessment of abdominal pain (11,12), end-of-week or end-of-day reports of stress are likely influenced by recall bias because stress (i.e., regarding daily life hassles) is a complex process subject to within-day fluctuations (13,14). This further emphasizes the need for studying the temporal interplay between stress and GI symptoms.
For this purpose, we previously developed an electronic smartphone-based patient-reported outcome measure specifically for the use of the Experience Sampling Methodology (ESM) in populations with IBS (15). The ESM is a momentary assessment method collecting repeated measurements randomly during the day, which concern the current status and natural environment of the subjects. These repeated in-the-moment assessments result in an extensive individual pattern of symptoms and provide insight into the complex interplay between this longitudinal symptom formation and possibly associated daily life factors.
Chan et al. (16) recently reported results of an ESM study evaluating the temporal relationship between psychological factors and bowel symptoms in patients with IBS with predominant diarrhea (IBS-D) and healthy volunteers. An interesting finding was a negative association between abdominal pain and preceding stress levels, whereas abdominal pain was predictive for the occurrence of daily life stress and negative effect. In this report, a potential association between concurrent abdominal and psychological symptoms was not evaluated, whereas Blanchard et al. (6) previously pointed toward an important association as such using daily reports.
Therefore, the aim of this study was to evaluate the association between stress and abdominal pain, using concurrent and time-lagged assessments, in patients with IBS and healthy controls (HC), using the ESM. A detailed interpretation of the association between daily life stress and abdominal pain on the subject level, taking into account between-subject heterogeneity, is provided.
METHODS
This prospective observational study was executed as part of a larger international, multicenter project. This report presents data of 1 center, the Maastricht University Medical Center+ (Maastricht UMC+), Maastricht, the Netherlands. The study protocol has been approved by the Maastricht UMC+ Committee of Ethics in November 2016 and was executed according to the tenets of the revised Declaration of Helsinki (64th World Medical Association General Assembly, Fortaleza, Brazil; October 2013). The study has been registered in the US National Library of Medicine (http://www.clinicaltrials.gov, NCT02880722).
Study participants
Recruitment of patients with IBS, aged between 18 and 70 years, took place at the outpatient clinic of Gastroenterology-Hepatology of Maastricht UMC+, a secondary/tertiary referral center. In addition, subjects who previously participated in the Maastricht IBS Cohort (17–19) were contacted to participate in this study. IBS, including subtype assignment, was diagnosed according to the Rome IV criteria (20,21), which were evaluated by a trained clinical researcher in a face-to-face interview. Reason for exclusion was abdominal surgery in the past (except for uncomplicated appendectomy, cholecystectomy, and/or hysterectomy).
HC, also aged between 18 and 70 years, were recruited through advertisements in Maastricht UMC+. Subjects were eligible if they did not have a past or present diagnosis of any GI disorder and did not fulfill Rome IV criteria for IBS. All study subjects could only participate if they could understand the Dutch language and were able to use the smartphone application. Subjects who did not have a smartphone but able to run the application were provided a suitable device for the duration of the study period. Participants were not allowed to change their medication use or start any nonpharmacological treatment within 1 month before the start of study participation until the end of the study period. All subjects gave written informed consent before participation.
Data collection
All study participants completed the ESM for 7 consecutive days during their regular daily life. A digital application (Maastricht Electronic Abdominal Symptom REcording [MEASuRE]), which was specifically developed for the use of ESM in patients with IBS (15), was downloaded on the participants' smartphones and was activated for the course of the study period. Subjects were instructed to carry their smartphone with them during the study week and to complete the real-time questionnaires as often as possible. The MEASuRE application was set to send out an auditory and written signal 10 times a day at random moments between 07:30 am and 10:30 pm, with at least 15 minutes and a maximum of 3 hours between subsequent signals. Every 10 minutes after a signal, the ESM questionnaire was available. Questionnaires that were not completed within 10 minutes after a signal were considered missing data. Therefore, subjects were instructed to complete as many questionnaires as possible each day, as soon as possible following each signal, but to skip questionnaires when considering completing impossible at that moment (e.g., when driving a car). Previous studies using this specific ESM algorithm have shown reasonably high completion rates (11,22).
The development of this ESM questionnaire for the momentary assessment of GI symptoms, the affective state, and environmental factors was described previously (15). This questionnaire was repeated in the same order at all measurement moments, and questions were scored on an 11-point Numeric Rating Scale (0 = not at all to 10 = very severely), according to the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) recommendations (23,24). Figure 1 shows a screenshot of the MEASuRE application for the items “abdominal pain” and “stress,” that were used in the current analyses.
Figure 1.
Screenshots of ESM questions for the assessment of abdominal pain (a) and stress (b). Questions were phrased in Dutch; in this study, it is translated into English.
At the end of the 7-day study period, the Patient Health Questionnaire (PHQ)-9 (0–3 scale; calculates a total composite score for severity of depressive symptoms; recall period of 2 weeks) (25), Generalized Anxiety Disorder (GAD)-7 (0–3 scale; calculates a total composite score for severity of anxious symptoms; recall period of 2 weeks) (26), and the Visceral Sensitivity Index (VSI) were completed (27,28). The 15-item VSI assesses GI-specific anxiety on a 6-point Likert scale. A composite sum score of all items was calculated; higher scores indicated more GI-specific anxiety. The item “In stressful situations, my belly bothers me a lot” was used in this study as the self-reported association between stress and abdominal symptoms.
Statistical analysis
All analyses were performed using R version 3.5.1 (R Core Team [2018]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria). Continuous outcomes are presented as mean ± SD, and differences between groups were tested using the independent samples t test. Proportions for categorical variables were tested using the χ2 test. A P value of <0.05 was considered statistically significant. Subjects who completed at least one-third of the total number of assessments (i.e., 23 of 70) were included in the analyses regarding ESM data (29,30).
Levels of abdominal pain and stress over the 7-day study period were compared between patients with IBS and HC using linear mixed-effects models because ESM data are hierarchical with repeated measures (level 1) nested within subjects (level 2). ESM scores (i.e., abdominal pain or stress) were used as the dependent variable and disease status (i.e., IBS or healthy) as the predictor variable. Models were corrected for repeated measures and autocorrelation using an autoregressive covariate (AR1) structure.
To evaluate associations between stress and abdominal pain, similar analyses were performed. First, the association between concurrent stress and abdominal pain was evaluated, using abdominal pain scores as dependent and stress scores as independent variables. A first model including the total study population included the interaction between stress and disease status as a predictor variable. When finding a significant association between this interaction term and abdominal pain scores, models were performed separately for patients with IBS and HC to further evaluate the association between stress and abdominal pain in each group. Because it was a priori hypothesized that stress and abdominal pain would particularly be related in subgroups of patients with IBS, the model was performed separately for each patient with IBS to obtain individual regression coefficients.
Second, we assessed whether stress scores at 1 point in time could predict subsequent abdominal pain scores, by using lagged scores (i.e., t = −1) of stress as the predictor variable. Similarly, lagged scores for abdominal pain were tested as predictor variables in models with stress scores as the dependent variable to assess a possible association between stress scores and preceding abdominal pain scores. In all models, random slopes for predictor variables were tested, but the models with the best model fit (i.e., based on Akaike Information Criterion) are reported in this study.
RESULTS
Study population
Thirty-seven patients with IBS and 36 HC were enrolled and completed the study. Female sex was predominant in both groups (70% in IBS and 67% in HC), and mean age was 36.7 ± 13.6 years in the IBS group and 31.2 ± 17.7 years in HC. These characteristics were not significantly different between the groups. In the IBS group, IBS subtypes were represented as follows: 14 diarrhea predominant (IBS-D), 10 constipation predominant, 8 mixed stool pattern, and 5 undefined predominant stool pattern. In both IBS and HC groups, most subjects scored only minimal or mild for both depressive (PHQ-9) and anxious (GAD-7) symptoms, i.e., total PHQ-9 or GAD-7 score of 0–9. These scores were not significantly different between the groups. Completion rate of ESM was 68.6% in the IBS group and 69.4% in the control group. All subjects completed at least one-third of the total number of assessments (70) and were, therefore, all included in the analyses.
Levels of abdominal pain and stress
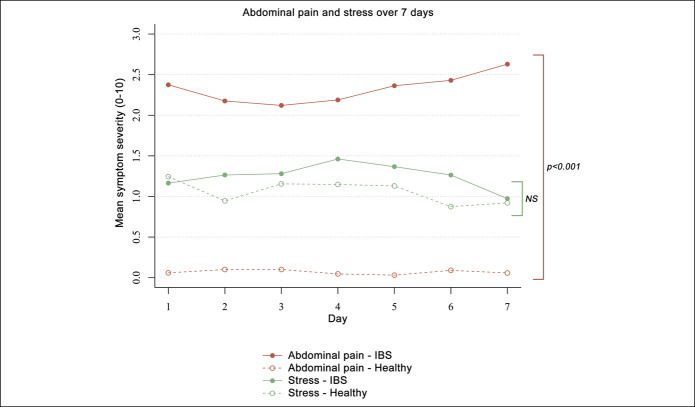
Overall, mean abdominal pain scores from day to day were significantly higher in the IBS group than pain scores reported by the HC. On average, patients with IBS reported abdominal pain scores of 2.21 points higher compared with HC (SE: 0.27, P < 0.001). However, mean stress levels were not significantly different between the groups (mean difference: 0.25, SE: 0.30, P = 0.406) (Figure 2).
Figure 2.
Symptom severity for abdominal pain and stress over the 7-day study period, separately for patients with irritable bowel syndrome and healthy subjects. Symptom scores are measured using ESM over 7 days; mean scores per day are presented. NS, not significant.
Association between stress and abdominal pain scores
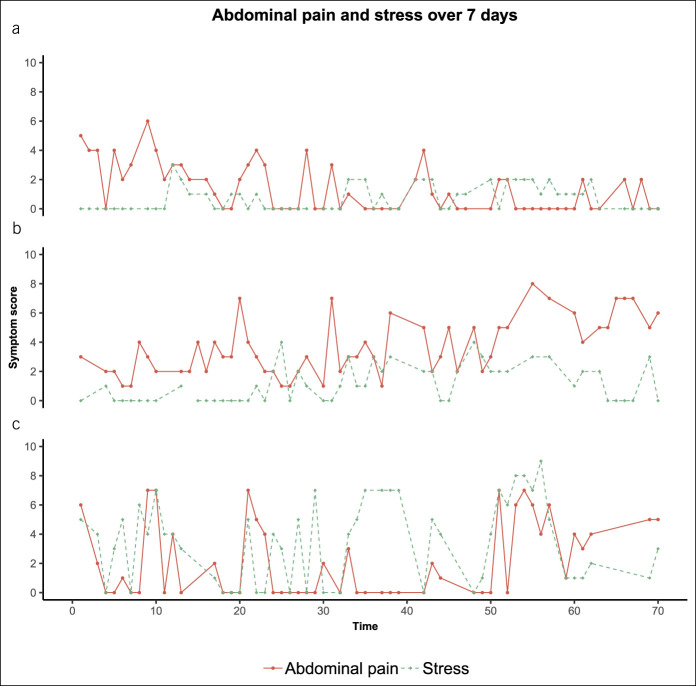
In the IBS group, a significant association was found between concurrent stress and abdominal pain scores. On average, a 1-point increase in stress was associated with 0.10 points increase in abdominal pain (SE: 0.04, P = 0.017). In HC, this was only 0.02 (SE: 0.01, P = 0.002). Figure 3 visualizes the repeated measures for abdominal pain and stress over the 7-day study period, separately for 3 of the patients with IBS, which were selected to illustrate between-subject differences in the association between stress and abdominal pain. The plots show that the interplay between stress and abdominal pain is different for each of the 3 individuals. This is confirmed when performing the above-described linear mixed-effects model, separately for each patient with IBS: the coefficients for the association between stress and abdominal pain range from −1.92 to 1.56. This means that, in 1 subject, a 1-point increase in stress corresponded to a decrease in abdominal pain of 1.92 points, whereas in another, this was associated with 1.56 points increase in abdominal pain.
Figure 3.
Symptom severity scores for abdominal pain and stress, on 70 random time points over 7 days, separately for 3 subjects with IBS. The x axis represents the 70 consecutive assessments: each within a 90-minute timeframe between 7:30 am and 10:30 pm for each day (day 1: 1–10; day 2: 11–20; day 3: 21–30; day 4: 31–40; day 5: 41–50; day 6: 51–60; and day 7: 61–70). The heterogeneity between subjects with IBS is shown by the different patterns of stress and abdominal pain; also indicated by the difference in corresponding regression coefficients for (a) −0.23, (b) 0.07, and (c) 0.28. IBS, irritable bowel syndrome.
Stress levels at t = −1 (i.e., lagged scores) were not a significant predictor for abdominal pain at t = 0 in both patients with IBS and HC. Similarly, abdominal pain scores at t = −1 could not predict stress levels at t = 0 in both groups.
Agreement with self-reported association between stress and IBS symptoms
The VSI item “In stressful situations, my belly bothers me a lot” can be interpreted as a representation of the subjectively experienced association between stress and GI symptoms (subjective association). If this corresponds well with the association as found in the current real-time ESM data (objective association), this would indicate that subjects are able to retrospectively report an association between stress and abdominal pain reliably.
In total, 81.1% of the subjects reported to have more GI symptoms during stressful situations to some extent (i.e., answering options “mildly agree,” “moderately agree,” or “strongly agree”). More than half of the study population (54.1%) strongly agreed with this statement. As described earlier, we found large heterogeneity in the magnitude of the association between stress and abdominal pain when assessing the real-time ESM data (objective association). Together with a correlation coefficient of only 0.125 between the subjective (i.e., VSI) and the objective (i.e., ESM) association, this suggests that patients with IBS retrospectively report an association between stress and abdominal pain as more important compared with when momentary assessed during daily life.
DISCUSSION
This ESM study demonstrates that concurrent levels of stress and abdominal pain are associated in patients with IBS but not in HC. High heterogeneity in the magnitude of this association was found, pointing toward different levels of stress sensitivity within the population with IBS. Although average abdominal pain scores over 7 days were significantly higher in patients with IBS compared with HC, stress levels did not differ between the groups. An association between stress and preceding abdominal pain scores could not be confirmed, and neither were stress levels predictive for subsequent abdominal pain scores. In addition, a subjectively reported relationship between stress and GI symptoms, represented by the VSI, was not in agreement with the association as found within the ESM data, suggesting overestimation of the role of stress in retrospective self-reported questionnaires.
As expected, reported abdominal pain was on average significantly lower in the HC compared with individuals diagnosed with IBS. By contrast, momentary stress levels were not significantly different over time between patients with IBS and their non-IBS counterparts. Both findings are consistent with a recent ESM study of Chan et al. (16) in patients with IBS-D. In addition to this, we could demonstrate a significant and clinically relevant association between stress levels and concurrent abdominal pain scores in the IBS but not the healthy group, which suggests that patients with IBS do not experience more stress per se but seem to be more susceptible to a greater impact of feeling stressed during daily life. This has been described previously in the context of early life stress events (31,32). It has been suggested that the association could work the other way around as well; in that case, abdominal pain would lead to (increasing) stress (2,7,33). This would most likely have been reflected by higher stress levels in patients with IBS compared with the control population, but because both abdominal pain and stress are transient symptoms, particularly in the context of momentary assessment, this effect could have been not apparent in the mean scores as presented. This ESM analyses do not allow us to draw conclusions for a causal relation, but based on the above-mentioned considerations, we assume that stress triggers the onset or worsening of abdominal pain in patients with IBS.
An interaction between concurrent stress and abdominal pain as such was previously demonstrated in a report by Blanchard et al. (6), in which weekly averages of daily symptom scores were analyzed. This study adds evidence to this by exploring short-term associations in more detail, that is, within the day. Our results underline that even within-day changes in stress can be associated with daily fluctuations in abdominal pain, of which the pattern could be overlooked when only analyzing on week level. This also illustrates the temporality of stress and abdominal pain, and the complexity of the interaction between the both over time, which should be considered when evaluating these symptoms in patients with IBS. Furthermore, we demonstrate high heterogeneity in the magnitude of the association between subjects, which points toward different levels of stress sensitivity regarding a stress–abdominal pain interaction within the patients with IBS. One explanation for this could be that each individual has different mechanisms of coping with stress (34). This finding emphasizes the need for an individualized approach in both research and clinical management regarding IBS. In addition, about understanding the impact of stress on IBS pain symptoms, future analyses of momentary assessment should be aimed at more in-depth analysis of contextual and ecological factors (including food intake), which we were currently unable to perform in a meaningful matter because of the fairly small sample size and the heterogeneity of the data.
Daily life stress did not predict subsequent abdominal pain scores, or vice versa, contradicting the findings of Chan et al. (16). Although, in this recent study, daily life stress was represented by the stressfulness of the major activity that took place since the previous entry, we used the scoring of to what extent a subject felt stressed at the current entry. Similarly, the reporting of abdominal pain was different: Chan et al. analyzed to what extent abdominal pain between the current and previous timepoint had affected the subject's daily activity, whereas our participants scored their abdominal pain on each assessment moment using an 11-point Numeric Rating Scale. These methodological differences in definitions of daily life stress and abdominal pain and the time span to which the assessments refer (i.e., “between the previous and current assessments” vs “at the current assessment”) might, therefore, be a reason for differences in results. Furthermore, slightly different timing of the assessments (i.e., 8 times a day for 14 consecutive days vs 10 times a day for 7 consecutive days) combined with unavoidable differences in missing data might lead to alterations in the time lags. Future research might benefit from taking into account the exact length of time between assessments, herewith providing a more reliable comparison between studies. Even though we did not find any significant associations in time-lagged analyses, this does not fully reject the potential presence of interactions as such, possibly only in a subgroup of patients with IBS. Lastly, the inclusion of all IBS subtypes compared with only IBS-D in the study of Chan et al. could explain differences between the studies. For instance, patients with IBS experiencing diarrhea and urgency compared with those with constipation, straining, and a feeling of incomplete evacuation could be characterized by different stress–pain relationships and the temporality of these interactions could also be different. The current data lack statistical power to perform any subtype-specific analyses, but future studies might look into differences in stress–abdominal pain interaction between IBS subtypes.
The disagreement between a self-reported relationship (using the VSI) between stress and abdominal pain and the association between these factors in our momentary data (using ESM) suggests that subjects do not reliably report this interplay between symptoms in retrospective questionnaires. This could be due to recall bias in which our autobiographical memory is not able to correctly recall the exact interaction between experiences. The reporting of peak rather than average levels of abdominal pain in patients with IBS has been linked to retrospective assessments in previous studies (10,11), which might also hold true for the stress–abdominal pain association. Being aware of the fact that patients might overreport an effect of stress on their abdominal symptoms is relevant for clinicians because subjects showing a considerable stress-related pain response might benefit from different management strategies than others (3). In addition, patients might benefit from education for providing insight into their individual, moment-to-moment flow of both psychological and abdominal symptoms, herewith creating awareness for their disease course and assisting therapeutic approaches such as cognitive behavioral therapy.
This study demonstrates that ESM is suitable to more deeply characterize individual symptom patterns using repeated, momentary assessments and taking into account psychosocial factors in patients with IBS. The inclusion of a HC group puts extra confidence to the conclusion that the presented results are reflecting the population with IBS, and by considering all 4 IBS subtypes, the results become generalizable to the heterogeneous population with IBS as a whole. Data were collected using a previously validated IBS-specific ESM tool that was developed according to FDA guidelines for the development of patient-reported outcome measures (15). All statistical models were corrected for autocorrelation of which the importance in this specific context was previously underlined by Blanchard et al. (6).
An inevitable limitation of using the ESM is the occurrence of missing data. However, this is accounted for as much as possible by only including subjects who complete at least one-third of the total number of assessments and by using advanced statistical modeling correction for repeated measures and within-subject autocorrelation. The inclusion of subjects in a secondary/tertiary center might result in a specific study population not fully generalizable to the entire population with IBS. However, subjects were previously also recruited through general practitioners' practices (i.e., for the Maastricht IBS Cohort), so this study population most likely represents a heterogeneous population with IBS. Furthermore, selection bias could have arisen by the fact that ESM might be experienced as burdensome. Because the current report only includes data from 1 of the 5 intended inclusion centers, sample size is relatively small, which impacts the strengths of the conclusions drawn from this analysis. Future analyses including more subjects, from different centers, could give additional insight into the heterogeneity in stress–abdominal pain interaction between subjects and between IBS subtypes and allow for assessment of cross-cultural differences.
In conclusion, using real-time symptom assessment, we demonstrated that stress levels are positively associated with concurrent abdominal pain scores, with high heterogeneity in the magnitude of this association between individual patients with IBS, pointing toward different levels of stress sensitivity regarding stress–abdominal pain interaction in IBS. Although patients with IBS and HC showed comparable levels of stress, the association between abdominal pain and stress was stronger in patients with IBS compared with that in HC, indicating a difference in response to stress rather than a difference in experienced stress per se. In addition, abdominal pain scores could not be predicted by preceding stress levels, and vice versa, suggesting an in-the-moment rather than a longitudinal association. This study underlines the importance of taking into account the individual flow of daily life when evaluating symptom patterns in patients with IBS and supports the use of real-time measurement when interpreting potential influencers of abdominal symptoms.
CONFLICTS OF INTEREST
Guarantor of the article: Lisa Vork, MD.
Specific author contributions: Study concept and design: L.V., D.K., Z.M., C.L., J.K., and A.A.M.M.; data collection: L.V.; data interpretation and statistical analysis: L.V., D.K., and S.K.; manuscript writing: L.V.; constructive review of manuscript: D.K., E.Q., H.T., M.S., Q.A., M.C., J.T., Z.M., C.L., J.K., and A.A.M.M. All authors approved the final manuscript.
Financial support: This study is investigator initiated and partly financially supported by Grünenthal GmBH, Aachen, Germany.
Potential competing interests: L.V.: None to declare. D.K.: Research funding from Will Pharma, Allergan, and Grünenthal; served as advisor for Will Pharma, Bayer, and Biocodex. S.M.J.v.K.: None to declare. E.G.Q.: Employee of Grünenthal. H.T: None to declare. M.S.: Unrestricted research grants of Danone Nutricia Research, Glycom, and Ferring Pharmaceuticals.;consultant/advisory board member of AstraZeneca, Danone Nutricia Research, Nestlé, Almirall, Allergan, Menarini, Biocodex, Genetic Analysis AS, Albireo, Glycom, Arena, and Shire; speakers' bureau of Tillotts, Menarini, Kyowa Kirin, Takeda, Shire, Allergan, Biocodex, Alimentary Health, AlfaSigma, and Almirall. Q.A.: Advisory board of Grünenthal and Allergan; conference support of Grünenthal and Alimentary health. M.C.: None to declare. J.T.: Scientific advice to AlfaWassermann, Allergan, Christian Hansen, Danone, Grünenthal, Ironwood, Janssen, Kyowa Kirin, Menarini, Mylan, Neutec, Novartis, Noventure, Nutricia, Shionogi, Shire, Takeda, Theravance, Tramedico, Tsumura, Zealand, and Zeria pharmaceuticals.;served on the speaker bureau for Abbott, Allergan, AstraZeneca, Janssen, Kyowa Kirin, Menarini, Mylan, Novartis, Shire, Takeda, and Zeria. Z.M.: None to declare. C.L.: None to declare. J.W.K.: None to declare. A.A.M.M.: Unrestricted grant from Grünenthal for development of ESM on IBS; grant from ZonMw and Will Pharma for RCT on peppermint oil in IBS.
Study Highlights.
WHAT IS KNOWN
✓ The common association between psychological factors and gastrointestinal symptoms in IBS has traditionally been ascertained using retrospective symptom reports.
✓ Real-time, electronic symptom assessment has the potential to reveal the interplay between gastrointestinal and affective symptoms more reliably in a longitudinal perspective.
WHAT IS NEW HERE
✓ Real-time stress levels are positively correlated with concurrent abdominal pain scores in patients with IBS but not in HC.
✓ High heterogeneity between patients with IBS points toward different levels of stress sensitivity within this population.
✓ Different phenotypes in stress–pain association can be identified using real-time measurement based on the experience sampling method by capturing the individual daily flow of IBS symptoms.
TRANSLATIONAL IMPACT
✓ ESM could be used in selecting subgroups of IBS patients that benefit from specific management strategies, for example targeting the abdominal pain-stress interaction.
ACKNOWLEDGEMENTS
We thank MEMIC (center for data and information management at Maastricht UMC+) for the development of the smartphone application and their support regarding data management. We also thank all patients with IBS and healthy volunteers who participated in this study.
References
- 1.Drossman DA. Functional gastrointestinal disorders: History, pathophysiology, clinical features and Rome IV. Gastroenterology 2016(150):1262–79. [DOI] [PubMed] [Google Scholar]
- 2.Van Oudenhove L, Crowell MD, Drossman DA, et al. Biopsychosocial aspects of functional gastrointestinal disorders. Gastroenterology 2016(150):1355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palsson OS, Drossman DA. Psychiatric and psychological dysfunction in irritable bowel syndrome and the role of psychological treatments. Gastroenterol Clin North Am 2005;34(2):281–303. [DOI] [PubMed] [Google Scholar]
- 4.Koloski NA, Jones M, Kalantar J, et al. The brain—Gut pathway in functional gastrointestinal disorders is bidirectional: A 12-year prospective population-based study. Gut 2012;61(9):1284–90. [DOI] [PubMed] [Google Scholar]
- 5.Jones MP, Tack J, Van Oudenhove L, et al. Mood and anxiety disorders precede development of functional gastrointestinal disorders in patients but not in the population. Clin Gastroenterol Hepatol 2017;15(7):1014–20.e1014. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard EB, Lackner JM, Jaccard J, et al. The role of stress in symptom exacerbation among IBS patients. J Psychosomatic Res 2008;64(2):119–28. [DOI] [PubMed] [Google Scholar]
- 7.Dancey CP, Taghavi M, Fox RJ. The relationship between daily stress and symptoms of irritable bowel: A time-series approach. J Psychosomatic Res 1998;44(5):537–45. [DOI] [PubMed] [Google Scholar]
- 8.Levy RL, Cain KC, Jarrett M, et al. The relationship between daily life stress and gastrointestinal symptoms in women with irritable bowel syndrome. J Behav Med 1997;20(2):177–93. [DOI] [PubMed] [Google Scholar]
- 9.Mearin F, Baro E, Roset M, et al. Clinical patterns over time in irritable bowel syndrome: Symptom instability and severity variability. Am J Gastroenterol 2004;99(1):113–21. [DOI] [PubMed] [Google Scholar]
- 10.Weinland SR, Morris CB, Hu Y, et al. Characterization of episodes of irritable bowel syndrome using ecological momentary assessment. Am J Gastroenterol 2011;106(10):1813–20. [DOI] [PubMed] [Google Scholar]
- 11.Mujagic Z, Leue C, Vork L, et al. The experience sampling method—a new digital tool for momentary symptom assessment in IBS: An exploratory study. Neurogastroenterol Motil 2015;27(9):1295–302. [DOI] [PubMed] [Google Scholar]
- 12.Stone AA, Schwartz JE, Broderick JE, et al. Variability of momentary pain predicts recall of weekly pain: A consequence of the peak (or salience) memory heuristic. Personal Soc Psychol Bull 2005;31(10):1340–6. [DOI] [PubMed] [Google Scholar]
- 13.Havermans R, Nicolson NA, Devries MW. Daily hassles, uplifts, and time use in individuals with bipolar disorder in remission. J Nervous Ment Dis 2007;195(9):745–51. [DOI] [PubMed] [Google Scholar]
- 14.DeLongis A, Folkman S, Lazarus RS. The impact of daily stress on health and mood: Psychological and social resources as mediators. J Personal Soc Psychol 1988;54(3):486–95. [DOI] [PubMed] [Google Scholar]
- 15.Vork L, Keszthelyi D, Mujagic Z, et al. Development, content validity, and cross-cultural adaptation of a patient-reported outcome measure for real-time symptom assessment in irritable bowel syndrome. Neurogastroenterol Motil 2018;30:e13244. [DOI] [PubMed] [Google Scholar]
- 16.Chan Y, So SH, Mak ADP, et al. The temporal relationship of daily life stress, emotions, and bowel symptoms in irritable bowel syndrome-diarrhea subtype: A smartphone-based experience sampling study. Neurogastroenterol Motil 2018:e13514. [DOI] [PubMed] [Google Scholar]
- 17.Ludidi S, Mujagic Z, Jonkers D, et al. Markers for visceral hypersensitivity in patients with irritable bowel syndrome. Neurogastroenterol Motil 2014;26(8):1104–11. [DOI] [PubMed] [Google Scholar]
- 18.Thijssen AY, Mujagic Z, Jonkers DM, et al. Alterations in serotonin metabolism in the irritable bowel syndrome. Aliment Pharmacol Ther 2016;43(2):272–82. [DOI] [PubMed] [Google Scholar]
- 19.Mujagic Z, Ludidi S, Keszthelyi D, et al. Small intestinal permeability is increased in diarrhoea predominant IBS, while alterations in gastroduodenal permeability in all IBS subtypes are largely attributable to confounders. Aliment Pharmacol Ther 2014;40(3):288–97. [DOI] [PubMed] [Google Scholar]
- 20.Palsson O, Whitehead W, van Tilburg MA, et al. Development and validation of the Rome IV diagnostic questionnaire for adults. Gastroenterology 2016;150:1481–91. [Google Scholar]
- 21.Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology 2016(150):1393–407. [DOI] [PubMed] [Google Scholar]
- 22.Vork L, Mujagic Z, Drukker M, et al. The experience sampling method-evaluation of treatment effect of escitalopram in IBS with comorbid panic disorder. Neurogastroenterol Motil 2019;31:e13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Department of Health and Human Services FaDA, Center for Drug Evaluation and Research (CDER). Guidance for Industry Irritable Bowel Syndrome—Clinical Evaluation of Drugs for Treatment, 2012. [Google Scholar]
- 24.EMA. Guideline on the Evaluation of Medicinal Products for the Treatment of Irritable Bowel Syndrome, 2014. [Google Scholar]
- 25.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med 2006;166(10):1092–7. [DOI] [PubMed] [Google Scholar]
- 27.Labus JS, Bolus R, Chang L, et al. The visceral sensitivity index: Development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther 2004;20(1):89–97. [DOI] [PubMed] [Google Scholar]
- 28.Labus JS, Mayer EA, Chang L, et al. The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: Further validation of the visceral sensitivity index. Psychosomatic Med 2007;69(1):89–98. [DOI] [PubMed] [Google Scholar]
- 29.Palmier-Claus JE, Myin-Germeys I, Barkus E, et al. Experience sampling research in individuals with mental illness: Reflections and guidance. Acta Psychiatrica Scand 2011;123(1):12–20. [DOI] [PubMed] [Google Scholar]
- 30.Delespaul P. Assessing Schizophrenia in Daily Life the Experience Sampling Method. UPM, Universitaire Pers Maastricht, Maastricht University: Maastricht, the Netherlands; 1995. [Google Scholar]
- 31.Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry 2001;62(suppl 8):28–36. [PubMed] [Google Scholar]
- 32.Whitehead WE, Crowell MD, Robinson JC, et al. Effects of stressful life events on bowel symptoms: Subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut 1992;33(6):825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craske MG, Wolitzky-Taylor KB, Labus J, et al. A cognitive-behavioral treatment for irritable bowel syndrome using interoceptive exposure to visceral sensations. Behav Res Ther 2011;49(6-7):413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazarus RS, Folkman S. Stress, Appraisal, and Coping . New York, NY: Springer Publishing Company; 1984. [Google Scholar]