Summary
Meiotic pairing between parental chromosomes (homologs) is required for generation of haploid gametes. Homolog pairing depends on recombination initiation via programmed double strand breaks (DSBs). Although DSBs occur prior to pairing, the homolog, rather than the sister chromatid, is used as repair partner for crossing over. Here, we show that DNA helicase Mph1, the budding yeast ortholog of human FANCM, prevents precocious DSB strand exchange between sister chromatids before homologs have completed pairing. By dissociating precocious DNA displacement loops (D-loops) between sister chromatids, Mph1FANCM ensures high levels of crossovers and non-crossovers between homologs. Later occurring recombination events are protected from Mph1FANCM-mediated dissociation by synapsis protein Zip1. Increased intersister repair in absence of Mph1FANCM triggers a shift among remaining interhomolog events from non-crossovers to crossover-specific strand exchange, explaining Mph1’s apparent anti-crossover function. Our findings identify temporal coordination between DSB strand exchange and homolog pairing as a critical determinant for recombination outcome.
eTOC Blurb
During meiosis, self-inflicted double strand breaks (DSBs) are required for pairing between parental chromosomes (homologs). Sandhu and colleagues now demonstrate that the yeast FANCM DNA helicase dissociates precocious DSB strand exchange between sister chromatids, thereby ensuring normal exchange between homologs.
Graphical Abstract

INTRODUCTION
During the first meiotic cell division homologs are separated, resulting in genome haploidization, a requirement for gamete formation and sexual reproduction. Crossovers (COs) in combination with sister chromatid cohesion, provide physical linkage between homologs, mediating their attachment to opposite spindle poles (Zickler and Kleckner, 2015). Crossovers are generated from programmed double strand breaks (DSBs) via homologous recombination, at different positions in different cells (Lam and Keeney, 2014). Close juxtaposition of homologs along their length (homolog pairing) is a precondition for recombinational exchange at allelic positions (Zickler and Kleckner, 2015).
DSBs are formed in excess over COs (Lam and Keeney, 2014). Recombination events not processed into COs undergo patch repair with the homolog without exchange of flanking chromosome arms, giving rise to non-crossovers (NCOs; Hunter, 2015).
Alternatively, the sister chromatid may serve as repair template (Schwacha and Kleckner, 1997). Crossovers are critical for homolog segregation. Intersister exchange allows DSB repair in absence of a template sequence on the homolog (Goldfarb and Lichten, 2010).
DSBs are induced by a multiprotein complex that includes Spo11 as its catalytic component (Lam and Keeney, 2014). Following DSB formation, resection rapidly removes 5’ ends carrying covalently attached Spo11, giving rise to extended single-ended 3’ overhangs competent for strand invasion (Mimitou et al., 2017). Strand exchange proteins include Rad51 and meiosis-specific Dmc1 (Bishop, 1994). Only Dmc1’s strand exchange activity, but not that of Rad51, is required for formation of COs, NCOs and most intersister exchanges (Schwacha and Kleckner, 1997; Cloud et al., 2012).
Partner choice between sister chromatid and homolog, and differentiation of interhomolog events into future COs and NCOs, occur no later than DSB first-end strand exchange (Allers and Lichten, 2001; Hunter and Kleckner, 2001; Börner et al., 2004). Both CO formation and intersister exchange entail two successive DSB strand invasions. Strand exchange by the DSB first-end generates single-end invasion (SEI) intermediates between homologs (IH-SEIs) or sister chromatids (IS-SEIs) (Hunter and Kleckner, 2001; Kim et al., 2010). Both types of SEIs subsequently initiate extension DNA synthesis to fill in nucleotides previously removed by 5’ resection. Single end invasions capture and become ligated to their cognate DSB second ends, giving rise to double Holliday junctions between homologs (IH-dHJs) or sister chromatids (IS-dHJs; Schwacha and Kleckner, 1995). All branched recombination intermediates are collectively referred to as “joint molecules” (JMs). Non-crossovers form via synthesis-dependent strand annealing where the DSB first-end transiently invades the homolog, followed by copying of homolog sequences, displacement of the invading strand and re-ligation with the DSB second end (Allers and Lichten, 2001; McMahill et al., 2007; Marsolier-Kergoat et al., 2018).
Whereas DSB repair in vegetative cells occurs between sister chromatids, meiotic recombination preferentially involves homologs (Kadyk and Hartwell, 1992; Schwacha and Kleckner, 1997; Bzymek et al., 2010). Homolog bias depends on several factors, including differential control of Rad51 and Dmc1, as well as structural chromosome features (Tsubouchi and Roeder, 2006; Kim et al., 2010). Inhibition of Rad51-mediated strand exchange occurs via Mek1, an ortholog of mammalian CHK2 kinase. Mek1 phosphorylation stabilizes Hed1, a meiosis-specific Rad51 inhibitor (Busygina et al., 2008; Lao et al., 2013; Callender et al., 2016).
Post-replicative homolog pairing and recombination are mutually dependent: Recombination initiation and strand exchange are required for pairing, yet pairing is also required for interhomolog recombination (Weiner and Kleckner, 1994; Peoples et al., 2002; Zickler and Kleckner, 2015). Following establishment of pairing at a distance of ~400 nm, homolog juxtaposition at ~100 nm is achieved via the synaptonemal complex (SC) involving e.g., yeast transverse filament protein Zip1 (Sym et al., 1993).
DSBs are induced gradually in each nucleus and, depending on their abundance, recombination intermediates are processed differently (Joshi et al., 2015; Mimitou et al., 2017). During early meiosis at low DSB abundance, “scout” DSBs prefer the sister chromatid as repair template. Increasing DSB abundance results in preferential recombination with the homolog (Joshi et al., 2015). Abundance of recombination events also affects CO/NCO differentiation. At lower DSB abundance, COs are increased at the expense of NCOs, a phenomenon called crossover homeostasis (Martini et al., 2006).
Conserved yeast DNA helicases Mph1, Sgs1, and Srs2 have been implicated in DSB strand exchange in both mitotic and meiotic cells (Ira et al., 2003; Bernstein et al., 2010). Sgs1 is the ortholog of human BLM (Watt et al., 1996), and Srs2 is related to bacterial UvrD (Bernstein et al., 2010). The Mph1 ortholog FANCM is part of a DNA repair complex defective in Fanconi anemia patients (Deans and West, 2009). While FANCM orthologs in fission yeast and plants limit meiotic COs (Lorenz et al., 2012; Crismani et al., 2012; Blary et al., 2018), no MPH1 role has been identified in budding yeast, even though the protein is meiotically expressed (Wild et al., 2019).
Here, we have examined the temporal and functional integration of homolog pairing, recombination partner choice, and CO/NCO differentiation. We show that the DNA helicase Mph1FANCM acts as a timing factor, preventing DSB strand exchange before homologs have undergone pairing and/or synapsis. Increased intersister exchange in absence of Mph1 affects the frequency of interhomolog recombination and CO/NCO differentiation. Together, our findings suggest that temporal coordination between DSB strand exchange and homolog pairing is critical in determining recombination outcome and segregation of parental chromosomes.
RESULTS
Interdependence of homolog pairing and recombination.
In many organisms, homolog pairing depends on recombination, yet the spatiotemporal relationship between these processes has not been examined. To define DNA helicase roles in DSB strand exchange, we first analyzed the timing of transitions in recombination and pairing during WT meiosis.
Assay system. –
Temporal resolution of recombination steps relevant to DSB strand exchange was achieved by inducing synchronized yeast meiosis at the lower temperature of 23ºC (Börner et al., 2004). Pairing was monitored using GFP-tetR fusion proteins tethered to tetO arrays proximal to centromere 3, under conditions that block homolog separation (ndt80Δ; Brar et al., 2009). GFP focus distances were measured in surface-spread nuclei. If two closely juxtaposed foci or a single focus was detected, nuclei were scored as paired and/or synapsed.
Recombination was examined at the HIS4::LEU2 recombination hotspot (HIS4LEU2 hereafter). Polymorphic XhoI sites flanking HIS4LEU2 allow detection of restriction fragments derived from “Mom” and “Dad” homologs (Fig. 1A,B; Oh et al., 2007). DSBs and crossovers (CO-1 and CO-2) as well as non-crossovers are detected by one-dimensional (1D-) gel analysis (Fig. 1A,D). 2D-gel analysis detects five JM signals: Interhomolog signals comprise two single end invasion intermediates (IH-SEIs) and one double Holliday junction (IH-dHJ) (Fig. 1B,C-i; Schwacha and Kleckner, 1995; Hunter and Kleckner, 2001). Corresponding intermediates between sister chromatids (presumed IS-SEIs and IS-dHJs) are also detectable. Only IS-dHJs can be identified unambiguously in WT, whereas IS-SEIs are obscured by comigrating IH-SEIs (Kim et al., 2010). When intersister recombination predominates, IS-SEIs are detected as two crescent-shaped signals (below). Pairing at HIS4LEU2 is inferred from pairing of homologous GFP signals which are located within ~30 kb along chromosome 3 (corresponding to 60 nm SC contour length; see Kleckner, 2006).
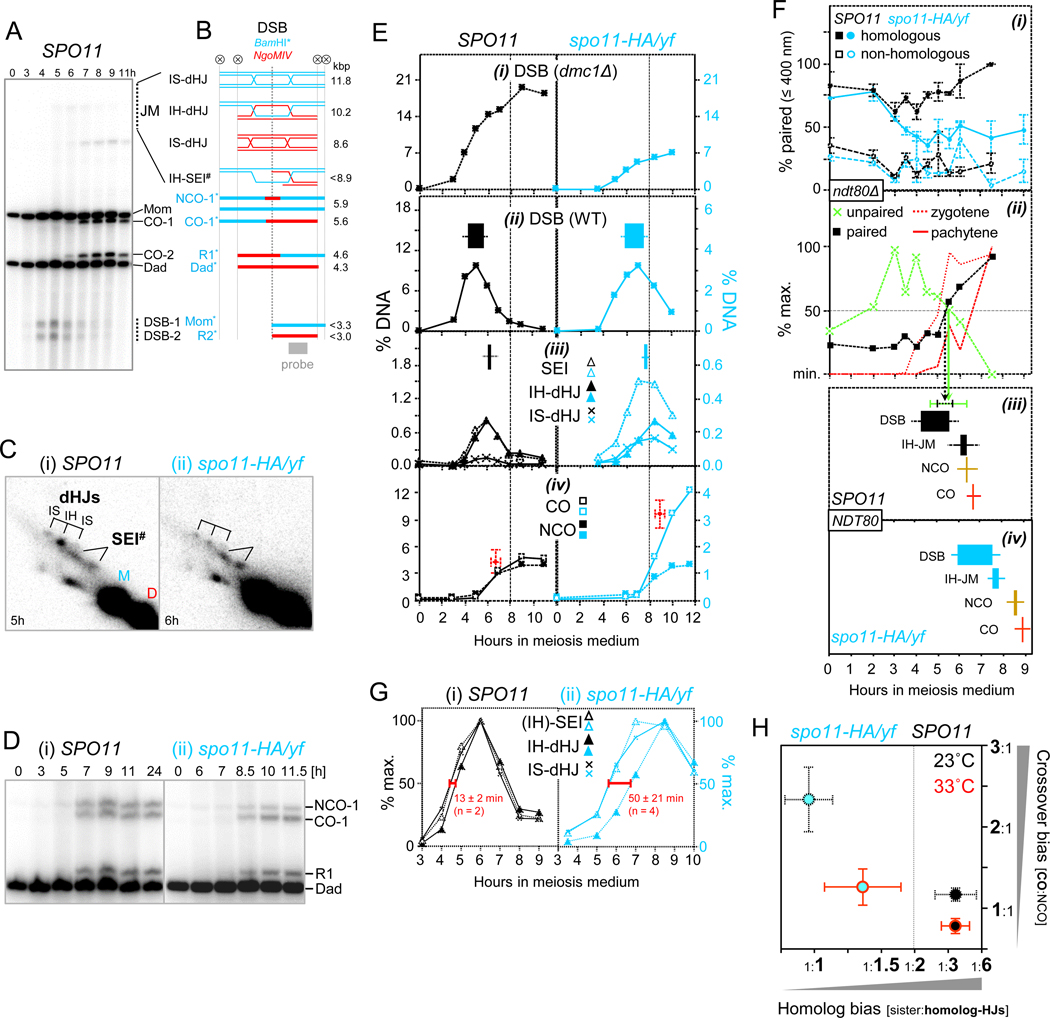
Fig. 1. Weak Homolog Bias prior to Pairing Completion is Compensated for by Enhanced Crossover-Specific Strand Exchange (23˚C).
(A) DSBs and crossovers at the HIS4::LEU2 hotspot. 1D gel Southern blot analysis of XhoI-digested genomic DNA.
(B) Schematic depiction of recombination intermediates and products at HIS4::LEU2. Circled X indicates XhoI sites. #, only one of four IH-SEI intermediates is shown (Hunter and Kleckner, 2001); *, double digestion with XhoI and BamHI detects subsets of crossovers (CO-1) and non-crossovers (NCO-1) as well as a subset of CO-2 (R1) and a mixture of COs and NCOs (R2; see Fig. S1C).
(C) 2D gel Southern blot analyses of JMs in (i) SPO11 and (ii) spo11-HA/yf.
(D) 1D gel Southern blot analysis of COs and NCOs in (i) SPO11 and (ii) spo11-HA/yf.
(E) Quantitative analysis in SPO11 (left) and spo11-HA/yf (right) of (i) DSBs in dmc1Δ; and (ii) DSBs, (iii) SEIs, IH-dHJs and IS-dHJs and (iv) COs and NCOs in WT. Filled rectangles indicate 50% entry and 50% exit times, perpendicular error bars indicate SDs (see Table S1). In (iii), the rectangle indicates the combined lifespan of IH-SEIs and IH-dHJs. In (iv), the red dot indicates average maximum levels and 50% entry times of COs, error bars indicate SDs. Vertical dashed lines mark t = 8 h.
(F) Homolog pairing and recombination at normal (SPO11) or reduced DSB abundance (spo11-HA/yf). (i) Nuclei exhibiting two GFP dot(s) within ≤ 400 nm or one GFP dot in SPO11 ndt80Δ and spo11-HA/yf ndt80Δ. GFP-tetR marks homologous positions at LEU2 (n = 2) or non-homologous positions on different chromosomes (LEU2 and LYS2; n = 2). See Fig. S2A. (ii) Unpaired (green): Average frequencies of SPO11 ndt80Δ nuclei with homologous GFP foci separated by > 400 nm. Paired (black): Single GFP focus or focus pairs within ≤ 400 nm arising from homologous GFP tag positions adjusted for non-homologous GFP pairs (n = 3). Dotted and continuous red lines indicate zygotene and pachytene entry, respectively (n = 2). Also see Fig. S3B. Vertical arrows indicate average times of 50% exit from the “unpaired” stage (green) and 50% entry into the “paired” stage (black). Error bars (SD) are shown at the end of arrows in (iii).<\p>Recombination kinetics in (iii) SPO11 NDT80 and (iv) spo11-HA/yf NDT80. Filled rectangles are from Fig. 1E.
(G) Kinetic analysis of IH-SEI, IH-dHJ and IS-dHJ in (i) SPO11 and (ii) spo11-HA/yf. Red horizontal bars indicate the time interval between IH-SEIs and IH-dHJs.
(H) Inverse correlation between homolog bias and CO bias. IS:IH ratios are from the work presented here (23˚C) and from Joshi et al. (2015) (33˚C). IS:IH and CO:NCO ratios at the time of their respective maximum levels were determined in the same DNA samples. Error bars, SD (see Fig. S1C-ii, Table S1 for details).
To compare the kinetics of cumulative processes (e.g., COs and NCOs as well as pairing in ndt80Δ) with transient recombination stages, cumulative analysis was used to determine the times when 50% of intermediates had entered into a stage or had exited from it one life span later (see Data S1; Methods; Padmore et al., 1991). Meiosis was investigated at both normal and decreased DSB levels, in WT (SPO11) and spo11-HA/yf hypomorphic backgrounds (Diaz et al., 2002). In spo11 hypomorphs, short-lived features of the earliest cohort of “scout” recombination events, including low nucleus-wide DSB abundance and loose pairing, are temporally extended compared to WT facilitating analysis (below; Joshi et al., 2015).
DSB abundance and pairing. –
To investigate the temporal and functional relationship between DSB appearance and homolog pairing, we first determined maximum DSB levels in a dmc1Δ background. In SPO11, DSBs reach ~22% of hybridization signal, whereas in spo11-HA/yf, DSBs accumulate to ~1/3 of those levels (Fig. 1E; Fig. S1A). Timing of DSB formation relative to homolog pairing was determined using parallel analyses or analysis under identical conditions (Fig. 1F).
In SPO11, homologous GFP foci are frequently associated at t = 0 h, consistent with somatic homolog pairing, followed by unpairing during pre-meiotic S-phase as indicated by increased frequencies of inter-focus distances > 400 nm (t = 0 h to 3 h; Fig. S2A; Weiner and Kleckner, 1994). Pairing is progressively restored, as indicated by appearance of focus pairs within ≤ 400 nm, followed by single foci in ≥ 95% of nuclei by t = 7 h (i.e. the time when prophase I normally is completed). In contrast, non-homologous loci remain dispersed at all times (Fig. 1F-i). In hypomorphic spo11, pairing is reduced, as indicated by persistence at low levels of homologous GFP associations, even though pairing exceeds fortuitous colocalization (Fig. 1F-i). Thus, homolog pairing in spo11-HA/yf is unstable, either because it has been established only in a subset of cells, is frequently disrupted (“homolog kissing”; Kleckner and Weiner, 1993), or a combination thereof. Hereafter, this status is referred to as loose pairing.
Pairing kinetics in SPO11 were analyzed by two complementary approaches (see Methods): First, association frequencies between non-homologous loci at ≤ 400 nm were subtracted from those between homologous loci. Second, the timing of pairing was inferred from the disappearance kinetics of unpaired homologous GFP foci thereby avoiding locus-dependent effects of non-homologous GFP focus pairs. Both approaches yielded similar results. Post-replicative homolog pairing normalized for fortuitous associations reaches 50% of maximum levels by t = 5.3 (± 0.28) h, whereas nuclei containing unpaired homologous GFP dots have declined to 50% of maximum levels by t = 5.4 (± 0.88) h (Fig. 1F-ii).
To determine the temporal relationship between homolog pairing and SC central region assembly, appearance of linear Zip1 staining was monitored in the same cells. Zip1 lines along a minority or a majority of the 16 yeast chromosomes indicate entry into the zygotene or pachytene stages, respectively (Fig. S3). Zygonema and pachynema reached half-maximum levels at 5.0 (± 0.25) h and 6.6 (± 0.05) h, respectively. Thus, homolog pairing in ndt80Δ is temporally associated with synapsis onset (zygotene entry), as reported earlier for NDT80, whereas synapsis completion (pachytene entry) occurs later (Fig. 1F-ii; Weiner and Kleckner, 1994).
Recombination analysis provided the following insights: In WT (SPO11), DSBs become detectable as fuzzy bands at t ~ 3h, indicating rapid resection and generation of single-stranded 3’ overhangs competent for strand exchange (Fig. 1A). DSBs peak at t ~ 5 h and have disappeared by t ~ 8 h (Fig. 1A, E-ii; Fig. S1B). In spo11-HA/yf compared to SPO11, DSBs peak with a ~1.5 h delay, likely due to impaired DSB formation by catalytically deficient Spo11 (Fig. 1E-ii). In spo11-HA/yf compared to SPO11, DSB entry and subsequent recombination events are delayed by ~1.7 h (Fig. 1E-ii to iv). DSB lifespans (indicated by filled rectangles) are somewhat longer in spo11-HA/yf, possibly due to a delay in DSB first- and/ or second-end strand exchange (Fig. 1F-iii,iv).
Temporal comparison of recombination and pairing reveals that in SPO11, invasion-competent DSBs persist for up to half of their lifespan along homologs that have not completed pairing (Fig. 1F-iii). Notably, cumulative analysis provides average entry and exit times, but does not take into account that DSBs at a given locus occur earlier or later in different cells (Joshi et al., 2015). Accordingly, earlier and later DSBs may be present along unpaired and paired homologs, respectively. Consistent with the presence along unpaired homologs of invasion-competent DSBs, Dmc1 abundantly localizes to chromatin while centromeres are unpaired (Obeso and Dawson, 2010).
DSB first-end and second-end strand exchange. –
Our analysis further reveals that loose pairing is associated with a shift in partner bias during strand exchange. JMs in SPO11 appear and disappear with expected levels and timing, yet IS-dHJs are more abundant earlier, whereas IH-dHJs predominate later (Fig. 1E-iii; Joshi et al., 2015).
Accordingly, IS:IH ratios are ~1:1 when dHJs first appear and ~1:3 when dHJs reach peak levels, indicating progression from sister to homolog bias (Fig. S1B; IS:IH ratios > 1:2 indicate sister bias given availability of one sister chromatid and two homologous chromatids).
In spo11-HA/yf, dHJs are reduced to ~1/3 of WT, and IH-JMs appear with a 1.4 h delay, likely due to reduced and delayed DSB formation (Fig. 1E-iii; above). Joint molecules in spo11-HA/yf compared to SPO11 exhibit three additional features. First, homolog bias is weakened at all times, as indicated by increased IS:IH ratios (Fig. S1B). Increased IS-dHJ abundance in spo11-HA/yf is due to their increased formation rather than delayed turnover (Joshi et al., 2015). Second, IH-SEI steady state levels are 2–3 fold higher compared to IH-dHJs in spo11-HA/yf, whereas in SPO11 these intermediates exhibit similar levels (Fig. 1C; 1E-iii). Third, the temporal separation between IH-SEIs and IH-dHJs is increased ~3.5-fold in spo11-HA/yf compared to SPO11, even though IS-dHJs are not delayed (Fig. 1G).
One explanation for these results is that in spo11-HA/yf, crossover-specific DSB first-end strand exchange (i.e. IH-SEI formation) occurs at increased frequencies, an inference supported by a corresponding shift from NCOs to COs (below). IH-SEIs may also accumulate transiently due to delayed capture of the DSB second end, possibly due to dependence of this transition on pairing (Discussion). We conclude that DSB strand exchange is initiated while chromosomes are paired loosely. Loose pairing is further associated with frequent intersister strand exchange and enhanced formation of crossover-specific IH-SEIs.
Crossovers and non-crossovers. –
Further analysis revealed that increased recombination with the sister chromatid is compensated for by high CO levels at the expense of NCOs among the remaining interhomolog recombination events. In WT (SPO11), COs and NCOs accumulate to similar maximum levels. DSB reduction in spo11-HA/yf disproportionally affects NCOs (Fig. 1D, E-iv). Consequently, COs compared to NCOs are more than two-fold overrepresented among interhomolog recombination products, as indicated by CO-to-NCO ratios of ~2.3 in spo11-HA/yf compared to ~1.2 in SPO11 (Fig. 1H). The same DNA samples also exhibit weaker homolog bias as suggested by IS:IH ratios of ~ 1.0 in spo11-HA/yf compared to ~ 0.30 in SPO11. Thus, homolog bias and CO bias are inversely correlated (Fig. 1H). Crossover homeostasis was previously observed at another locus, yet appeared to be absent at HIS4LEU2 (Martini et al., 2006). The discrepancy between our results and the earlier analysis could be due to differences in incubation conditions as also suggested by CO:NCO ratios in SPO11 and spo11-HA/yf closer to parity at 33ºC compared to 23ºC (Fig. 1H).
Together, these results indicate that lack of homolog bias associated with loose pairing is compensated for by enhanced crossover-specific strand exchange. Early DSBs that arise along unpaired chromosomes tend to engage in first-end strand exchange with the readily available sister chromatid followed by rapid progression to IS-dHJs, as suggested by absence of detectable IS-SEIs in spo11-HA/yf (Fig. S1D). Less frequently, strand exchange occurs between homologs, possibly during transient homolog association.
Decreased interhomolog recombination due to sister bias is compensated for by a bias among remaining interhomolog events towards COs over NCOs. Importantly, crossover homeostasis appears to respond to increased intersister exchange and/or a lack of stable homolog pairing, rather than to decreased DSBs (Discussion).
Mph1FANCM dissociates DSB first-end strand exchange in vivo.
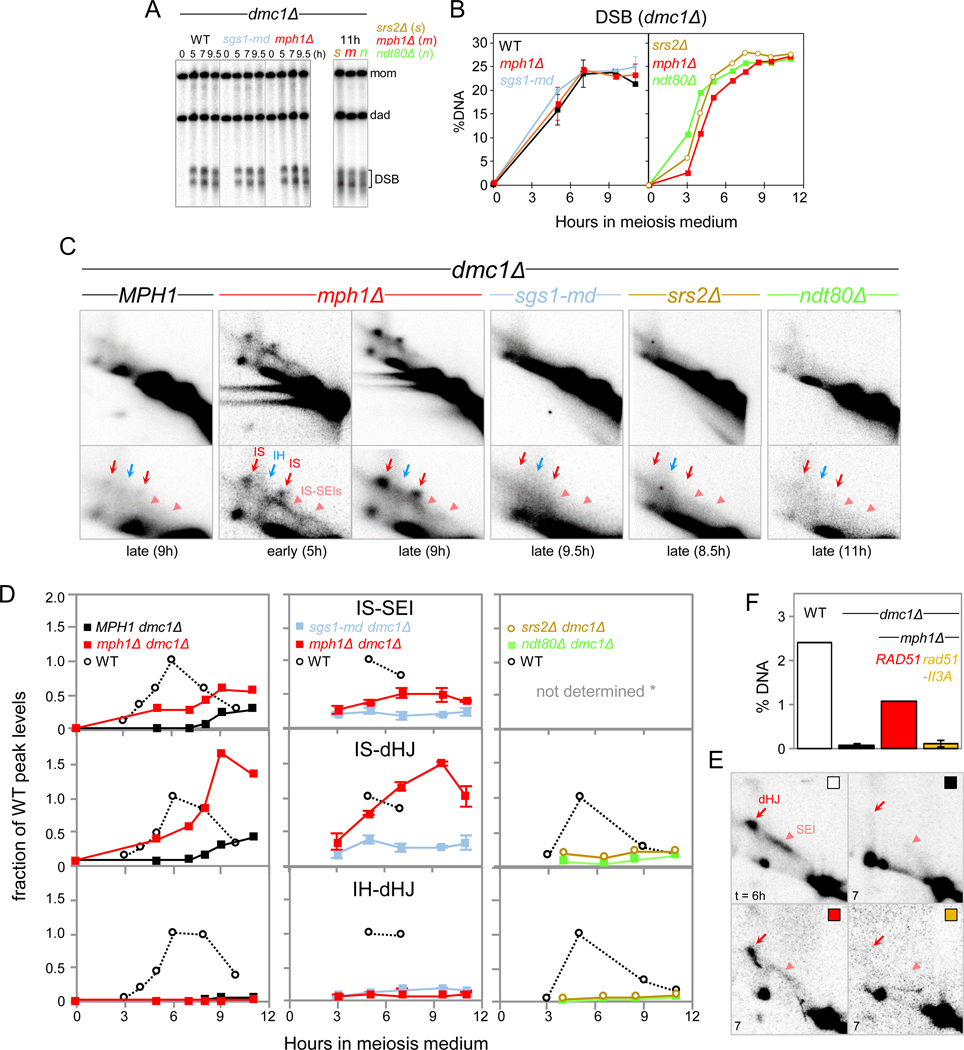
DNA helicases Srs2UvrD, Mph1FANCM, and Sgs1BLM are attractive candidates for controlling DSB strand exchange as they can dissociate stalled three-armed intermediates (Dupaigne et al., 2008; Prakash et al., 2009; Cejka and Kowalczykowski, 2010; Ira et al., 2003; Mazón and Symington, 2013; Piazza et al., 2019). To examine their role in DSB strand exchange, SRS2 and MPH1 were deleted whereas SGS1 was expressed under control of the meiotically inactive CLB2 promoter (sgs1-md; Oh et al., 2008). DNA helicase mutants were initially examined in a dmc1Δ background where DSB first end strand exchange intermediates are undetectable (Hunter and Kleckner, 2001).
In double mutants, DSBs accumulate to similar levels as in a dmc1Δ single mutant (Fig. 2A, B). In dmc1Δ, JMs remain undetectable, yet at late time points, intersister dHJs appear at ~10% of WT maximum levels, indicating low levels of strand exchange (Fig. 2C,D). When compared to srs2Δ and sgs1-md, mph1Δ distinctly affects JM formation: In mph1Δ dmc1Δ, JMs between sister chromatids are detected at levels comparable to WT peak levels (Fig. 2C,D). Specifically, at t = 5 h, the crescent-shaped IS-SEI signal is detected together with low levels of IS-dHJs. Later (t = 9 h), the IS-dHJ signal is more prominent compared to IS-SEIs, consistent with progression of IS-SEIs to IS-dHJs. JMs have turned over by t = 24 h (Fig. S4B). Finally, CO and NCO products are essentially absent in both dmc1Δ and mph1Δ dmc1Δ (Fig. S4C).
Fig. 2. DNA Helicase Mph1 Dissociates DSB First End Strand Exchange Intermediates.
(A) 1D gel Southern blot analyses of DSBs in a dmc1Δ background also carrying mutations sgs1-md or mph1Δ (left panel), or srs2Δ (s), mph1Δ (m), or ndt80Δ (n) (right panel).
(B) Quantitation of DSBs in Fig. 2A.
(C) Excerpts of 2D gel analyses in dmc1Δ background. Lower panels show enlarged excerpts from upper panels. Also see Fig. S4A.
(D) Quantitation of SEIs, IS-dHJs and IH-dHJs. Intermediates are expressed as fractions of maximum levels in parallel WT cultures. Asterisk, for srs2Δ and ndt80Δ, SEIs were not quantitated due to weak signal intensities.
(E) Excerpts of 2D gel analyses of WT, dmc1Δ, dmc1Δ mph1Δ, and dmc1Δ mph1Δ rad51-II3A following PvuII digestion. Parents carry identical PvuII sites separated by 5.5 kb at HIS4LEU2, and IS- and IH-dHJs comigrate. Also see Fig. S4D.
(F) Quantitation of dHJs at t = 6 h (WT) or t =7 h (other genotypes) in dmc1Δ mph1Δ in presence (RAD51) or absence of Rad51 strand exchange activity (rad51-II3A). Error bars indicate range (n = 2).
Appearance of JMs in mph1Δ dmc1Δ may indicate that Mph1 plays roles (i) in dissociating DSB first-end strand exchange intermediates and/or (ii) in resolving IS-dHJs. We distinguished between these possibilities by testing whether JMs are detectable in ndt80Δ dmc1Δ. The Ndt80 transcription factor mediates activation of meiotic JM resolution pathways (Zakharyevich et al., 2012). Although NDT80 is not induced in dmc1Δ (Hepworth et al., 1998), JM resolution in dmc1Δ could be mediated by low Ndt80 levels. Yet, no JMs are detectable in ndt80Δ dmc1Δ (Fig 2A-D). Thus, JM appearance in mph1Δ dmc1Δ is not due to a role of MPH1 in resolving JMs, instead indicating a role in dissociating D-loop intermediates.
We conclude that in absence of DMC1, DSB first-end strand exchange occurs at substantial frequencies, yet resulting IS-SEIs are normally dissociated by Mph1. Mph1 dissociates SEIs formed by Rad51, as Rad51’s strand exchange activity is required for their formation (Fig. 2E,F). Appearance in ordered succession of IS-SEIs and IS-dHJs suggests that Mph1 acts on DSB first-end strand exchange intermediates (i.e., IS-SEIs), whereas IS-dHJs appear as a secondary consequence. It is unlikely that Mph1 also interferes with DSB second-end strand exchange since in that case, IS-SEIs should be undetectable in mph1Δ dmc1Δ due to accelerated progression to IS-dHJs. Preferential strand exchange with the sister chromatid in mph1Δ dmc1Δ could be due to defective homolog pairing in dmc1Δ (Brar et al., 2009). It is unlikely that Mph1 specifically acts upon IS-JMs, as Mph1 can also dissociate IH-JMs (below). Finally, persistence in mph1Δ dmc1Δ of most DSBs indicates that Mph1 dissociates strand exchange for a relatively small DSB subset, consistent with additional mechanisms for suppression of strand exchange in dmc1Δ (Fig. 2A-i; Niu et al., 2009; Callender et al., 2016).
MPH1 minimizes intersister recombination.
Following identification of functions in dmc1Δ we wanted to determine Mph1’s role during WT meiosis. In mph1Δ DMC1, DSBs appear with essentially WT kinetics (Fig. S5A, B).
Decreased DSB steady-state levels in mph1Δ are likely due to faster turnover rather than an overall reduction since DSBs accumulate normally in mph1Δ dmc1Δ (Fig. 2B).
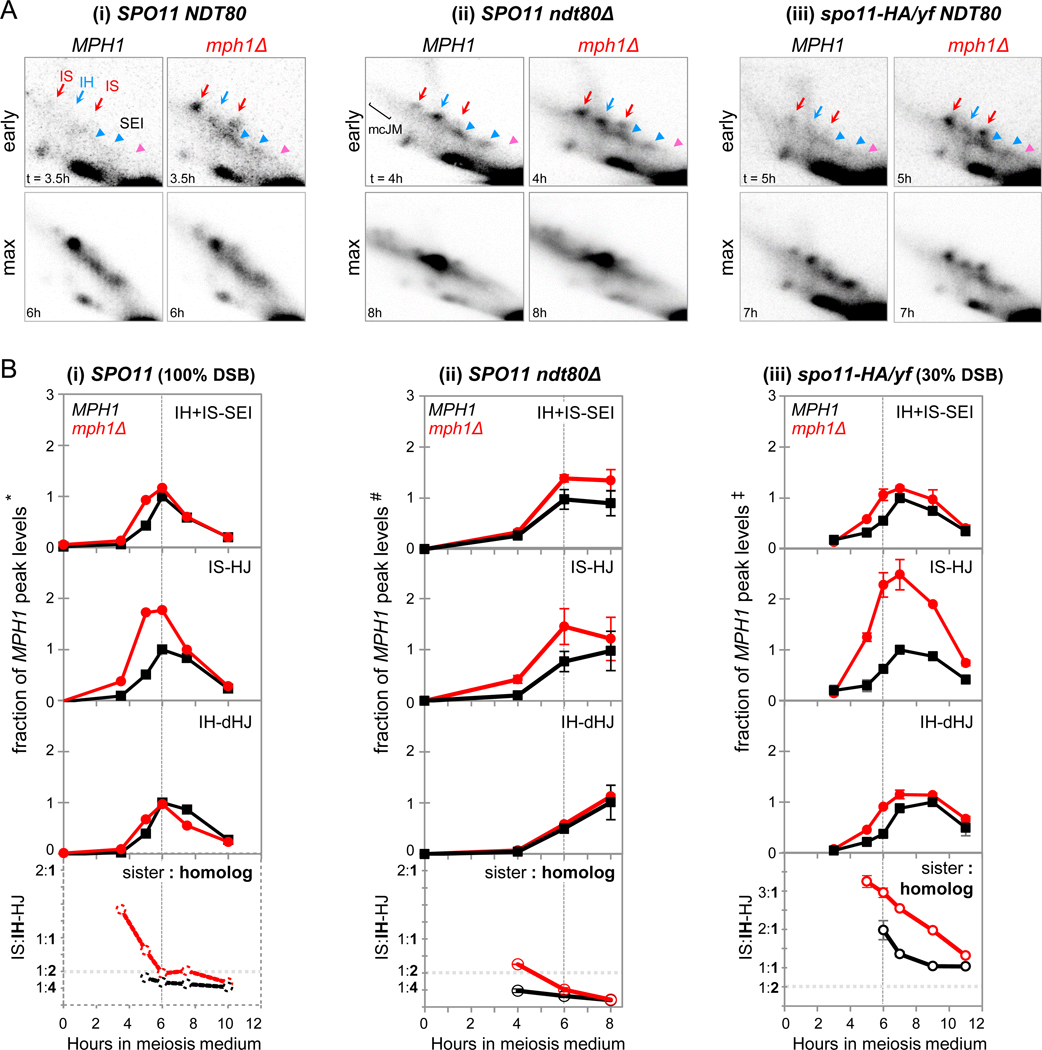
Two prominent effects on JM kinetics and levels are apparent. First, JMs appear ~1 h earlier in mph1Δ compared to WT (Fig. 3A-i, B-i). Earlier JM appearance is not due to an overall increase which should increase JMs uniformly at all times. Instead, JMs in mph1Δ are disproportionally increased at the earliest time point when intermediates are detectable in both genotypes, 2.6 (± 0.53)-fold above WT, compared to an increase of only 1.3 (± 0.08)-fold at JM peak times (n = 4; Fig. S5B). Second, precocious JM appearance predominantly involves strand exchange between sister chromatids, whereas IH-dHJs appear with ~ normal timing. Thus, IS-dHJs and IH-dHJs appear in rapid succession in WT, as indicated by their separation by only 14 (± 10) min (n = 5; see also Fig. 1G). This time interval is 4-fold increased in mph1Δ, to 53 (± 19) min (n = 3).
Fig. 3. Mph1 Delays Stable Strand Invasion to Minimize Exchange between Sister Chromatids.
(A) Excerpts from 2D gel Southern blot analyses in MPH1 and mph1Δ at times of earliest JM detection (“early”) and times of maximum JM levels (“max”). (i) NDT80 SPO11, (ii) ndt80Δ SPO11, and (iii) NDT80 spo11-HA/yf. For complete 2D gels, see Fig. S5
(B) Top: Quantitative analysis of JMs in MPH1 and mph1Δ in the indicated strain backgrounds. JM levels are expressed as fractions of peak levels of the respective species in the parallel MPH1 culture. Bottom: Ratios of IS-dHJs to IH-dHJs. Dotted vertical lines indicate the time of maximum JMs in WT.
(i) NDT80 SPO11. *, JM peak levels in MPH1 are 1.6 % (IH + IS-SEIs), 0.60 % (IS-dHJs) and 0.99 % (IH-dHJs).
(ii) ndt80Δ SPO11. #, JM average maximum levels at t = 8 h in MPH1 are 1.3 ± 0.26 % (IH + IS-SEIs), 0.49 ± 0.17 % (IS-dHJs) and 4.9 ± 1.2 % (IH-dHJs), n = 3; error bars, SD.
(iii) NDT80 spo11-HA/yf. ‡, JM average peak levels in MPH1 are 0.54 ± 0.01 % (IH + IS-SEIs), 0.23 ± 0.05 % (IS-dHJs) and 0.19 ± 0.04 % (IH-dHJs). n = 2; error bars indicate ranges.
Thus, in mph1Δ versus WT, IS-dHJs reach half-maximum levels at t = 3.8 (± 0.16) h and 4.7 (± 0.57) h, whereas IH-dHJs appear with similar timing in both genotypes, at 4.7 (± 0.15) h and 4.9 (± 0.42) h. As a consequence of earlier appearance and higher abundance of IS-dHJs, ratios of IS:IH-dHJs in mph1Δ are increased at all times, albeit with more pronounced effects at earlier time points (Fig. 3B-i; Fig. S5B, bottom panels). Thus, at earlier time points in mph1Δ, sister chromatids are 4-fold preferred over homologs as recombination partners.
Increased IS-dHJ steady state levels could indicate their formation at higher frequencies in mph1Δ, or an MPH1 requirement for IS-dHJ, but not IH-dHJ resolution. Only increased formation of IS-dHJs should be detectable in an ndt80Δ background. Indeed, IS-dHJs are increased in mph1Δ ndt80Δ, again with more pronounced effects at earlier time points (Fig. 3A-ii, B-ii). Thus, MPH1 interferes with strand exchange not only in dmc1Δ, but also in WT, thereby minimizing IS-dHJ formation.
During WT meiosis, “scout” DSBs are processed under conditions of low nucleus-wide DSB abundance and incomplete homolog pairing (Fig. 1; Joshi et al., 2015). To test whether MPH1’s role in delaying JM formation and reducing IS exchange during early meiosis is related to low DSB abundance and correlated effects, we examined MPH1’s role in spo11 hypomorphic recombination. When DSB abundance is permanently decreased, JMs in mph1Δ again appear earlier compared to MPH1 (Fig. 3A-iii; B-iii).
Moreover, IS-dHJs are substantially increased in mph1Δ, whereas SEIs and IH-dHJs appear at similar levels as in MPH1. Sister-to-homolog ratios of ~3:1 in mph1Δ spo11-HA/yf indicate a 6-fold preference for exchange with the sister chromatid over the homolog (Fig. 3B-iii, bottom panel). Exchange with the sister chromatid is preferred in mph1Δ spo11-HA/yf throughout meiosis and not only at early time points. Finally, occurrence of substantial IH-dHJ levels, despite increased intersister exchange, may indicate preferential processing of interhomolog events via CO-specific strand exchange (IH-SEIs and IH-dHJs) instead of via (undetectable) NCO-specific intermediates (below).
We conclude that MPH1 mediates dissociation of DSB first-end strand exchange during early WT meiosis, when DSB abundance is low and pairing is incomplete. As a consequence, JMs appear earlier in mph1Δ than in WT. At the time of JM appearance in mph1Δ, homolog pairing is close to its minimum levels in the SPO11 background (t = 3.5 h; Fig. 1F). These findings suggest that Mph1 normally dissociates precocious strand exchange between sister chromatids prior to pairing completion thereby making available recombination intermediates for interhomolog exchange at later times.
MPH1 contributes to crossover and non-crossover formation.
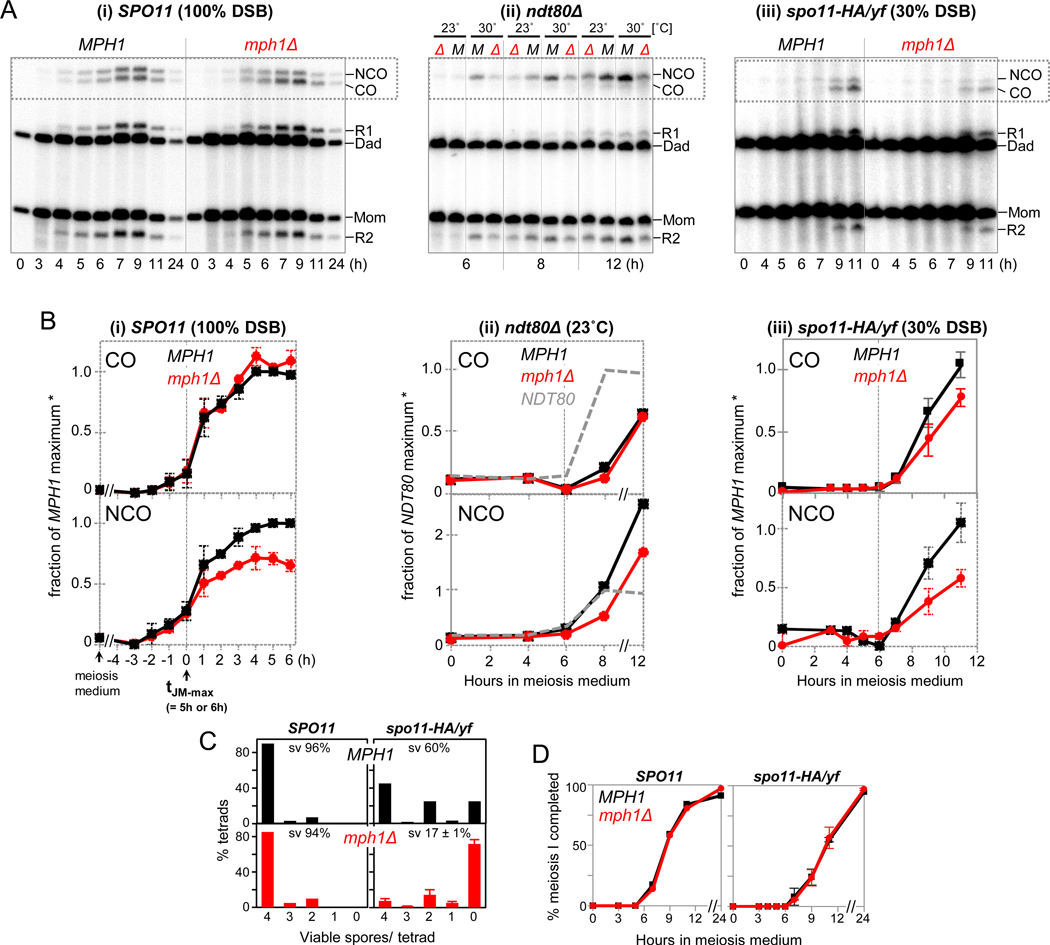
To examine whether mph1Δ also affects product formation, we monitored COs and NCOs in the same cultures analyzed for DSBs and/or JMs. Comparison of multiple experiments was facilitated by setting as t = 0 h the time of JM peak levels (Fig. 4A,B). In mph1Δ, COs are unchanged or marginally increased (Fig. 4A-i; B-i; Fig. S6A). By contrast, NCOs are decreased to ~70% of WT levels. COs and NCOs were also examined in other backgrounds where mph1Δ increases intersister exchange. While absence of MPH1 in ndt80Δ has no major effects on COs, NCOs are decreased to ~70% of WT levels (Fig. 4B-ii). Thus, MPH1 is required for NCO formation regardless whether Ndt80 is expressed, distinguishing it from SGS1 (below; De Muyt et al., 2012).
Fig. 4. MPH1-Mediated Interhomolog Recombination Contributes to Crossovers and Non-Crossovers.
(A) 1D gel Southern blot analyses of COs and NCOs in MPH1 and mph1Δ in the following backgrounds: (i) SPO11 at 23˚C, (ii) ndt80Δ at 23˚C and 30˚C, and (iii) spo11-HA/yf at 23˚C.
(B) Quantitative analyses of COs and NCOs. (i) In SPO11, multiple pairs of MPH1 and mph1Δ cultures are shown. Timing of all species is shown relative to the JM peak time (dotted line; see Fig. S5B). Signals are expressed as fractions of maximum levels in a parallel WT culture. Average maximum levels in MPH1 are 4.2 ± 1.5% (COs) and 3.4 ± 1.4% (NCOs). n = 5; error bars, SD.
(ii) In ndt80Δ, CO and NCO levels are expressed as fractions of maximum levels in a parallel WT culture (NDT80), i.e. 6.5% (COs) and 5.6% (NCOs). Also see Fig. S6B.
(iii) In spo11-HA/yf, COs and NCOs are expressed as fractions of the average maximum levels in spo11-HA/yf MPH1 [2.8 ± 0.2 % (CO) and 1.1 ± 0.1 % (NCO), respectively]. n = 2; error bars indicate range.
(C) Spore viabilities in MPH1 and mph1Δ at normal (left) and decreased DSB levels (right). Numbers of spores analyzed are n = 400 (MPH1 SPO11); n = 240 (mph1ΔSPO11); n = 240 (MPH1 spo11-HA/yf); n = 480 (mph1Δ spo11-HA/yf, from N = 2 meiotic cultures).
(D) Meiotic progression in MPH1 and mph1Δ in SPO11 and spo11-HA/yf.
In spo11-HA/yf, absence of MPH1 results in a decrease of both COs and NCOs, to ~75% and 50% of MPH1 levels, respectively (Fig. 4A-iii; B-iii). Thus, increased recombination with the sister chromatid in mph1Δ spo11-HA/yf is associated with a decrease of both CO and NCO interhomolog products, with more substantial effects on NCOs. Non-crossovers in spo11-HA/yf are already reduced 3-fold due to effects of crossover homeostasis (Fig. 1H). When combined with mph1Δ, NCOs are ≥ 6-fold reduced compared to wild type, to ~0.5% of hybridization signal, close to background levels (compare Fig. 4A-iii to Fig. 4A-i).
Decreased COs in mph1Δ spo11-HA/yf are associated with a substantial reduction in spore viability, whereas mph1Δ spore viability is normal in the SPO11 background (Fig. 4C). Viability patterns in mph1Δ spo11-HA/yf are characterized by an increase of zero viable spores at the expense of 4 and 2 viable spores per tetrad, consistent with viability patterns caused by homolog nondisjunction, e.g. due to CO reduction below a critical threshold (Nishant et al., 2010). Furthermore, homolog pairing in mph1Δ SPO11 is delayed and decreased, even though it eventually reaches normal levels (Fig. S6C; Fig. S2B,C). Pairing defects in mph1Δ are readily explained if an appropriate level of interhomolog strand exchange and/or NCO formation is required for normal homolog pairing. The opposite relationship is less likely as mph1Δ affects recombination even in maximally pairing-defective dmc1Δ (Fig. 2; Brar et al., 2009). Finally, normal meiotic divisions in mph1Δ exclude premature meiotic progression as a cause for reduced spore viability (Fig. 4D).
One explanation for these findings is Mph1 as its primary function dissociates precocious strand exchange, thereby minimizing intersister recombination. As secondary consequence of increased intersister exchange, remnant interhomolog events are redistributed, resulting in a relative increase of COs at the expense of NCOs. In mph1Δ at normal DSB abundance, high CO levels are maintained by crossover homeostasis, whereas in mph1Δ spo11-HA/yf, NCO levels are insufficient to fully compensate for the further loss of interhomolog recombination. We infer that MPH1 ensures normal levels of all interhomolog recombination events, both COs and NCOs. Alternatively, MPH1 may perform four separate functions i.e., delaying strand exchange, mediating homolog bias, enhancing NCO formation under all conditions, and enhancing CO formation specifically at low DSB abundance. This scenario is less attractive as it fails to take into account effects of crossover homeostasis (Fig. 1H).
ZIP1 antagonizes Mph1-mediated JM dissociation.
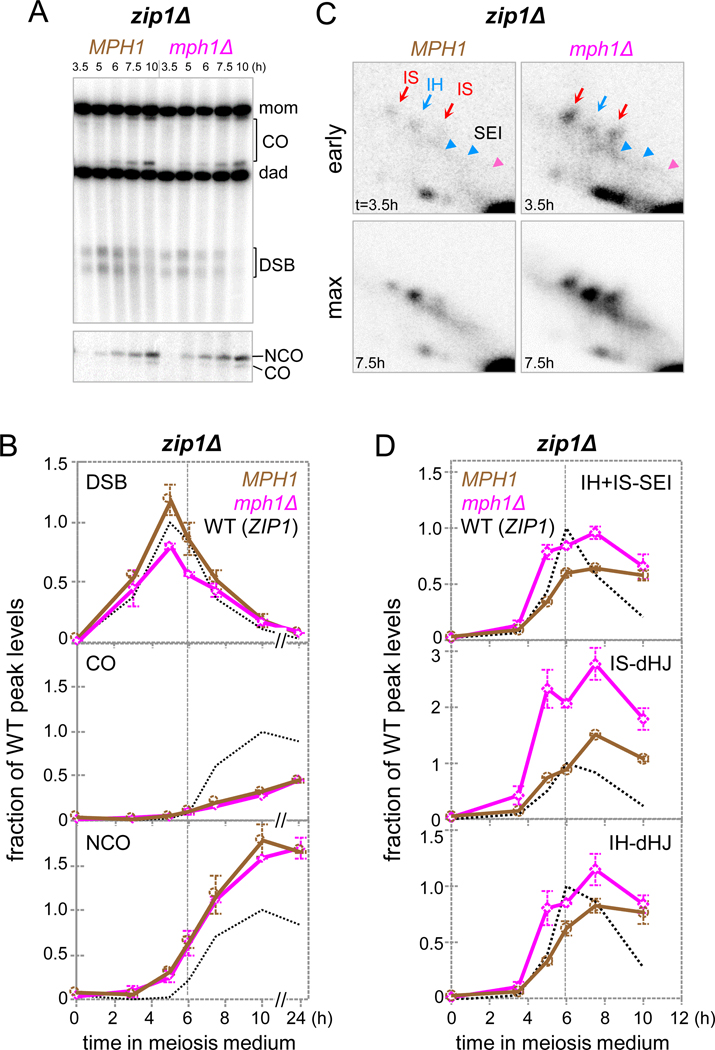
To explain how Mph1 specifically dissociates early D-loops between sister chromatids, but not later ones between homologs, we hypothesized that Mph1’s activity could be negatively regulated by the onset of synapsis. To explore this possibility, mph1Δ effects on recombination were examined in absence of Zip1. In zip1Δ, homologs become coaligned, but fail to undergo synapsis (Sym et al., 1993). Interhomolog strand exchange is delayed, yet eventually reaches substantial levels, with a decrease in COs and an increase in NCOs (Fig. 5A-D; Börner et al., 2004).
Fig. 5. ZIP1 Antagonizes Mph1-Mediated Dissociation of Late JM Intermediates.
(A) 1D gel Southern blot analyses of DSBs and COs (top) as well as COs and NCOs (bottom) in WT and mph1Δ in a zip1Δ background.
(B) Quantitative analysis of blots in Fig. 5A. Duplicate cultures were compared to a parallel WT culture (MPH1 ZIP1).
(C) Excerpts from 2D gel analyses in MPH1 and mph1Δ in a zip1Δ background at times of earliest JM detection (“early”) and times of maximum JM levels (“max”).
(D) Quantitative analysis of JMs in MPH1 and mph1Δ in a zip1Δ background. Dotted vertical lines indicate time of maximum JMs in WT. *, JM maximum levels in MPH1 are0.5 % (IH + IS-SEIs), 0.23 % (IS-dHJs) and 0.68 % (IH-dHJs). n = 2; error bars indicate range.
Three mph1Δ-induced effects in zip1Δ are qualitatively similar to those in a WT background, indicating precocious strand exchange with the sister chromatid. Accordingly, DSB steady-state levels are decreased, JMs appear early, and IS-dHJs are ~2-fold increased (Fig. 5A-D). Three additional mph1Δ effects are distinct for zip1Δ. First, increased JM levels are observed at all times, not just early. Second, IH-dHJs and bona fide IH-SEIs are increased (Fig. 5D). Third, at 33ºC, where zip1Δ causes a more severe strand exchange defect, both IS- and IH-dHJs are dramatically increased in mph1Δ, consistent with a pronounced MPH1 contribution in dissociating both IS and IH intermediates (Fig. S7A,B; Börner et al., 2004).
Together, these findings suggest that Mph1 exhibits no intrinsic specificity for intersister D-loops. Rather, this apparent preference can be attributed to an antagonistic role of Zip1 and/ or synapsis. In WT, earlier occurring exchanges between sister chromatids are dissociated by Mph1 because they precede Zip1 recruitment, whereas later strand exchanges between homologs are protected by Zip1 or a ZIP1-mediated process.
Accordingly, both IS- and IH-JMs are similarly increased when Mph1 is absent in spo11-HA/yf, a strain background that also exhibits impaired synapsis (Fig. 3B-iii; Henderson and Keeney, 2004). Mechanisms of Zip1 antagonism may include ZIP1-mediated displacement of Mph1 from chromosomes and/ or protection of JM intermediates.
Mph1FANCM controls homolog bias independent of Mek1.
The mammalian Mph1 ortholog FANCM is among the first proteins recruited to stalled replication forks (Huang et al., 2010). Analogous to FANCM-induced activation of ATM/ATR (Huang et al., 2010), Mph1 could ensure homolog bias by activating checkpoint kinases Tel1ATM /Mec1ATR and effector kinase Mek1, thereby e.g., stabilizing Rad51-antagonist Hed1 (Callender et al., 2016). As a readout of Mek1 activity, we examined Hed1 phosphorylation in the spo11-HA/yf background where mph1Δ dramatically affects homolog bias. No mph1Δ effects on Mek1 activation were detectable. Thus, Mph1 controls homolog bias by mechanism(s) other than activation of this signal-transduction cascade (Fig. S7D).
Distinct roles of MPH1FANCM and SGS1BLM in meiotic recombination.
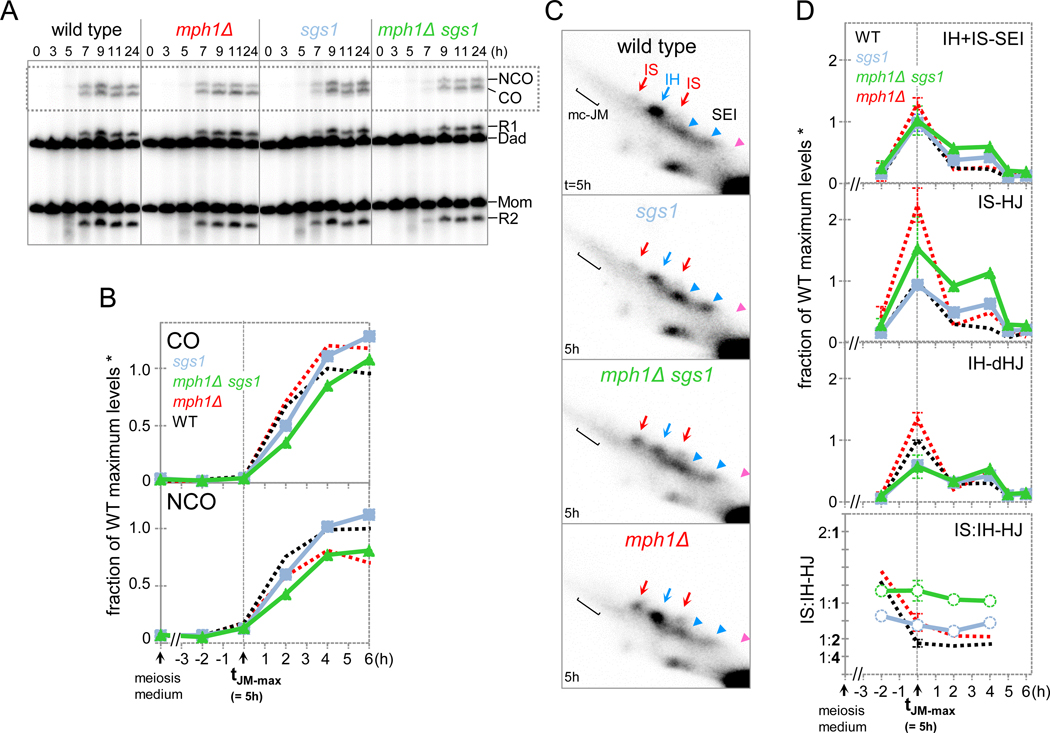
Mph1 ensures interhomolog exchange by delaying DSB strand exchange until homolog pairing has been completed (above). Conversely, Sgs1BLM maintains the fate of NCO-designated intermediates by channeling them to JM-independent NCO formation (De Muyt et al., 2012). To better understand contributions to recombination of these DNA helicases we directly compared mph1Δ and sgs1-md.
Whereas NCOs are decreased in mph1Δ, both COs and NCOs form at ~WT levels in sgs1-md (Fig. 6A,B). Distinct contributions of MPH1 and SGS1 to meiotic recombination are further supported by our finding that mph1Δ reduces NCOs irrespective of NDT80 status, whereas in sgs1, NCOs are decreased only when NDT80-dependent JM resolution is disrupted (Fig. 4B-ii; de Muyt et al., 2012). IS:IH ratios are increased in both sgs1-md and mph1Δ, yet distinct effects are responsible for these changes: Whereas IS-dHJ steady-state levels are increased in mph1Δ, they are unchanged in sgs1-md, yet IH-dHJs are decreased (Fig. 6 C,D). Effects on progression kinetics likely are responsible for these changes, as reduction of IH-dHJs in sgs1-md is not associated with reduced IH recombination products (Fig. S8A).
Fig. 6. Independent Contributions of MPH1 and SGS1 to Recombination.
(A) 1D gel Southern blot analysis of COs and NCOs in WT, mph1Δ, sgs1-md, and sgs1-md mph1Δ cultures at 23˚C.
(B) Quantitative analysis of COs and NCOs. The dotted line indicates the time of JM maximum levels (t = 5 h). Levels are expressed as fraction of WT maximum levels. Also see Fig. S8.
(C) Excerpts of 2D gel analyses at maximum JM levels at 23˚C in sgs1-md and mph1Δ single and double mutants.
(D) Top: Quantitative analysis of JMs. The dotted line indicates the JM peak time in WT. Average WT peak levels are 1.2 ± 0.58 % (IH + IS-SEIs), 0.65 ± 0.27 % (IS-dHJs) and 1.6 ± 0.80 % (IH-dHJs). Bottom: Average ratios of IS-dHJs to IH-dHJs. n = 2; error bars indicate range.
Single mutants contribute additively to recombination in mph1Δ sgs1-md. Accordingly, as in mph1Δ, IS-dHJ steady state levels are increased, and NCOs are decreased.
Moreover, only SGS1, but not MPH1 suppresses multichromatid (mc-)JM formation, as indicated by analysis at 33ºC (see legend, Fig. S8E). mc-JMs arise from aberrant strand exchange of SEIs with a partner other than the cognate DSB second-end (Oh et al., 2007). Finally, ectopic COs are increased, similar to sgs1-md (Fig. S8A,B). Together, these findings indicate additive roles of Mph1 and Sgs1 in meiotic recombination.
DISCUSSION
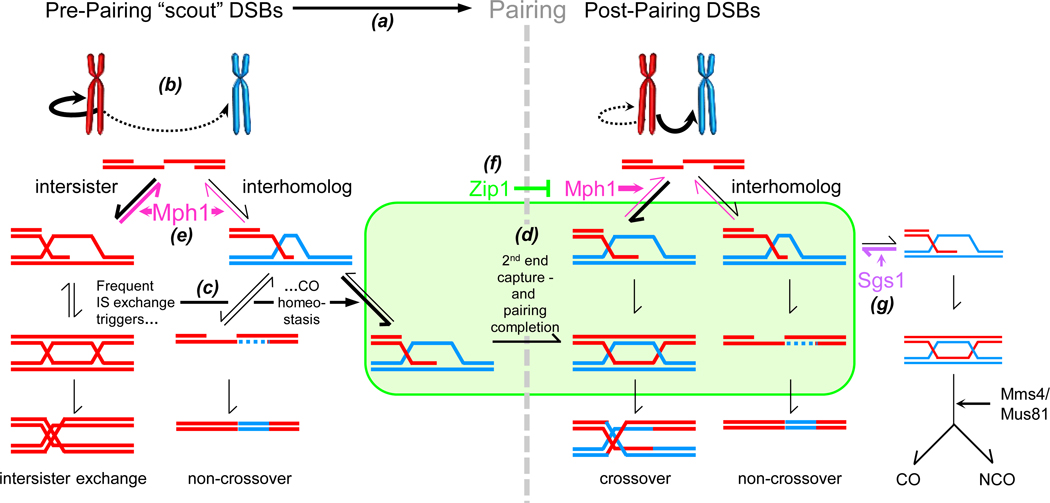
Our findings indicate that recombination outcome is determined by the temporal relationship between DSB strand exchange and homolog pairing/synapsis onset. Invasion-competent DSBs appear along chromosomes that are incompletely or unstably paired, prior to synapsis onset (Fig. 7b). Loose pairing is associated with frequent intersister exchange, but also enhanced crossover-specific strand invasion at the expense of NCOs (Fig. 7c). DNA helicase Mph1FANCM dissociates premature intersister strand exchange prior to pairing completion, enhancing interhomolog recombination (Fig. 7e). Preferential loss of NCOs in mph1Δ likely is a secondary consequence of crossover homoeostasis.
Fig. 7. Coordination between DSB Strand Exchange and Homolog Pairing during Meiosis.
(a) Recombination events along unpaired homologs mediate pairing. (b) Early DSBs are preferentially repaired with the sister chromatid. (c) Intersister exchange is compensated for by crossover-specific strand invasion. (d) Crossover-specific IH-SEIs are stabilized during loose pairing, whereas DSB second end capture depends on pairing completion. (e) DNA helicase Mph1 FANCM dissociates DSB first end strand exchange prior to pairing/synapsis. (f) Zip1, acting locally or via the SC (symbolized by rounded rectangle), protects exchanges between homologs and sister chromatids (not shown) from dissociation by Mph1 FANCM. (g) Roles of Mph1 FANCM and Sgs1BLM (see text for details).
Timing of strand exchange determines recombination outcome.
Pairing status during strand exchange affects both recombination partner choice and CO/NCO differentiation. Since DSBs form at normal levels in mph1Δ (Fig. 2B), weakened homolog bias rather than low DSB abundance appears to be responsible for a homeostatic CO increase (see also Lao et al., 2013). Finally, we show that the shift from NCOs to COs occurs no later than DSB first end-strand exchange (Fig. 1E). By implication, crossover homeostasis stabilizes IH-SEIs at the expense of SDSA intermediate(s) thereby increasing COs over NCOs (Fig. 7C).
These findings have implications for CO/NCO differentiation during WT meiosis. Early DSBs occur in presence of low DSB abundance, along unpaired or incompletely paired homologs. We infer that early occurring “scout” DSBs tend to recombine with the sister chromatid, but those recombining with the homolog preferentially give rise to COs (Fig. 1; Joshi et al., 2015). One key determinant of DSB timing is passing of the replication fork (Murakami and Keeney, 2014). By implication, early replicating regions would tend to recombine with the sister chromatid, but remnant interhomolog events may preferentially generate COs. Such effects could result in genome-wide variations in CO:NCO ratios (Mancera et al., 2008; Chen et al., 2008; de Boer et al., 2015).
Our findings also provide insights into the interplay between pairing and DSB second-end capture. During WT meiosis, IH-SEIs and IH-dHJs appear in quick succession even though these steps are functionally distinct (Hunter and Kleckner, 2001; Lao et al., 2008). In hypomorphic spo11, IH-dHJs appear with a delay despite timely and efficient formation of IH-SEIs and IS-dHJs (Fig. 1). Thus, recombination progression at the IH-SEI to IH-dHJ transition may be coordinated with pairing status. Notably, homolog pairing and synapsis onset might license CO formation. DSB second-end capture gives rise to ligated IH-dHJs, a step that constitutes bona fide irreversible CO commitment. By contrast, IH-SEIs represent yet reversible intermediates readily converted into intersister or NCO intermediates (Kim et al., 2010). Coordinating second-end capture and CO commitment with homolog pairing minimizes the potential for meiotic translocations.
Mph1FANCM dissociates DSB first-end strand exchange in vivo.
Mph1 and its ortholog FANCM dissociate three-armed strand exchange intermediates in vitro in addition to branch-migrating Holliday junctions (Gari et al., 2008; Prakash et al., 2009; Cejka and Kowalczykowski, 2010). Our results show that Mph1 dissociates D-loops during ongoing homologous recombination in vivo, thereby preventing their progression to dHJs (Fig. 2). Mph1 dissociates Rad51-mediated DSB strand exchange events in the dmc1Δ background (Fig. 2E,F), but it may also act upon strand exchange intermediates formed by Dmc1. Accordingly, MPH1 delays appearance of JMs during WT and spo11 hypomorphic meiosis, when Dmc1 carries out the bulk of strand exchange (Fig. 3). These findings further indicate that Rad51-mediated strand exchange is suppressed incompletely in dmc1Δ. Inhibition of Rad51 may be intrinsically leaky, requiring Mph1 as a cleanup system. Alternatively, nucleus-wide DSB abundance may have to cross a certain threshold to fully activate Mec1ATR/Tel1ATM-Mek1-mediated Rad51 inhibition (see Joshi et al., 2015).
Mph1 temporally coordinates strand exchange with homolog pairing.
MPH1 under all conditions delays DSB first-end strand exchange, yet its effects on recombination partner choice depend on chromosomal context. When pairing and SC assembly proceed normally, MPH1 interferes with early occurring intersister exchanges only. When pairing and/or SC assembly are delayed or absent, MPH1 also diminishes later occurring interhomolog exchange. Thus, Mph1 has the potential to interfere with both IS and IH strand exchange, yet synapsis limits Mph1’s activity to early times when IS exchange dominates. Mechanistically, synapsis could inhibit or displace Mph1, or protect strand exchange intermediates from Mph1’s unwinding activity.
Our findings are not easily explained by Mph1 dissociating specifically intersister exchanges as MPH1 reduces both interhomolog and intersister exchanges in mutant backgrounds defective for pairing/SC assembly. Our findings also do not support a role of Mph1 as anti-CO recombinase thereby mediating NCO formation (Prakash et al., 2009). Notably, Mph1 contributes to formation of both COs and NCOs in a spo11 hypomorph, consistent with a general role in enhancing interhomolog recombination. At the same time, Mph1 may be directed to distinct substrates via interaction with antagonists that depend on cellular background as demonstrated here for Zip1. Finally, the current analysis does not address possible locus-specific mph1Δ effects.
Our results also exclude the possibility that Mph1 controls homolog bias by activating the Mec1ATR/Tel1ATM-dependent signaling cascade that inhibits Rad51-mediated strand exchange (Carballo et al., 2008; Callender et al., 2016, Fig. S7D). Notably, Mph1 and Hed1 control strand exchange at different stages. Whereas Hed1/Rad54 inhibit Rad51 prior to strand exchange, Mph1 dissociates D-loops after their formation (Fig. 2; Busygina et al., 2008; Prakash et al., 2009). At the same time, the two pathways exhibit intriguing similarities. First, Rad51-mediated meiotic recombination is characterized by high levels of intersister exchange, a CO increase at the expense of NCOs and low spore viability at reduced DSBs (Lao et al., 2013). Second, Mek1 has been proposed to minimize intersister recombination by delaying DSB processing at the strand invasion step (Goldfarb and Lichten, 2010; Subramanian et al., 2016).
The following model summarizes meiotic Mph1 functions. Pre-pairing “scout” DSBs lack homolog bias (Fig. 7a; Joshi et al., 2015). At this stage, Mph1 prevents precocious strand exchange with the sister chromatid (Fig. 7e). Lack of temporal control of DSB strand exchange results in precocious strand exchange with the sister chromatid which in turn triggers crossover homeostasis (Fig. 7c,d). When DSBs are limiting, loss of interhomolog recombination in mph1Δ results in chromosome missegregation and reduced gamete viability. Our results suggest that partner choice is a dynamic process: Precocious exchanges dismantled by Mph1 may undergo repeated rounds of abortive exchange with sister chromatid and homolog, possibly involving DNA synthesis at the invading 3’ end. Such transient interactions with multiple DNA templates may explain complex gene conversion patterns distal from the DSB site, in both vegetative and meiotic cells (Štafa et al., 2014, Piazza et al., 2017; Marsolier-Kergoat et al., 2018).
Distinct roles of DNA helicases Mph1FANCM and Sgs1BLM.
Mutations in MPH1 or SGS1 decrease NCOs under certain conditions, yet these DNA helicases perform distinct roles during meiosis. SGS1 maintains the fate of NCO intermediates by channeling them to JM-independent NCO formation (Fig. 7g; De Muyt et al., 2012). MPH1, by contrast, generates preconditions for high levels of interhomolog recombination but is not specifically required along the NCO pathway (Fig. 7e).
SC protein Zip1 acts as an antagonist of both Sgs1 and Mph1: Zip1 in conjunction with other ZMM proteins appears to prevent dissociation by Sgs1 of crossover-designated intermediates (Fig. 7f; De Muyt et al., 2012). Zip1 also prevents Mph1 from dissociating later-appearing stable strand exchange intermediates between homologs and sister chromatids (Fig. 7e; Fig. 5). In both cases, helicase specificity is achieved by antagonistic interactions with a chromosome structure protein which may make intermediates inaccessible to DNA helicase and/or promote conversion into dissociation-resistant intermediates.
FANCM ortholog roles in DSB processing.
In fancm deficient S. pombe, COs are increased over NCOs, without an overall increase in interhomolog recombination at most loci (Lorenz et al., 2012; Lorenz et al., 2014; Brown et al., 2019). In inbred Arabidopsis and Brassica, loss of FANCM results in a 3-fold CO increase, yet effects on NCOs are unknown (Crismani et al., 2012; Blary et al., 2018). One explanation for diverse effects is that FANCM-type helicases are controlled differently in other species. Depending on antagonists in the relevant cellular context, they may act on intersister and/or interhomolog JMs. Alternatively, FANCM may as its primary function also delay strand exchange in these organisms, yet with different effects on recombination outcome. For example, crossover homeostasis in fission yeast (Kan et al., 2011) and plants may overcompensate for precocious intersister strand exchange resulting in a CO increase. Alternatively, precocious strand exchange in absence of FANCM may bypass mechanisms that normally limit interhomolog recombination.
Notably, in Arabidopsis fancm mutants COs are increased only in inbred crosses, but not in hybrids (Girard et al., 2015). Thus, other factors that delay strand exchange such as mismatch detection may substitute for FANCM functions (see Tian and Loidl, 2019).
Precocious strand exchange may also result in increased COs in mitotically dividing mph1Δ cells (Prakash et al., 2009; Mazón and Symington, 2013; Piazza et al., 2019). MPH1 functions in these cells were examined in assay systems that select against intersister repair products (i.e. due to recleavage by a sequence-specific endonuclease). mph1Δ-induced precocious strand exchange could result in concurrent cleavage of invading 3’ DSB end and sister chromatid repair template. Such truncations may leave one-sided crossing over with the homolog or ectopic sequences as the only repair option (Mazón and Symington, 2013; Piazza et al., 2019). Notably, ectopic COs appear with advanced timing in mph1Δ (see Fig. S2C in Mazón and Symington, 2013).
Concluding remarks
DSBs serve both as precursors for meiotic recombination and as signals that trigger multiple downstream events in a dose-dependent manner (Henderson and Keeney, 2004; Cole et al., 2012; Joshi et al., 2015; Prugar et al., 2017). The current study demonstrates that Mph1FANCM controls DSB first end stand exchange of early, low abundance DSBs. Notably, distinct factors also control resection (Tel1ATM), and/ or homolog bias (Pch2TRIP13) of the same DSB cohort (Joshi et al., 2015).
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, G. Valentin Börner (g.boerner@csuohio.edu).
Materials Availability
Yeast strains generated in this study are available by request from the lead contact.
Data and Code Availability
The published article includes all datasets and code generated during this study.
Experimental Model and Subject Details
All experiments were performed using isogenic Saccharomyces cerevisiae SK1 strains. Gene deletions and mutations were introduced either by PCR-based transformation into diploids (Goldstein and McCusker, 1999) or by mating followed by tetrad dissection and replica plating to selective media. Gene deletions were marked by kanMX4 or hphMX4 drug resistance cassettes. All gene deletions were confirmed by Southern blot analyses or PCR. The sgs1-md and rad51-II3A strains were gifts from Dr. Neil Hunter (UC Davis) and Dr. Doug Bishop (University of Chicago), respectively. Strains carrying TetR-GFP fusion protein and tetO arrays at homologous or non-homologous genomic loci were gifted by Dr. Angelika Amon (MIT). spo11-HA and spo11-Y135F-HA strains were previously described (Joshi et al., 2015). Hed1 and Ndt80 antibodies were gifts from Dr. Patrick Sung (Yale) and Dr. Michael Lichten (National Cancer Institute), respectively.
Method details
Synchronous meiotic cultures (time courses)
Synchronous meiotic time courses were performed as described (Ahuja and Börner, 2011). Briefly, strains frozen at −80°C were patched on YPG plates (1% Bacto yeast extract, 2% Bacto peptone, 3% glycerol, 2% agar) and incubated at 30°C for ~16 hrs. Patches were streaked for single colonies on YPD plates (1% Bacto yeast extract, 2% Bacto peptone, 2% dextrose, 2% agar) and incubated at 30°C for ~54 h. Single colonies were inoculated in 4 ml YPD and incubated on a roller drum at 30°C for 26 h. Saturated cultures were inoculated at dilutions ranging between 1:100 to 1:200 in 150 ml pre-warmed YPA (1% Bacto yeast extract, 2% Bacto peptone, 1% potassium acetate) and incubated with vigorous shaking at 30°C for 13.5 h. Following growth and G1 arrest in YPA, cultures exhibiting ODs of ~1.2–1.5 were selected. Cultures were washed and resuspended in sporulation medium (SPM, 0.5 % potassium acetate, 0.02 % raffinose, 1:1,000 antifoam) and incubated at 23°C. This time point was set as 0 h. For time courses performed at 33°C, cultures were incubated at 30°C for 2 hours following transfer to the sporulation medium to ensure completion of pre-meiotic S-phase.
Incubator temperature was increased to 33°C thereafter, resulting in warming of the medium to 33°C within ~30 min. For time courses performed at 30°C, SPM was pre-warmed to 30°C while for time courses performed at 23°C, SPM was kept at room temperature.
Meiotic progression
Culture aliquots were removed at various time points during time course. Aliquots were mixed at a 1:1 ratio with fixative (80% ethanol, 100 mM sorbitol, 0.5 mM EDTA) and stored at −20°C. Prior to counting, fixed cultures were stained with DAPI dye (1 μg/ml) and the number of nuclei per cell was counted at each time point using a fluorescence microscope. At least 100 cells per time point of each culture were counted.
Tetrad dissection
Samples were collected at t = 24 h following transfer of cells to liquid sporulation medium. Tetrads were analyzed from the same cultures used for physical analysis and progression analysis. Cells were digested with zymolyase for 30 min at 37°C followed by dissection of yeast asci. Plates were incubated at 30°C for ~3 days, until colonies were visible. Spore viability was calculated by dividing the number of viable spores by the total number of spores dissected.
Homolog pairing
Strains containing TetR-GFP fusion protein tethered to tetO repeat arrays inserted at homologous positions at LEU2, approximately 30 kb from CEN3 were used to monitor homolog pairing. TetR-GFP tethered to arrays of tetO repeats inserted at two non-homologous positions (LEU2 and LYS2 on chromosomes 3 and 2, respectively) were used as a background for fortuitous locus association. Images of surface-spread nuclei were collected using a Deltavision Imaging System. Distances between GFP foci were measured using the SoftWoRx imaging software. Zip1 antibody staining was performed as described (Joshi et al., 2015).
Meiotic chromatin spreads and immunocytology
Samples from meiotic cultures were collected at the indicated time points and chromatin spreads were prepared as described (Börner et al., 2004). Briefly, cells were washed in Tris buffer and spheroplasted using Zymolyase 20T. After spheroplasting, cells were washed and resuspended in MES buffer. Spheroplasted cells were crosslinked on pre-cleaned glass slides using 3% paraformaldehyde/sucrose solution. After crosslinking, cells were lysed using 1% lipsol. Lysed cells were spread on the slide using a glass serological pipette. Spreads were allowed to dry overnight in the fume hood.
For immunocytology, spread slides were washed once with TBS. Non-specific binding of antibody was minimized by blocking spreads with 1% BSA in a moist chamber for 10 minutes. Following blocking, appropriate primary antibodies in TBS/BSA solution were used to immunodecorate samples. For Zip1 detection, goat anti-Zip1p antibody was used (YC-19; Santa Cruz). Spreads were incubated with primary antibodies overnight at 4°C. Slides were subsequently washed with TBS and probed with appropriate secondary antibodies conjugated to Alexa 594 or Alexa 488. Secondary antibody incubation was performed for 2 hours at room temperature. All incubations were carried out in a moist chamber. Following secondary antibody incubation, slides were washed with TBS and stained with DAPI. Spreads were then mounted in ProLong Diamond and imaged using a Deltavision imaging system.
Focus distances were measured from intrinsic GFP signals using SoftWorx or FiJi software. Distances from centers of the two GFP signals were measured. A minority of nuclei exhibited 3 or more GFP signals. The longest interfocus distances were assumed to represent inter-homolog associations, with closer distances representing inter-sister distances. Specifically, in ndt80Δ, mph1Δ ndt80Δ and spo11-HA/yf ndt80Δ, ≥ 3 GFP foci were observed in 0.9 (± 0.5) %, 1.5 (± 1.0) % and 1.8 (± 0.7) % of nuclei carrying GFP at LEU2-LEU2 and in 4.2 (± 1.7) %, 5.1 (± 2.9) % and 5.2 (± 2.1) % of nuclei carrying non-homologous GFP at LEU2-LYS2 (n ≥ 11 samples; errors indicate 2 SEM). Higher average frequencies of 3 foci in cells carrying GFP at LEU2-LYS2 likely are due to unstable sister chromatid cohesion at the LYS2 locus making them susceptible to minor variations in sample processing, as indicated by substantial variance between samples.
Physical Analysis
Crosslinking and DNA extraction
Cultures collected during time courses were pelleted and crosslinked using trioxsalen and long wave (360 nm) UV treatment for 10 minutes. For DNA extraction, cells were spheroplasted using zymolyase and lysed using the SDS lysis method. RNase A, proteinase K and phenol chloroform treatments were also performed as described (Ahuja and Börner, 2011). Following phenol chloroform extraction and ethanol precipitation, DNA pellets were resuspended in TE buffer and allowed to rehydrate overnight at 4°C prior to restriction digestion. For analysis of DSBs, crossovers, and joint molecules, ¼ of DNA was digested with 60 units of XhoI overnight at 37°C. To analyze NCOs, DNA digested overnight with XhoI was further digested with HF-BamHI (New England Biolabs) for ~5 h. Digested DNA was precipitated using standard ethanol/sodium acetate precipitation.
One-dimensional and two-dimensional gel electrophoresis
To detect DSBs, COs, and NCOs, appropriately restriction digested DNA was run on 0.6 % agarose gels without ethidium bromide at 60 V for 26 h. Southern blotting was performed using alkaline transfer, and DNA was transferred to neutral nylon membrane (Amersham N). For joint molecule detection, 2D gel electrophoresis was performed as described (Ahuja and Börner, 2011). Briefly, XhoI digested DNA was separated on a 0.4 % Seakem Gold Agarose without ethidium bromide at 35 V for 17.5 h. Following staining with ethidium bromide, gel slices ranging from 12 to 3 kb were excised as indicated by a comigrating molecular weight marker using long wave UV light. Gel slices were placed horizontally on a separate gel tray. Following lane excision, 0.8 % agarose containing ethidium bromide was poured around the gel slices in a cold room. Electrophoresis was run in the second dimension for ~5 h at 150 V in a coldroom. Following electrophoresis, Southern blotting was performed using SSC transfer of DNA to uncharged nylon membrane. After transfer, the membrane was crosslinked with UV light.
Membranes were hybridized with “probe 4” for the HIS4::LEU2 hotspot (Börner et al., 2004) which was 32P-dCTP labelled using the Prime-It II Random Primer Labeling Kit (Stratagene). Following hybridization, membranes were exposed to imaging plates and scanned using a Typhoon phosphoimager. Non-overexposed scans of 1D and 2D Southern blots were used for quantitation using Quantity One software (BioRad).
Protein extraction and western blotting
Protein extracts from time course cultures were prepared using the tri-chloroacetic acid (TCA) method (Prugar et al., 2017). Protein extracts were electrophoretically separated on SDS-PAGE gels and transferred to PVDF membrane. Western blotting was performed using rabbit anti-Hop1 (N.M.H.), guinea pig anti-Mek1 (N.M.H.), rabbit anti-Ndt80 (Michael Lichten), rabbit anti-Hed1 (Patrick Sung), rabbit anti-Hed1 p-T40 rabbit (N.M.H.), rabbit anti-Rad51 (N.M.H.), and goat anti-Arp7 (Santa-cruz sc-8961) antibodies. HRP-labelled secondary antibodies were from Santa Cruz.
Quantification, Statistics and Kinetic Analyses
Statistical details can be found in the figure legends. For duplicate cultures analyzed in the same experiment, error bars represent top and bottom ranges (n = 2) or standard deviations (n > 2). To facilitate comparison of meiotic cultures analyzed on different days (Figures 3,4,and 6), the time at which maximum joint molecules were observed (5 or 6 h) in a parallel WT culture was set as t = 0 h. To account for day-to-day variability, DSBs and joint molecules were expressed as a fraction of the peak values in a parallel wild-type culture.
For intrinsically cumulative events (i.e. homolog pairing, crossovers, and non-crossovers), the 50% entry time was determined by plotting events as a percentage of minimum and maximum levels and graphically interpolating the time when 50% of the respective event had occurred. Calculation with the custom Excel Macro gave identical 50% entry times. For pairing, an average was determined for cells exhibiting a single GFP focus emanating from non-homologous loci. This value was subtracted from pairing levels in parallel cultures carrying GFP foci at homologous positions.
For recombination intermediates (DSBs, IH-JMs), average durations (lifespans) and 50% entry and exit times were calculated as described (Padmore et al., 1991), using a custom Excel Macro. Briefly, the average lifespan of a stage is given by the area under the corresponding steady state curve, divided by the total number of events. On the curve of measured values, values are interpolated at one-lifespan intervals in both directions from the peak time point. The first non-zero value at an interpolated time point is transferred as is into the cumulative curve. The cumulative value at the next interpolated time point equals the value at that time point plus the preceeding cumulative value (see Info tab in Kinetics Macro). The average 50% entry time corresponds to the time when the resulting cumulative curve reaches 50% of its maximum value. The 50% exit time corresponds to that time point plus one lifespan.
For DSBs, total recombination events were determined in a dmc1Δ background as ~22.5% (SPO11) and ~7.0% (spo11-HA/yf). Thus, recombination is initiated in spo11-HA/yf with similar efficiencies at 23ºC and 33ºC, contrary to the suggestion that DSB formation in spo11-HA/yf is cold-sensitive (Diaz et al., 2002; Joshi et al., 2015).Five assumptions were made to calculate 50% entry and 50% exit times for combined interhomolog strand exchange stages (i.e. IH-SEI plus IH-dHJs) in SPO11 and spo11-HA/yf: First, the majority of SEI signals are derived from IH-SEIs, i.e. IS-SEIs contribute negligibly to the SEI signal. This assumption is supported by absence of the crescent-shaped signal characteristic for IS-SEIs and appearance of SEI signals in spo11-HA/yf concurrent with, rather than prior, to IS-dHJs, as would be expected for a IS-dHJ precursor (see Fig. 1G). Second, IH-SEIs and IH-dHJs progress exclusively to COs (e.g. Börner et al., 2004). Third, all COs are formed via IH-SEIs and IH-dHJs. Fourth, the two IH-SEI that are well-resolved in typical 2D blots (SEI-3 [7.4 kb] and SEI-4 [8.7 kb] in Hunter and Kleckner (2001)) can be used to quantitatively account for two additional IH-SEIs (SEI-1 and SEI-2) that are predicted to occur at HIS4LEU2, but are not well resolved. Fifth, intermediate levels in spo11-HA/yf are assumed to amount to 1/3 of SPO11 levels, as suggested by DSB levels in a dmc1Δ background. For simplicity of the kinetic estimates performed here, opposite effects of increased intersister exchange and increased CO abundance among the remaining interhomolog events at the expense of NCOs (Fig. 1H) are assumed to be similar in extent and thus cancel each other out.
Based on these assumptions, the total number of events passing through the IH-SEI and IH-dHJ stages was derived from the average maximum CO band intensity (~22%; Fig. S1E; Table S1). Quantitated steady state levels of SEIs and IH-dHJs for all cultures were added for each time point to obtain total levels of IH-strand exchange intermediates and cumulative curves were determined under the assumption that in SPO11, the total number of crossover-specific IH-JMs should amount to ~33% (i.e. 22% IH-dHJs plus 11% SEIs [i.e. half of the detected SEIs]). Cumulative IH-JM levels in spo11-HA/yf were assumed to be 11% [i.e. 1/3 of 33%]. To exclude abnormally early or late meiotic cultures from analysis, only cultures were included in the kinetic analyses if (i) timing of 50% CO formation resembled that of SPO11 and spo11-HA/yf cultures shown in Fig. 1E and (ii) aliquots had been taken with close spacing for the relevant events.
Supplementary Material
Data S1: CumKin 2.0 Kinetics Macro with Instructions and Sample Data (Related to Fig. 1).
Highlights.
-
-
Meiotic double strand breaks form D-loops following pairing of parental chromosomes
-
-
DNA helicase Mph1FANCM dissociates precocious D-loops between sister chromatids
-
-
Intersister repair due to lack of Mph1 triggers a non-crossover-to-crossover shift
-
-
Synapsis protein Zip1 protects D-loops between parental chromosomes from Mph1
ACKNOWLEDGMENTS
This research was supported by NIH grants R01GM125800 to G.V.B. and R01GM050717 to N.M.H.. We thank N. Hunter, D. Bishop and A. Amon for strains and N. Hunter and N. Kleckner for an earlier version of the Kinetics macro, A. Lorenz, J. Ahuja and M. Lichten for data sharing and discussion, J. Ahuja, N. Joshi, M. Assar, I. Kuragayala and F. El Bach for experimental assistance, and A. Toth, R. Mercier, J. Yanowitz, A. Severson, B. Li, A. Tartakoff and Börner lab members for discussion.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahuja JS, and Börner GV (2011). Analysis of meiotic recombination intermediates by two-dimensional gel electrophoresis. Methods Mol. Biol. 745, 99–116. [DOI] [PubMed] [Google Scholar]
- Allers T, and Lichten M. (2001). Differential timing and control of noncrossover and crossover recombination during meiosis. Cell 106, 47–57. [DOI] [PubMed] [Google Scholar]
- Bernstein KA, Gangloff S, and Rothstein R. (2010). The RecQ DNA helicases in DNA repair. Annu. Rev. Genet. 44, 393–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DK (1994). RecA homologs Dmc1 and Rad51 interact to form multiple nuclear complexes prior to meiotic chromosome synapsis. Cell 79, 1081–1092. [DOI] [PubMed] [Google Scholar]
- Blary A, Gonzalo A, Eber F, Bérard A, Bergès H, Bessoltane N, Charif D, Charpentier C, Cromer L, Fourment J, et al. (2018). FANCM limits meiotic crossovers in Brassica crops. Front. Plant Sci. 9, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börner GV, Kleckner N, and Hunter N. (2004). Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell 117, 29–45. [DOI] [PubMed] [Google Scholar]
- Brar GA, Hochwagen A, Ee LS, and Amon A. (2009). The multiple roles of cohesin in meiotic chromosome morphogenesis and pairing. Mol. Biol. Cell. 20, 1030–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SD, Mpaulo SJ, Asogwa MN, Jézéquel M, Whitby MC, and Lorenz A. (2019). DNA sequence differences are determinants of meiotic recombination outcome. Sci Rep. 11, 16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busygina V, Sehorn MG, Shi IY, Tsubouchi H, Roeder GS, and Sung P. (2008). Hed1 regulates Rad51-mediated recombination via a novel mechanism. Genes Dev. 22, 786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzymek M, Thayer NH, Oh SD, Kleckner N, and Hunter N. (2010). Double Holliday junctions are intermediates of DNA break repair. Nature 464, 937–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callender TL, Laureau R, Wan L, Chen X, Sandhu R, Laljee S, Zhou S, Suhandynata RT, Prugar E, Gaines WA, et al. (2016). Mek1 down regulates Rad51 activity during yeast meiosis by phosphorylation of Hed1. PLoS Genet. 12, e1006283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo JA, Johnson AL, Sedgwick SG, and Cha RS (2008) Phosphorylation of the axial element protein Hop1 by Mec1/Tel1 ensures meiotic interhomolog recombination. Cell 132, 758–770. [DOI] [PubMed] [Google Scholar]
- Cejka P, and Kowalczykowski SC (2010). The full-length Saccharomyces cerevisiae Sgs1 protein is a vigorous DNA helicase that preferentially unwinds Holliday junctions. J. Biol. Chem. 285, 8290–8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Tsubouchi T, Rockmill B, Sandler JS, Richards DR, Vader G, Hochwagen A, Roeder GS, and Fung JC (2008). Global analysis of the meiotic crossover landscape. Dev. Cell 15, 401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloud V, Chan YL, Grubb J, Budke B, and Bishop DK (2012). Rad51 is an accessory factor for Dmc1-mediated joint molecule formation during meiosis. Science 337, 1222–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole F, Kauppi L, Lange J, Roig I, Wang R, Keeney S, and Jasin M. (2012). Homeostatic control of recombination is implemented progressively in mouse meiosis. Nat. Cell Biol. 14, 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crismani W, Girard C, Froger N, Pradillo M, Santos JL, Chelysheva L, Copenhaver GP, Horlow C, and Mercier R. (2012). FANCM limits meiotic crossovers. Science 336, 1588–1590. [DOI] [PubMed] [Google Scholar]
- De Boer E, Jasin M, and Keeney S. (2015). Local and sex-specific biases in crossover vs. noncrossover outcomes at meiotic recombination hot spots in mice. Genes Dev. 29, 1721–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Muyt A, Jessop L, Kolar E, Sourirajan A, Chen J, Dayani Y, and Lichten M. (2012). BLM helicase ortholog Sgs1 is a central regulator of meiotic recombination intermediate metabolism. Mol. Cell 46, 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans AJ, and West SC (2009). FANCM connects the genome instability disorders Bloom’s Syndrome and Fanconi Anemia. Mol. Cell 36, 943–953. [DOI] [PubMed] [Google Scholar]
- Diaz RL, Alcid AD, Berger JM, and Keeney S. (2002). Identification of residues in yeast Spo11p critical for meiotic DNA double-strand break formation. Mol. Cell. Biol. 22, 1106–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupaigne P, Le Breton C, Fabre F, Gangloff S, Le Cam E, and Veaute X. (2008). The Srs2 helicase activity is stimulated by Rad51 filaments on dsDNA: implications for crossover incidence during mitotic recombination. Mol. Cell 29, 243–254. [DOI] [PubMed] [Google Scholar]
- Gari K, Décaillet C, Stasiak AZ, Stasiak A, and Constantinou A. (2008). The Fanconi anemia protein FANCM can promote branch migration of Holliday junctions and replication forks. Mol. Cell 29, 141–148. [DOI] [PubMed] [Google Scholar]
- Girard C, Chelysheva L, Choinard S, Froger N, Macaisne N, Lehmemdi A, Mazel J, Crismani W, and Mercier R. (2015). AAA-ATPase FIDGETIN-LIKE 1 and helicase FANCM antagonize meiotic crossovers by distinct mechanisms. PLoS Genet. 11, e1005369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb T, and Lichten M. (2010). Frequent and efficient use of the sister chromatid for DNA double-strand break repair during budding yeast meiosis. PLoS Biol. 8, e1000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AL, and McCusker JH (1999) Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15, 1541–1553. [DOI] [PubMed] [Google Scholar]
- Henderson KA, and Keeney S. (2004). Tying synaptonemal complex initiation to the formation and programmed repair of DNA double-strand breaks. Proc. Natl. Acad. Sci. USA 101, 4519–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth SR, Friesen H, Segall J. (1998). NDT80 and the meiotic recombination checkpoint regulate expression of middle sporulation-specific genes in Saccharomyces cerevisiae. Mol Cell Biol. 10, 5750–5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Kim JM, Shiotani B, Yang K, Zou L, and D’Andrea AD (2010). The FANCM/FAAP24 complex is required for the DNA interstrand crosslink-induced checkpoint response. Mol. Cell 39, 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N, and Kleckner N. (2001). The single-end invasion: an asymmetric intermediate at the double-strand break to double-Holliday junction transition of meiotic recombination. Cell 106, 59–70. [DOI] [PubMed] [Google Scholar]
- Hunter N. (2015). Meiotic recombination: the essence of heredity. Cold Spring Harb. Perspect. Biol. 7, a016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ira G, Malkova A, Liberi G, Foiani M, and Haber JE (2003). Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell 115, 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi N, Brown MS, Bishop DK, and Börner GV (2015). Gradual implementation of the meiotic recombination program via checkpoint pathways controlled by global DSB levels. Mol. Cell 57, 797–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadyk LC, and Hartwell LH (1992). Sister chromatids are preferred over homologs as substrates for recombinational repair in Saccharomyces cerevisiae. Genetics 132, 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan F, Davidson MK, and Wahls WP (2011). Meiotic recombination protein Rec12: Functional conservation, crossover homeostasis and early crossover/non-crossover decision. Nucleic Acids Res. 39, 1460–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KP, Weiner BM, Zhang L, Jordan A, Dekker J, and Kleckner N. (2010). Sister cohesion and structural axis components mediate homolog bias of meiotic recombination. Cell 143, 924–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. (2006). Chiasma formation: chromatin/axis interplay and the role(s) of the synaptonemal complex. Chromosoma 115, 175–94. [DOI] [PubMed] [Google Scholar]
- Kleckner N, and Weiner BM (1993). Potential advantages of unstable interactions for pairing of chromosomes in meiotic, somatic, and premeiotic cells, Cold Spring Harb. Symp. Quant. Biol. 58 553–565. [DOI] [PubMed] [Google Scholar]
- Lam I, and Keeney S. (2014). Mechanism and regulation of meiotic recombination initiation, Cold Spring Harb. Perspect. Biol. 7 a016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao JP, Cloud V, Huang CC, Grubb J, Thacker D, Lee CY, Dresser ME, Hunter N, and Bishop DK (2013). Meiotic crossover control by concerted action of Rad51-Dmc1 in homolog template bias and robust homeostatic regulation. PLoS Genet. 9 e1003978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao JP, Oh SD, Shinohara M, Shinohara A, and Hunter N. (2008). Rad52 promotes postinvasion steps of meiotic double-strand-break repair. Mol. Cell 29, 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz A, Osman F, Sun W, Nandi S, Steinacher R, and Whitby MC (2012). The fission yeast FANCM ortholog directs non-crossover recombination during meiosis. Science 336, 1585–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz A, Mehats A, Osman F, and Whitby MC (2014). Rad51/Dmc1 paralogs and mediators oppose DNA helicases to limit hybrid DNA formation and promote crossovers during meiotic recombination. Nucleic Acids Res. 42, 13723–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancera E, Bourgon R, Brozzi A, Huber W, and Steinmetz LM (2008). High-resolution mapping of meiotic crossovers and non-crossovers in yeast. Nature 454, 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsolier-Kergoat MC, Khan MM, Schott J, Zhu X, and Llorente B. (2018). Mechanistic view and genetic control of DNA recombination during meiosis. Mol. Cell 70, 9–20, e6. [DOI] [PubMed] [Google Scholar]
- Martini E, Diaz RL, Hunter N, and Keeney S. (2006). Crossover homeostasis in yeast meiosis. Cell 126, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazón G, and Symington LS (2013). Mph1 and Mus81-Mms4 prevent aberrant processing of mitotic recombination intermediates. Mol. Cell 52, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahill MS, Sham CW, and Bishop DK (2007). Synthesis-dependent strand annealing in meiosis. PLoS Biol. 5, e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimitou EP, Yamada S, and Keeney S. (2017). A global view of meiotic double-strand break end resection. Science 355, 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H, and Keeney S. (2014). Temporospatial coordination of meiotic DNA replication and recombination via DDK recruitment to replisomes. Cell 158, 861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishant KT, Chen C, Shinohara M, Shinohara A, and Alani E. (2010). Genetic analysis of baker’s yeast Msh4-Msh5 reveals a threshold crossover level for meiotic viability. PLoS Genet. 6, e1001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Wan L, Busygina V, Kwon YH, Allen JA, Li X, Kunz RC, Kubota K, Wang B, Sung P, et al. (2009). Regulation of meiotic recombination via Mek1-mediated Rad54 phosphorylation. Mol. Cell 36, 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso D, and Dawson DS (2010). Temporal characterization of homology-independent centromere coupling in meiotic prophase. PLoS One 5, 10336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SD, Lao JP, Hwang PYH, Taylor AF, Smith GR, and Hunter N. (2007). BLM ortholog, Sgs1, prevents aberrant crossing-over by suppressing formation of multichromatid joint molecules. Cell 130, 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SD, Lao JP, Taylor AF, Smith GR, and Hunter N. (2008). RecQ Helicase, Sgs1, and XPF family endonuclease, Mus81-Mms4, resolve aberrant joint molecules during meiotic recombination. Mol. Cell 31, 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmore R, Cao L, and Kleckner N. (1991). Temporal comparison of recombination and synaptonemal complex formation during meiosis in S. cerevisiae. Cell 66, 1239–1256. [DOI] [PubMed] [Google Scholar]
- Pan J, Sasaki M, Kniewel R, Murakami H, Blitzblau HG, Tischfield SE, Zhu X, Neale MJ, Jasin M, Socci ND, et al. (2011). A hierarchical combination of factors shapes the genome-wide topography of yeast meiotic recombination initiation. Cell 144, 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples TL, Dean E, Gonzalez O, Lambourne L, and Burgess SM (2002). Close, stable homolog juxtaposition during meiosis in budding yeast is dependent on meiotic recombination, occurs independently of synapsis, and is distinct from DSB-independent pairing contacts. Genes Dev. 16, 1682–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza A, Wright WD, and Heyer WD (2017). Multi-invasions are recombination byproducts that induce chromosomal rearrangements. Cell 170, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza A, Shah SS, Wright WD, Gore SK, Koszul R, and Heyer WD (2019). Dynamic processing of displacement loops during recombinational DNA repair. Mol Cell 73, 1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R, Satory D, Dray E, Papusha A, Scheller J, Kramer W, Krejci L, Klein H, Haber JE, Sung P, et al. (2009). Yeast Mph1 helicase dissociates Rad51-made D-loops: implications for crossover control in mitotic recombination. Genes Dev. 23, 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prugar E, Burnett C, Chen X, and Hollingsworth NM (2017). Coordination of double strand break repair and meiotic progression in yeast by a Mek1-Ndt80 negative feedback loop. Genetics 206, 497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacha A, and Kleckner N. (1995). Identification of double Holliday junctions as intermediates in meiotic recombination. Cell 83, 783–791. [DOI] [PubMed] [Google Scholar]
- Schwacha A, and Kleckner N. (1997). Interhomolog bias during meiotic recombination: meiotic functions promote a highly differentiated interhomolog-only pathway. Cell 90, 1123–1135. [DOI] [PubMed] [Google Scholar]
- Štafa A, Donnianni RA, Timashev LA, Lam AF, and Symington LS (2014). Template switching during break-induced replication is promoted by the Mph1 helicase in Saccharomyces cerevisiae. Genetics 196, 1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian VV, MacQueen AJ, Vader G, Shinohara M, Sanchez A, Borde V, Shinohara A, and Hochwagen A. (2016). Chromosome synapsis alleviates Mek1-dependent suppression of meiotic DNA repair. PLoS Biol. 14, e1002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sym M, Engebrecht JA, and Roeder GS (1993). ZIP1 is a synaptonemal complex protein required for meiotic chromosome synapsis. Cell 72, 365–378. [DOI] [PubMed] [Google Scholar]
- Tian M, and Loidl J. (2019). An MCM family protein promotes interhomolog recombination by preventing precocious intersister repair of meiotic DSBs. PLoS Genet. 9, e1008514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi H, and Roeder GS (2006). Budding yeast Hed1 down-regulates the mitotic recombination machinery when meiotic recombination is impaired. Genes Dev. 20, 1766–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt PM, Hickson ID, Borts RH, and Louis EJ (1996). SGS1, a homologue of the Bloom’s and Werner’s syndrome genes, is required for maintenance of genome stability in Saccharomyces cerevisiae. Genetics 144, 935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner BM, and Kleckner N. (1994). Chromosome pairing via multiple interstitial interactions before and during meiosis in yeast. Cell 77, 977–991. [DOI] [PubMed] [Google Scholar]
- Wild P, Susperregui A, Piazza I, Dörig C, Oke A, Arter M, Yamaguchi M, Hilditch AT, Vuina K, Chan KC, et al. (2019). Network rewiring of homologous recombination enzymes during mitotic proliferation and meiosis. Mol Cell 75, 859–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharyevich K, Tang S, Ma Y, and Hunter N. (2012). Delineation of joint molecule resolution pathways in meiosis identifies a crossover-specific resolvase. Cell 149, 334–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickler D, and Kleckner N. (2015). Recombination, pairing, and synapsis of homologs during meiosis. Cold Spring Harb. Perspect. Biol. 7, 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: CumKin 2.0 Kinetics Macro with Instructions and Sample Data (Related to Fig. 1).
Data Availability Statement
The published article includes all datasets and code generated during this study.