Abstract
INTRODUCTION:
Besides Helicobacter pylori and Epstein-Barr virus, other viruses might play potential roles in gastric carcinogenesis. This systematic review and meta-analysis was conducted to compare the prevalence of the viruses between gastric cancer (GC) and any controls.
METHODS:
Comprehensive literature was searched up to January 25, 2019, and search was updated on April 6, 2020. The studies that compared the prevalence of viruses other than Epstein-Barr virus between GC and healthy or nonmalignant controls were eligible. Stata 12.0 software was used for heterogeneity tests and meta-analyses. Meanwhile, subgroup analysis, sensitivity analysis, and publication bias evaluation were performed where applicable. The power (1–β) was estimated by the PASS 11 software for each individual study.
RESULTS:
A total of 41 eligible studies were included, concerning 11 kinds of viruses. Prevalence were significantly higher in GC for hepatitis B virus (odds ratio [OR] = 1.39, 95% confidence interval [CI] 1.11–1.75), human cytomegalovirus (OR = 2.25, 95% CI 1.14–4.43), human papillomavirus (HPV) (OR = 1.63, 95% CI 1.05–2.54), and John Cunningham virus (OR = 2.52, 95% CI 1.26–5.04). In subgroup analyses, HPV-16 infection was significantly associated with GC (OR = 2.42, 95% CI 1.00–5.83).
DISCUSSION:
This study demonstrated that hepatitis B virus, human cytomegalovirus, HPV, and John Cunningham virus were more prevalent in GC. However, the causal relationship between their infection and risk of GC remains inconclusive, and further investigations are required.
INTRODUCTION
It is estimated that up to 50% of human cancers are caused by infectious agents. Viruses are responsible for 10%–15% particularly (1). As one of the most common cancers worldwide, the pathogenesis of gastric cancer (GC) remains unclear (2). Helicobacter pylori and Epstein-Barr virus (EBV) have been recognized as infectious agents, and the potential mechanisms inducing gastric carcinogenesis are well established (3–7). Almost 50% of the global population is infected with H. pylori, and 1% of them develop GC (7). Similarly, EBV is estimated to be responsible for 5.6%–19.5% of cases with GC globally (8).
In addition to H. pylori and EBV, other viruses might play potential roles in gastric carcinogenesis. A potential association between hepatitis B virus (HBV) and the risk of GC was found in a case–control study (9). Del Moral-Hernández et al. (10) considered human cytomegalovirus (HCMV) infection might contribute to gastritis and GC etiology. The role of HPV in GC is also indicated in several studies (11–14). Several sporadic studies sought to attach viruses other than EBV to the risk of GC; however, there is no comprehensive review to identify any potentially causative virus until now. Therefore, we performed this systematic review and meta-analysis to clarify the associations between the prevalence of viruses other than EBV and the risk of GC based on epidemiological studies.
METHODS
Reporting
This meta-analysis was conducted according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) 2000 statements, and a flow diagram was plotted (15,16). Details of study design have been reported elsewhere (17).
Literature search
PubMed, Embase, Cochrane Library, and Web of Science databases were searched up to January 25, 2019, and search was updated on April 6, 2020. The PubMed was searched with the following search terms, ((“stomach neoplasms”[MeSH Terms] OR (“stomach”[All Fields] AND “neoplasms”[All Fields]) OR “stomach neoplasms”[All Fields] OR (“gastric”[All Fields] AND “cancer”[All Fields]) OR “GC”[All Fields]) AND (“viruses”[MeSH Terms] OR “viruses”[All Fields] OR “virus”[All Fields])) NOT (“animal”[Filter] OR “review”[Publication Type] OR “case reports”[Publication Type] OR “letter”[Publication Type] OR “comment”[Publication Type]) (17). A similar search strategy was applied in searching published literature in Embase, Cochrane Library, and Web of Science databases.
Eligibility
The studies that compared the positivity of any virus between cases with histologically proven GC with healthy or nonmalignant controls were eligible. The patients with gastric lymphoma, gastrointestinal stromal tumor, and squamous cell carcinoma were ineligible. Any type of cohort study, cross-sectional study, or case–control study was considered. The detection of viruses was performed in cases and controls based on either blood/serum or tissue specimens. Any targeted virus other than EBV was eligible in this systematic review. There was no limitation on the race, sex, age, cancer stage, and treatment strategy. Only those studies with extractable data were considered for analysis (17). For overlap study, only the most recent study or the study with the larger number of participants was included.
Selection and quality assessment
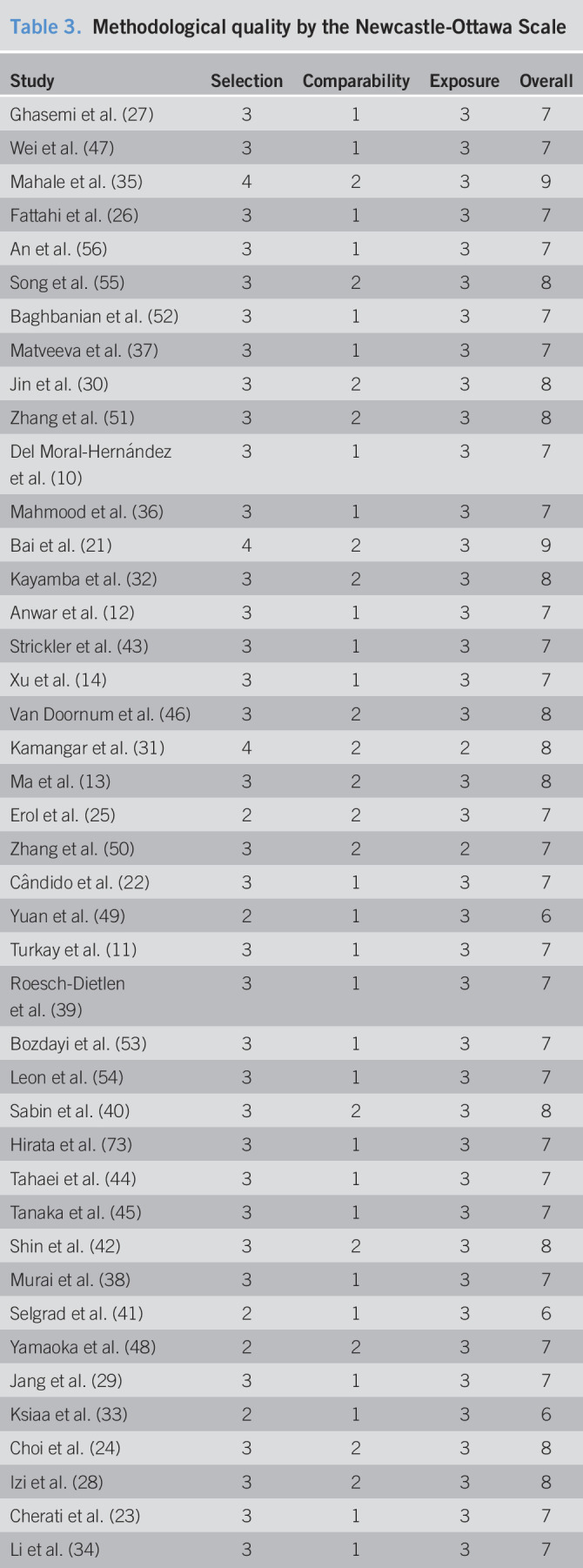
Two reviewers separately browsed the titles/abstracts and assessed the potentially eligible full-texts according to the predefined inclusion and exclusion criteria. Discrepancies were resolved by consensus with a third reviewer. Risk of bias assessment of all included studies was independently performed by 2 reviewers, according to the Newcastle-Ottawa Scale (18).
Data extraction
Data were extracted independently by 2 reviewers. Extracted items included general study characteristics (publication year, country, and study design), characteristics of the study population (sample size, sex, and age), and types of measurements (specimen types and viruses detection methods). Numbers of cases and controls were extracted from publications. Included study where different test methods were applied or various types of viruses were investigated was treated as more than 1 study. For study that had taken paired corresponding normal tissues (PCNTs) from the same patients with GC and noncancer gastric mucosa samples from patients without cancer as controls simultaneously, only data about patients without cancer were extracted. Potential discrepancies in extracted items if any were resolved by further review and discussion (17).
Statistical analysis
The STATA 12.0 and the PASS 11 software were used for statistical analysis (19,20). All analysis was based on individual virus. The pooled prevalence of any virus infection in patients with GC was combined for rate, with 95% confidence intervals (CIs). The pooled odds ratios (ORs) and their 95% CIs for virus prevalence were calculated between patients with GC and any controls by fixed or random effect model where suitable, and reanalysis was conducted after excluding subjects with known H. pylori and EBV positive. Two-sided P values for the pooled ORs < 0.05 were considered as statistical significance. I2 was estimated to evaluate the heterogeneity. Any P value < 0.05 of Begg or Egger test was considered as significance of publication bias. The leave-one-out method was applied for sensitivity analysis. In addition, L'Abbé plot and Galbraith plot were used to observe the heterogeneity. For an individual study, the power (1–β) was estimated by the PASS 11 software (20). Two-sided Z test (pooled) was provided with α = 0.05.
RESULTS
Systematic literature search and general information
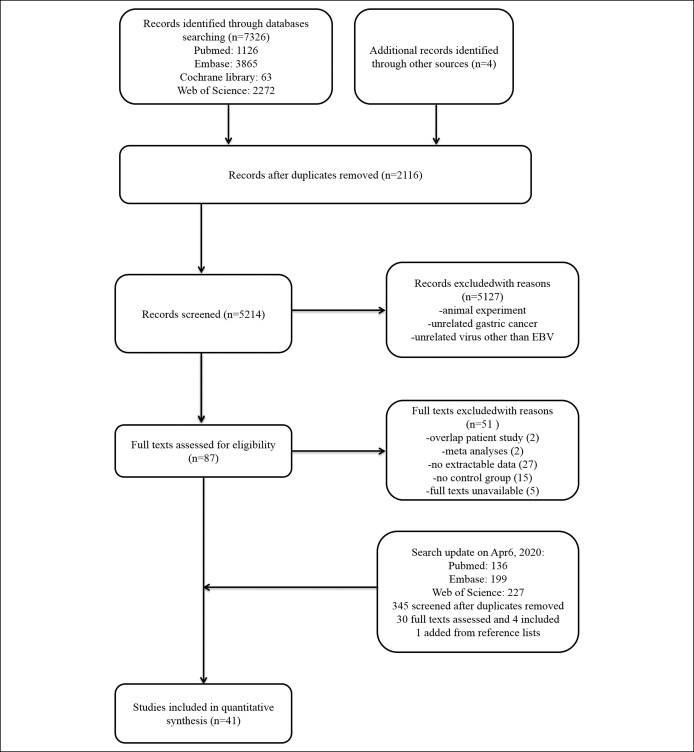
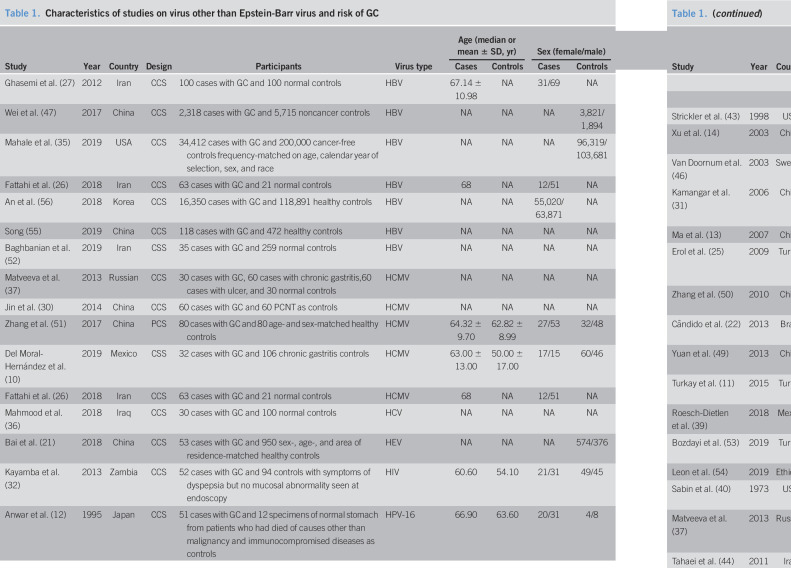
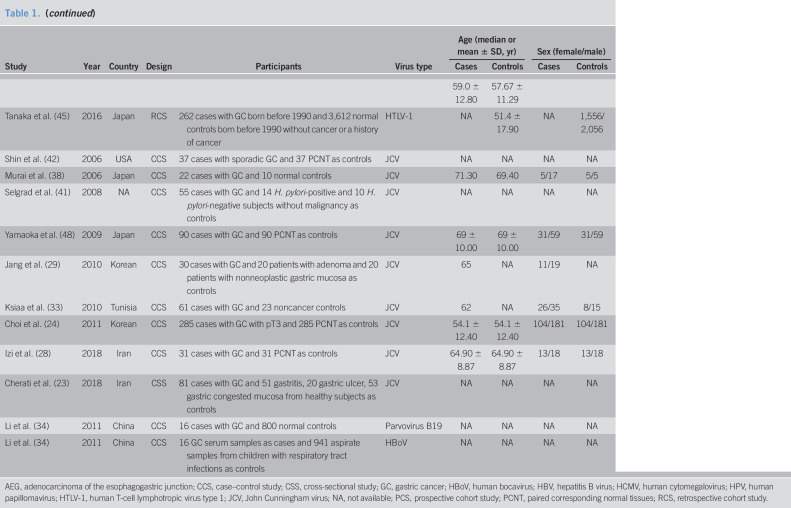
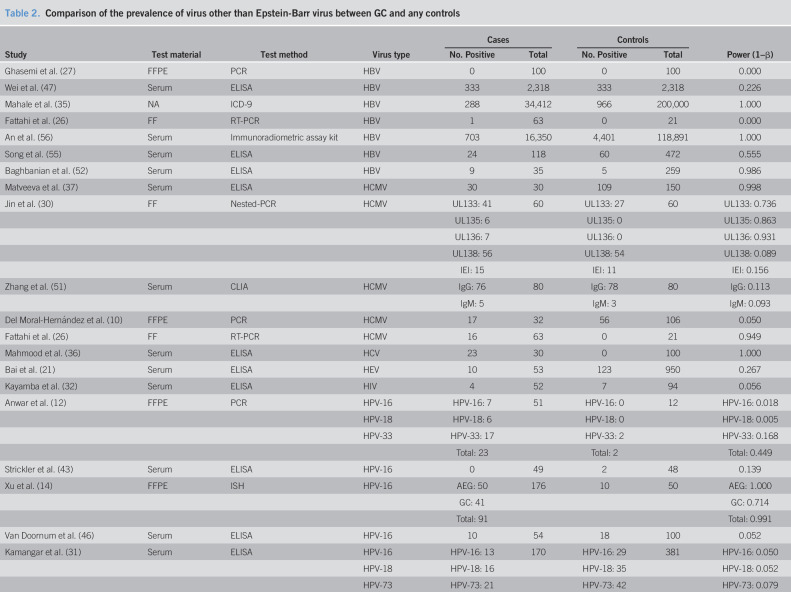
Forty-one studies were included in this meta-analysis finally (Figure 1) (10–14,21–56). Eleven kinds of viruses were involved including HBV, hepatitis C virus (HCV), hepatitis E virus (HEV), HCMV, HIV, human papillomavirus (HPV), herpes simplex virus, human T-cell lymphotropic virus type 1 (HTLV-1), John Cunningham virus (JCV), and human parvovirus (B19 and human bocavirus) (Table 1). The comparison of prevalence viruses between patients with GC and any controls with corresponding estimated power is summarized in Table 2. Quality assessment scoring of studies is tabulated in Table 3 with Newcastle-Ottawa Scale scores ranging from 6 to 9. Meta-analysis was conducted in studies involving HBV, HCMV, HPV, HTLV-1, and JCV.
Figure 1.
The flowchart of the literature search.
Table 1.
Characteristics of studies on virus other than Epstein-Barr virus and risk of GC
Table 2.
Comparison of the prevalence of virus other than Epstein-Barr virus between GC and any controls
Table 3.
Methodological quality by the Newcastle-Ottawa Scale
HBV and GC
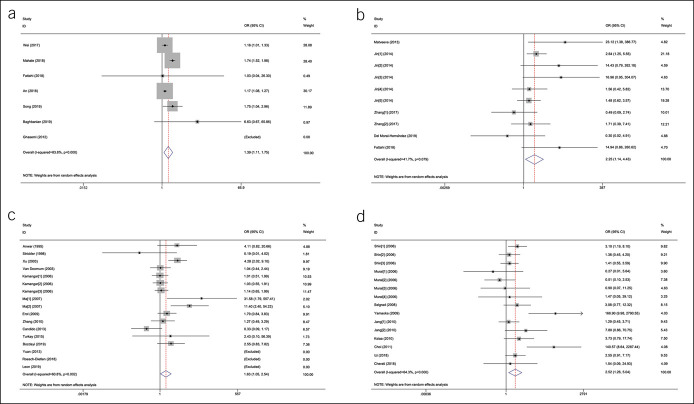
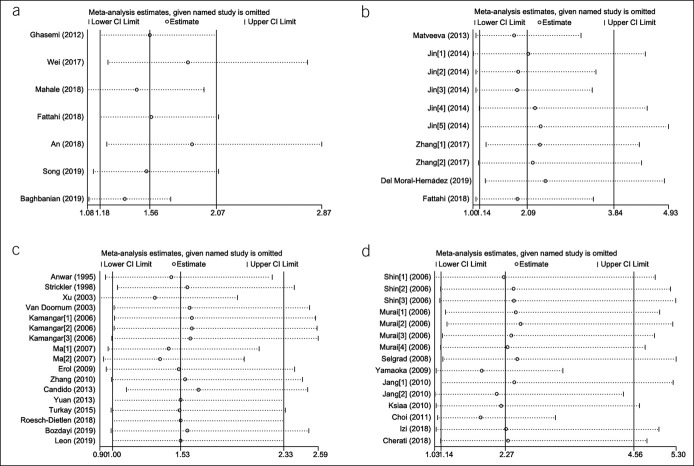
The 7 studies that investigated the relationship between HBV and GC involved 53,396 patients with GC and 325,458 any controls (26,27,35,47,52,55,56). The pooled HBV prevalence in GC was 7.6% (95% CI 4.6%–10.6%, random model, heterogeneity P < 0.001). Association between HBV infection and the risk of GC was observed (OR = 1.56, 95% CI 1.18–2.07, random model, heterogeneity P < 0.001) (see Figure 1a, Supplementary Digital Content 1, http://links.lww.com/CTG/A299). Two studies investigated the coinfection of H. pylori (26,52), and data could be extracted completely for cases and controls from only 1 study (52). We reanalyzed after excluding subjects with known H. pylori positive, and association between HBV infection and the risk of GC was observed (OR = 1.39, 95% CI 1.11–1.75, random model, heterogeneity P < 0.001) (Figure 2a).
Figure 2.
The forest plots of the comparison on virus prevalence between patients with gastric cancer and any controls after excluding subjects with known H. pylori and EBV positive: (a) the forest plot for HBV; (b) HCMV: Jin [1–5]: different sequences of HCMV genome were detected; Zhang [1–2]: IgG or IgM was detected; (c) the forest plot for HPV: Kamangar [1–3]:different subtypes of HPV were investigated; Ma [1–2]:different test methods were used; and (d) the forest plot for JCV: Jang [1–2]: different test methods were used; Murai [1–3]: different sequences of JCV genome were detected by Southern blot; Murai [4]: T-Ag were detected by immunohistochemistry; Shin [1–3]: different sequences of JCV genome were detected. CI, confidence interval; EBV, Epstein-Barr virus; HCMV, human cytomegalovirus; HPV, human papillomavirus; JCV, John Cunningham virus; OR, odds ratio; T-Ag, transforming antigen.
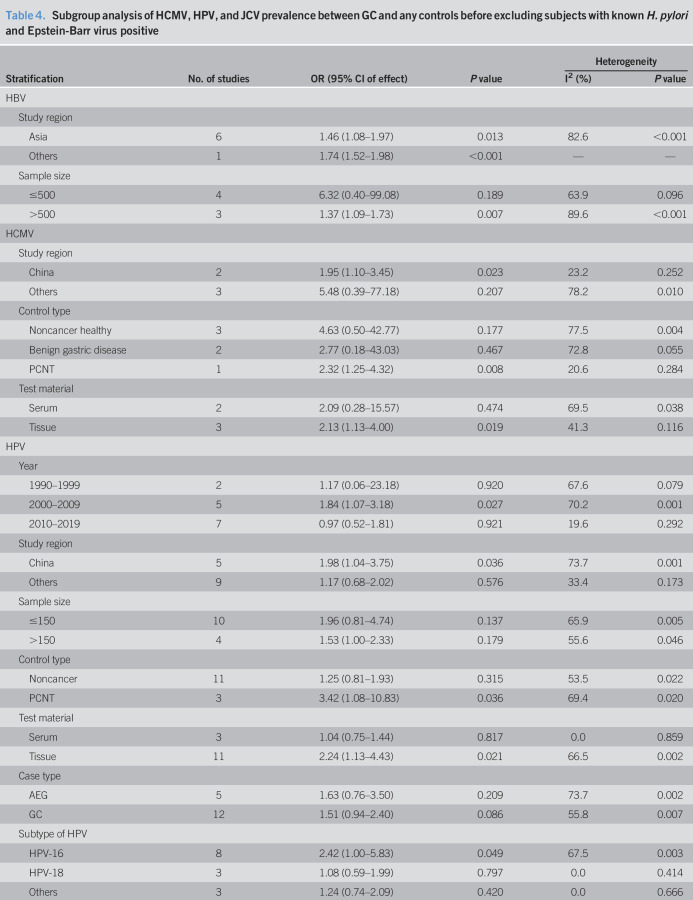
According to the different study regions and sample size, subgroup analysis was conducted before and after excluding subjects with known H. pylori positive (Tables 4 and 5). The result showed that HBV infection was still related to the risk of GC after 1 study from United States was dropped along with a decrease of heterogeneity, which indicated that study location might be responsible for the heterogeneity (OR = 1.18, 95% CI 1.08–1.29, random model, heterogeneity P = 0.344) (Table 5).
Table 4.
Subgroup analysis of HCMV, HPV, and JCV prevalence between GC and any controls before excluding subjects with known H. pylori and Epstein-Barr virus positive
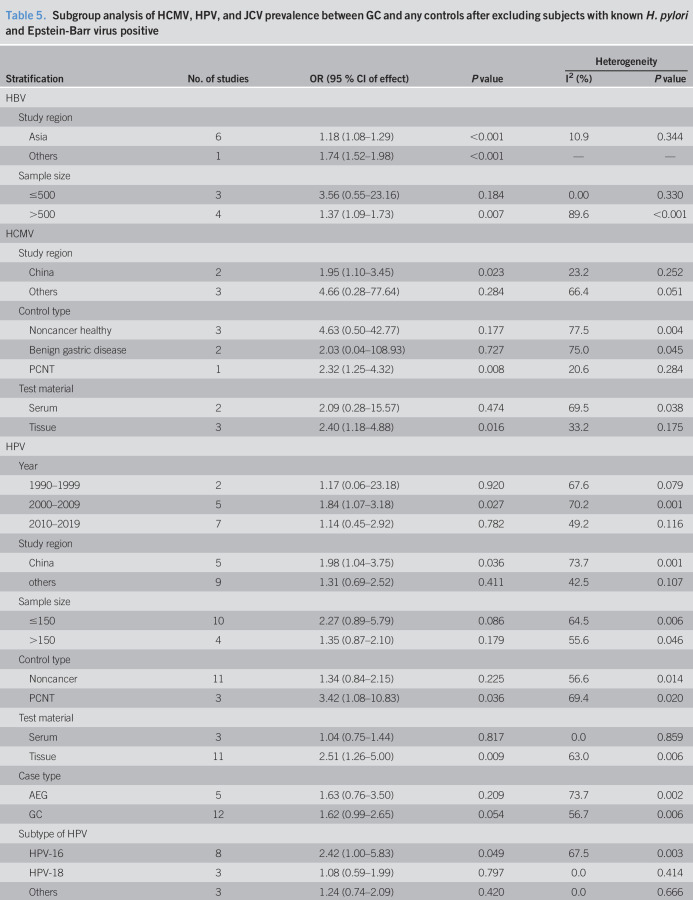
Table 5.
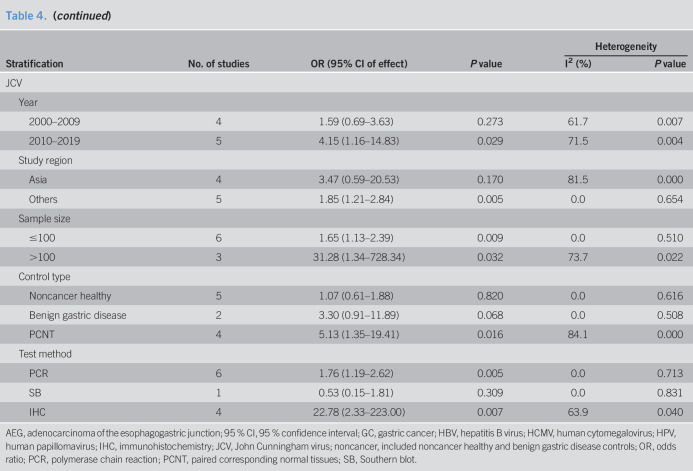
Subgroup analysis of HCMV, HPV, and JCV prevalence between GC and any controls after excluding subjects with known H. pylori and Epstein-Barr virus positive
HCMV and GC
Five studies sought to detect HCMV in GC with 682 samples (265 for cases with GC, 417 for noncancer controls) (10,26,30,37,51). The pooled HCMV prevalence in GC was 43.1% (95% CI 14.2%–72.0%, random model, heterogeneity P < 0.001). The pooled OR showed that HCMV infection was highly related to the risk of GC (OR = 2.09, 95% CI 1.14–3.84, random model, heterogeneity P = 0.058) (see Figure 1b, Supplementary Digital Content 1, http://links.lww.com/CTG/A299). Two studies investigated the coinfection of H. pylori and EBV (10,26), and data could been extracted completely for cases and controls from only 1 study (10). We reanalyzed after excluding subjects with known H. pylori and EBV positive, and association between HCMV infection and the risk of GC was observed (OR = 2.25, 95% CI 1.14–4.43, random model, heterogeneity P = 0.079) (Figure 2b).
According to the different study regions, control type, and test material, subgroup analysis was conducted before and after excluding subjects with known H. pylori and EBV positive (Tables 4 and 5). Compared with noncancer healthy and benign gastric disease controls, the prevalence of HCMV in patients with GC was higher than that in PCNT controls significantly. By comparison with serum samples, association between HCMV infection and the risk of GC was observed in gastric tissues. Study location might be responsible for the heterogeneity among the studies from China (I2 = 23.2%, P = 0.252) and other regions (I2 = 66.4%, P = 0.051) (Table 5).
HPV and GC
Fourteen studies investigated the prevalence of HPV including 901 patients with GC and 1,205 any controls (11–14,22,25,31,39,43,46,49,50,53,54). The pooled HPV prevalence in GC was 23.6% (95% CI 15.5%–31.6%, random model, heterogeneity P < 0.001). Association between HPV infection and the risk of GC was observed (OR = 1.53, 95% CI 1.00–2.33, random model, heterogeneity P = 0.002) (see Figure 1c, Supplementary Digital Content 1, http://links.lww.com/CTG/A299). Five studies investigated the coinfection of H. pylori or EBV without extractable data for cases and controls (12,13,49,53,54). We reanalyzed after excluding controls with H. pylori-positive gastritis in the study by Bozdayi et al., and association still existed (OR = 1.63, 95% CI 1.05–2.54, random model, heterogeneity P = 0.002) (Figure 2c) (53).
According to publication year, study regions, sample size, control type, test material, case type, and subtype of HPV, subgroup analysis was conducted before and after excluding subjects with known H. pylori positive (Tables 4 and 5). HPV infection was associated with GC for studies from China, publication year from 2000 to 2009, and if tissue specimens were tested. Compared with noncancer healthy and benign gastric disease controls, the prevalence of HPV in patients with GC was higher than that in PCNT controls significantly. Statistically significant association between HPV-16 infection and the risk of GC was also observed (OR = 2.42, 95% CI 1.00–5.83, random model, heterogeneity P = 0.003) (Table 5).
HTLV-1 and GC
Serum antibodies to HTLV-1 were measured in 4,293 serum samples (463 cases with GC, 3,831 noncancer controls) in 2 studies (44,45). The pooled HTLV-1 prevalence was 6.20% (95% CI −5.3% to 17.7%, random model, heterogeneity P < 0.001). No association was observed between HTLV-1 infection and the risk of GC (OR = 0.89, 95% CI 0.61–1.30, fixed model, heterogeneity P = 0.272) (see Figure 1d, Supplementary Digital Content 1, http://links.lww.com/CTG/A299).
JCV and GC
Nine studies involving 1,356 tissue samples (692 for GC and 664 for any controls) investigated the association between JCV and GC (23,24,28,29,33,38,41,42,48). The pooled JCV prevalence was 35.6% (95% CI 23.2%–48.1%, random model, heterogeneity P < 0.001). Significant correlation between JCV infection and the risk of GC was revealed (OR = 2.28, 95% CI 1.14–4.56, random model, heterogeneity P < 0.001) (see Figure 1e, Supplementary Digital Content 1, http://links.lww.com/CTG/A299). Three studies investigated the coinfection of H. pylori or EBV, and data could be extracted completely for cases and controls from only 1 study (23,41,48). Subjects with JCV infection were not accompanied with EBV coinfection in the study by Cherati et al. (23). We reanalyzed after excluding H. pylori-positive controls in the study by Selgrad et al. and subjects with EBV positive in the study by Yamaoka et al., and association still existed (OR = 2.52, 95% CI 1.26–5.04, random model, heterogeneity P < 0.001) (Figure 2d) (41,48).
According to the publication year, study regions, sample size, control type, and the test method, subgroup analysis was conducted before and after excluding subjects with known H. pylori and EBV positive (Tables 4 and 5). JCV infection was associated with GC for studies from non-Asian regions, publication year from 2010 to 2019, and using polymerase chain reaction method or immunohistochemistry method. Compared with noncancer healthy and benign gastric disease controls, the prevalence of JCV in GC was higher than PCNT controls significantly (Table 5).
Sensitivity, publication bias, and heterogeneity
Sensitivity and publication bias were conducted for studies involving HBV, HCMV, HPV, and JCV. In the leave-one-out analysis, we found that the pooled ORs were always consistent by excluding any single study, which verified the reliability of this meta-analysis statistically (Figure 3). Because all P values for Begg test and Egger test were >0.05, it meant that no evidence for publication bias was indicated. The L'Abbé plot and the Galbraith plot demonstrated the existence of heterogeneity in this meta-analysis for HBV, HCMV, HPV, and JCV. The Begg funnel plot, Egger regression plot, L'Abbé plot, and Galbraith plot of the all-included meta-analysis are shown in Supplementary Figures 2–5, respectively (Supplementary Digital Content 2–5, http://links.lww.com/CTG/A294, http://links.lww.com/CTG/A295, http://links.lww.com/CTG/A296, http://links.lww.com/CTG/A297).
Figure 3.
Sensitivity analysis of the meta-analysis: (a) HBV; (b) HCMV; (c) HPV; and (d) JCV. CI, confidence interval; HBV, hepatitis B virus; HCMV, human cytomegalovirus; JCV, John Cunningham virus.
DISCUSSION
GC is one of the infection-associated malignancies, and the potential mechanisms inducing gastric carcinogenesis are well established for H. pylori and EBV (3–7). However, the potential carcinogenesis of viruses other than EBV associated with GC risk remains unclear until now; thus, this systematic review and meta-analysis was conducted to clarify this issue (see Figure legend Supplementary Digital Content 6, http://links.lww.com/CTG/A298). This systematic review and meta-analysis demonstrated that HBV, HCMV, HPV, and JCV are each associated with a statistically significantly increased risk of GC.
As a causative pathogen for hepatocellular carcinoma, HBV infection was shown to be associated with the risk of GC in some sporadic studies (9,57). One meta‐analysis that included 3 case–control studies and 5 cohort studies also found that HBV infection is associated with a higher risk for GC (OR = 1.23, 95% CI 1.10–1.37) (58). As shown in this study, HBV DNA was investigated directly in gastric mucosa tissue by polymerase chain reaction method only in 2 studies with the low positive rate (0%–3.2% in GC), which was consistent with a previous study (59). In addition, the prevalence of HBV might be underestimated for studies that applied serologic assay due to the existence of occult HBV infection in which circulating hepatitis B surface antigen is absent (60). However, HBV serologic assay are still irreplaceable effective method to evaluate the status of HBV infection currently, and low prevalence rates of occult HBV infection has been reported in Asia, where most of the studies included in this meta-analysis were performed (61–63).
As one of the 8 well-known herpes viruses, HCMV infection has been reported in immunocompetent patients with gastrointestinal disease such as gastritis, and its appearance was also detected in patients with carcinomas such as colon cancer (64–66). Michaelis et al. reported that HCMV might accelerate malignant process by strengthening tumor inflammation, which indicated its role in cancer (65). Our study demonstrated that the HCMV is associated with the risk of GC and the positivity of HCMV in GC tissues was higher than any controls especially. HCMV infection might contribute to gastritis and GC subsequently (10).
The role of HPV in the etiology of cervical cancer has been established, in addition to anal and colorectal cancers potentially (67–69). Our study verified the association between the prevalence of HPV (especially HPV-16, a high-risk subtype of HPV) and GC, and the pooled HPV prevalence in GC was 23.6%, no obvious difference from the previous report (70). Although HPV serologic test was considered as a good marker for HPV detection, significant association between HPV infection and the risk of GC was observed in gastric tissues by comparison with serum samples (71). Mechanisms of HPV escaping from the host immune system might explain this result (72). As a risk factor of malignant neoplasia of the digestive tract with epithelial mucosa, HPV-16 might play a role in the development of GC initiate with infecting gastric glandular epithelium cells (13).
Significant association between infection with HTLV-1 and reduced risk of GC was suggested in several studies (73–75). In our meta-analysis, no causal association between HTLV-1 infection and GC was found. However, correlation between HTLV-1 and gastric carcinogenesis was verified in another meta‐analysis, which included 3 cohort studies that inferred that HTLV-1 infection reduces the risk of GC by intervening H. pylori infection and proliferation possibly (76).
In addition, there are some other viruses, such as JCV, which belong to the polyomavirus family, were included in our study. The presence of JCV has been detected in the normal gastrointestinal tract and colon cancer in humans (77,78). In our study, the prevalence of JCV was associated with increased risk of GC. The presence of JCV transforming antigen was identified in all included studies, because transforming antigen, which is encoded by all polyomaviruses, was supposed to bind and inactivate tumor suppressor genes resulting in genomic instability, an important event in the oncogenesis progression (23,24,28,29,33,38,41,42,48).
Strengths and Limitations
To our knowledge, this study was the first meta-analysis to systematically evaluate the associations between the prevalence of viruses other than EBV and GC till now. However, because of limited published data, differences in sensitivity of test methods and test samples, sample size, and environmental or geographical factors, significant heterogeneity existed. Because of the insufficient number of included studies, meta-analysis was impossible for some viruses. Moreover, because of the property of study design and almost included studies did not take H. pylori or EBV coinfection into consideration, it was hard to determine the causal relationship between infection of viruses and risk of GC.
However, as one of the infection-associated malignancies, the development of GC is a multifactorial and multistep process. Several studies have shown that significant difference in H. pylori prevalence between the GC and any controls was not found, although H. pylori is a well-known carcinogenic associated with GC, and its prevalence is very common (3,4,26,32,79–81). We reanalyzed after excluding subjects with known H. pylori and EBV positive for studies involving HBV, HCMV, HPV, and JCV, and their relationships with the increased risk of GC had not changed. Wei et al. believed that evidence supporting the interaction between HBV and H. pylori was insufficient (9,82,83). Fattahi et al. found that HCMV viral loads were much higher in H. pylori-negative tissues compared with those in H. pylori-positive tissues, and the prevalence of H. pylori was lower in tumor tissues than that in nontumor tissues for GC (26,84). No correlation between HPV and H. pylori or EBV infection in gastric carcinogenesis was found in previous studies too (12,13,49).
CONCLUSION
In short, this study demonstrated that HBV, HCMV, HPV, or JCV were more prevalent in GC. However, the causal relationship between infection of HBV, HCMV, HPV, or JCV and risk of GC remains inconclusive, and further investigations are required (see PRISMA checklist 2009, Supplementary Digital Content 7, http://links.lww.com/CTG/A333).
CONFLICTS OF INTEREST
Guarantor of the article: Xin-Zu Chen, MD, PhD, and Jian-Kun Hu, MD, PhD, FRCS, gave final approval of the version to be published.
Specific author contributions: Hui Wang and Xiao-long Chen, MD, PhD, contributed equally to this work. H.W., X.-L.C., X.-Z.C., and J.-K.H.: made substantial contributions to conception and design of the study. H.W., X.-L.C., K.L., D.B., and W.-H.Z.: participated in this systematic review for literature search, selection, quality assessment, and data extraction. H.W. and X.-L.C.: drafted this systematic review. H.W., X.-L. C., and J.-K.H.: gave critical revision for important intellectual content. K.L., and W.-H.Z., X.-Z.C., and J.K.H: participated in critical revision for important intellectual content.
Financial support: (1)The 1•3•5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZY2017304); (2) National Natural Science Foundation of China, (No. 81702366); (3) Sichuan Science and Technology Program (No.2019YFS0255); (4) Wu Jieping Medical Foundation (No. 320.2710.1815, No.320.2710.1865).
Potential competing interests: None to report.
Ethics and dissemination: The meta-analysis was not submitted for any ethical approval due to the literature-based nature.
Registration: The present systematic review (registration number: CRD42015029703) was registered in the PROSPERO International Prospective Register of Systematic Reviews supported by the National Institute for Health Research of the National Health Service (NHS), UK (85).
Supplementary Material
Acknowledgment
The work is a joint with the Sichuan Gastric Cancer Early Detection and Screening (SIGES) research project. The authors thank the substantial work of the Volunteer Team of Gastric Cancer Surgery (VOLTGA), West China Hospital, Sichuan University, China.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at https://links.lww.com/CTG/A299, links.lww.com/CTG/A294, links.lww.com/CTG/A295, links.lww.com/CTG/A296, links.lww.com/CTG/A297, http://links.lww.com/CTG/A298, links.lww.com/CTG/A333
REFERENCES
- 1.Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer 2010;10:878–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohnishi N, Yuasa H, Tanaka S, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci U S A 2008;105:1003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wroblewski LE, Peek RM, Jr, Wilson KT. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin Microbiol Rev 2010;23:713–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang Q, Yao X, Tang S, et al. Integrative identification of Epstein-Barr virus-associated mutations and epigenetic alterations in gastric cancer. Gastroenterology 2014;147:1350–62.e4. [DOI] [PubMed] [Google Scholar]
- 6.Fukayama M. Epstein-Barr virus and gastric carcinoma. Pathol Int 2010;60:337–50. [DOI] [PubMed] [Google Scholar]
- 7.Herrera LA, Benitez-Bribiesca L, Mohar A, et al. Role of infectious diseases in human carcinogenesis. Environ Mol Mutagen 2005;45:284–303. [DOI] [PubMed] [Google Scholar]
- 8.Faghihloo E, Saremi MR, Mahabadi M, et al. Prevalence and characteristics of Epstein-Barr virus-associated gastric cancer in Iran. Arch Iran Med 2014;17:767–70. [PubMed] [Google Scholar]
- 9.Wei XL, Qiu MZ, Jin Y, et al. Hepatitis B virus infection is associated with gastric cancer in China: An endemic area of both diseases. Br J Cancer 2015;112:1283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Moral-Hernández O, Castañón-Sánchez CA, Reyes-Navarrete S, et al. Multiple infections by EBV, HCMV and Helicobacter pylori are highly frequent in patients with chronic gastritis and gastric cancer from Southwest Mexico: An observational study. Medicine (Baltimore) 2019;98:e14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turkay DO, Vural C, Sayan M, et al. Detection of human papillomavirus in esophageal and gastroesophageal junction tumors: A retrospective study by real-time polymerase chain reaction in an instutional experience from Turkey and review of literature. Pathol Res Pract 2015;212:77–82. [DOI] [PubMed] [Google Scholar]
- 12.Anwar K, Nakakuki K, Imai H, et al. Infection of human papillomavirus (HPV) and Epstein-Barr virus (EBV) and p53 overexpression in human gastric carcinoma. Int J Oncol 1995;7:391–7. [DOI] [PubMed] [Google Scholar]
- 13.Ma TY, Liu WK, Chu YL, et al. Detection of human papillomavirus type 16 DNA in formalin-fixed, paraffin-embedded tissue specimens of gastric carcinoma. Eur J Gastroenterol Hepatol 2007;19:1090–6. [DOI] [PubMed] [Google Scholar]
- 14.Xu WG, Zhang LJ, Lu ZM, et al. Detection of human papillomavirus type 16 E6 mRNA in carcinomas of upper digestive tract [Chinese]. Zhonghua Yi Xue Za Zhi 2003;83:1910–4. [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med 2009;151:W65–94. [DOI] [PubMed] [Google Scholar]
- 17.Wang R, Liu K, Chen XZ. Associations between gastric cancer risk and virus infection other than Epstein-Barr virus: The protocol of a systematic review and meta-analysis based on epidemiological studies. Medicine (Baltimore) 2019;98:e16708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. (http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm). Accessed December 29, 2018. [Google Scholar]
- 19.StataCorp LP. Stata/SE 12.0 for Windows. College Station, Texas: StataCorp LLC, 2011. (www.stata.com). [Google Scholar]
- 20.Hintze J. PASS 11. NCSS, LLC. Kaysville, Utah, 2011. (www.ncss.com). [Google Scholar]
- 21.Bai MJ, Zhou N, Dong W, et al. Seroprevalence and risk factors of hepatitis E virus infection in cancer patients in Eastern China. Int J Infect Dis 2018;71:42–7. [DOI] [PubMed] [Google Scholar]
- 22.Cândido ACL, de Lima Filho JL, Martins DBG, et al. Association of human papillomavirus genomic sequences by polymerase chain reaction in gastric carcinomas in Brazil. Anal Quant Cytol Histpathol 2013;35:1–6. [PubMed] [Google Scholar]
- 23.Cherati AY, Yahyapour Y, Ranaee M, et al. No evidence for an association between JC polyomavirus infection and gastroduodenal diseases. Gastrointest Tumors 2018;5:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi SH, Choi MG, Noh JH, et al. The expression of jamestown canyon virus(JCV) T-antigen and clinical manifestation in pT3 gastric cancer. Eur J Cancer 2011;47:S479. [Google Scholar]
- 25.Erol D, Bulut Y, Yüce H, et al. Investigation of the presence of human papillomavirus DNA in various gastrointestinal carcinoma samples [Turkish]. Mikrobiyol Bul 2009;43:259–68. [PubMed] [Google Scholar]
- 26.Fattahi S, Nikbakhsh N, Taheri H, et al. Prevalence of multiple infections and the risk of gastric adenocarcinoma development at earlier age. Diagn Microbiol Infect Dis 2018;92:62–8. [DOI] [PubMed] [Google Scholar]
- 27.Ghasemi M, Larijani LV, Abediankenari S. Investigation of relationship between hepatitis B virus and gastric adenocarcinoma. Iranian Red Crescent Med J 2012;14:453–4. [PMC free article] [PubMed] [Google Scholar]
- 28.Izi S, Youssefi M, Rahmani IF, et al. Detection of JC polyomavirus tumor antigen in gastric carcinoma: A report from Iran. Iranian J Microbiol 2018;10:266–74. [PMC free article] [PubMed] [Google Scholar]
- 29.Jang EJ, Jang JS, Kim JH, et al. Detection of JC virus T-Ag in early gastric cancer. Korean J Pathol 2010;44:456–61. [Google Scholar]
- 30.Jin J, Hu C, Wang P, et al. Latent infection of human cytomegalovirus is associated with the development of gastric cancer. Oncol Lett 2014;8:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamangar F, Qiao YL, Schiller JT, et al. Human papillomavirus serology and the risk of esophageal and gastric cancers: Results from a cohort in a high-risk region in China. Int J Cancer 2006;119:579–84. [DOI] [PubMed] [Google Scholar]
- 32.Kayamba V, Asombang AW, Mudenda V, et al. Gastric adenocarcinoma in Zambia: A case-control study of HIV, lifestyle risk factors, and biomarkers of pathogenesis. South Afr Med J 2013;103:256–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ksiaa F, Ziadi S, Mokni M, et al. The presence of JC virus in gastric carcinomas correlates with patient's age, intestinal histological type and aberrant methylation of tumor suppressor genes. Mod Pathol 2010;23:522–30. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Dong Y, Jiang J, Yang Y, Liu K, Li Y. High prevelance of human parvovirus infection in patients with malignant tumors. Oncol Lett 2012;3:635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahale P, Engels EA, Koshiol J. Hepatitis B virus infection and the risk of cancer in the elderly US population. Int J Cancer 2019;144:431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahmood EA. Relationship between Hepatitis C virus and cancer. Res J Pharm Biol Chem Sci 2018;9:1532–5. [Google Scholar]
- 37.Matveeva LV. Detection of the markers of herpesvirus infections in stomach diseases of inhabitants of the Republic of Mordovia. Vopr Virusol 2013;58:46–8. [PubMed] [Google Scholar]
- 38.Murai Y, Zheng HC, Osman H, et al. High JC virus load in gastric cancer and adjacent non-cancerous mucosa. Cancer Sci 2007;98:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roesch-Dietlen F, Cano-Contreras AD, Sanchez-Maza YJ, et al. Frequency of human papillomavirus infection in patients with gastrointestinal cancer. Rev Gastroenterol Mex 2018;83:253–8. [DOI] [PubMed] [Google Scholar]
- 40.Sabin AB, Tarro G. Herpes simplex and herpes genitalis viruses in etiology of some human cancers. Proc Natl Acad Sci U S A 1973;70:3225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selgrad M, Wex T, Kuester D, et al. JC virus is present in H. pylori-induced gastritis and gastric cancer. Helicobacter 2008;13:403–. [Google Scholar]
- 42.Shin SK, Goel A, Li MS, et al. Oncogenic T-antigen of Jc virus is present in the human gastric cancers. Gastroenterology 2005;128:A402. [Google Scholar]
- 43.Strickler HD, Schiffman MH, Shah KV, et al. A survey of human papillomavirus 16 antibodies in patients with epithelial cancers. Eur J Cancer Prev 1998;7:305–13. [DOI] [PubMed] [Google Scholar]
- 44.Tahaei SME, Mohebbi SR, Fatemi SR, et al. Low frequency of human T-cell lymphotropic virus 1 antibodies in Iranian gastric cancer patients in comparison to controls. Asian Pac J Cancer Prev 2011;12:2447–50. [PubMed] [Google Scholar]
- 45.Tanaka T, Hirata T, Parrott G, et al. Relationship among strongyloides stercoralis infection, human T-cell lymphotropic virus type 1 infection, and cancer: A 24-year cohort inpatient study in Okinawa, Japan. Am J Trop Med Hyg 2016;94:365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Doornum GJJ, Korse CM, Buning-Kager JCGM, et al. Reactivity to human papillomavirus type 16 Ll virus-like particles in sera from patients with genital cancer and patients with carcinomas at five different extragenital sites. Br J Cancer 2003;88:1095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei XL, Luo HY, Li CF, et al. Hepatitis B virus infection is associated with younger median age at diagnosis and death in cancers. Int J Cancer 2017;141:152–9. [DOI] [PubMed] [Google Scholar]
- 48.Yamaoka S, Yamamoto H, Nosho K, et al. Genetic and epigenetic characteristics of gastric cancers with JC virus T-antigen. World J Gastroenterol 2009;15:5579–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan XY, Wang MY, Wang XY, et al. Non-detection of Epstein-Barr virus and human papillomavirus in a region of high gastric cancer risk indicates a lack of a role for these viruses in gastric carcinomas. Genet Mol Biol 2013;36:183–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J, Tian XY, Wu XJ, et al. Role of papillomavirus in adenocarcinoma of esophagogastric junction [Chinese]. Zhonghua Yi Xue Za Zhi 2010;90:2259–62. [PubMed] [Google Scholar]
- 51.Zhang L, Guo GQ, Xu JF, et al. Human cytomegalovirus detection in gastric cancer and its possible association with lymphatic metastasis. Diagn Microbiol Infect Dis 2017;88:62–8. [DOI] [PubMed] [Google Scholar]
- 52.Baghbanian M, Hoseini Mousa SA, Doosti M, et al. Association between gastric pathology and hepatitis B virus infection in patients with or without Helicobacter pylori. Asian Pac J Cancer Prev 2019;20:2177–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bozdayi G, Dinc B, Avcikucuk H, et al. Is human papillomavirus and Helicobacter pylori related in gastric lesions? Clin Lab 2019;65:1807–12. [DOI] [PubMed] [Google Scholar]
- 54.Leon ME, Kassa E, Bane A, et al. Prevalence of human papillomavirus and Helicobacter pylori in esophageal and gastroesophageal junction cancer biopsies from a case-control study in Ethiopia. Infect Agents Cancer 2019;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song C, Lv J, Liu Y, et al. Associations between hepatitis B virus infection and risk of all cancer types. JAMA Netw Open 2019;2:e195718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.An J, Kim JW, Shim JH, et al. Chronic hepatitis B infection and non-hepatocellular cancers: A hospital registry-based, case-control study. PLoS One 2018;13:e0193232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewis R. Possible link between hepatitis B infection and gastric cancer. Lancet Oncol 2015;16:e159. [DOI] [PubMed] [Google Scholar]
- 58.Cui H, Jin Y, Chen F, et al. Clinicopathological evidence of hepatitis B virus infection in the development of gastric adenocarcinoma. J Med Virol 2020;92:71–7. [DOI] [PubMed] [Google Scholar]
- 59.Chen XZ, Wang R, Hu JK. Hepatitis B virus infection and gastric cancer risk: Pitfalls in the potential association. Br J Cancer 2015;112:1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raimondo G, Pollicino T, Cacciola I, et al. Occult hepatitis B virus infection. J Hepatol 2007;46:160–70. [DOI] [PubMed] [Google Scholar]
- 61.Zheng X, Ye X, Zhang L, et al. Characterization of occult hepatitis B virus infection from blood donors in China. J Clin Microbiol 2011;49:1730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuen MF, Lee CK, Wong DK, et al. Prevalence of occult hepatitis B infection in a highly endemic area for chronic hepatitis B: A study of a large blood donor population. Gut 2010;59:1389–93. [DOI] [PubMed] [Google Scholar]
- 63.Behzad-Behbahani A, Mafi-Nejad A, Tabei SZ, et al. Anti-HBc & HBV-DNA detection in blood donors negative for hepatitis B virus surface antigen in reducing risk of transfusion associated HBV infection. Indian J Med Res 2006;123:37–42. [PubMed] [Google Scholar]
- 64.Boeckh M, Geballe AP. Cytomegalovirus: Pathogen, paradigm, and puzzle. J Clin Invest 2011;121:1673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michaelis M, Doerr HW, Cinatl J. The story of human cytomegalovirus and cancer: Increasing evidence and open questions. Neoplasia 2009;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harkins L, Volk AL, Samanta M, et al. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet 2002;360:1557–63. [DOI] [PubMed] [Google Scholar]
- 67.Valentino K, Poronsky CB. Human papillomavirus infection and vaccination. J Pediatr Nurs 2016;31:e155–66. [DOI] [PubMed] [Google Scholar]
- 68.Harper DM, Vierthaler SL. Who should be targeted for vaccination against anal cancer? Lancet Oncol 2011;12:828–9. [DOI] [PubMed] [Google Scholar]
- 69.Chen H, Chen XZ, Waterboer T, et al. Viral infections and colorectal cancer: A systematic review of epidemiological studies. Int J Cancer 2015;137:12–24. [DOI] [PubMed] [Google Scholar]
- 70.Zeng ZM, Luo FF, Zou LX, et al. Human papillomavirus as a potential risk factor for gastric cancer: A meta-analysis of 1,917 cases. OncoTargets Ther 2016;9:7105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dillner J. The serological response to papillomaviruses. Semin Cancer Biol 1999;9:423–30. [DOI] [PubMed] [Google Scholar]
- 72.Bard E, Riethmuller D, Meillet D, et al. High-risk papillomavirus infection is associated with altered antibody responses in genital tract: Non-specific responses in HPV infection. 2004;17:381–9. [DOI] [PubMed] [Google Scholar]
- 73.Hirata T, Nakamoto M, Nakamura M, et al. Low prevalence of human T cell lymphotropic virus type 1 infection in patients with gastric cancer. J Gastroenterol Hepatol 2007;22:2238–41. [DOI] [PubMed] [Google Scholar]
- 74.Arisawa K, Sobue T, Yoshimi I, et al. Human T-lymphotropic virus type-I infection, survival and cancer risk in southwestern Japan: A prospective cohort study. Cancer Causes Control 2003;14:889–96. [DOI] [PubMed] [Google Scholar]
- 75.Matsumoto S, Yamasaki K, Tsuji K, et al. Human T lymphotropic virus type 1 infection and gastric cancer development in Japan. J Infect Dis 2008;198:10–5. [DOI] [PubMed] [Google Scholar]
- 76.Schierhout G, McGregor S, Gessain A, et al. Association between HTLV-1 infection and adverse health outcomes: A systematic review and meta-analysis of epidemiological studies. Lancet Infect Dis 2020;20:133–43. [DOI] [PubMed] [Google Scholar]
- 77.Boland CR, Bigler J, Newcomb PA, et al. Evidence for an association between JC virus and colorectal neoplasia. Cancer Epidemiol Biomarkers Prev 2004;13:2285–6. [PubMed] [Google Scholar]
- 78.Laghi L, Randolph AE, Chauhan DP, et al. JC virus DNA is present in the mucosa of the human colon and in colorectal cancers. Proc Natl Acad Sci U S A 1999;96:7484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev 2006;19:449–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Estevens J, Fidalgo P, Tendeiro T, et al. Anti-Helicobacter pylori antibodies prevalence and gastric adenocarcinoma in Portugal: Report of a case-control study. Eur J Cancer Prev 1993;2:377–80. [DOI] [PubMed] [Google Scholar]
- 81.Peleteiro B, Lunet N, Barros R, et al. Factors contributing to the underestimation of Helicobacter pylori-associated gastric cancer risk in a high-prevalence population. Cancer Causes Control 2010;21:1257–64. [DOI] [PubMed] [Google Scholar]
- 82.Chen NL, Bai L, Deng T, et al. Expression of hepatitis B virus antigen and Helicobacter pylori infection in gastric mucosa of patients with chronic liver disease. Hepatobiliary Pancreat Dis Int 2004;3:223–5. [PubMed] [Google Scholar]
- 83.Kirchner GI, Beil W, Bleck JS, et al. Prevalence of Helicobacter pylori and occurrence of gastroduodenal lesions in patients with liver cirrhosis. Int J Clin Exp Med 2011;4:26–31. [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang L, Guo G, Xu J, et al. Human cytomegalovirus detection in gastric cancer and its possible association with lymphatic metastasis. Diagn Microbiol Infect Dis 2017;88:62–8. [DOI] [PubMed] [Google Scholar]
- 85.Chen XZ, Hu JK. Associations between Gastric Cancer Risk and Virus Infection Other than EBV: A Systematic Review and Meta-Analysis Based on Epidemiological Studies. PROSPERO International Prospective Register of Systematic Reviews 2015; (Registration number: CRD42015029703). (http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015029703). Accessed July 22, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.