Abstract
Background and aims
Despite anticoagulation, usually with heparin, mortality for thromboembolic events in COVID-19 remains high. Clinical efficacy of heparin is due to its interaction with antithrombin (AT) that may be decreased in COVID-19. Therefore, we correlated AT levels with outcomes of COVID-19.
Methods and results
We recruited 49 consecutive patients hospitalized for COVID-19. AT levels were significantly lower in 16 non-survivors than in 33 survivors (72.2 ± 23.4 versus 94.6 ± 19.5%; p = 0.0010). A multivariate Cox regression analysis showed that low AT (levels below 80%) was a predictor of mortality (HR:3.97; 95%CI:1.38 to 11.43; p = 0.0103). BMI was the only variable that showed a significant difference between patients with low and those with normal AT levels (32.9 ± 7.9 versus 27.5 ± 5.9%; p = 0.0104). AT levels were significantly lower in obese patients than in subjects with normal weight or overweight (77.9 ± 26.9 versus 91.4 ± 26.9 versus 91.4 ± 17.1%; p = 0.025). An inverse correlation between AT levels and BMI was documented (r:-0.33; p = 0.0179).
Conclusions
Our data first suggest that AT is strongly associated with mortality in COVID-19. In addition, AT may be the link between obesity and a poorer prognosis in patients with COVID-19. Other studies should confirm whether AT may become a prognostic marker and a therapeutic target in COVID-19.
Keywords: COVID-19, Thrombosis, Antithrombin, Death, Obesity
Highlights
-
•
Reduced Antithrombin is associated to mortality in COVID-19.
-
•
Reduced Antithrombin is associated to need for mechanical ventilation in COVID-19.
-
•
Reduced Antithrombin is associated to obesity in COVID-19.
-
•
Reduced antithrombin is associated to heparin resistance.
Introduction
Coronavirus Disease 2019 (COVID-19) is characterized by an elevated risk for thromboembolic events that can lead to death [1]. This risk is attributed to a procoagulant state due to hyperinflammation [1,2]. So prophylactic doses of heparin are recommended in all the hospitalized patients and a therapeutic anticoagulation, mainly with heparin, is used when thromboembolic events occur [1,2]. However, despite anticoagulation, mortality in COVID-19 remains high [[1], [2], [3], [4]]. The clinical efficacy of heparin is mainly due to its interaction with antithrombin (AT), a powerful natural anticoagulant [5]. Hyperinflammation can markedly decrease AT levels and can reduce its physiological actions [5]. This suggests that anticoagulation may be ineffective in patients with low AT levels (heparin resistance) [5]. Nevertheless, whether COVID-19 affects AT levels and whether there is an association between AT and outcomes of COVID-19 remains unclear, considering that specific studies were never carried out. Therefore, we evaluated AT levels in patients hospitalized for COVID-19 and correlated AT levels with the outcomes of COVID-19.
Methods
We retrospectively enrolled 49 consecutive patients admitted to our hospital for Severe Acute Respiratory Syndrome–Coronavirus 2 (SARS-CoV2) pneumonia. Pneumonia was documented by Chest Computed Tomography and infection was established by Real Time-Polymerase Chain Reaction. On admission, AT was measured with a chromogenic method (HemosIL, Werfen, Barcelona, Spain) together with all the other laboratory tests routinely made in patients hospitalized for COVID-19. All the patients received standard treatment for SARS-CoV2 pneumonia according to the protocol of our hospital: hydroxychloroquine, lopinavir/ritonavir, antibiotics (doxiciclin and ceftriaxone) and a prophylactic dose of enoxaparin. Other tailored treatments, including tocilizumab, therapeutic anticoagulation, steroids and/or AT concentrate, were made on the basis of clinical courses. The study was carried out according to the ethical standards of the institutional research committee and with the 1964 Helsinki declaration.
In this retrospective study of prospectively collected data the primary outcome was death. A combined outcome of death or need for mechanical ventilation in survivors was taken into account.
Statistical analysis
Univariate analysis was carried out by using Student t-test to find significant differences and the exact Fisher's test for frequency comparisons. To find differences among three groups ANOVA was used. The Pearson's correlation was used to analyze statistical associations. Nonnormally distributed variables were log-transformed before the analysis. Survival curves were estimated by the Kaplan–Meier test and compared by the Mantel log-rank test. The effect of the variables with a p < 0.1 at the univariate analysis on the primary outcome and on the combined outcome was tested in a multivariate Cox regression analysis. Variables were dichotomized before the analysis. A p < 0.05 was considered statistically significant.
Results
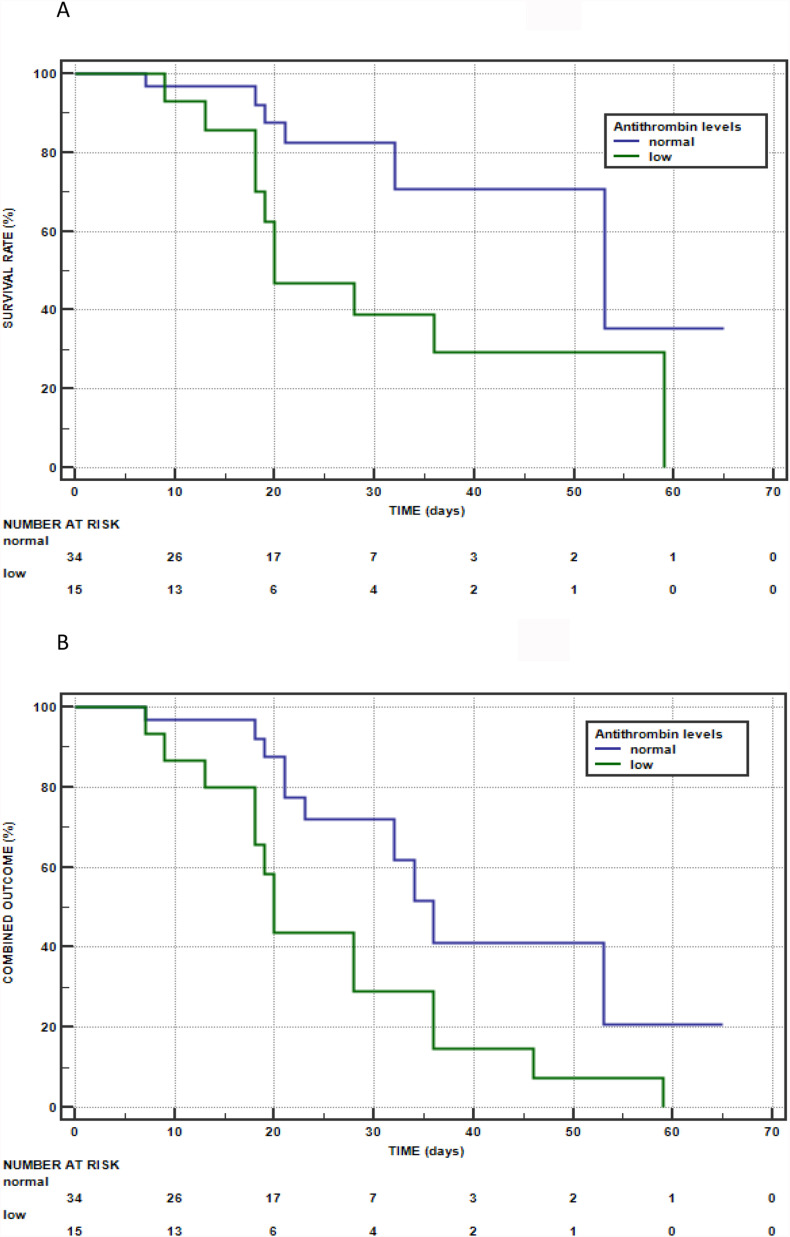
Table 1 shows that non-survivors had troponin levels significantly higher and AT levels and oxygen saturation (SaO2) significantly lower than survivors. The Kaplan Meier method showed that AT was associated with a higher mortality (log-rank test:5.5398; p = 0.0186) (Fig. 1 Panel A). A multivariate analysis with the primary outcome as the dependent variable was exploited. The following variables were tested and dichotomized before the analysis: history of diabetes (yes or not), BMI (<25 or ≥ 25), high-sensitivity troponin: (<20 or ≥ 20 pg/ml), Oxygen Saturation (Sa02) (<93 or ≥93%), AT (<80 or ≥ 80%). A multivariate Cox regression analysis showed that low AT (levels below 80%) was a predictor of mortality (HR:3.97; 95%CI:1.38 to 11.43; p = 0.0103) together with a history of diabetes (HR:4.40; 95%CI:1.30 to 1.82; p = 0.0167) and a normal BMI (HR:0.22; 95%CI: 0.05 to 0.90; p = 0.0351). When the combined outcome of death or need for mechanical ventilation in survivors was considered, the difference in AT levels between the patients with the combined outcome (n = 24) and survivors who did not require mechanical ventilation (n = 25) remained significant (73.7 ± 20.6 versus 100.4 ± 17.5%; p < 0.0001). The Kaplan Meier method showed that AT was associated with the combined outcome (log-rank test:5.8272; p = 0.0158) (Fig. 1 Panel B). The variables with p < 0.1 at the univariate analysis were history of diabetes, BMI, troponin, ScO2 and AT and therefore were tested in a multivariate analysis. A multivariate Cox regression analysis showed that low AT was the only predictor of the combined outcome (HR: 2.56; 95%CI:1.13 to 5.81; p = 0.0238). Table 2 reports the patients stratified by normal/low AT levels; as shown, the two groups did not significantly differ in any variable except for BMI that was significantly higher in patients with reduced AT levels. When the patients were divided into three subgroups according to the presence of normal weight (BMI<25; n = 11), overweight (BMI 25–29.9; n = 23) and obesity (BMI≥30; n = 15), AT levels were significantly lower in obese patients (77.9 ± 26.9%) than in subjects with normal weight (91.4 ± 26.9%) and overweight (91.4 ± 17.1%): ANOVA p = 0.025. An inverse correlation between AT levels and BMI was documented in the whole population (r:-0.33; p = 0.0179). Mortality and the percentage of patients with the combined outcome were significantly higher in patients with low than in those with normal AT levels.
Table 1.
Features of the whole study population and of survivors and non-survivors.
| variable | Total patients (n = 49) | Survivors (n = 33) | Non-survivors (n = 16) | p-value |
|---|---|---|---|---|
| Age (years) | 69.4 ± 14.8 | 67.1 ± 14.5 | 74.1 ± 14.9 | 0.1245 |
| Males (%) | 49.0 | 45.4 | 56.2 | 0.5512 |
| History of Diabetes (%) | 20.4 | 12.1 | 37.5 | 0.0596 |
| History of Hypertension (%) | 34.7 | 33.3 | 37.5 | 0.9989 |
| History of CVD (%) | 16.3 | 12.1 | 25.0 | 0.4105 |
| History of Lung Disease (%) | 16.3 | 12.1 | 25.0 | 0.4105 |
| BMI | 29.1 ± 7.0 | 28.0 ± 6.4 | 31.5 ± 7.8 | 0.0975 |
| eGFR (ml/min) | 70.6 ± 27.0 | 70.4 ± 25.3 | 70.8 ± 31.1 | 0.9667 |
| CRP (mg/L) | 122.5 ± 85.5 | 116.1 ± 79.8 | 135.8 ± 97.6 | 0.9245 |
| D-dimer (ng/ml) | 1848.7 ± 5949.4 | 1436.8 ± 3902.6 | 2698.2 ± 8923.6 | 0.6387 |
| High-sensitivity Troponin (pg/ml) | 20.1 ± 25.2 | 14.1 ± 17.7 | 32.4 ± 33.5 | 0.0246 |
| AT (%) | 87.3 ± 23.2 | 94.6 ± 19.5 | 72.2 ± 23.4 | 0.0010 |
| Prothrombin time (sec) | 13.1 ± 3.1 | 12.8 ± 2.3 | 13.8 ± 4.3 | 0.3035 |
| activated partial-thromboplastin time (sec) | 32.6 ± 5.4 | 33.3 ± 5.5 | 31.2 ± 5.2 | 0.2207 |
| SaO2 (%) | 90.5 ± 5.8 | 92.6 ± 4.3 | 87.6 ± 7.2 | 0.0043 |
| Lactate dehydrogenase (U/L) | 344.7 ± 161.4 | 326.6 ± 149.3 | 382.0 ± 183.3 | 0.3669 |
AT: antithrombin; SaO2: oxygen saturation; BMI: body mass index. CVD: cardiovascular Disease; CRP: C-reactive Protein.
Figure 1.
Kaplan–Meier survival curve according to normal/low antithrombin levels in patients hospitalized for COVID-19 (PANEL A) and Kaplan–Meier curve of the combined outcome of death or the need for mechanical ventilation in survivors according to normal/low antithrombin levels in patients hospitalized for COVID-19 (PANEL B).
Table 2.
Features and outcomes of the patients stratified by normal/low antithrombin levels.
| variable | Total patients (n = 49) | Patients with normal AT (n = 34) | Patients with low AT (n = 15) | p-value |
|---|---|---|---|---|
| Age (years) | 69.4 ± 14.8 | 71.0 ± 13.8 | 65.7 ± 16.9 | 0.2507 |
| Males (%) | 49.0 | 47.1 | 53.3 | 0.7624 |
| History of Diabetes (%) | 20.4 | 23.5 | 13.3 | 0.7021 |
| History of Hypertension (%) | 34.7 | 35.3 | 33.3 | 0.9999 |
| History of CVD (%) | 16.3 | 17.6 | 13.3 | 0.9898 |
| History of Lung Disease (%) | 16.3 | 17.6 | 13.3 | 0.9898 |
| BMI | 29.1 ± 7.0 | 27.5 ± 5.9 | 32.9 ± 7.9 | 0.0104 |
| eGFR (ml/min) | 70.6 ± 27.0 | 68.1 ± 28.8 | 76.1 ± 22.2 | 0.3448 |
| CRP (mg/L) | 122.5 ± 85.5 | 121.7 ± 83.8 | 124.4 ± 92.3 | 0.8530 |
| D-dimer (ng/ml) | 1848.7 ± 5949.4 | 2455.5 ± 7084.2 | 433.3 ± 375.2 | 0.7065 |
| High-sensitivity Troponin (pg/ml) | 20.1 ± 25.2 | 18.4 ± 25.1 | 23.8 ± 25.9 | 0.1789 |
| AT (%) | 87.2 ± 23.2 | 99.6 ± 15.2 | 59.2 ± 9.4 | <0.0001 |
| Prothrombin time (sec) | 13.1 ± 3.1 | 12.9 ± 2.3 | 13.6 ± 4.4 | 0.4948 |
| activated partial-thromboplastin time (sec) | 32.6 ± 5.4 | 33.3 ± 5.6 | 31.1 ± 5.0 | 0.2050 |
| SaO2 (%) | 90.5 ± 5.8 | 91.8 ± 5.2 | 89.1 ± 7.0 | 0.1342 |
| Lactate dehydrogenase (U/L) | 344.7 ± 161.4 | 333.7 ± 159.0 | 369.7 ± 169.6 | 0.3794 |
| Non-survivors (%) | 32.6 | 17.6 | 66.7 | 0.0019 |
| Survivors who required mechanical ventilation (%) | 24.3 | 14.3 | 80 | 0.0076 |
| Non survivors and survivors who required mechanical ventilation (%) | 49.0 | 29.4 | 93.3 | <0.0001 |
AT: antithrombin; SaO2: oxygen saturation; BMI: body mass index. CVD: cardiovascular Disease; CRP: C-reactive Protein.
Discussion
We found AT levels below the lower limit of the normal range in 30.6% of our patients hospitalized for COVID-19; among them, 66.7% died. Among survivors with low AT levels, 80% of them required mechanical ventilation. Our preliminary data first show that low AT levels seem to be associated with a high mortality and need for mechanical ventilations in patients with COVID-19. This independent association may be at least partially due to the fact that anticoagulation, mainly with heparin, may be ineffective [5]. Several studies showed that thrombosis, both in the venous and arterial circulations, seems to be the main cause of death in patients with COVID-19 [1,2]. On the other hand, microthrombi were regularly found within small lung arteries of all the patients who died because of COVID1-19 [6]. The high thromboembolic risk is due to a procoagulant state that is caused by an excessive inflammatory response through several mechanisms [1,2]. Abnormal coagulation parameters [3], presence of lupus anticoagulant [7] and antiphospholipid antibodies [8] have been described in patients with severe COVID-19. To prevent and treat thromboembolic events in patients with COVID-19 prophylactic or therapeutic anticoagulation is recommended [1,2]. However, despite the anticoagulation, mortality in COVID-19 is very high [[1], [2], [3], [4]]. Heparin is usually used for anticoagulation in these patients [[1], [2], [3], [4]]. The clinical efficacy of heparin is mainly due to its interaction with AT [5], but severe inflammatory states markedly decrease AT levels because of a reduced synthesis [5]. Then, inflammation promotes degradation of elastase from activate neutrophils and causes a consumption due to thrombin generation [9]. In addition, the function of AT depends on glycosaminoglycans of the endothelial surface, but their synthesis is reduced by inflammatory mediators [10]. Therefore, reduced levels and impaired function of AT may explain why heparin is ineffective in some patients with COVID-19.
Our study shows that in hospitalized patients with severe COVID-19 the proportion of subjects with low AT levels is quite high, but this finding is in agreement with previous studies [3,11,12]. Tang found that AT levels were lower in non-survivors than in survivors even if difference did not attain statistical significance [3]. Panigada found low mean AT levels in 11 patients admitted to the Intensive Care Unit [11]. Ranucci observed that among 16 patients admitted to Intensive Care Unit 25% of them had AT levels below the lower limit of the normal range [12]. Taken together, these data suggest that a high proportion of patients with COVID-19 can have low AT levels. A recent study suggests that acutely low AT levels are even common in severe forms of COVID-19 [13]. However, it is unclear why the acute decrease in AT regards only a proportion of patients. Inflammation can acutely and markedly reduce AT levels [5], but our data did not show any difference in inflammatory factors or other variables between patients with low and those with normal AT levels. BMI is the only variable that shows a significant difference between the two groups. In particular, BMI was significantly higher in patients with reduced AT levels. In addition, we have also found an inverse correlation between AT levels and BMI in the whole population. To better evaluate the relationship between BMI and AT in patients with COVID-19, they were stratified according to the presence of normal weight, overweight and obesity; AT levels were significantly lower in obese patients than in the other two subgroups. It is important to remember that obesity is a recognized powerful risk factor for a worse prognosis in COVID-19 [14]. Our data seems to suggest that AT may be the link between obesity and poorer prognosis, including death [14]. It is of interest to note that obesity is characterized by chronic inflammation and a pro-thrombotic state [15], but decreased AT levels were not clearly documented [16]. However, obese patients seem to be more prone to a marked reduction in AT levels when hyperinflammation of COVID-19 occurs. The reasons remain unclear.
In our study troponin levels were significantly higher in non-survivors, even if they did not maintain the statistical significance in multivariate analysis. This may be due to the relatively small study population. However, elevated troponin levels are expression of cardiac impairment and therefore they may be reliable predictors of worse outcomes of COVID-19, as recently documented [17]. This may imply that troponin should be considered as a predictor of survival in severe COVID-19 together with other biological parameters [17], including AT.
If confirmed, our findings can have important clinical implications. First, AT may become a strong predictor of worse outcomes of COVID-19 and therefore it may be used in the stratification of the risk for thrombosis and death. AT levels should be measured on admission and monitored over time, as they can be reduced by heparin itself [5]. In addition, patients with low AT levels should be treated with AT concentrate to face heparin resistance [5,18]. On the other hand, AT has also anti-inflammatory properties and therefore it may reduce hyperinflammation due to COVID-19 [11]. We used AT concentrate together with heparin in patients with low AT and worsening disease progression, but mortality was high. Specific studies should evaluate whether an earlier use of AT concentrate may improve outcomes. Alternatives to AT concentrate may be fresh frozen plasma [18] or anticoagulants other than heparin [13]. However, we cannot exclude that also patients with normal AT levels may have heparin resistance due to an impaired function of AT, as inflammation acts not only on the synthesis but also on the function [5].
The main limitations of our study are the relatively small population and the retrospective design. However, even if it is a retrospective analysis all data are available and no patient was lost to the follow-up.
In conclusion, our data first suggest that AT is strongly associated with mortality in COVID-19. In addition, AT may be the link between obesity and a poorer prognosis in patients with COVID-19. Other studies should confirm whether AT may become a prognostic marker and a therapeutic target in severe COVID-19.
Funding
None.
Declaration of Competing Interest
None.
Handling Editor: D. Noto
References
- 1.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020 Apr 15;(20):35008–35017. doi: 10.1016/j.jacc.2020.04.031. pii: S0735–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020 Apr 27 doi: 10.1182/blood.2020006000. blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemostasis. 2020 Apr;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llitjos J.F., Leclerc M., Chochois C., Monsallier J.M., Ramakers M., Auvray M. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemostasis. 2020 Jul;18(7):1743–1746. doi: 10.1111/jth.14869. Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy J.H., Sniecinski R.M., Welsby I.J., Levi M. Antithrombin: anti-inflammatory properties and clinical applications. Thromb Haemostasis. 2016;115(4):712–728. doi: 10.1160/TH15-08-0687. [DOI] [PubMed] [Google Scholar]
- 6.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020 May 6 doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazzaruso C., Carlo Stella N., Mariani G., Nai C., Coppola A., Naldani D. High prevalence of antinuclear antibodies and lupus anticoagulant in patients hospitalized for SARS-CoV2 pneumonia. Clin Rheumatol. 2020 Jul;39(7):2095–2097. doi: 10.1007/s10067-020-05180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N Engl J Med. 2020 Apr 23;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levi M., van der Poll T., Büller H.R. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109(22):2698–2704. doi: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi M., Shimada K., Ozawa T. Human recombinant interleukin-1 beta- and tumor necrosis factor Alpha-mediated suppression of heparin-like compounds on cultured porcine aortic endothelial cells. J Cell Physiol. 1990;144(3):383–390. doi: 10.1002/jcp.1041440304. [DOI] [PubMed] [Google Scholar]
- 11.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V. Hypercoagulability of COVID-19 patients in intensive Care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemostasis. 2020 Jul;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranucci M., Ballotta A., Di Dedda U. The procoagulant pattern of patients with COVID-19 acute respiratory distress Syndrome. J Thromb Haemostasis. 2020 Apr 17 doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arachchillage D.J., Remmington C., Rosenberg A., Xu T., Passariello M., Hall D. Anticoagulation with argatroban in patients with acute antithrombin deficiency in severe COVID-19. Br J Haematol. 2020 Jun 9 doi: 10.1111/bjh.16927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrakis D., Margină D., Tsarouhas K., Tekos F., Stan M., Nikitovic D. Obesity - a risk factor for increased COVID-19 prevalence, severity and lethality. Mol Med Rep. 2020 Jul;22(1):9–19. doi: 10.3892/mmr.2020.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samad F., Ruf W. Inflammation, obesity, and thrombosis. Blood. 2013;122(20):3415–3422. doi: 10.1182/blood-2013-05-427708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pardina E., Ferrer R., Rivero J., Baena-Fustegueras J.A., Lecube A., Fort J.M. Alterations in the common pathway of coagulation during weight loss induced by gastric bypass in severely obese patients. Obesity. 2012 May;20(5):1048–1056. doi: 10.1038/oby.2011.361. [DOI] [PubMed] [Google Scholar]
- 17.Qin J.J., Cheng X., Zhou F., Lei F., Akolkar G., Cai J. Redefining cardiac biomarkers in predicting mortality of inpatients with COVID-19. Hypertension. 2020 Jul 14 doi: 10.1161/HYPERTENSIONAHA.120.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiess B.D. Treating heparin resistance with antithrombin or fresh frozen plasma. Ann Thorac Surg. 2008 Jun;85(6):2153–2160. doi: 10.1016/j.athoracsur.2008.02.037. [DOI] [PubMed] [Google Scholar]