Abstract
Background:
Periodontal ligament fibroblasts (PDLF) play a key role in periodontal wound healing and tooth-supporting structures. Various approaches have been tried to enhance the fibroblastic activity such as laser irradiation or doxycycline application. The current study explored the influence of laser irradiation and doxycycline application on human PDLF. The aim of the study was the effect of low-level laser treatment and doxycycline application on the expression of collagen I and matrix metalloproteinase-8 (MMP8) from cultured human periodontal ligament cells.
Materials and Methods:
In this experimental study After preparation of human PDLF in three replications, they were divided into five treatment groups. The first group was day 0, which was used for standardization. The second group was the control group, which received no treatment within 4 days of the study. The third group was treated with doxycycline 30,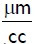 daily for 4 consecutive days. The fourth group was treated with diode laser 2
daily for 4 consecutive days. The fourth group was treated with diode laser 2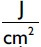 daily for 4 consecutive days. The fifth group was treated with both doxycycline and laser irradiation pertaining to the third and fourth groups. After 4 days of treatment, cells were tested for collagen I and MMP-8 secretion through real-time-polymerase chain reaction and ELISA reader. The data were analyzed using the ANOVA and least significant difference pair tests ( P < 0.05 ).
daily for 4 consecutive days. The fifth group was treated with both doxycycline and laser irradiation pertaining to the third and fourth groups. After 4 days of treatment, cells were tested for collagen I and MMP-8 secretion through real-time-polymerase chain reaction and ELISA reader. The data were analyzed using the ANOVA and least significant difference pair tests ( P < 0.05 ).
Results:
Treatment of human PDLF either with diode laser or doxycycline reduced the secretion of MMP-8 significantly. The maximum reduction was related to doxycycline application. Regarding collagen, I, only doxycycline application significantly increased collagen I secretion. Other groups showed no significant increase in collagen I secretion.
Conclusion:
This study showed that treatment of human PDLF either with diode laser or doxycycline significantly reduced MMP-8. Treatment with doxycycline significantly increased the secretion of collagen I.
Key Words: Collagen, diode laser, doxycycline, matrix metalloproteinases-8
INTRODUCTION
Periodontitis is a destructive inflammatory disease of the supporting tissues of the teeth and is caused either by specific microorganisms or by a group of specific microorganisms.[1] Periodontitis has been proposed as having an etiological or modulating role in cardiovascular and cerebrovascular disease, diabetes, respiratory disease, and adverse pregnancy outcome, and several mechanisms have been proposed to explain or support such theories and oral lesions are indicators of disease progression and oral cavity can be a window to overall health and body systems.[2]
Fibroblasts of the periodontal ligaments (PDLs) play a critical role in periodontal treatment with the production of macromolecules like collagen. Furthermore, they contribute in wound healing process and protection of periodontium, particularly after surgeries or conservative periodontal treatments by producing elastin, fibronectin, proteoglycans, enzymes, and growth factors. Fibroblast cells of PDLs are considered to be important for the production and degeneration of collagen; collagen production is essential for the attachment of periodontal tissue, and collagen degeneration is responsible for the loss of attachment.
Type I collagen constitutes the major parts of periodontal tissue and is also used for determining the tensile strength of the periodontal tissues. Type I collagen production is primarily intracellular, and in the tropocollagen form, and its further completion is extracellular.[3] After periodontal surgeries, controlled increase of collagen production with normal positioning helps in protection of the gingival tissue.
Matrix metalloproteinases (MMPs) are proteolytic enzymes produced by periodontal fibroblasts, which degenerate the extracellular matrix of connective tissues[4,5] and act reversely with respect to the tissue metalloproteinase inhibitors in PDL.[6] MMP-8 causes degeneration of collagen in periodontal tissues.[3]
Tetracyclines are administered as inhibitors of MMP synthesis to prevent periodontal remodeling during orthodontic tooth movements. Doxycycline has more inhibitory potential compared to tetracycline and reduces the velocity in orthodontic tooth movements by decreasing bone and root loss activity.[7,8,9,10] Administration of tetracycline may change the role of MMPs in PDL remodeling at primary stages, and as a result, it provides a basis for the progression of biologic retention protocol concerning the control of MMPs and collagenases in PDL.
Low-level laser therapy (LLLT) is utilized to control the inflammation and accelerating the wound healing, pain reduction, and controlling the chronic mucosal diseases. Applying LLLT in conservative periodontal treatments after removal of calculus and microbial plaque decreases the need for invasive treatments, which in turn increases the wound healing ability.
LLLT influences the velocity of tooth movements by stimulating MMP gene expression in the periodontium.[11,12] LLLT also increases the velocity of relapse in the shifted teeth without braces, whereas it keeps the balance between collagen degeneration and production during the remodeling phase.[11] However, it accelerates collagen production and degeneration in teeth with retainer, determined by the partial overexpression of MMP and collagen in PDLs.[11,12]
Cell culture studies have better evaluated the effects of LLLT on gingival fibroblasts in an experimental environment by eliminating most of intervening factors.
In a study by Gavish et al.,[13] LLLT caused overexpression of MMP-6 and MMP-2 along with the increase in the collagen production in smooth muscle cells, whereas in a study by Gwack et al.[14] LLLT caused overexpression of MMP-6, MMP-8, and MMP-13 along with a decrease in collagen production in PDLs of the moved teeth with orthodontic.
Since fiberotomy is one of the routine treatments used for the prevention of relapse after orthodontic treatments, more investigations are needed concerning novel methods to be used along with fiberotomy and adjacent therapies. In this regard, LLLT is used together with adjunctive therapy of MMP inhibitors; but there are a limited number of studies regarding this approach, especially using cell culture methods, and in some cases, they are controversial. Therefore, the present study was aimed to compare the levels of MMP-8 and Type I collagen produced by the cultured PDL fibroblasts, after treatment with LLLT and doxycycline.
MATERIALS AND METHODS
In this experimental study, human PDLs fibroblasts were studied. Initially, the fibroblasts were prepared and were cultured in an especial culture media. Then, the cells were counted and a certain number of cells were extracted for sampling. Five cell groups were selected for evaluating the effect of doxycycline and LLLT on PDL fibroblasts, including the Group 1 (day 0), in which the cells were harvested after passage and proliferation without any intervention or exchange of the culture medium. No intervention was administered on Group 2 (control). Group 3 was treated with 30 μg/mL of doxycycline, and the medium was changed every 24 h. Group 4 was treated using a diode low-level laser with a wavelength of 810 nm and a power of 0.2 w, continuously for 5 s, and the medium was changed every 24 h. Group 5 was treated with both doxycycline (i e, conditions of Group 3) and laser (i.e., conditions of Group 4), and its medium was changed every 24 h.
The 100 mW GaAlAs Doctor Smile dental laser was used with a wavelength of 810 nm (LAMBDA Spa, Brandola, Italy) and by 300 μm fiber. The Lasers' wavelength and the range of power were equal to 810 nm and 0.1–7 W, respectively. The type of radiation was continuous, and a power of 0.2 w was used in our study. The daily radiation dose used for the proliferation group was equal to 10 j/cm2 during all 4 days.
During the 4-days laser radiation phase, the culture media of the proliferation groups were exchanged every day before radiation or doxycycline treatment. The groups were subjected to radiation dose of 2 j/cm2(with a power of 0.2 mw for 10 s) in a continuous manner, the cross section of optical fiber was equal to 8 mm, and the distance from the device was equal to 10 mm; these conditions continued for 4 successive days (with total power of 6 j/cm2) within 24-h intervals.
Samples were taken from all five cell groups to be tested by polymerase chain reaction and special kits (BIOTECON Diagnostics, Germany) for the evaluation of Type I collagen and MMP-8 levels; the secretion levels were determined in all culture media and were measured by ELISA reader (eBioscience, Germany) and special kit.
The data were analyzed using the ANOVA and least significant difference pair tests in the SPSS software version 22 (IBM Corporation, USA). P < 0.05 was considered as statistically significant.
RESULTS
According to Figure 1, there was a statistically significant difference (P < 0.001) in the mean value of MMP-8 secretion between the cultured cells of five groups (day 0, control, doxycycline, laser, doxycycline + laser); the maximum and minimum levels of secretion belonged to the control group and day 0 group, respectively.
Figure 1.
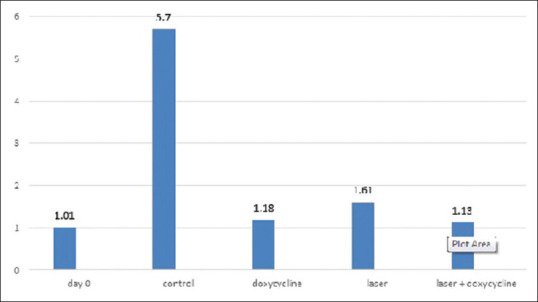
The mean value of matrix metalloproteinases-8 (Ng/dl) in different groups
Results of the pairwise comparison of the groups showed a significant difference in MMP-8 secretion only for the control group (P < 0.001) compared to other groups (day 0, doxycycline, laser, doxycycline + laser). However, the remaining groups were not significantly different from each other (P > 0.05) [Table 1].
Table 1.
The pairwise comparison of the groups for matrix metalloproteinases-8 (Ng/dl) secretion
| Groups | Day 0 | Control | Doxycycline | Laser | Doxycycline + laser |
|---|---|---|---|---|---|
| Day 0 | - | 0.001 | 0.83 | 0.43 | 0.88 |
| Control | 0.001 | - | 0.001 | 0.001 | 0.001 |
| Doxycycline | 0.83 | 0.001 | - | 0.56 | 0.94 |
| Laser | 0.43 | 0.001 | 0.56 | - | 0.52 |
| Doxycycline + laser | 0.88 | 0.001 | 0.94 | 0.52 | - |
According to Figure 2, there was a significant difference in mean Type I collagen secretion in the cultured cell of five groups (day 0, control, doxycycline, laser, doxycycline + laser) (P < 0.001); the maximum and minimum levels of secretion belonged to the doxycycline group and the control group, respectively.
Figure 2.
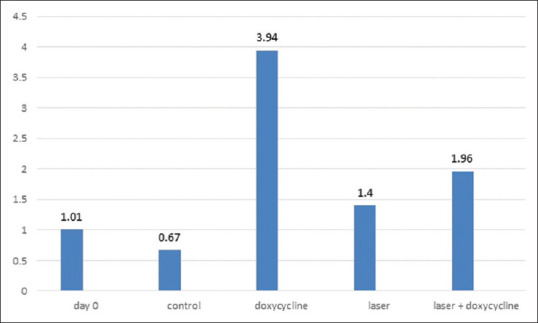
The mean value of collagen I (Nmol/μg) in different groups
Results of the pairwise comparison of the groups showed a significant difference in Type I collagen secretion for the doxycycline group (P < 0.001) compared to other groups (day 0, control, laser, doxycycline + laser). There was a statistically significant difference between the doxycycline + laser and the control group (P < 0.01), and the doxycycline (P < 0.001) group. The remaining groups were not significantly different from each other (P > 0.05) [Table 2].
Table 2.
The pairwise comparison of the groups for collagen I (Nmol/μg) secretion
| Groups | Day 0 | Control | Doxycycline | Laser | Doxycycline + laser |
|---|---|---|---|---|---|
| Day 0 | - | 0.46 | 0.001 | 0.41 | 0.65 |
| Control | 0.46 | - | 0.001 | 0.13 | 0.01 |
| Doxycycline | 0.001 | 0.001 | - | 0.001 | 0.001 |
| Laser | 0.41 | 0.13 | 0.001 | - | 0.25 |
| Doxycycline + laser | 0.65 | 0.01 | 0.001 | 0.25 | - |
DISCUSSION
The present study was aimed to evaluate the effects of doxycycline and laser on secretion levels of MMP-8, in this regard the maximum reduction of secretion belonged to the doxycycline, laser + doxycycline, and laser groups, respectively. Seemingly, compared to laser doxycycline has a stronger inhibitory effect on decreasing the levels of MMP-8, as a collagenase secreted by the fibroblasts. This is consistent with the findings of the studies by Smith et al.[15] and Choi et al.[16] Qadri et al.[17] in a study investigated the effects of laser on secretion levels of MMP-8, and they showed that low-level laser treatment leads to the reduction of periodontal inflammation, attributed to the reduction of IL-1, MMP-8, and elastase activities.
However, radiation parameters were found to vary in different studies, which include the type of laser, continuous or discontinuous manner of radiation, the site and surface of radiation, power density, and the radiation regimen.
Kreisler et al.[18] in another study evaluated the effects of diode low-level laser in the proliferation of PDL fibroblasts, and they found that laser radiation treatment for the cultured cells resulted in extremely high proliferation of fibroblasts compared to the control group after 24 h. Although this difference was also observed after 48 and 72 h of radiation, it reduced afterward. They attributed this to the gradual reduction of laser effect and/or the fibroblasts reaching to proliferation saturation point after 3 days.
The results of analyzing the effects of doxycycline + laser on levels of Type I collagen production demonstrated that the maximum reduction of secretion levels belonged to the laser, laser + doxycycline, and doxycycline groups, respectively. Fibroblasts seem to play a central role in the maintenance of biologic balance around the tooth by producing both collagen and collagenase.
The study revealed no finding regarding the laser efficiency in overexpression of collagen. However, findings demonstrated its effects on the reduction of inflammation. Basso et al.[19] evaluated the effects of diode laser in three-dimensional cell culture method and found that laser radiation can cause overexpression of Type I collagen. Martignago et al.[20] investigated the effects of low-level laser radiation treatment on Type I collagen and vascular endothelial growth factor (VEGF) secretion levels and found that the radiation causes an increase in the secretion of Type I collagen and VEGF which is not consistent with the results of the present study. Frozanfar et al.[21] in a study evaluated the effects of diode low-level laser on the proliferation and expression of Type I collagen, and they found that the laser radiation leads to overexpression of Type I collagen which is in accordance with our results.
The results of the study showed that doxycycline did not have any significant effects on the reduction of Type I collagen levels, while Xi et al.[22] in their study demonstrated that metacycline, a member of the tetracycline family, inhibits TGFβ activity and Smad signaling pathway and causes an increase in the Type I collagen level, which is not consistent with our results, but it is in line with the results of the study by Grieve et al.[23]
It seems that the mechanism of low-level laser referred to as “proliferative stimulation, ” is influenced by the cytochrome reductase enzyme. The enzyme participates in the mitochondrial respiration cycle and alters the gene expression levels by changing the oxidative situations.
Mayahara et al.[24] cultured the PDL fibroblasts under mechanical stress condition; three time-groups were subjected to low-level laser treatment (wavelength of 830 nm and radiation dose of 3.82 j/cm2): 6 h before force application, 1 h before force application, and immediately after force application. They found that low-level laser radiation significantly reduced the cox-2 and CPL-A[4] expression, and the maximum reduction was obtained as the laser utilized immediately after the force application, which is consistent with our results.
Fibroblasts seem to have the ability of accumulating some of the antibiotics such as doxycycline and ciprofloxacin inside themselves. In a study by Lavda et al.,[25] the candidate patients for periodontal surgery were treated with the two aforementioned antibiotics 3 days prior to surgery, and samples of gingival crevicular fluid (GCF), gingival connective tissue (GCT), and serums of the patients were obtained during the surgery. They found that the concentration of antibiotics was high in GCF fibroblasts, GCT, and the serum, respectively. The accumulation of doxycycline in fibroblasts was also confirmed in the present study.
Ozawa et al.[26] utilized a plasminogen-activator degenerating marker to evaluate the effects of the diode laser. The PDL fibroblasts under stretching condition were treated with radiation doses of 3.95 and 7.9 j/cm2. They found that laser radiation results in the reduction of plasminogen activator in a dose-dependent manner. It should be noted that plasminogen activator participates in the degeneration of the extracellular matrix. The reduction of inflammation factors caused by the laser radiation was also reported in the current study.
It should be mentioned that the presented results were obtained in a stress-free environment. The culture media are classified into two groups according to the nutritional requirements of PDL fibroblasts. The medium with adequate concentrations of nutrient components contains 10% of fetal bovine serum, and the stress medium with low concentrations of nutrient components contains 5% of serum. The culture medium used for osteogenic differentiation of PDL fibroblasts contains mineral trioxide aggregate (MTA).
CONCLUSION
The secretion levels of MMP-8 decreased by PDL fibroblasts when treated with laser + doxycycline. Doxycycline treatment was not found to increase the Type I collagen secreted from PDL fibroblasts. Moreover, laser radiation did not increase the Type I collagen secreted from PDL fibroblasts.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Saini R. Ozone therapy in dentistry: A strategic review. J Nat Sci Biol Med. 2011;2:151–3. doi: 10.4103/0976-9668.92318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saini R, Saini S, Sharma S. Periodontal disease linked to cardiovascular disease. J Cardiovasc Dis Res. 2010;1:161–2. doi: 10.4103/0975-3583.70925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman MG, Takei HH, Klokkevold PR, Carranza FA. Carranza's Clinical Periodontology. 12th ed. St. Louis: Elsevier; 2015. [Google Scholar]
- 4.Stamenkovic I. Extracellular matrix remodelling: The role of matrix metalloproteinases. J Pathol. 2003;200:448–64. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi I, Onodera K, Nishimura M, Mitnai H, Sasano Y, Mitani H. Expression of genes for gelatinases and tissue inhibitors of metalloproteinases in periodontal tissues during orthodontic tooth movement. J Mol Histol. 2006;37:333–42. doi: 10.1007/s10735-006-9060-7. [DOI] [PubMed] [Google Scholar]
- 6.Verstappen J, Von den Hoff JW. Tissue inhibitors of metalloproteinases (TIMPs): Their biological functions and involvement in oral disease. J Dent Res. 2006;85:1074–84. doi: 10.1177/154405910608501202. [DOI] [PubMed] [Google Scholar]
- 7.Bildt MM, Bloemen M, Kuijpers-Jagtman AM, Von den Hoff JW. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in gingival crevicular fluid during orthodontic tooth movement. Eur J Orthod. 2009;31:529–35. doi: 10.1093/ejo/cjn127. [DOI] [PubMed] [Google Scholar]
- 8.Bildt MM, Henneman S, Maltha JC, Kuijpers-Jagtman AM, Von den Hoff JW. CMT-3 inhibits orthodontic tooth displacement in the rat. Arch Oral Biol. 2007;52:571–8. doi: 10.1016/j.archoralbio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Golub LM, Wolff M, Roberts S, Lee HM, Leung M, Payonk GS. Treating periodontal diseases by blocking tissue-destructive enzymes. J Am Dent Assoc. 1994;125:163–9. doi: 10.14219/jada.archive.1994.0261. [DOI] [PubMed] [Google Scholar]
- 10.Hudson JB, Hatch N, Hayami T, Shin JM, Stolina M, Kostenuik PJ, et al. Local delivery of recombinant osteoprotegerin enhances postorthodontic tooth stability. Calcif Tissue Int. 2012;90:330–42. doi: 10.1007/s00223-012-9579-4. [DOI] [PubMed] [Google Scholar]
- 11.Obradović RR, Kesić LG, Pesevska S. Influence of low-level laser therapy on biomaterial osseointegration: A mini-review. Lasers Med Sci. 2009;24:447–51. doi: 10.1007/s10103-008-0573-z. [DOI] [PubMed] [Google Scholar]
- 12.Bicakci AA, Kocoglu-Altan B, Toker H, Mutaf I, Sumer Z. Efficiency of low-level laser therapy in reducing pain induced by orthodontic forces. Photomed Laser Surg. 2012;30:460–5. doi: 10.1089/pho.2012.3245. [DOI] [PubMed] [Google Scholar]
- 13.Gavish L, Perez L, Gertz SD. Low-level laser irradiation modulates matrix metalloproteinase activity and gene expression in porcine aortic smooth muscle cells. Lasers Surg Med. 2006;38:779–86. doi: 10.1002/lsm.20383. [DOI] [PubMed] [Google Scholar]
- 14.Gwack CK, Park SB, Son WS, Kim YD, Jun ES, Park MH. The expression of MMP-1, -8, and -13 mRNA in the periodontal ligament of rats during tooth movement with cortical punching. Korean J Orthod. 2008;38:187–201. [Google Scholar]
- 15.Smith GN, Jr, Mickler EA, Hasty KA, Brandt KD. Specificity of inhibition of matrix metalloproteinase activity by doxycycline: Relationship to structure of the enzyme. Arthritis Rheum. 1999;42:1140–6. doi: 10.1002/1529-0131(199906)42:6<1140::AID-ANR10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Choi DH, Moon IS, Choi BK, Paik JW, Kim YS, Choi SH, et al. Effects of sub-antimicrobial dose doxycycline therapy on crevicular fluid MMP-8, and gingival tissue MMP-9, TIMP-1 and IL-6 levels in chronic periodontitis. J Periodontal Res. 2004;39:20–6. doi: 10.1111/j.1600-0765.2004.00696.x. [DOI] [PubMed] [Google Scholar]
- 17.Qadri T, Miranda L, Tunér J, Gustafsson A. The short-term effects of low-level lasers as adjunct therapy in the treatment of periodontal inflammation. J Clin Periodontol. 2005;32:714–9. doi: 10.1111/j.1600-051X.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 18.Kreisler M, Christoffers AB, Willershausen B, d'Hoedt B. Effect of low-level gaAlAs laser irradiation on the proliferation rate of human periodontal ligament fibroblasts: An in vitro study. J Clin Periodontol. 2003;30:353–8. doi: 10.1034/j.1600-051x.2003.00001.x. [DOI] [PubMed] [Google Scholar]
- 19.Basso FG, Soares DG, de Souza Costa CA, Hebling J. Low-level laser therapy in 3D cell culture model using gingival fibroblasts. Lasers Med Sci. 2016;31:973–8. doi: 10.1007/s10103-016-1945-4. [DOI] [PubMed] [Google Scholar]
- 20.Martignago CC, Oliveira RF, Pires-Oliveira DA, Oliveira PD, Pacheco Soares C, Monzani PS, et al. Effect of low-level laser therapy on the gene expression of collagen and vascular endothelial growth factor in a culture of fibroblast cells in mice. Lasers Med Sci. 2015;30:203–8. doi: 10.1007/s10103-014-1644-y. [DOI] [PubMed] [Google Scholar]
- 21.Frozanfar A, Ramezani M, Rahpeyma A, Khajehahmadi S, Arbab HR. The effects of low level laser therapy on the expression of collagen type I gene and proliferation of human gingival fibroblasts (Hgf3-pi 53):In vitro study. Iran J Basic Med Sci. 2013;16:1071–4. [PMC free article] [PubMed] [Google Scholar]
- 22.Xi Y, Tan K, Brumwell AN, Chen SC, Kim YH, Kim TJ, et al. Inhibition of epithelial-to-mesenchymal transition and pulmonary fibrosis by methacycline. Am J Respir Cell Mol Biol. 2014;50:51–60. doi: 10.1165/rcmb.2013-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grieve WG, 3rd, Johnson GK, Moore RN, Reinhardt RA, DuBois LM. Prostaglandin E (PGE) and interleukin-1 beta (IL-1 beta) levels in gingival crevicular fluid during human orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1994;105:369–74. doi: 10.1016/s0889-5406(94)70131-8. [DOI] [PubMed] [Google Scholar]
- 24.Mayahara K, Yamaguchi A, Sakaguchi M, Igarashi Y, Shimizu N. Effect of Ga-Al-As laser irradiation on COX-2 and cPLA2-alpha expression in compressed human periodontal ligament cells. Lasers Surg Med. 2010;42:489–93. doi: 10.1002/lsm.20871. [DOI] [PubMed] [Google Scholar]
- 25.Lavda M, Clausnitzer CE, Walters JD. Distribution of systemic ciprofloxacin and doxycycline to gingiva and gingival crevicular fluid. J Periodontol. 2004;75:1663–7. doi: 10.1902/jop.2004.75.12.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozawa Y, Shimizu N, Abiko Y. Low-energy diode laser irradiation reduced plasminogen activator activity in human periodontal ligament cells. Lasers Surg Med. 1997;21:456–63. doi: 10.1002/(sici)1096-9101(1997)21:5<456::aid-lsm7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]


