Abstract
Background:
Usage of chemical mouthwashes for controlling dental caries can end to some side effects such as oral biological imbalance. Recently, using natural derivatives such as herbs, presented to overcome such adverse effects. Due to antibacterial property of olive leaf extracts (Olea europaea), this study conducted in order to evaluate bacteriocidal, anti-acid production, and anti-adhesion effects of olive leaf ethanolic, methanolic, and hydroalcoholic extracts on Streptococcus mutans.
Materials and Methods:
In this in vitro study, nine strains of S. mutans (PTCC1683) were used. Maceration methods were done in order to provide the olive leaf ethanolic, methanolic, and hydroalcoholic extracts. The antibacterial activities evaluated by macrodilution and disc diffusion method in different concentrations (3.25%–100%). Acid production and adhesion of bacterial strains also were evaluated. The obtained data were analyzed by analysis of variance method using SPSS software. P < 0.05 was considered as statistically significant.
Results:
The minimum inhibitory concentration and minimum bactericidal concentration for ethanolic, methanolic, and hydroalcoholic olive leaf extracts on S. mutans are 12%–25%, 50%–75%, and 12%–25%, respectively. In addition, inhibition zone of S. mutans significantly increased in higher concentration (ethanolic and methanolic extracts: P = 0.004; hydroalcoholic extract: P = 0.003). The acid production and adhesion significantly decreased by increase in the concentration (P < 0.001).
Conclusion:
In general, olive leaf ethanolic, methanolic, and hydroalcoholic extracts induce growth inhibition, acid production, and adhesion of S. mutans. Consequently, it can be used as a natural preservative in the food industry, as well as in the production of commercial products such as chewing gum, chocolate, and toothpaste to prevent dental caries.
Key Words: Bacterial adhesion, microbial sensitivity tests, Olea, Streptococcus mutans
INTRODUCTION
Dental caries is the most prevalent chronic bacterial disease observed in people worldwide. It forms through a complex interaction over time between acid-producing bacteria and fermentable carbohydrate, and the host factors including saliva and teeth.[1,2] Dental caries is caused by the bacterial activity that can efficiently create an acidic environment to remove the dental minerals. This gelatinous mass of bacteria that binds to the surface of the tooth is called dental plaque.[3] Several (above 330) bacterial species may colonize the buccal cavity of the adults; however, only a small group of bacteria can produce acid and cause caries. The main strains involved in this process are Streptococci (Streptococcus mutans and Streptococcus sobrinus) and Lactobacillus.[4,5]S. mutans is known to be the main etiologic factor of decay[6] and is naturally present in the mouth as a small group of oral microbial complexes.[7]
The routine methods of decay prevention include mechanical methods (toothbrush–dental floss) and the use of chemical agents with antimicrobial features, such as mouthwashes. The use of chemical agents may lead to microbial resistance and can also cause certain complications by disturbing the biological equilibrium of the mouth.[8]
In recent years, due to the increasing resistance of pathogenic microorganisms to the chemical antimicrobials and the side effects of these compounds, researches on medicinal plants have been considered in order to discover new antibacterial sources.[9] Medicinal plants are a rich source of active biological compounds with advanced antibacterial properties.[10] The most known cause of antimicrobial activity in medicinal plants is the presence of phytoalexins compounds, which are small antibiotics with <500 molecular weight, and are divided to several groups including polyphenols, flavonoids, terpenoids, and glycosteroids.[11] Recently, efforts have been increased to identify new therapeutic strategies against oral microbes, using plant compounds. Regarding the presence of secondary metabolites, plants have significant pharmacological effects toward different microorganisms and their synergistic patterns.[10]
Olive (Olea europaea) leaf have been widely used in folk medicine over centuries.[12] Olive leaf extract contains a noticeable amount of polyphenols, which is responsible for its various medicinal properties.[13] Several studies have reported the antimicrobial activity of different medicinal plants and its biological compounds.[14,15,16,17] However, very limited studies have been done on antimicrobial effects of olive leaf on bacteria in oral flora.[10]
Consequently, the aim of this study was to investigate the inhibitory effects of ethanolic, methanolic, and hydroalcoholic extracts of O. europaea leaf on growth, acid production, and adhesion of S. mutans.
MATERIALS AND METHODS
Preparation of extract
This study was approved by the research and ethics committee of Isfahan university of medical sciences No: 295045. In this in vitro study, the fresh olive leaves were collected from olive gardens in Shahin Shahr, Isfahan province, Iran, in 2015. The obtained olive leaf dried in 3 days at room temperature and then powdered. The preparation of plant extracts was performed by maceration method.[18] About 500 ml of solvent was added to 100 g of olive leaf powder and stirred for 5 days at room temperature. The obtained solutions were filtered and the remaining plant was pressed by press instrument. To prevent chemical changes of extracts, due to exposure to sunlight, preparation of extracts was carried out in a dark place. The obtained extracts were kept at sterilized vials at 4°C.
Preparation of bacterial strains
In this study, nine strains of S. mutans PTCC1683 (Persian Type Culture Collection, Tehran) were purchased from microbial collection of Iranian Research Organization for Science and Technology, and bacterial suspension was prepared according to the 0.5 McFarland standard (1.5 × 108 CFU/ml).
Evaluation of antimicrobial activity by macrodilution method
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) determination, was performed according to the Clinical and Laboratory Standards Institute (CLSI) guide.[19,20] The Brain Heart Infusion broth (BHI) (Merck, Darmstadt, Germany) was added (1 ml) to each tube. Then, 100% concentration of leaf extracts was added to the first well and other dilutions (10 concentrations) were prepared by serial macrodilution method. Following this, 1 ml bacterial suspension equivalent (1.5 × 108 CFU/ml) was added to each tubes. Furthermore, two tubes were considered as positive (medium with bacterial suspension) and negative (medium with extracts) controls, respectively. The tubes were incubated for 18–24 h at 37°C and then its opacity was evaluated.
The first tube without opacity was considered as MIC and was cultured on a BHI broth and incubated for 18–24 h at 37°C. The lowest concentration at which an antimicrobial agent will kill a particular microorganism was considered MBC. This experiment was repeated three times for each ethanolic, methanolic, and hydroalcoholic extracts.
Evaluation of antimicrobial activity by disc diffusion
According to the CLSI guidelines,[21] using a sterile inoculating loop, four or five isolated colonies were cultured uniformly on Mueller–Hinton agar. The blank discs containing prepared concentrations of extracts are placed on inoculated Mueller–Hinton agar and incubated at 37°C during the night. The diameter of inhibition zone was measured and result read from the Kirby–Bauer chart as sensitive, intermediate, or resistant.[22]
Evaluation of acid production
The method described by Matsumoto et al.[23] was used to investigate the effect of ethanolic, methanolic, and hydroalcoholic extracts of olive leaf on acid production by S. mutans. The sterile extracts of olive leaf (0.1–0.5 mg/ml) and bacterial suspension (0.05 ml) were added to 0.95 ml phenol red solution containing 1% glucose. After 24 h incubation at 37°C, the pH was measured. The solution of sodium fluoride 1% was used as a positive control. This experiment was repeated three times for each ethanolic, methanolic, and hydroalcoholic extracts.
Evaluation of adhesion
The method described by Köhler et al.[24] was used to investigate the effect of ethanolic, methanolic, and hydroalcoholic extracts of olive leaf on adhesion by S. mutans to the glass. The suspension of S. mutans was cultured in test tubes containing ethanolic, methanolic, and hydroalcoholic extracts of olive leaf (3.125%–100%) for 18 h at 37°C. Then, the contents of the tubes were discarded and the sticked to bacteria to the tube were suspended in distilled water by a shaker. The bacterial colonies were measured by WPA Biowave II spectrophotometer (Biochrom UK) at 550 nm. This experiment was repeated three times for each ethanolic, methanolic, and hydroalcoholic extracts.
Statistic analysis
Statistical analysis of obtained data was performed using the Statistical Package for the Social Sciences software version 22.0 (Chicago, IL, USA). The experiments were repeated three times and obtained data were analyzed by analysis of variance (ANOVA) method and Bonferroni test. P < 0.05 was considered as statistically significant.
RESULTS
In this study, the antibacterial effects of ethanolic, methanolic, and hydroalcoholic extracts of olive leaf were determined using MIC, MBC, and inhibition zone diameter. The MIC and MBC values of the extracts are shown in Table 1.
Table 1.
The minimum inhibitory concentration and minimum bactericidal concentration of olive leaf ethanolic, methanolic, and hydroalcoholic extracts on Streptococcus mutans (%)
| Extract type | MIC (%) | MBC (%) |
|---|---|---|
| Ethanolic extracts | 12 | 25 |
| Methanolic extracts | 50 | 75 |
| Hydroalcoholic extracts | 12 | 25 |
MIC: Minimum inhibitory concentration; MBC: Minimum bactericidal concentration
Analysis of various concentrations effects of ethanolic, methanolic, and hydroalcoholic extracts of olive leaf on bacterial growth (inhibition zone) of S. mutans was performed using one-way ANOVA. The results showed [Table 2] that the inhibition zone had a significant correlation with concentration in all three extracts at higher than 6.25% concentrations (ethanolic and methanolic extracts: P = 0.004; hydroalcoholic extract: P = 0.003).
Table 2.
The mean and standard deviation of Streptococcus mutans inhibition zone in the effects of different concentrations of ethanolic, methanolic, and hydroalcoholic extracts of olive leaf (mm)
| Concentrations (%) | Ethanolic extracts |
Methanolic extracts |
Hydroalcoholic extracts |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| 100 | 21.93 | 2.12 | 20.50 | 1.55 | 14.63 | 1.24 |
| 50 | 14.97 | 4.06 | 9.73 | 0.46 | 8.39 | 0.80 |
| 25 | 9.07 | 1.63 | 6.97 | 0.34 | 6.76 | 0.43 |
| 12.5 | 6.77 | 0.26 | 6.45 | 0.03 | 6.45 | 0.57 |
| 6.25 | 6.41 | 0.17 | 6.40 | 0.01 | 6.40 | 0.00 |
| 3.125 | 6.40 | 0.00 | 6.40 | 0.00 | 6.40 | 0.00 |
| Negative control | 6.41 | 0.12 | 6.40 | 0.00 | 6.40 | 0.00 |
| Statistically significant | <0.004 | <0.004 | <0.003 | |||
SD: Standard deviation
Effects of extract type on diameter of inhibition zone were analyzed using the Bonferroni test. The results showed that inhibition zone of ethanolic and methanol extracts was equal (P > 0.05) and higher than hydroalcoholic extract (P < 0.05), at 100% concentration. Furthermore, diameter of inhibition zone of methanolic and hydroalcoholic extracts was equal (P > 0.05) and less than ethanolic extract (P < 0.05), at 25% and 50% concentrations. There was no significant difference in inhibition zone of ethanolic, methanolic, and hydroalcoholic extracts, at 1.625%, 3.125%, 6.25%, and 12.5% concentrations (P > 0.05), respectively [Figure 1].
Figure 1.
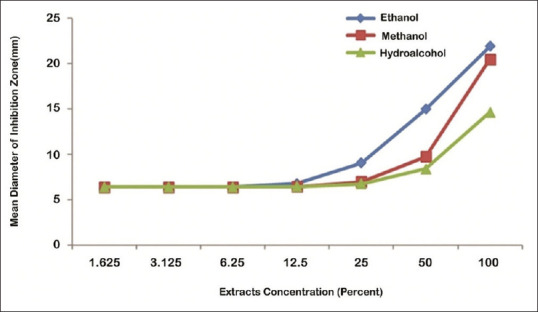
The mean of Streptococcus mutans inhibition zone in the effects of different concentrations of ethanolic, methanolic, and hydroalcoholic extracts of olive leaf
Analysis of various concentrations effects of ethanolic, methanolic, and hydroalcoholic extracts of olive leaf on adhesion of S. mutans was performed using one-way ANOVA. The results showed that increase of concentration of ethanolic, methanolic, and hydroalcoholic extracts decreases adhesion of S. mutans, significantly (P < 0.001) [Table 3].
Table 3.
The mean and standard deviation of Streptococcus mutans adhesion in the effects of different concentrations of ethanolic, methanolic, and hydroalcoholic extracts of olive leaf (colony number)
| Concentrations (%) | Ethanolic extracts |
Methanolic extracts |
Hydroalcoholic extracts |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Negative control | 1.73 | 0.10 | 1.73 | 0.10 | 1.73 | 0.10 |
| 3.125 | 1.17 | 0.06 | 1.51 | 0.12 | 1.35 | 0.06 |
| 6.25 | 0.79 | 0.07 | 1.44 | 0.16 | 1.10 | 0.06 |
| 12.5 | 0.75 | 0.01 | 1.36 | 0.01 | 0.91 | 0.03 |
| 25 | 0.61 | 0.07 | 1.14 | 0.10 | 0.87 | 0.04 |
| 50 | 0.52 | 0.04 | 0.94 | 0.10 | 0.79 | 0.02 |
| Adhesion mean | 0.93 | 0.43 | 1.36 | 0.29 | 1.12 | 0.34 |
| Statistically significant | <0.001 | <0.001 | <0.001 | |||
SD: Standard deviation
Effects of extract type on adhesion were analyzed using one-way ANOVA. The results showed that there was a significant difference in inhibition of bacterial adhesion by ethanolic, methanolic, and hydroalcoholic extracts (P = 0.003). The highest and the lowest inhibition of bacterial adhesion are related to ethanolic and methanolic extracts, respectively [Table 3].
Analysis of simultaneous effect of the concentration and type of extract on adhesion of S. mutans was performed using two-way ANOVA. The results showed that the difference of inhibition of bacterial adhesion mean by different concentrations of ethanolic, methanolic, and hydroalcoholic extracts was statistically significant (P < 0.001).
Effects of extract type on adhesion of S. mutans were analyzed using Bonferroni test. The results showed that inhibition of bacterial adhesion by methanolic extracts was significantly more than hydroalcoholic and ethanolic extracts (P < 0.05), at 50%, 25%, 12.5%, and 6.25% concentrations. Furthermore, inhibition of bacterial adhesion by methanolic and hydroalcoholic extract was equal (P = 0.053) and less than ethanolic extract (P < 0.05), at 3.125% concentration [Figure 2].
Figure 2.
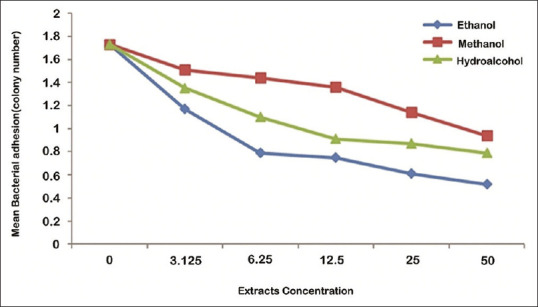
The mean of Streptococcus mutans adhesion in the effects of different concentrations of ethanolic, methanolic, and hydroalcoholic extracts of olive leaf
Effects of different concentrations of ethanolic, methanolic, and hydroalcoholic extracts of olive leaf on acid production by S. mutans were analyzed using one-way ANOVA. The results showed that increase of concentration of ethanolic, methanolic, and hydroalcoholic extracts decreases acid production by S. mutans, significantly (P < 0.001) [Table 4].
Table 4.
The mean and standard deviation of Streptococcus mutans acid production in the effects of different concentrations of ethanolic, , and hydroalcoholic extracts of olive leaf (pH)
| Concentrations (%) | Ethanolic extracts |
Methanolic extracts |
Hydroalcoholic extracts |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Negative control | 5.43 | 0.03 | 5.43 | 0.03 | 5.54 | 0.03 |
| 3.125 | 5.81 | 0.01 | 5.38 | 0.23 | 5.77 | 0.06 |
| 6.25 | 6.23 | 0.05 | 5.40 | 0.01 | 5.93 | 0.14 |
| 12.5 | 6.66 | 0.23 | 5.87 | 0.12 | 6.35 | 0.21 |
| 25 | 7.08 | 0.03 | 6.27 | 0.25 | 7.00 | 0.00 |
| 50 | 7.17 | 0.03 | 6.90 | 0.17 | 7.07 | 0.06 |
| pH mean | 6.40 | 0.66 | 5.87 | 0.58 | 6.25 | 0.63 |
| Statistically significant | <0.001 | <0.001 | <0.001 | |||
SD: Standard deviation
Effects of extract type on inhibition of acid production were analyzed using one-way ANOVA. The results showed that there was a significant difference in inhibition of acid production by ethanolic, methanolic, and hydroalcoholic extracts (P = 0.003). The highest and the lowest inhibition of acid production are related to ethanolic and methanolic extracts, respectively [Table 3].
Analysis of the simultaneous effect of the concentration and type of extract on an acid production was performed using two-way ANOVA. The results showed that the pH mean of medium was statistically significant at different concentrations (P < 0.001). The results showed that the difference of pH mean in different concentrations of ethanolic, methanolic, and hydroalcoholic extracts was statistically significant (P < 0.001).
Effects of extract type on inhibition of acid production were analyzed using the Bonferroni test. The results showed that inhibition of acid production by ethanolic and hydroalcoholic extracts was equal (P > 0.05) and more than methanolic extract (P < 0.05), at 50%, 25%, 12.5%, and 3.125% concentration. Furthermore, inhibition of acid production by ethanolic extracts was significantly more than hydroalcoholic and methanolic extracts (P < 0.05), at 6.25% concentrations [Figure 3].
Figure 3.
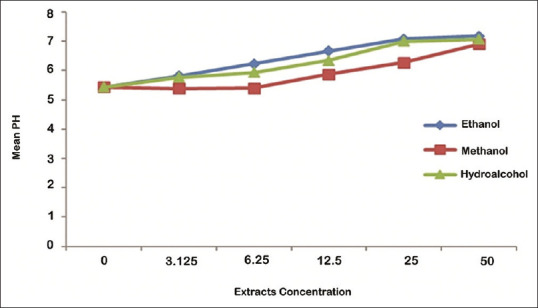
The mean of Streptococcus mutans acid production in the effects of different concentrations of ethanolic, methanolic, and hydroalcoholic extracts of olive leaf
DISCUSSION
The results of the present study showed that ethanolic, methanolic, and hydroalcoholic extracts of olive leaf inhibit growth, adhesion, and acid production by S. mutans. So far, several studies have investigated the antimicrobial effect of olive fruit on various bacterial strains, such as S. mutans;[10,25,26] however, very limited studies have investigated the antibacterial activity of olive leaf on S. mutans.[10] Olive leaf extract was effective against Gram-negative pathogens such as Porphyromonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum. Sudjana et al. reported antibacterial action of olive leaf against Campylobacter jejuni, Helicobacter pylori, and Staphylococcus aureus.[13]
Olive fruit and leaf have high antibacterial activity due to the presence of phenolic compounds in its extracts, including oleuropein and hydroxytyrosol.[27,28] In the study of Karygianni et al., oleuropein content of olive leaf and fruit extracts was reported 60% and 15%, respectively.[10] Regarding different levels of phenolic compounds in olive fruit and leaf, their antibacterial properties are also different, probably.
Pereira et al. reported an appropriate antimicrobial effects for olive leaf on Gram-positive bacteria, including Bacillus cereus.[29]
The cause of cell wall destruction of Gram-positive bacteria by olive extracts is probably due to the presence of antiquorum sensing (QS) compounds.[30] Other plants compounds with antibacterial effect against S. mutans are Thymus deanesis, Thymus valgaris, thymol, and carvacrol.[31]
Contrary to the present study, the results of Karygianni et al. showed that the olive leaf extract does not have a significant bactericidal effect on S. mutans and only inhibits its growth.[10] This variation may be due to differences amount of phenolic compounds in the studied olive leaf. Studies have shown that climate, weather conditions, and harvest time of a plant could effect on type and amount of extracts compounds.[32]
The results of this study showed that an increase in antibacterial properties of the studied extracts was associated by an increase in its concentration. However, this association was observed at higher than 6.25% concentrations. This is probably due to an increase in the amount of the extract compounds by increasing its concentration, which leads to a stronger antibacterial effect. Korukluoglu et al. showed that type of used solvent could effect on the amount of phenolic compounds and also antibacterial properties of extracts.[32] In the present study, it was observed that the type of used solvent (ethanol, methanol, and hydroalcohol) effects on inhibition of growth, acid production, and adhesion of S. mutans, so that similar concentrations of different extracts show a different effects on growth, acid production, and adhesion of S. mutans. S. mutans gradually cause damage to tooth enamel and dental caries, because of adhesion to dental surfaces and the production of acidic lactic after metabolizing foodstuffs.[33,34,35]
Casagrande et al. reported that the solvent type could effect on antimicrobial properties of plants extract, and ethanol is the best solvent to extract phenolic compounds.[36] The present study also showed that the ethanolic extract causes the most inhibition of adhesion and acid production of S. mutans. This is probably due to the difference in the amount of polyphenols extraction by various solvents.
Šikić Pogačar et al. showed that the extract of olive leaf (with 3.125–200 μg/mL-1 concentration) decreased 10% to 23% of C. jejuni adhesion.[37] The polyphenolic compounds can interact with proteins, enzymes, and membranes of microbes, and thus changes in cell permeability, and loss of protons, ions and macromolecules, which can lead to inhibition of bacterial adhesion.[38]
Contrary to the present study, Rafiei et al. showed that the methanolic extracts of olive leaf contain the highest amount of phenolic compounds and also has the highest bactericidal effects on S. aureus and Escherichia coli strains.[39]
The limitations of the present study are failure to determine of minimum bactericidal time, as well as efficacy of olive leaf extracts in inhibition of bacterial adhesion to the tooth surface (hydroxyapatite), denture prosthesis, and oral applicators. Furthermore, the present study performed in vitro and cannot show accurate inhibition of acid production by olive leaf extracts in a saliva-containing medium. Therefore, it is suggested that the bacterial growth curve based on time, as well as inhibition of adhesion to mentioned dental structures and inhibition of acid production in the presence of saliva, will be investigated by other studies.
CONCLUSION
In general, it can be concluded that methanolic, ethanolic, and hydroalcoholic extracts of olive leaf extracts have appropriate antibacterial activities due to their high phenolic content and can also reduce bacterial adhesion in dental plaque formation, as well as increases pH of the oral environment. Therefore, olive leaf extracts can be used as a natural preservative in the food industry, as well as in the production of commercial products such as chewing gum, chocolate, and toothpaste to prevent dental caries.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Byun R, Nadkarni MA, Chhour KL, Martin FE, Jacques NA, Hunter N, et al. Quantitative analysis of diverse Lactobacillus species present in advanced dental caries. J Clin Microbiol. 2004;42:3128–36. doi: 10.1128/JCM.42.7.3128-3136.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369:51–9. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 3.Caglar E, Kavaloglu SC, Kuscu OO, Sandalli N, Holgerson PL, Twetman S. Effect of chewing gums containing xylitol or probiotic bacteria on salivary mutans streptococciand lactobacilli. Clin Oral Investig. 2007;11:425–9. doi: 10.1007/s00784-007-0129-9. [DOI] [PubMed] [Google Scholar]
- 4.Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, et al. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008;46:1407–17. doi: 10.1128/JCM.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aklaghi N, Mortazavi S, Akhlaghi N. Relationship between salivary Streptococcus mutans and Lactobacillus counts and caries in adults with a high level of dental care. J Isfahan Dent Sch. 2011;6:750–9. [Google Scholar]
- 6.Nakano K, Nomura R, Nakagawa I, Hamada S, Ooshima T. Demonstration of Streptococcus mutans with a cell wall polysaccharide specific to a new serotype, k, in the human oral cavity. J Clin Microbiol. 2004;42:198–202. doi: 10.1128/JCM.42.1.198-202.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Söderling E, Hirvonen A, Karjalainen S, Fontana M, Catt D, Seppä L, et al. The effect of xylitol on the composition of the oral flora: A pilot study. Eur J Dent. 2011;5:24–31. [PMC free article] [PubMed] [Google Scholar]
- 8.Badet C, Quero F. The in vitro effect of manuka honeys on growth and adherence of oral bacteria. Anaerobe. 2011;17:19–22. doi: 10.1016/j.anaerobe.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Bonjar S. Evaluation of antibacterial properties of some medicinal plants used in Iran. J Ethnopharmacol. 2004;94:301–5. doi: 10.1016/j.jep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Karygianni L, Cecere M, Skaltsounis AL, Argyropoulou A, Hellwig E, Aligiannis N, et al. High-level antimicrobial efficacy of representative Mediterranean natural plant extracts against oral microorganisms. Biomed Res Int. 2014;2014:839019. doi: 10.1155/2014/839019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemaiswarya S, Kruthiventi AK, Doble M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine. 2008;15:639–52. doi: 10.1016/j.phymed.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Gucci R, Lombardini L, Tattini M. Analysis of leaf water relations in leaves of two olive (Olea europaea) cultivars differing in tolerance to salinity. Tree Physiol. 1997;17:13–21. doi: 10.1093/treephys/17.1.13. [DOI] [PubMed] [Google Scholar]
- 13.Sudjana AN, D'Orazio C, Ryan V, Rasool N, Ng J, Islam N, et al. Antimicrobial activity of commercial Olea europaea (Olive) leaf extract. Int J Antimicrob Agents. 2009;33:461–3. doi: 10.1016/j.ijantimicag.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Bisignano G, Tomaino A, Lo Cascio R, Crisafi G, Uccella N, Saija A. On the in vitro antimicrobial activity of oleuropein and hydroxytyrosol. J Pharm Pharmacol. 1999;51:971–4. doi: 10.1211/0022357991773258. [DOI] [PubMed] [Google Scholar]
- 15.Aziz NH, Farag SE, Mousa LA, Abo-Zaid MA. Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios. 1998;93:43–54. [PubMed] [Google Scholar]
- 16.Lee OH, Lee BY. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea l eaf extract. Bioresour Technol. 2010;101:3751–4. doi: 10.1016/j.biortech.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 17.Sousa A, Ferreira IC, Calhelha R, Andrade PB, Valentão P, Seabra R, et al. Phenolics and antimicrobial activity of traditional stoned table olives 'alcaparra'. Bioorg Med Chem. 2006;14:8533–8. doi: 10.1016/j.bmc.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 18.United Nations Industrial Development Organization. Handa SS, Khanuja SP, Longo G, Rakesh DD. Extraction Technologies for Medicinal and Aromatic Plants: Earth, Environmental and Marine Sciences and Technologies. United Nations Industrial Development Organization. 2008 [Google Scholar]
- 19.Jones RN, Pfaller MA, Rhomberg PR, Walter DH. Tiamulin activity against fastidious and nonfastidious veterinary and human bacterial isolates: Initial development of in vitro susceptibility test methods. J Clin Microbiol. 2002;40:461–5. doi: 10.1128/JCM.40.2.461-465.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wikler MA. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: Approved standard. CLSI (NCCLS) 2006;26:M7–A. [Google Scholar]
- 21.Forster H, Marotta JS, Heseltine K, Milner R, Jani S. Bactericidal activity of antimicrobial coated polyurethane sleeves for external fixation pins. J Orthop Res. 2004;22:671–7. doi: 10.1016/j.orthres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Biemer JJ. Antimicrobial susceptibility testing by the Kirby-Bauer disc diffusion method. Ann Clin Lab Sci. 1973;3:135–40. [PubMed] [Google Scholar]
- 23.Matsumoto M, Minami T, Sasaki H, Sobue S, Hamada S, Ooshima T. Inhibitory effects of oolong tea extract on caries-inducing properties of mutans streptococci. Caries Res. 1999;33:441–5. doi: 10.1159/000016549. [DOI] [PubMed] [Google Scholar]
- 24.Köhler B, Krasse B, Carlén A. Adherence and Streptococcus mutans infections:In vitro study with saliva from noninfected and infected preschool children. Infect Immun. 1981;34:633–6. doi: 10.1128/iai.34.2.633-636.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bafna HP, Krishnan CA, Kalantharakath T, Kalyan P, Arhi RP. An in vitro evaluation of the effect of anti-candidal herb (Olive) on Streptococcus mutans. Indian J Oral Health Res. 2015;1:20–6. [Google Scholar]
- 26.Kubo A, Lunde CS, Kubo I. Antimicrobial activity of the olive oil flavor compounds. J Agric Food Chem. 1995;43:1629–33. [Google Scholar]
- 27.Ranalli A, Contento S, Lucera L, Di Febo M, Marchegiani D, Di Fonzo V. Factors affecting the contents of iridoid oleuropein in olive leaves (Olea europaea L.) J Agric Food Chem. 2006;54:434–40. doi: 10.1021/jf051647b. [DOI] [PubMed] [Google Scholar]
- 28.Obied HK, Prenzler PD, Omar SH, Ismael R, Servili M, Esposto S, et al. Pharmacology of olive biophenols. Advances in molecular toxicology. 6: Elsevier. 2012:195–242. [Google Scholar]
- 29.Pereira AP, Ferreira IC, Marcelino F, Valentão P, Andrade PB, Seabra R, et al. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. cobrançosa) leaves. Molecules. 2007;12:1153–62. doi: 10.3390/12051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray B, Hall P, Gresham H. Targeting agr- and agr-like quorum sensing systems for development of common therapeutics to treat multiple gram-positive bacterial infections. Sensors (Basel) 2013;13:5130–66. doi: 10.3390/s130405130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khozeimeh F, Golestannejhad Z, Hossein Moaddabi A, Hassanabadi ME, Farokhi H, Shiva A. In vitro comparison of anti-bacterial effect of Shirazian and T. deanesis essential oil and their effective ingredients thymol and carvacrol on S. mutans strain. J Res Med Dent Sci. 2018;6:188–98. [Google Scholar]
- 32.Korukluoglu M, Sahan Y, Yigit A, Ozer ET, Gucer S. Antibacterial activity and chemical constitutions of Olea europaea L. leaf extracts. J Food Process Preservation. 2010;34:383–96. [Google Scholar]
- 33.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–80. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamrani YY, Amanlou M, Esmaeelian B, Bidhendi SM, Jamei MS. Inhibitory effects of a flavonoid-rich extract of Pistacia vera hull on growth and acid production of bacteria involved in dental plaque. Int J Pharmacol. 2007;3:219–26. [Google Scholar]
- 35.Finkelstein P, Yost KG, Grossman E. Mechanical devices versus antimicrobial rinses in plaque and gingivitis reduction. Clin Prev Dent. 1990;12:8–11. [PubMed] [Google Scholar]
- 36.Casagrande M, Zanela J, Júnior AW, Busso C, Wouk J, Iurckevicz G, et al. Influence of time, temperature and solvent on the extraction of bioactive compounds of Baccharis dracunculifolia: In vitro antioxidant activity, antimicrobial potential, and phenolic compound quantification. Ind Crops Prod. 2018;125:207–19. [Google Scholar]
- 37.Šikić Pogačar M, Klančnik A, Bucar F, Langerholc T, Smole Možina S. Anti-adhesion activity of thyme (Thymus vulgaris L.) extract, thyme post-distillation waste, and olive (Olea europea L.) leaf extract against Campylobacter jejuni on polystyrene and intestine epithelial cells. J Sci Food Agric. 2016;96:2723–30. doi: 10.1002/jsfa.7391. [DOI] [PubMed] [Google Scholar]
- 38.Hattori M, Kusumoto IT, Namba T, Ishigami T, Hara Y. Effect of tea polyphenols on glucan synthesis by glucosyltransferase from Streptococcus mutans. Chem Pharm Bull (Tokyo) 1990;38:717–20. doi: 10.1248/cpb.38.717. [DOI] [PubMed] [Google Scholar]
- 39.Rafiei Z, Jafari SM, Alami M, Khomeiri M. Evaluation of antimicrobial activity of olive leaf extracts by ELISA method. Iran J Med Aromatic Plants. 2012;28:280–92. [Google Scholar]


