Abstract
With the emergence of COVID-19 extensive research began to identify medications, candidate compounds and other therapeutic approaches. The complex virology of COVID-19 may provide multiple potential target points for antiviral therapy, and vaccines; extensive global research is underway to exploit these potential opportunities. The complex pathophysiology, pulmonary and extrapulmonary disease, and immune mediated effects such as cytokine storm, make medical management more challenging than many viral illnesses. Non medication based interventions including hyperbaric oxygen (HBOT), extracorporeal membrane oxygenation (ECMO), aggressive dialysis, and other interventions, all with various degrees of clinical success, and will be discussed in this section. Several antivirals approved for other clinical indications were studied for repurposing against COVID-19, which we highlight, again with varying results. In addition to therapeutics, concern was raised over potential risks associated with ACE inhibitors and ARB use, which is presented. Often the timing of the medication determined its clinical benefit as will be discussed with dexamethasone and other medications. As such, this Therapeutics Review will present prominent and/or promising medications and therapeutic approaches with the caveats that 1. To date, none are FDA approved beyond emergency use authorization (EUA), and 2. Although a comprehensive look at various classes of interventions, it is by no means a complete list of every compound trialed against COVID-19. Recognizing the knowledge basis upon which we treat COVID-19 patients, develop therapeutics, and vaccines continues to evolve as new information is presented, every effort nevertheless has been made to provide as timely information as possible. It is hoped that the information shared can help guide the clinician in terms of potential options to treat this complex group of patients.
Introduction
Extensive research is underway to identify and validate a wide variety of potential interventions to treat COVID-19, as well as other coronaviruses, in addition to evaluating best practices in aggressive symptomatic and supportive care. What follows is an overview of the medications, other therapeutic agents, and a variety of interventions, including hyperbaric oxygen therapy (HBOT), that have shown some clinical benefit, and emerged as possible treatment candidates.
It is worth noting that the use of interventions discussed may be predicated on the level of illness and stage of severity, extrapulmonary involvement and other factors. Moreover some may play a role in combination instead of as single agents. For example, a recent British study suggests dexamethasone may have a role for treating severe or ventilator dependent patients, but little clinical utility in less severely ill patients.
Every effort has been made to provide the clinician with a list of available and potentially useful medications as possible agents against COVID-19. To be sure what follows is not the complete list, given the unprecedented worldwide efforts of research laboratories, academic, and the pharmaceutical industry, in terms of new medications being designed to treat coronaviruses. Nor does it exhaust the list of therapeutics currently approved for other indications now being repurposed as a potential treatment for COVID-19 or other pathogenic coronaviruses. And as of 06/09/20 there are no US Food and Drug Administration (FDA) medications approved specifically for the treatment of COVID-19. However there are limited candidates, such as remdesivir that have been given FDA emergency use authorization or other FDA special use guidance, such as convalescent plasma.
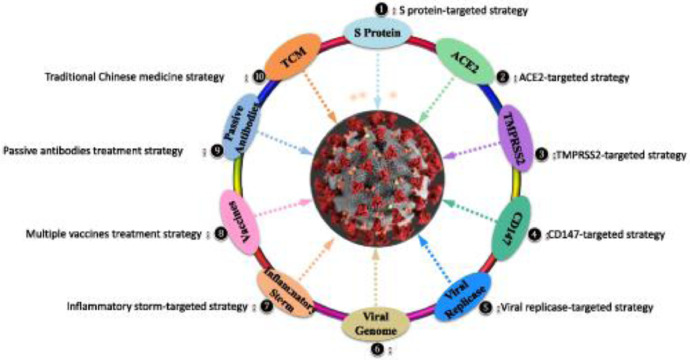
Fig. 1 (1) represents the various, and complex pathways involved in the viral life cycle, along with virus -host interactions, and with it potential opportunities for antivirals and vaccines. Several of the therapeutics discussed in the following section may be found in this figure.
Fig. 1.
COVID-19 Viral structure and protein targeting strategy for potential therapeutics (1).
References
-
1.
Zhou H, Fang Y, Xu T, Ni WJ, et al. Potential therapeutic targets and promising drugs for combating SARS‐CoV‐2. British Journal of Pharmacology 05/05/20 https://doi.org/10.1111/bph.15092. Last accessed 07/29/20.
FDA Approves Remdesivir for use as a treatment for COVID-19
On 10/22/20 The U.S. Food and Drug Administration (FDA) approved the antiviral drug Veklury (Remdesivir) for use in adult and pediatric patients 12 years of age and older and weighing at least 40 kilograms (about 88 pounds) for the treatment of COVID-19 requiring hospitalization.
Per FDA Veklury (Remdesivir) should only be administered in a hospital or in a healthcare setting capable of providing acute care comparable to inpatient hospital care.
Veklury (Remdesivir) is the first treatment for COVID-19 to receive FDA approval.
RE: Other pediatric patients, the FDA guidance states the approval does not include the entire population that had been authorized to use Veklury (Remdesivir) under an Emergency Use Authorization (EUA) originally issued on May 1, 2020. Per FDA “In order to ensure continued access to the pediatric population previously covered under the EUA, the FDA revised the EUA for Veklury (Remdesivir) to authorize the drug’s use for treatment of suspected or laboratory confirmed COVID-19 in hospitalized pediatric patients weighing 3.5 kg to less than 40 kg or hospitalized pediatric patients less than 12 years of age weighing at least 3.5 kg. Clinical trials assessing the safety and efficacy of Veklury in this pediatric patient population are ongoing.”
Safety information is available in the prescribing information associated with the approval but similar safety information about using Veklury (Remdesivir) to treat COVID-19 in certain hospitalized pediatric patients under the EUA is available in the fact sheets for health care providers (https://www.fda.gov/media/137566/download)
Remdesivir
Remdesivir (GS 5734) is a monophasic nucleotide analogue prodrug, which metabolizes to an active C adenosine nucleoside triphosphate analogue (1–19). It is an inhibitor of the viral RNA-dependent, RNA polymerase that was found to interrupt viral replication (Fig. 1 ) (1, 5, 7–10, 14–20). Nucleoside analogues are a class of antiviral therapeutics that are utilized clinically to treat several viruses, including hepatitis B, hepatitis C, and HIV (1, 3–7).
Among the potential antivirals, Remdesivir (GS-5734) has demonstrated clinical benefit against coronaviruses, with some positive results noted with SARS and MERS (4, 11, 18, 20).
Remdesivir has been studied as a potential coronavirus replication inhibitor, including of late multiple clinical trials involving use against COVID-19 (1, 6, 11, 14). Noted to have inhibitory activity against SARS and the Middle East respiratory syndrome (MERS),(1, 4, 14, 18, 20) was identified early as a promising therapeutic candidate for Covid-19 because of its ability to inhibit SARS-CoV-2 in vitro (5, 10). In addition, in nonhuman primate studies, when Remdesivir was initiated 12 h after inoculation with MERS-CoV9,(5, 11, 14), there was a noted reduction in lung virus levels and lung damage. In murine lung MERS infection models, Remdesivir prevented lung hemorrhage as well as reduced lung titers of virus compared to other agents (1, 21)
In various clinical trials Remdesivir demonstrated not only activity against RNA viruses, including SARS, MERS, but zoonotic coronaviruses, and human coronaviruses HCoV-OC43 and HCoV-229E, which are among the various viruses that cause what is referred to as “the common cold.”
As reported by de Wit et al, remdesivir revealed in a nonhuman primate model in vivo activity (prophylactic and therapeutic) against the MERS coronavirus (14). Remdesivir was noted in non human primate study, when initiated 12 h after inoculation with MERS-CoV, it reduced lung virus levels, as well as lung damage. More recently multiple studies have demonstrated clinical benefit from Remdesivir. In human trials, Remdesivir has shown in vitro and in vivo benefit against COVID-19.
Clinical improvement in COVID-19 patients.(REM 107) has been demonstrated with Remdesivir. Moreover early analysis of the Adaptive COVID-19 Treatment Trial (NCT04280705) demonstrated improvement in the primary endpoint for patients receiving remdesivir, compared to control. Remdesivir resulted in a 31% faster time to recovery. Extensive research continues geoglobally.
Owing to the clinical benefit associated with early test results involving Remdesivir, the U.S. Food and Drug Administration issued an Emergency Use Authorization which allows the emergency use of Remdesivir with an indication for the treatment of hospitalized COVID-19 patients. This is the first FDA authorization of an investigational therapeutic for use in treating SARS-CoV-2 (22–24).
In May 2020, the results from a multicenter study involving 1063 hospitalized patients with laboratory test confirmed COVID – 19 infection, who had clinical signs and symptoms of lower respiratory tract involvement randomly selected for treatment with either Remdesivir or placebo were published (5). Clinical benefit was noted early in the study, such that the data and safety monitoring board recommended early unmasking of the study. The primary outcome was time to recovery, and the secondary outcome was odds of improvement.
Patients were randomly assigned to Remdesivir, with dosing schedule of a 200 mg loading dose on day 1, followed by 100 mg daily for up to 9 additional days, or placebo for up to 10 days. Their primary outcome measure was time to recovery, defined by either discharge from the hospital or hospitalization for infection control purposes only. A total of 1059 patients were involved in the study, of which 538 were assigned to Remdesivir, and 521 to placebo.
The Remdesivir group experienced shortened time to recovery, compared with placebo group (5). Of note, patients receiving Remdesivir treatment had a median recovery time of 11 days (95% confidence interval [CI], 9 to 12), compared with 15 days (95% CI, 13 to 19) administered the placebo, with a rate ratio for recovery, 1.32; 95% CI 1.12 to 1.55; p<0.001). The Kaplan-Meier estimates of death by 14 days were 7.1% in the Remdesivir group, and 11.9% for those receiving placebo. Serious adverse events were reported for 114 of the 541 patients receiving Remdesivir group (21.1%) compared to 141 of the 522 patients in the placebo group (27.0%). The most common adverse events in the remdesivir group were anemia or decreased hemoglobin (43 events [7.9%], as compared with 47 [9.0%] Of note not all patients in either group completed the trial for a variety of reasons.
Results were variable depending upon level of illness, underlying comorbid conditions, and other issues. According to researchers, the benefit was most apparent in patients with a baseline ordinal score of 5 (requiring oxygen). Unknowns remain given the number of patients completing the study, variations in healthcare delivery across test sites, and other potential limitations to the study.
The authors also cite a randomized trial from China involved 237 patients where 158 received Remdesivir and 79 placebo (13). The time to clinical improvement, which involved a two-point improvement in score on the ordinal scale, was 21.0 days (95% CI, 13.0 to 28.0) for the Remdesivir group and 23.0 days (95% CI, 15.0 to 28.0) for the placebo group, with a hazard ratio for clinical improvement of 1.23 (95% CI, 0.87 to 1.75). There were also challenges noted with this study (5).
The researchers disclose several important issues to consider, including an early change in primary outcome during the study. Nevertheless, the FDA is allowing Remdesivir to be made available for clinical use under an emergency-use authorization for the treatment of adults and children with severe Covid-19 disease (5). Severity defined as having an oxygen saturation of less than 94 percent, requiring supplemental oxygen, mechanical ventilation or a heart-lung bypass machine, ECMO (5).
Their conclusion - Remdesivir was superior to placebo in shortening the time to recovery in adults hospitalized with Covid-19 and evidence of lower respiratory tract infection. The authors also note the data release are preliminary findings, but assert their study supports the use of Remdesivir for patients who are hospitalized with Covid-19 and require supplemental oxygen therapy. They further state “…. given high mortality despite the use of Remdesivir, it is clear that treatment with an antiviral drug alone is not likely to be sufficient. Future strategies should evaluate antiviral agents in combination with other therapeutic approaches or combinations of antiviral agents to continue to improve patient outcomes in Covid-19.” (5).
Beigel et al report other important outcomes. The odds of improvement in the ordinal scale score were higher in the Remdesivir group at day 15 visit, than in the placebo group. Mortality was lower in the remdesivir group than placebo group, but the difference was not significant (hazard ratio for death, 0.70; 95% CI, 0.47 to 1.04; 1059 patients). The Kaplan–Meier estimates of mortality by 14 days were 7.1% and 11.9% in the Remdesivir and placebo groups, respectively. The Kaplan– Meier estimates of mortality by 28 days are not reported in this preliminary analysis, given the large number of patients that had yet to complete day 29 visits. An analysis with adjustment for baseline ordinal score as a stratification variable showed a hazard ratio for death of 0.74 (95% CI, 0.50 to 1.10) (5).
Owing to the recent FDA emergency use approval of Remdesivir (23, 24), it is important to share the safety outcomes in Beigel et al (5). They report serious adverse events in 114 patients (21.1%) in the remdesivir group and 141 patients (27.0%) in the placebo group. They note 4 events (2 in each group) were judged by site investigators related to either remdesivir or placebo. No deaths were attributed to treatment assignment, as judged by the site investigators. Grade 3 or 4 adverse events occurred in 156 Remdesivir patients (28.8%), and 172 in placebo group (33.0%).
The most commonly noted adverse events in the Remdesivir group (R group) or Placebo (P group) (5):
-
•
Anemia or decreased hemoglobin (R group 43 events [7.9%] P group 47 [9.0%]
-
•
Acute kidney injury (AKI) - decreased estimated glomerular filtration rate (eGFR) or creatinine clearance, or increased blood creatinine (Cr) Remdesivir [7.4%], Placebo [7.3%])
-
•
Pyrexia R group 27 events [5.0%], P group 17 [3.3%])
-
•
Hyperglycemia or increased blood glucose level, R group 22 events [4.1%], P group 17 [3.3%])
-
•
Elevated LFTs - increased aminotransferase levels including alanine aminotransferase, aspartate aminotransferase, or both R group 22 events [4.1%], P group 31 [5.9%]).
Beyond these, the authors report the incidence of adverse events was not found to be significantly different between Remdesivir or placebo groups.
The study conclusion, which the FDA seems to agree, suggest a 10- day course of treatment with Remdesivir was superior to placebo in the treatment of hospitalized patients with Covid-19. The early success led them to un-blind the research. The authors revealed the results of the study earlier than planned due to early positive clinical results. These findings were deemed to be of immediate importance for the care of patients still participating in the trial as well as for those outside the trial who might benefit from treatment with Remdesivir (5).
In other research, such as Phase 1 studies (1, 25) IV infusions ranging in dose from 3 mg to 225 mg were well tolerated w/out evidence of hepatic or renal toxicity. The pharmacokinetics were linear within those dose ranges, and revealed an intracellular t ½ of 35 h. In multi-dose studies, reversible aspartate aminotransferase and alanine transaminase elevations were reported. Caution is raised in administering to patients with eGFR less than 30 mL/min. The use in women who are pregnant and children should be through compassionate use, or clinical trial.
According to the FDA, Health and Human Services Emergency Use Authorization (EUA) “Remdesivir is authorized for use under an EUA only for the treatment of patients with suspected or laborator confirmed SARS Cov2 infection and severe COVID19. Severe disease is defined as patients with an oxygen saturation (SpO2) less than or equal to 94% on room air or requiring supplemental oxygen, mechanical ventilation, and/or extracorporeal membrane oxygenation (ECMO). Remdesivir is authorized for adult or pediatric patients who are admitted to a hospital and for whom use of an IV agent is clinically appropriate. Remdesivir must be administered intravenously.” (23, 24)
Remdesivir is not currently known to be a significant inducer or inhibitor of the cytochrome P450 (CYP450) enzymes, but monitoring when coadministered with strong inducers/inhibitors is still recommended (1, 21, 26, 27). Elevated transaminases with treatment is known, but considered reversible upon treatment cessation. Kidney injury has been reported (1).
Remdesivir is administered via an intravenous injection (IV) as follows*:
-
•Day 1- loading dose 200 mg in adults,
-
○Adjusted for body weight in pediatric patients
-
○
-
•
Day 2 up to Day 10 – administer 100 mg every 24 h in adults
*At the time of publication this information is correct. As with any therapeutic agent trialed against COVID-19 be alert to adaptations in dosing schedules.
References
-
1.
Sanders JM Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus dieseas (COVID-19): A Review. Jama 2020;323 (18):1824-1836 doi:10.1001/jama.2020.6019. Last accessed 06/05/20
-
2.
Eastman, RT, Roth, JS, Brimacombe, KR, Simeonov, A. ACS Cent. Sci. 2020, 672-683
-
3.
Riva, L. A Large-scale Drug Repositioning Survey for SARS-CoV-2 Antivirals. bioRxiv, 2020, 2020.04.16.044016
-
4.
Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERSCoV. Nat Commun 2020;11:222.
-
5.
Beigel JH, Tomashek, KM, Dodd, LE, Mehta, AK, et al. Remdesivir for the Treatment of Covid-19 — Preliminary Report NE JM 2020; May
-
6.
Agostini ML, Andres EL, Sims AC, et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio 2018;9(2):e00221- 18.
-
7.
Brown AJ, Won JJ, Graham RL, et al. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res 2019;169:104541.
-
8.
Sheahan TP, Sims AC, Graham RL, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 2017;9:eaal3653.
-
9.
https://www.drugtargetreview.com/news/56798/mechanism-of-action-revealed-for-remdesivir-potential-coronavirus-drug/. Last accessed 05/31/20
-
10.
Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30: 269-71.
-
11.
de Wit E, Rasmussen AL, Falzarano D, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc Natl Acad Sci U S A 2013; 110:16598-603.
-
12.
ACS Cent Sci. 2020 May 4: acscentsci.0c00489.Published online 2020 May 4. doi:10.1021/acscentsci.0c00489.
-
13.
Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19
-
14.
de Wit E, Feldmann F, Cronin J, et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A 2020;117:6771-6
-
15.
Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395:1569-78.
-
16.
Varga, A.; Lionne, C.; Roy, B. Intracellular Metabolism of Nucleoside/Nucleotide Analogues: a Bottleneck to Reach Active Drugs on HIV Reverse Transcriptase. Curr. Drug Metab. 2016, 17, 237−252.
-
17.
Brown, A. J.; et al. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019, 169, 104541.
-
18.
Agostini ML, Andres EL, Sims AC, et al. Coronavirus susceptibility to the antiviral remdesivir (GS=5734) is mediated by the viral polymerase and the proofreading exoribonuclear. MBio 2018;9 pii:e00221-18. doi:10.1128/mbio.00221-18.
-
19.
Grein, J. et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020, doi:10.1056/NEJMoa2007016.
-
20.
Li H, Liu SM, Yu XH, et al. Coronavirus disease 2019)COVID-19): current status and future perspectives. Int J Antimicrobial Agents 55 (2020) 105951
-
21.
Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):222. doi:10.1038/s41467-019-13940-6.
-
22.
NIAID News Release, April 29, 2020. NIH Clinical Trial Shows Remdesivir Accelerates Recovery from Advanced COVID-19. (https://www.niaid.nih.gov/news-events/nih-clinical-trial-showsremdesivir-accelerates-recovery-advanced-covid-19).
-
23.
FDA News Release, May 1, 2020. Remdesivir EUA Letter of Authorization. (https://www.fda.gov/media/137564/download).
-
24.
www.fda.gov. Emergency Use Authorization of Remdesivir. Last accessed 06/07/20
-
25.
World Health Organization. WHO R&D blueprint: ad-hoc expert consultation on clinical trials for Ebola therapeutics. Published October 2018. Accessed March 20, 2020. https://www.who.int/ebola/drc-2018/summaries-of-.
-
26.
Siegel D, Hui HC, Doerffler E, et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J Med Chem. 2017;60(5): 1648-1661. doi:10.1021/acs.jmedchem.6b01594.
-
27.
Al-Tawfiq JA, Al-Homoud AH, Memish ZA. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis. Published online March 5, 2020. doi:10.1016/j.tmaid.2020.101615.
Hydroxychloroquine
Hydroxychloroquine is an aminoquinoline class drug with extensive use in the treatment of rheumatologic disorders such as rheumatoid arthritis, chronic inflammatory diseases include systemic lupus erythematosus (SLE), as well as malaria (1–3).
Hydroxychloroquine and chloroquine have gained significant attention during the COVID-19 pandemic with various claims of clinical success emanating primarily from small trials, and anecdotal reports.
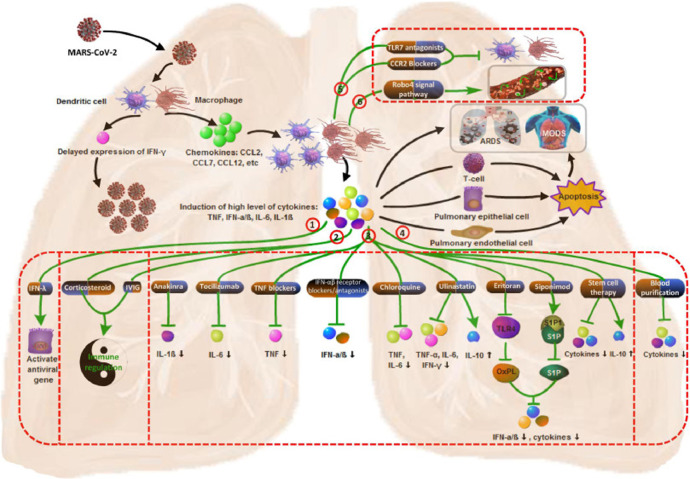
Chloroquine is noted to inhibit the production/release of TNF and IL-6, both associated with cytokine storm (Fig. 2 ) (4). It has been postulated that chloroquine and hydroxychloroquine can attenuate the cytokine storm associated with COVID-19 (4, 5).
Fig. 2.
Mechanism of cytokine storm in COVID-19 and potential therapy.
In addition to immunomodulatory effects achieved by attenuating cytokine production, inhibiting autophagy and lysosomal activity in host cells is also noted. They also appear to block viral entry into cells by inhibiting glycosylation of host receptors, prteoloytic processing and endosomal acidification. In vitro studies with choloroquine demonstred inhibition of COVID-19, as did hydroxycholoroquine.
Reports from China suggest good results and clinical benefit, but detailed information about some of the research have been unavailable for peer review (1). An open-label study from France involving 36 patients also revealed superior clinical results in the hydroxychloroquine group, but also note azithromycin was added to this group in several patients, which resulted in improved viral clearance. There were some limitations to this study, including removal of 6 patients in the treatment group resulting from critical illness or treatment associated adverse events. Over the last several months there have been reports of clinical benefit from Hydroxychloroquine.
A small study from Shanghai where patients were treated with 400 mg hydroxychloroquine x 5 days compared with a control group receiving conventional treatment only, revealed by day 7, nearly 90% of both treatment and control groups had negative viral throat swabs. Of note, all the patients in the study received aerosolized interferon alpha by nebulizer (3, 6).
Recent research suggests hydroxychloroquine may be a more potent antiviral than chloroquine (3, 7). The safety profile of both drugs is well known in the treatment of non-COVID-19 patients, having been prescribed in large numbers both in the US and other countries.
Recently a large observational study involving the use of hydroxychloroquine in the treatment of COVID – 19 was published (3). The authors reviewed the association between hydroxychloroquine use and intubation or death at a large advanced care medical center in New York City. They hypothesized those treated with hydroxychloroquine would demonstrate lower risk of intubation or death when adjusted for major predictors of respiratory failure, and weighted to propensity scores to assess probability of hydroxychloroquine use.
Of 1446 consecutive patients, 1376 were reviewed for inclusion into the study. In their conclusion the authors stated hydroxychloroquine administration was not associated with either a greatly lowered or an increased risk of the composite end point of intubation or death. Random controlled trials of hydroxychloroquine in patients with COVID-19 are needed (3). The authors note the observational design and wide confidence intervals (CI) do not commend the study to recommend use or avoidance of hydroxychloroquine. The authors caution that hydroxychloroquine should be administered only in clinical trial settings. They also state that at their healthcare facility, hydroxychloroquine is not suggested for the treatment of COVID019 at this time.
In spite of complex biostatistical modeling and approaches, this study also had multiple variables and confounding issues, that could have impacted the results, including a not insignificant number of patients that came out of the study for various reasons. Multiple interventions were also administered, and while good effort to match the various groups was evident, as the authors state, this was not a randomized controlled prospective clinical trial such that the results must be interpreted with caution.
While there is of yet no optimum dose established for chloroquine or hydroxychloroquine in the treatment of COVID-19, dosing recommendations for hydroxychloroquine have been based upon their use treating rheumatologic illness such as SLE - 400 mg orally daily (1, 8). Other dosing has been suggested, positing the question about high versus low doses (9). Using pharmacokinetic modeling, one study recommended administering a loading dose of 400 mg twice daily for day 1, followed by 200 mg twice daily on subsequent days to treat COVID-19 (1, 10). Other doses based upon other clinical experiences have been posited as well.
In some Chinese trials using chloroquine as a treatment for COVID-19, the dosing was as follows (4, 5):
Patient weight more than 50 kg = administered 500 mg twice a day, × 7 days
Patient weight less than 50 kg = administered 500 mg twice a day × 2 days, then 500 mg once a day x 5 days
Clearly further studies are needed not just to determine the clinical role of hydroxychloroquine in the treatment of COVID-19, but also to identify the optimal dose.
It should be noted that while there are isolated reports of benefit from hydroxychloroquine, and that generally speaking the aminoquinolines are relatively safe to use, there are adverse side effect risks. QTc prolongation has been reported, along with hypoglycemia, neuropsychiatric effects, and retinopathy (1, 11, 12). If hydroxychloroquine is considered for use, preferably as part of a clinical trial, baseline electrocardiogram (EKG) and regular cardiac monitoring should be obtained. Caution should be given when co-administering other potential QT interval prolonging agents, such as azithromycin, and fluoroquinolones (1, 13). Hydroxychloroquine is also a CYP 2D6, CYP 3A4, CYP 3A5, and CYP 2C8 substrate (1, 14).
Hydroxychloroquine seems to have had some beneficial effects on some patients, but data remain equivocal. Because the issue of hydroxychloroquine in the treatment of COVID-19 has yet to be settled, and acknowledging there are risks from adverse events, the FDA has discouraged the use of Hydroxychloroquine and chloroquine for the treatment of COVID-19 except in a monitored health care setting and/or clinical trial.
Currently there are multiple randomized control trials (RCT) underway involving chloroquine, and hydroxychloroquine, to further evaluate their potential effectiveness in the treatment of COVID - 19 (1). Additionally, there are studies either in process or soon to be that are assessing the role of chloroquine as prophylaxis for health care workers, and hydroxychloroquine for post exposure prophylaxis associated with high risk exposures (1, 14).
Perhaps the ideal role for aminoquinolines will be as part of a therapeutic cocktail utilizing multiple medications with different mechanisms of action for synergistic effect in the treatment of COVID-19, or perhaps certain subpopulations – by demographic or clinical category may proved a better fit for hydroxychloroquine and chloroquine. Further study is required (15).
Most recently prior to completion of this article a study was reported (16). A double blind placebo RCT study involving various regions of the United States and Canada was conducted to study the potential role of hydroxychloroquine as possible post-exposure prophylaxis (PEP). Adults with a known household or occupational exposure with someone who tested positive for Covid-19 defined as a distance of less than 6 ft for more than 10 min while wearing neither a face mask nor an eye shield (high-risk exposure) or while wearing a face mask but no eye shield (moderate-risk exposure). Participants within 4 days post exposure were randomly assigned to receive placebo or hydroxychloroquine (800 mg once, followed by 600 mg in 6 to 8 h, then 600 mg daily for 4 additional days). The primary outcome was the incidence of either laboratory-confirmed Covid-19 or illness compatible with Covid-19 within 14 days.
They enrolled 821 asymptomatic participants. Overall, 87.6% of the participants (719 of 821) reported a high-risk exposure to a confirmed Covid-19 contact. The study results revealed an incidence of new illness compatible with Covid-19 did not differ significantly between participants receiving hydroxychloroquine (49 of 414 [11.8%]) and those receiving placebo (58 of 407 [14.3%]); the absolute difference was −2.4 percentage points (95% confidence interval, −7.0 to 2.2; P=0.35). Side effects were more common with hydroxychloroquine than with placebo (40.1% vs. 16.8%), but no serious adverse reactions were reported (16).
It should also be noted that while in March 2020 the FDA issued an Emergency Use Authorization (EUA) allowing hydroxychloroquine and chloroquine to be administered in COVID-10 patients, owing to anti-inflammatory and antiviral potential, but as of 15 June 2020 the FDA revoked the EUA (17, 18). “FDA has concluded that, based on this new information and other information discussed in the attached memorandum, it is no longer reasonable to believe that oral formulations of HCQ and CQ may be effective in treating COVID-19, nor is it reasonable to believe that the known and potential benefits of these products outweigh their known and potential risks.” (18).
References
-
1.
Sanders JM Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus dieseas (COVID-19): A Review. Jama 2020;323 (18):1824-1836 doi:10.1001/jama.2020.6019. Last accessed 06/05/20.
-
2.
Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3(11):722-727. doi:10.1016/S1473-3099(03)00806-5.
-
3.
Geleris J, Sun Y, Platt J, Zucker J, et al. Observational study of Hydroxychloroquine in hospitalized patients with COVID-19. NE JM 2020. doi:10.1056/nejmoa2012410.
-
4.
Ye Q, Wang B, Mao J The pathogenesis and treatment of the cytokine storm in COVID-19. J Infect https://doi.org/10.1016/j.jinf2020.03.037.
-
5.
J G, Z T, X Y . Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 2020; 14 (1):72–3 PubMed PMID: 32074550.
-
6.
Chen J, Liu D, Liu L, et al. A pilot study of hydroxychloroquine tin treatment of patients with common coronavirus disease-19 (COVID-19). J Zhejiang Uni (MED Sci) 2020 http://www.zjujournals.com/med/EN/10.3785/j.issn.1008-9292.2020.03.03.
-
7.
Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin infect Dis 2020 March 0 Epub ahead of print.
-
8.
Hydroxychloroquine [database online].Hudson, OH: Lexicomp Inc; 2016. Accessed March 17, 2020. http://online.lexi.com.
-
9.
Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open 2020;3(4): e208857.
-
10.
Yao X, Ye F, ZhangM, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. Published online March 9, 2020. doi:10.1093/cid/ciaa237.
-
11.
Kalil AC. Treating COVID-19—off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA. Published March 24, 2020. doi:10.1001/jama.2020.4742.
-
12.
Interview with David Juurlink. Coronavirus (COVID-19) update: chloroquine/ hydroxychloroquine and azithromycin. JAMA. March 24, 2020. Accessed April 3, 2020. https://edhub.ama-assn.org/jn-learning/audio-player/18337225ho-translation.pdf.
-
13.
Chloroquine [database online]. Hudson, OH: Lexicomp Inc; 2016. Accessed March 17, 2020. http://online.lexi.com.
-
14.
Zhou D, Dai SM, Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. [published online March 20, 2020]. J Antimicrob Chemother. 2020;dkaa114. doi:10.1093/jac/dkaa114.
-
15.
ClinicalTrials.gov. Accessed March 18, 2020.https://clinicaltrials.gov/.
-
16.
Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med. 2020 Jun 3. doi:10.1056/NEJMoa2016638.
-
17.
Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and. Last accessed 06/17/20
-
18.
FDA Memorandum RE: Revocation of EUA for chloroquine and hydroxychloroquine in the treatment of COVID-19 https://www.fda.gov/media/138945/download. Last accessed 06/17/20
Lopinavir-Ritonavir
Is an oral antiviral medication FDA approved for the treatment of HIV (1–5). Studies have shown Lopinavir/Ritonavir had in vitro activity against coronaviruses through inhibition of 3 chymotrypsin-like protease (3 CL protease). Most studies utilized Lopinavir/Ritonavir against SARS, with a few against MERS. Retrospective studies with SARS patients revealed a decreased mortality and use of intubation, but these were retrospective, and observational, so their generalizability is limited. Of note, treatment must begin early; in cases with delayed treatment using Lopinavir/Ritonavir, they had no beneficial effect on outcome (1, 6, 7).
As a possible treatment for COVID-19 infection there have been case reports and small retrospective studies. Cao et al revealed results from an open label RCT that compared lopinavir/ritonavir with standard care in 199 patients infected with COVID019 (1, 8). Median time from symptom onset to randomization was 13 days. Primary outcome was time to clinical improvement or hospital discharge. Viral clearance was also assessed. A subgroup analysis looking at patients who received treatment within 12 days was also implemented. No significant differences in viral clearance, or 28 day mortality rates were observed. Results were similar between groups in other outcomes measures.
The doses most frequently recommended for lopinavir/ritonavir for the treatment of COVID-19 is 400 mg/100mg twice daily for up to 14 days. Of note, there remains potential for significant drug-drug interactions, and adverse drug reactions. These can be severe, and include nausea, vomiting and diarrhea in upward of 28% of patients, and hepatotoxicity, which has been reported in 2 – 10% (1, 9). An important consideration – reports suggest approximately 20% to 30% of patients infected with COVID-19 have elevated transaminases at presentation (1, 10). COVID-19 may cause liver damage; these antivirals could exacerbate the hepatic injury. And in multiple COVID-19 studies, elevated transaminase levels are an exclusion criterion. Pancreatitis is also possible, along with cardiac conduction abnormalities. Moreover an RCT revealed ~50% of lopinavir/ritonavir patients suffer from an adverse effect, with 14% discontinued treatment due to gastrointestinal side effects (1,8). Careful review of medications and patient comorbidities, as well as close monitoring should be done if this combination drug is considered.
In terms of pharmacology considerations, lopinavir/ritonavir therapy is a CYP 3A4 inhibitor and substrate, multiple other CYP enzyme inducer and substrate, p-GP substrate, and UGT1A1 inducer (1).
References
-
1.
Sanders JM Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus dieseas (COVID-19): A Review. Jama 2020;323 (18):1824-1836. doi:10.1001/jama.2020.6019. last accessed 06/05/20.
-
2.
Chu CM, Cheng VC, Hung IF, et al; HKU/UCH SARS Study Group. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252-256. doi:10.1136/thorax.2003.012658.
-
3.
de Wilde AH, Jochmans D, Posthuma CC, et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014; 58(8):4875-4884. doi:10.1128/AAC.03011-14.
-
4.
Hung IFN, Lung KC, Tso E YK, Liu R, et al. Triple combination of interferon beta 1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open lable, randomized phase 2 trial. The Lancet 2020 (395) May 20. https://doi.org/10.10116/S0140-6736.(20)31042-4 Last accessed 06/01/20
-
5.
Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; 382:1787-99.
-
6.
Yao TT, Qian JD, ZhuWY,Wang Y,Wang GQ. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-A possible reference for coronavirus disease-19 treatment option. [published online February 27, 2020]. J Med Virol. 2020. doi:10.1002/jmv.25729.
-
7.
Chan KS, Lai ST, Chu CM, et al. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9(6):399-406.\
-
8.
Cao B,Wang Y,Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. Published online March 18, 2020. 10.1056/NEJMoa2001282.
-
9.
Lopinavir/ritonavir [database online]. Hudson (OH): Lexicomp Inc; 2016. Accessed March 17, 2020. http://online.lexi.com.
-
10.
Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia inWuhan, China. JAMA Intern Med. Published online March 13, 2020.
Lopinavir/Ritonavir, Ribavirin and interferon beta 1b combination treatment
Hung and colleagues recently reported on an open label, multicenter, randomized, phase 2 trial evaluating triple combination therapy involving interferon beta-1b, lopinavir-ritonavir, and ribavirin to treat patients infected with COVID-19, and admitted to the hospital (1).
Lopinavir-ritonavir combination is an oral protease inhibitor. Ribavirin is an oral nucleoside analogue (1-9).
The rationale for the study design was partly based on early success with treating SARS in 2003, using lopinavir/ritonavir and ribavirin (1, 10). Mortality and need for intensive respiratory support was noted (1, 10). In an animal study involving marmosets, lopinavir-ritonavir or interferon beta – 1b reduced viral load and improved lung pathology (1, 11). Combination antiviral therapy for patients hospitalized with severe influenza seemed to confer greater results, for those with high viral loads at presentation (1, 12, 13).
Studies have shown both SARS and MERS viral loads peak at ~7 – 10 days after symptoms begin compared to COVID-19 which seems to peak at the time of clinical presentation (1, 13–15). This makes time sensitive administration of medications a challenge.
The study involved patients randomly assigned to the triple combination (lopinavir-ritonavir, ribavirin, and interferon beta 1b) or control group (lopinavir-ritonavir), in a 2:1 ratio, using simple randomization with no stratification. Treatment was less than 7 days from symptom onset – triple combination 14 days of oral lopinavir 400 mg-ritonavir 100 mg every 12 h. If patients were intubated, it was administered through nasogastric tube), and ribavirin 400 mg every 12 h, and one to three doses of interferon beta-1b 1 mL (8 million IU) with each dose given on alternate days. Of note, if patients were recruited between days 7 and 14, interferon beta-1b injection was not provided owing to risk of proinflammatory risk.
Patients in the control group received only oral lopinavir-ritonavir at the same dose as treatment group, every 12 h for 14 days. Lopinavir-ritonavir was adjusted depending upon cardiac and hepatic status. Treatment had to be initiated within 48 h of hospital admission.
Other care was implemented as needed, which included oxygen, ventilator support, ECMO if needed, as well as availability to dialysis, and antimicrobials for secondary bacterial infection. Extensive laboratory and radiographic testing, EKG and regular monitoring were provided. CBC, LFTs, RFTs, C reactive protein, ESR and cytokine profile labs were obtained regularly.
All patients in the study had to have laboratory confirmation by reverse transcriptase polymerase chain reaction (RT-PCR) in nasopharyngeal swab. Primary outcome measures were time to achieve a negative RT-PCR, and secondary endpoints were time to symptom resolution (NEWS2 and SOFA scores of “0”), length of hospital stay, 30 – day mortality, time to achieve negative RT-PCR. Regular monitoring for adverse events was provided.
The results revealed patients in the combination group had a shorter average time to negative nasopharyngeal swab; triple therapy 7 days, control 12 days. This effect was also seen in time to negative viral load from all specimens – nasopharyngeal, posterior oropharyngeal saliva, throat swab, stool and urine specimens. Clinical improvement was better in the triple therapy group as well, where there is a shorter average time to alleviation of symptoms (NEWS2 Score 0); triple therapy 4 days, control 8 days. Shorter average median hospital stay was realized in the triple therapy group (9 days) compared to control group (14. 5 days). Interestingly the Il-6 concentration was significantly lower in the triple therapy group compared with control group, but TNFa and IL-10 concentrations were not significantly different between the groups, and no significant nsp5 mutations were identified.
Adverse events were reported in 48% of triple therapy group and 49% of control group patients. The most commonly noted adverse events were, diarrhea 41%, fever 38%, nausea 34%, elevated alanine transaminase 14%, and sinus bradycardia (3%); no significant difference between groups referable to adverse events, and these mostly resolved within 72 h after drug initiation, although 1 patient in the control group had a severe event of impaired liver enzymes, and treatment was discontinued.
Of note, patients who started treatment less than 7 days after the onset of symptoms demonstrated better clinical and virological outcomes in the triple therapy group compared with the control group. There was significant difference between the two treatment groups in the various outcomes among those who were treated 7 or more days after onset of symptoms.
The authors note that lopinavir/ritonavir, ribavirin and interferon beta 1b combination therapy holds promise in the treatment of COVID-19 when administered early after symptom onset, while lopinavir-ritonavir alone has similar effects to placebo on reducing viral load when treatment initiation is 13 days after symptom onset, with only some improvement in symptoms (8). They also point out that not all patients received interferon beta 1 b, which may be a key component of the combination treatment (Fig. 2) (17). Also critically ill patients were not enrolled in the study, limiting the generalizability of findings to severe cases.
Another combination study involving the use of arbidol and lopinavir-ritonavir, compared with lopinavir-ritonavir alone showed a negative viral load at day 7 post treatment in 75% of COVID-19 patients treated with the two drugs, compared with 35% who only received lopinavir-ritonavir, but it was a small cohort (1, 16).
References
-
1.
Hung IFN, Lung KC, Tso E YK, Liu R, et al. Triple combination of interferon beta 1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open lable, randomized phase 2 trial. The Lancet 2020 (395) May 20. https://doi.org/10.10116/S0140-6736.(20)31042-4 Last accessed 06/01/20
-
2.
Sanders JM Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus dieseas (COVID-19): A Review. Jama 2020;323 (18):1824-1836. 10.1001/jama.2020.6019. last accessed 06/05/20.
-
3.
Chu CM, Cheng VC, Hung IF, et al; HKU/UCH SARS Study Group. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252-256. doi:10.1136/thorax.2003.012658.
-
4.
de Wilde AH, Jochmans D, Posthuma CC, et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014; 58(8):4875-4884. doi:10.1128/AAC.03011-14.
-
5.
Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; 382:1787-99.
-
6.
Yao TT, Qian JD, ZhuWY,Wang Y,Wang GQ. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-A possible reference for coronavirus disease-19 treatment option. [published online February 27, 2020]. J Med Virol. 2020. doi:10.1002/jmv.25729.
-
7.
Chan KS, Lai ST, Chu CM, et al. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9(6):399-406.\
-
8.
Cao B,Wang Y,Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. Published online March 18, 2020. doi:10.1056/NEJMoa2001282.
-
9.
Lopinavir/ritonavir [database online]. Hudson (OH): Lexicomp Inc; 2016. Accessed March 17, 2020. http://online.lexi.com.
-
10.
Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir inthe treatment of SARS: initial virological and clinical findings.Thorax 2004; 59: 252–56.
-
11.
Chan JF, Yao Y, Yeung ML, et al. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis 2015; 212: 1904–13.
-
12.
Dunning J, Baillie JK, Cao B, Hayden FG. Antiviral combinations for severe influenza. Lancet Infect Dis 2014; 14: 1259–70.
-
13.
Hung IFN, To KKW, Chan JFW, et al. Efficacy of clarithromycin - naproxen-oseltamivir combination in the treatment of patients hospitalized for influenza A(H3N2) infection: an open-label randomized, controlled, phase IIb/III trial. Chest 2017; 151: 1069–80.
-
14.
To KK-W, Tsang OT-Y, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020; published online March 23. https://doi.org/10.1016/S1473-3099.(20)30196-1.
-
15.
Cheng VC, Tang BS, Wu AK, Chu CM, Yuen KY. Medical treatment of viral pneumonia including SARS in immunocompetent adult. J Infect 2004; 49: 262–73. 24
-
16.
Deng L, Li C, Zeng Q, et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J Infect 2020; published March 11. doi:10.1016/j.jinf.2020.03.002.
-
17.
Ye, I, Wang B, Mao J. The pathogenesis and treatment of the cytokine storm in COVID-19. J Infect https://doi.org/10.1016/j.jinf2020.03.037.
Ribavirin
Ribavirin is a guanine, oral nucleoside analogue, which inhibits viral RNA-dependent RNA polymerase. It has shown some in vitro activity against SARS, where high concentrations/high doses were required to inhibit viral replication (1.2 g to 2.4 g PO every 8 h), and combination therapy via intravenous or enteral administration (1, 2). Some studies have trialed inhaled ribavirin preparations to treat various viral illnesses. A study reviewing ribavirin in the treatment of respiratory syncytial virus found no significant benefit of inhalation administration over intravenous or enteral (1, 3).
Ribavirin research has been ongoing against coronaviruses. Studies on the use of Ribavirin as a treatment for SARS were either inconclusive in terms of clinical benefit, or suggested possible harm referable to adverse events, which included hepatic and hematologic deleterious effects (1, 2). As an intervention against MERS, studies usually involved Ribavirin in combination with other therapeutics, often interferons, and did not reveal significant clinical benefit on outcomes or viral clearance (1, 4, 5).
Ribavirin is associated with dose-dependent hematological toxicity, which can be severe. It has been reported from some SARS studies that the high doses utilized resulted in ~60% of the patients developing hemolytic anemia (1, 2). It has been reported in one study of ribavirin as a treatment for SARS, ~75% of the patients had elevated transaminase levels (1, 2). In a large MERS observational study, ~40% of the patients on a ribavirin plus interferon intervention required blood transfusions (1, 5). Ribavirin is a teratogen (1, 6).
To date the beneficial use of ribavirin as monotherapy, including as inhalation treatment against COVID-19 has not been reported. Research into the use of Ribavirin in the treatment of COVID-19 is ongoing, and to date the data do not commend the use of this antiviral as monotherapy.
Ribavirin may have a role as part of an antiviral cocktail in the treatment of COVID-19. As monotherapy it may not have significant clinical effect in safe dose ranges, but might contribute synergistically, and help confer some clinical benefit, as was suggested in the study described earlier where lopinavir/ritonavir, ribavirin, and interferon beta 1b were used in combination (7). Further study is needed.
References
-
1.
Sanders JM Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus dieseas (COVID-19): A Review. Jama 2020;323 (18):1824-1836. doi:10.1001/jama.2020.6019. last accessed 06/05/20.
-
2.
Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi:10.1371/journal.pmed.0030343.
-
3.
Foolad F, Aitken SL, Shigle TL, et al. Oral versus aerosolized ribavirin for the treatment of respiratory syncytial virus infections in hematopoietic cell transplant recipients. Clin Infect Dis. 2019;68(10):1641-1649. doi:10.1093/cid/ciy760.
-
4.
Morra ME, Van Thanh L, Kamel MG, et al. Clinical outcomes of current medical approaches for Middle East respiratory syndrome: a systematic review and meta-analysis. Rev Med Virol. 2018;28 (3):e1977. doi:10.1002/rmv.1977.
-
5.
Arabi YM, Shalhoub S, Mandourah Y, et al. Ribavirin and interferon therapy for critically ill patients with Middle East respiratory syndrome: a multicenter observational study. Clin Infect Dis. Published online June 25, 2019. doi:10.1093/cid/ciz544.
-
6.
Altınbas S, Holmes JA, Altınbas A. Hepatitis C virus infection in pregnancy: an update. Gastroenterol Nurs. 2020;43(1):12-21. doi:10.1097/SGA.0000000000000404.
-
7.
Hung IFN, Lung KC, Tso E YK, Liu R, et al. Triple combination of interferon beta 1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open lable, randomized phase 2 trial. The Lancet 2020 (395) May 20. https://doi.org/10.10116/S0140-6736.(20)31042-4 Last accessed 06/01/20
Favipiravir
Previously referred to as T-705, it is a purine nucleotide prodrug – favipiravir ribofuranosyl-5’-triphosphate, and when activated inhibits the RNA polymerease, halting viral replication. It has been initially trialed against influenza and Ebola viruses (1–4), but has shown broader activity against other RNA virues (1, 5). Dose has been influenced by the target virus being treated. Based upon previous experience with various infections, it is postulated that higher dose ranges should be considered if favipiravir is trialed against COVID-19. It has a t ½ of 5 h (1, 6).
A prospective, multicenter RCT clinical trial compared favipiravir to arbidol in the treatment of moderate and severe COVID-19 infections (1, 7). A total of 120 patients were in each arm of the study. Clinical recovery by day 7 for patients suffering moderate severity illness was 71.4% in the favipiravir group, and 55.9% in the arbidol group. In both the severely ill, and combined group of moderate with severely ill patients, no significant difference between the two medications were noted (7).
From a clinical trial perspective suggested dosage is:
Loading dose 2400 mg to 3000 mg every 12 h × 2 doses, followed by
Maintenance dose 1200 mg to 1800 mg × 12 h)
While favipiravir has shown potential benefit, it is cautioned this therapeutic agent is still investigational, continues being studied, and is not currently FDA approved for routine treatment of COVID-19 (2–4). Adverse event profiles seem mild at lower doses, and not well described at the higher end of the dose range, which would likely be required for COVID-19 (1, 8–11). Prior experience suggests it is relatively well tolerated by patients. Again further research is needed, and ongoing.
References
-
1.
Sanders JM Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus dieseas (COVID-19): A Review. Jama 2020;323 (18):1824-1836 doi:10.1001/jama.2020.6019. last accessed 06/05/20.
-
2.
Furuta Y, Gowen BB, Takahashi K, Shiraki K, et al. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir Res 2013;100(2):446-54
-
3.
Goldhill DH, Te Velthuis AJW, Fletcher RA, Langart P, et al. The mechanism of resistatnce to favipiravir in influenza. P Natl Acad Sci USA 12018;115 (45):11613-8
-
4.
AP, Warren TK, Martins KA, REisler RB, et al. Will there be a cure for eEbola? Annu Rev Pharmacol 2017;57:329.48
-
5.
Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017; 93(7):449-463. doi:10.2183/pjab.93.027.
-
6.
Shiraki K, Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. [published online February 22, 2020]. Pharmacol Ther. 2020;107512. doi:10.1016/j.pharmthera.2020.107512.
-
7.
Chen C, Huang J, Cheng Z, et al. Favipiravir versus Arbidol for COVID-19: a randomized clinical trial. medRxiv. Preprint posted March 27, 2020. doi:10.1101/2020.03.17.20037432.
-
8.
Sissoko D, Laouenan C, Folkesson E, et al; JIKI Study Group. Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19) Review Clinical Review & Education a historically controlled, single-arm proof-of-concept trial in Guinea [published correction appears in PLoS Med. 2016;13(4): e1002009]. PLoS Med. 2016;13(3):e1001967. doi:10.1371/journal.pmed.1001967.
-
9.
Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther. 2020;14(1):58-60. doi:10.5582/ddt.2020.01012.
-
10.
Chinello P, Petrosillo N, Pittalis S, Biava G, Ippolito G, Nicastri E; INMI Ebola Team. QTc interval prolongation during favipiravir therapy in an Ebolavirus-infected patient. PLoS Negl Trop Dis. 2017;11(12):e0006034. doi:10.1371/journal.pntd.0006034.
-
11.
Kumagai Y, Murakawa Y, Hasunuma T, et al. Lack of effect of favipiravir, a novel antiviral agent, on QT interval in healthy Japanese adults. Int J Clin Pharmacol Ther. 2015;53(10):866-874. doi:10.5414/CP202388.
Umifenovir (Arbidol)
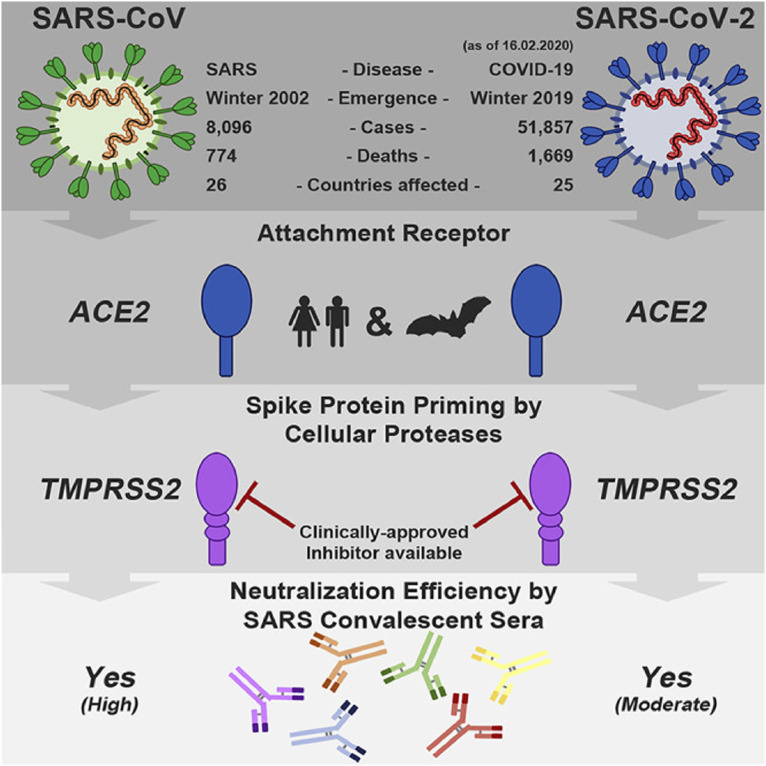
Arbidol is an antiviral that may prove promising given the mechanism of action seems aligned with the manner in which COVID-19 interacts with the host cell (1–4). Arbidol is an S protein/ACE2 membrane fusion inhibitor that is postulated to help prevent COVID-19 viral fusion to host cells. (1, 5–9) (Fig. 3 ) (9).
Fig. 3.
SARS and COVID-19 Infectivity/Response Comparisons ((From Hoffman, et al - (9).
Arbidol is an anti-influenza drug that targets the influenza virus hemagglutinin (HA), and according to a May 2020 article in Cell Discovery, is being used in a clinical trial against COVID-19 (ChiCTR2000029573) and has been recently added to the Guidelines for the Diagnosis and Treatment of COVID-19 (sixth and seventh editions) in China (1, 3). They report that a recent retrospective study suggested the use of arbidol treatment showed a tendency to improve discharge rate and decreased mortality rate in COVID-19 patients4. They rightly point out that there is a need for greater research in terms of the effectiveness of anti-influenza drugs against COVID-19 (1, 2, 4).
It is also approved in The Russian Federation as both prophylaxis, and treatment of influenza (1). In vitro research suggests it has antiviral activity against SARS (1, 6).
Based on limited data using Arbidol in China, the recommended dose for influenza (200 mg orally every 8 h) is being used for the treatment of COVID-19 in studies. Recently reported from China, a non RCT study of 67 patients infected with COVID-19 using Umifenovir for an average treatment time of 9 days was associated with lower mortality rates compared to patients who were not treated with this medication (1, 7). It was also noted that patients treated with umifenovir had higher discharge rates from hospital. This was a small study, and not an RCT, so results should be taken with caution. Ongoing RCT are being conducted to further evaluate the role of this antiviral in the treatment of COVID-19 (1, 7).
Xu et al reported a retrospective cohort study of NCP patients who received empiric al antiviral regimens with or without Arbidol. A total of 111 patients from two clinical centers in China were enrolled (2). The arbital plus symptomatic care group (A+ER) seemed to do better clinically as suggested by the decreased need for high flow nasal catheter (HFNC) oxygen therapy compared to the symptomatic treatment only group ER (P=0.002). About 55.1% patients in group A+ER had focal absorption on chest CT images, higher than 32.2% in group ER (P=0.016). They note the beneficial effect of Arbidol was more apparent in patients with mild illness severity at admission. Side effects of the Arbidol were minimal in this study. Their conclusion: study results suggest Arbidol could accelerate viral clearance, improve focal absorption on radiologic images, and reduce demand for HFNC oxygen therapy during hospitalization. These effects were pronounced in patients with mild illness at admission. The authors assert their results provide a basis for clinical use of Arbidol and supports for further randomized controlled trials in patients with COVID-19 pneumonia.
Chinese research was described in the European Pharmaceutical Review concerning an exploratory Phase IV randomized, open-label, controlled study on the safety and efficacy of either lopinavir/ritonavir (LPV/r) or Arbidol (8). The study assessed 86 patients with mild-to-moderate COVID-19, with 34 randomly assigned to receive LPV/r, 35 to receive Arbidol and 17 with no antiviral medication as a control. All three groups showed similar outcomes at seven and 14 days, with no differences between groups in the rates of fever reduction, cough alleviation or improvement of chest CT scan. Patients in both drug groups experienced adverse events such as diarrhoea, nausea and loss of appetite during the follow-up period, while no apparent adverse event occurred in the control group (8).
As noted earlier, these antivirals are approved as treatments for HIV-1 and influenza, respectively in certain nations. Some in vitro studies and reports suggested possible clinical benefit from these therapies as treatments for COVID-19. But results from this study suggest neither drug improves the clinical outcome of patients hospitalized with mild-to-moderate cases of COVID-19 compared to supportive care only (8).
Allergic reaction, gastrointestinal upset, elevated transaminases are known adverse events. Arbidol is metabolized through CYP3A4; requiring awareness of other meds administered that are strong inducers/inhibitors (1, 5 – 7).
References
-
1.
Sanders JM Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus dieseas (COVID-19): A Review. Jama 2020;323 (18):1824-1836 doi:10.1001/jama.2020.6019. last accessed 06/05/20.
-
2.
Xu K, Chen Y. Clinical Efficacy of Arbidol in Patients with 2019 Novel Coronavirus-Infected Pneumonia: A Retrospective Cohort Study Lancet 2020 Feb 25. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3542148. Last accessed 06/08/20
-
3.
Wang XI, Cao R, Zhang H, Liu J et al. Cell Discovery 2020 May 02. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. https://www.nature.com/articles/s41421-020-0169-8. Last accessed 06/08/20
-
4.
Xu P, Huang J, Fan Z, Huang W, et al. Arbidol/IFN-α2b therapy for patients with corona virus disease 2019: a retrospective multicenter cohort study Microbes Infect 2020 May-June: 22 (4): 200 – 205. Published online 2020 May 20. doi:10.1016/j.micinf.2020.05.012. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7238991/. Last accessed 06/15/20
-
5.
Kadam RU, Wilson IA. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc Natl Acad Sci U S A. 2017;114(2):206-214. doi:10.1073/pnas.1617020114.
-
6.
Khamitov RA, Loginova SIa, Shchukina VN, Borisevich SV, Maksimov VA, Shuster AM. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures [in Russian]. Vopr Virusol. 2008;53(4):9-13.
-
7.
Wang Z, Yang B, Li Q,Wen L, Zhang R. Clinical Features of 69 cases with coronavirus disease 2019 inWuhan, China. Clin Infect Dis. Published online March 16, 2020. doi:10.1093/cid/ciaa272.
-
8.
Trial finds lopinavir/ritonavir and Arbidol ineffective for mild-to-moderate COVID-19 European Pharmaceutical Review 2020 April 21 https://www.europeanpharmaceuticalreview.com/news/117273/trial-finds-lopinavir-ritonavir-and-arbidol-ineffective-for-mild-to-moderate-covid-19/. Last accessed 06/08/20.
-
9.
Hoffmann M, Kleine-Weber H, Schroeder S, Muller MA, et al. SARS - CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181: 271–280. doi:10.1016/j.cell.2020.02.052.
Anticytokine therapy
As discussed earlier in the Immune Response and Clinical Sections, the phenomenon of “cytokine storm” (Fig. 2) has been well reported and documented in multiple severely ill COVID-19 infected patients (1). Various approaches in the prevention and/or attenuation of this phenomenon are essential for the critically ill patient (1–4).
As a brief review, the use of anticytokine and immunomodulatory therapeutics is based upon evidence that the severe lung damage and extrapulmonary organs results from an amplified immune response that leads to significant cytokine release – what has been referred to as “cytokine storm.” Early research has implicated IL-6 as a primary contributor in this inflammatory cascade (1–4).
References
-
1.
Ye Q, Wang B, Mao J The pathogenesis and treatment of the cytokine storm in COVID-19. J Infect https://doi.org/10.1016/j.jinf2020.03.037.
-
2.
Sanders JM Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus dieseas (COVID-19): A Review. Jama 2020;323 (18):1824-1836. doi:10.1001/jama.2020.6019. last accessed 06/05/20.
-
3.
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395(10229):1033-1034. doi:10.1016/S0140-6736(20)30628-0.
-
4.
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3.
Tocilizumab
Tocilizumab is an IL-6 antagonist and can cause immune-suppression (1–4). IL-6 is a proinflammatory cytokine, associated with cytokine storm (3–8). Therefore one approach to this immune mediated cytokine damage is the use of monoclonal antibodies that target IL-6, with the hope this could attenuate the cascade safely, without interfering with other and beneficial host immunity, leading to clinical improvement. Tocilizumab as a monoclonal antibody IL-6 receptor antagonist, and FDA approved therapeutic used in the treatment of rheumatoid arthritis, and cytokine release syndrome which can result after chimeric antigen receptor T cell therapy, it seems a potential candidate in the treatment of COVID-19 (4, 9). And with the need to address the well described cytokine storm impacting severely ill COVID-19 infected patients, tocilizumab has been utilized in a variety of clinical trials.
In a small study involving 21 patients infected with COVID-19, 91% of those who received tocilizumab, showed clinical improvement (4, 10). characterized as improved respiratory function, rapide defervescence, and discharge from health care facility. Of note, most patients received 1 dose 400 mg (4, 10).
In another COVID-19 study evaluating the use of tocilizumab as a treatment for the hyperimmune state associated with cytokine storm, following initiation, there is elevation in the IL-6 levels and CRP levels dramatically decreased (3).
There have been Chinese studies that suggest Tocilizumab is beneficial treating severely ill patients who have extensive bilateral lung involvement, with elevated Il-6 levels. They administered Tocilizumab with a first dose 4 – 8 mg/kg. The recommended dose was 400 mg with 0.9% saline diluted to 100 ml, and an infusion time of more than 1 hour. Patients without good initial response, an additional and identical dose as above was given 12 h after the first one, with a total recommended maximum in their experience of 2 doses (3).
These results are encouraging but should be cautiously interpreted; these were small trials. In March 2020 the FDA approved a phase III study involving the use of tocilizumab in a COVID -19 study. Additional and larger trials are underway, including RCTs, where tocilizumab is being studied alone, and as combination therapy (4, 11).
References
-
1.
Antwi-Amoabeng D, Kanji Z, Ford B, Beutler BD, et al. Clinical Outcomes in COVID-19 Patients Treated With Tocilizumab: An Individual Patient Data Systematic Review J Med Virol 2020;May 21 https://pubmed.ncbi.nlm.nih.gov/32436994/. Last accessed 06/08/20
-
2.
Biggioggero M, Crotti C, Becciolini A, Favalli EG . Tocilizumab in the treatment of rheumatoid arthritis: an evidence-based review and patient selection. DrugDesign, Devel Therapy 2018; 13:57–70 PubMed PMID: 30587928. eng.
-
3.
Ye Q, Wang B, Mao J The pathogenesis and treatment of the cytokine storm in COVID-19. J Infect https://doi.org/10.1016/j.jinf2020.03.037.
-
4.
Sanders JM Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease (COVID-19): A Review. Jama 2020;323 (18):1824-1836. doi:10.1001/jama.2020.6019. last accessed 06/05/20.
-
5.
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395(10229):1033-1034. doi:10.1016/S0140-6736(20)30628-0.
-
6.
Law HKW, Cheung CY, Ng HY, Sia SF, Chan YO, Luk W, et al. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood 2005; 106 (7):2366–74 PubMed PMID: 15860669. Epub 2005/04/28. eng .
-
7.
Cheung CY, Poon LLM, Ng IHY, Luk W, Sia S-F, Wu MHS, et al. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J Virol 2005; 79 (12):7819–26 PubMed PMID: 15919935. eng.
-
8.
Lau SKP, Lau CCY, Chan K-H, Li CPY, Chen H, Jin D-Y, et al. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J Gen Virol 2013; 94 (Pt 12):2679–90 PubMed PMID:24077366. Epub 2013/09/28. eng .
-
9.
Kumagai Y, Murakawa Y, Hasunuma T, et al. Lack of effect of favipiravir, a novel antiviral agent, on QT interval in healthy Japanese adults. Int J Clin Pharmacol Ther. 2015;
-
10.
Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. chinaXiv. Preprint posted March 5, 2020. doi:10.12074/202003.00026.
-
11.
National Health Commission and State Administration of Traditional Chinese Medicine. Diagnosis and treatment protocol for novel coronavirus pneumonia. Accessed March 18, 2020. https://www.chinalawtranslate.com/wp-content/uploads/2020/03/Who-translation.pdf.
Anakinra
This is an IL-1B antagonist, and has been posited as a possible intervention against COVID-19 or other infection related cytokine storm (1–3). In a study involving patients with severe sepsis, Anakinra improved 28 day survival (3). As with tocilizumab, further study is needed.
References
-
1.
Ye Q, Wang B, Mao J The pathogenesis and treatment of the cytokine storm in COVID-19. J Infect https://doi.org/10.1016/j.jinf2020.03.037.
-
2.
Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA,Schlaak M, et al. Cytokine release syndrome. J ImmunoTherapy Cancer2018; 6 (1):56 2018/06/15.
-
3.
Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA,et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Critical Care Med 2016; 44 (2):275–81 PubMed PMID:26584195. eng.
Sarilumab
Another IL-6 receptor antagonist, also approved for the treatment of Rrheumatoid arthritis, is currently being evaluated for the treatment of hospitalized patients severely ill by COVID-19, as part of a multicenter, double blind research study (1,2).
Other candidate monoclonal antibodies are also being studied in the US, China, and elsewhere, including bevacizumab, which is an anti-vascular endothelial growth factor medication, fingolimod, which is an immunomodulatory approved for the treatment of multiple sclerosis (MS), and eculizumab, which is an antibody inhibiting terminal complement therapeutic (1, 3).
Research to develop antibodies that target specific aspects of the viral lifecycle and virus-host interaction is underway. For example, a human monoclonal antibody is being tested in preclinical research targeting a common epitope, which may allow it to block COVID-19 infection. It may work on SARS coronavirus as well (1, 4).
The clinician is recommended to be alert to these interventions as newer data are released; as adjunctive they may confer clinical benefit for the moderately to severely ill COVID-19 patient. Again further research is required.
References
-
1.
Sanders JM Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease (COVID-19): A Review. Jama 2020;323 (18):1824-1836 doi:10.1001/jama.2020.6019. last accessed 06/05/20.
-
2.
ClinicalTrials.gov. Accessed March 18, 2020. https://clinicaltrials.gov/.
-
3.
Sanofi. Sanofi and Regeneron begin global Kevzara (sarilumab) clinical trial program in patients with severe COVID-19 [news release]. Published March 16, 2020. Accessed March 18, 2020. http://www.news.sanofi.us/2020-03-16-Sanofi-and-Regeneron-begin-global-Kevzara-R-sarilumabclinical-trial-program-in-patients-with-severe-COVID-19.
-
4.
Wang C, LiW, Drabek D, et al. A human monoclonal antibody blocking SARS-CoV-2 infection. bioRxiv. Preprint posted March 11, 2020. doi:10.1101/2020.03.11.987958.2020.
Interferon
Type 1 interferons (IFN–I) are a diverse group of cytokines that are further distinguished as α and β, ε, ω and κ subtypes. They are involved in a complex immune response to pathogens. In terms of viral illness, upon early infection, IFN-I are among the first cytokines produced (1–7). Through a complex cascade of host-viral interaction, and can contribute to defeating the viral pathogen, as well as being involved in inflammation. Interferons are designed to interfere with viral replication and spread by multiple mechanisms, resulting in the activation of adaptive immunity. As immunomodulators, IFN-I have been used clinically, including multiple sclerosis with varying degrees of success.
The use of interferons in the treatment of COVID-19 remains investigational (2). Some studies have shown benefit when utilized in combination therapy, but methodological differences, patient populations and combination therapies utilized make recommendations about the use of interferons premature. Though promising, further research is needed.
References
-
1.
Blazek K, Eames HL, Weiss M, Byrne AJ, Perocheau D, Pease JE, et al. IFN- λ resolves inflammation via suppression of neutrophil infiltration and IL-1 β production. J Exper Med 2015; 212 (6):845–53 PubMed PMID: 25941255. Epub 2015/05/04. eng.
-
2.
Hung IFN, Lung KC, Tso E YK, Liu R, et al. Triple combination of interferon beta 1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open lable, randomized phase 2 trial. The Lancet 2020 (395) May 20. https://doi.org/10.10116/S0140-6736.(20)31042-4 Last accessed 06/01/20
-
3.
A.T.Huang, B.Garcia-Carreras, M.D.T.Hitchings, B.Yang, et al. A systematic review of antibody mediated immunity to coronaviruses: antibody kinetics, corre-lates of protection, and association of antibody responses with severity of disease Infectious Diseases (exceptHIV/AIDS)(2020). https://doi.org/10.1101/2020.04.14.20065771.
-
4.
Megaco JG, Azamor T, Bom A Protective immunity after COVID-19 has been questioned: What can we do without SARS-CoV-2-IgG detection? Cellular Immunology 2020 (353) July. https://doi.org/10.1016/j.cellimm.2020.104114. Last accessed 06/13/20
-
5.
F.Wu, A.Wang, M.Liu, Q.Wang, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. Infectious Diseases (exceptHIV/AIDS)(2020). https://doi.org/10.1101/2020.03.30.20047365.
-
6.
D.Weiskopf, K.S.Schmitz, M.P.Raadsen, A.Grifoni, et al. Phenotype of SARS-CoV-2-specific T-cells in COVID-19 patients with acute respiratory distress syndrome. Infectious Diseases(exceptHIV/AIDS)(2020). https://doi.org/10.1101/2020.04.11.20062349.
-
7.
Xu P, Huang J, Fan Z, Huang W, et al. Arbidol/IFN-α2b therapy for patients with corona virus disease 2019: a retrospective multicenter cohort study Microbes Infect 2020 May-June: 22 (4): 200 – 205. Published online 2020 May 20. doi:10.1016/j.micinf.2020.05.012. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7238991/. Last accessed 06/15/20.
Convalescent plasma - immunoglobulin therapy
The use of convalescent plasma or hyperimmune globulins – immunoglobulin therapy is based upon the notion that antibodies obtained from patients who have recovered from the illness in question may confer benefit with the circulating virus, and cell immune clearance (1, 2).
Administering convalescent plasma is not a novel concept new to COVID-19 treatment. This has been used for other viral illnesses, including as rescue therapy for persons infected with SARS MERS and H1N1 influenza (1, 3–5). For example, a prospective observational study involving 93 critically ill patients infected with H1N1, convalescent plasma was administered to 20 patients. Those who received it had a reduction in mortality (20%) compared to ones who did not receive it (54.8%) with p=.01. (5).
In another post hoc meta-analysis of observational studies involving SARS or severe influenza, administration of hyperimmune globulin and convalescent plasma was associated with reduced mortality. The authors of this meta-analysis caution the studies included were not uniformly high rigor projects, and there was risk of bias (1, 6).
IVIG has been utilized in China as well (7, 8) to treat COVID-19, due to the potential for immune substitution and immune-modulation. As an intervention for COVID-19 there are emerging data from China, and the United States commending the use of convalescent plasma, such that the FDA in March 2020 released guidance on screening donors for COVID-19 convalescent plasma (9), as well as information to request emergency investigational new drug application (INDA).
References
-
1.
Sanders JM Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease (COVID-19): A Review. Jama 2020;323 (18):1824-1836 doi:10.1001/jama.2020.6019. last accessed 06/05/20.
-
2.
Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20(4):398-400. doi:10.1016/S1473-3099(20)30141-9.
-
3.
Soo YO, Cheng Y,Wong R, et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect.2004;10(7):676-678. doi:10.1111/j.1469-0691.2004.00956.x.
-
4.
Arabi Y, Balkhy H, Hajeer AH, et al. Feasibility,safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus. 2015;4:709. doi:10.1186/s40064-015-1490-9.
-
5.
Hung IF, To KK, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447-456. doi:10.1093/cid/ciq106.
-
6.
Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al; Convalescent Plasma Study Group. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratorymeta-analysis. J Infect Dis. 2015;211(1):80-90. doi:10.1093/infdis/jiu396.
-
7.
Ye Q, Wang B, Mao J The pathogenesis and treatment of the cytokine storm in COVID-19. J Infect https://doi.org/10.1016/j.jinf2020.03.037.
-
8.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395 (10223):507–13 2020/02/15/.
-
9.
US Food and Drug Administration. Investigational COVID-19 Convalescent plasma: emergency INDs. Updated April 3, 2020. Accessed 06/04/20. https://www.fda.gov/vaccinesblood-biologics/investigational-new-drug-ind-ordevice-exemption-ide-process-cber/investigational-covid-19-convalescent-plasmaemergency-inds.
Corticosteroids
Corticosteroids are well known as immunomodulators, and can reduce the inflammatory response, something well described in highly pathogenic coronavirus infections, including COVID-19 infection, and well as severe influenza and other illnesses (1–10). The benefit of reducing the often significant inflammation associated with COVID-19 illness should be balanced against the risk of delaying viral clearance, and increasing the risk of a secondary infection (1–5, 8–10).
A retrospective study looking at the role of corticosteroids in the treatment of SARS showed a reduction in mortality rate and shortened hospital stay, with secondary infections and other complications uncommon (1, 6). There are data where the use of corticosteroids in SARS patients led to adverse events, too (1, 7). Early administration with corticosteroids increased plaszma viral load in non ICU patients with resultant exacerbation of illness.
Owing to the immune modulating effect of corticosteroid – and they have been utilized for many years to treat a variety of clinical illnesses, timing is important. It is postulated that administering corticosteroids prematurely may inhibit or deleteriously influence the immune defense system, resulting in an increased viral load, which can lead to progressive clinical consequences. Because of this, glucorticoids have been reserved to critically ill patients experiencing cytokine storm. Moreover high doses have not been encouraged in the setting of coronavirus, as it may cause a level of immunosuppression that delays viral clearance (1, 8).
Although corticosteroids have been utilized in the setting of critical care patients under selected circumstances, the use of these medications in COVID – 19 warrant caution.
A 2019 meta-analysis of patients with influenza pneumonia treated with corticosteroids revealed an increased risk of mortality, and secondary infection (2, 9). A Chinese study showed some clinical benefit from corticosteroids in COVID-19 but it was not an RCT, and there were methodological limitations, such that the results should be considered with caution (2, 10).
In mid June 2020 a group of British researchers announced their findings from an RCT involving the use of dexamethasone in the treatment of COVID-19 patients. Although the study results at the time of the announcement had not been published in peer reviewed journals for assessment by the wider medical community, these are the results they provided. They are listed for information only, especially in the absence of peer review citation at the time of this writing.
The Co-Chief study investigator, Dr. Peter Horby, Nuffield Department of Medicine, as part of the British National Health Service (NHS) announced the results mid June 2020: A total of 2104 patients were randomized to receive dexamethasone 6 mg once per day by mouth or by intravenous injection for 10 days and were compared with 4321 patients randomized to usual care alone. Dexamethasone reduced deaths by one-third in ventilated patients (rate ratio 0.65 [95% confidence interval 0.48 to 0.88]; p=0.0003) and by one fifth in other patients receiving oxygen only (0.80 [0.67 to 0.96]; p=0.0021) (11).
Because the actual study has yet to be published at the time of this manuscript – the specifics, such as “usual care alone,” any confounding in terms of variable use of other interventions, timing of administration, and other considerations are not available for wider scrutiny or assessment at the time of this writing. Therefore any new enthusiasm for corticosteroids must be measured.
Overall the data are lacking in terms of supporting the routine use of corticosteroids in the treatment of COVID-19. Will there be subpopulations that may benefit from the addition of corticosteroids to the treatment regimen, perhaps. This may include those experiencing cytokine storm, or mechanical ventilation, or pre-ventilation oxygen therapy. But the data remain to be seen and assessed.
As with any clinical decision, the status, comorbidities, and level of illness, multiorgan involvement and other considerations must be taken into account, as patients circumstance reveal. Timing within the known pathogenesis of the illness, current clinical condition, risks and benefits should be weighed to provide optimal care for each patient. Caution should be taken when considering routine administration of corticosteroids in the clinical setting.
References
-
1.
Ye Q, Wang B, Mao J The pathogenesis and treatment of the cytokine storm in COVID-19. J Infect https://doi.org/10.1016/j.jinf2020.03.037.
-
2.
Moccia F, Gerbino, A, Lionetti V, Miragoli M, et al. COVID-19 associated cardiovascular morbidity in older adults: a position paper from the Italian Society of Cardiovascular Researches. GeroScience 2020; 28 April https://doi.org/10.1007/s11357-020-00198-w.
-
3.
Sanders JM Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease (COVID-19): A Review. Jama 2020;323 (18):1824-1836. doi:10.1001/jama.2020.6019. last accessed 06/05/20.
-
4.
Chen R-C, Tang X-p, Tan S-y, Liang B-l, Wan Z-y, Fang J-q, et al. Treatment of Severe Acute Respiratory Syndrome With Glucosteroids: The Guangzhou Experience. Chest 2006; 129 (6):1441–52 2006/06/01/.
-
5.
Zhao J-p, Hu Y, Du R-h, Chen Z-s, Jin Y, Zhou M, et al. Expert consensus on the use of corticosteroid in patients with 2019-nCoV pneumonia. Chin J Tuberc Respir Dis 2020(00) E007-E. chi
-
6.
Chen R-c, Tang X-p, Tan S-y, Liang B-l, Wan Z-y, Fang J-q, et al. Treatment of Severe Acute Respiratory Syndrome With Glucosteroids: The Guangzhou Experience. Chest 2006; 129 (6):1441–52 2006/06/01/.
-
7.
Auyeung TW, Lee JSW, Lai WK, Choi CH, Lee HK, Lee JS, et al. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect 2005; 51 (2):98–102 PubMed PMID: 16038758.eng.
-
8.
Zhou Y-H, Qin Y-Y, Lu Y-Q, Sun F, Yang S, Harypursat V, et al. Effectiveness of glucocorticoid therapy in patients with severe novel coronavirus pneumonia: protocol of a randomized controlled trial. Chin Med J 2020(00) E020-E. chi.
-
9.
Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23(1):99. doi:10.1186/s13054-019-2395-8.
-
10.
Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia inWuhan, China. JAMA Intern Med. Published online March 13, 2020.
-
11.
UK COVID-19 Update: Dexamethasone ‘Breakthrough’, BAME Report Part https://www.medscape.com/viewarticle/932390. last accessed 06/17/20
Vaccine research
To date there the US Food and Drug Administration (FDA) has approved two COVID - 19 vaccines for use in the United States - one by Pfizer and one by Moderna. Additionally there are a significant number of vaccine candidates in various stages of design, and clinical trials – from bench to Phase III pre-approval. As discussed in the SARS and MERS section, coronaviruses pose complex challenges to treatment as well as vaccine development, with questions persisting in terms of immune response, and duration of protection, as well as basic effectiveness and safety considerations.
A more in depth look at the other many candidates, (several of which are based upon the same science discussed in the SARS and MERS sections of this article) would make this article prohibitively long. The authors recommend visiting the FDA COVID-19 vaccine site for updates.
ACE inhibitors/ARBs
The use of angiotensin converting enzyme inhibitors (ACE I), and angiotensin receptor blockers (ARBs) for hypertension management has been raised as an issue of potential concern, but the American Heart Association (AHA), American College of Cardiology (ACC) and the Heart Failure Society of America (HFSA), three organizations dedicated to various forms of heart disease have come out with a joint statement cautioning against the abrupt cessation of these medications, and counseled that patients with underlying cardiovascular comorbidities being managed with these classes of medications should continue with ongoing treatment in spite of the possible COVID-19 risk from ACEi and ARBs (1).
In their joint statement they offer the following "The continued highest standard of care for cardiovascular disease patients diagnosed with COVID-19 is top priority, but there are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-I or ARB medications," said Richard J. Kovacs, MD, FACC. "We urge urgent, additional research that can guide us to optimal care for the millions of people worldwide with cardiovascular disease and who may contract COVID-19. These recommendations will be adjusted as needed to correspond with the latest research." They further recommend tailoring medical care in the event that a cardiovascular patient is infected with COVID-19, on an individual basis(1).
Individuals who seem susceptible to more severe outcomes from COVID-19 include those with underlying cardiovascular disease, and diabetes. Modifiable risks for cardiovascular disease are well described, and include hypertension and obesity – two clinical conditions that have also been associated with increased risks of greater severity COVID-19 illness. ACE inhibitors and ARBs have become important options for treating hypertension. Is there an association between ACE I or ARB use and worse outcomes with COVID-19? (2). One concern associated with ACE-inhibitors is the potential upregulation of angiotensin converting enzyme 2 (ACE2) receptor allowing greater COVID-19 entry.
As discussed earlier, host cell proteins ACE2 and transmembrane protease serine S2 (TMPRSS2) are essential for COVID-19 entry into cells. Physiologically ACEi and ACE2 act in somewhat oppositional function, albeit a simplistic comparison does not reveal the complex and dynamic interplay or range of characteristics, let it suffice that ACE 2 works more in a vasodilatory fashion, and ACE plays a vasoconstrictive role (3).
Animal experiments demonstrated ACE I and ARBs up-regulated ACE2 in cell membranes (4). This upregulation contributed to concerns that these medications might increase human susceptibility to COVID-19 infection, and lead to more severe illness. A question posited to be addressed – does the increased ACE2 automatically lead to an increase in TMPRSS2?
A recent commentary in JAMA also raise the notion, might not ACE2 upregulation confer some beneficial effect on the pulmonary manifestations of COVID-19, specifically inflammation associated with SARS2 pneumonia, and could this lead to an improvement in clinical status (3)?
There is ongoing research looking at the potential therapeutic risks or benefits that may be associated with these medications in the context of COVID-19 (2, 3, 5). The question arises might ACE-inhibitors confer some clinical benefit against COVID-19 given this virus utilizes the ACE2 receptor for cell entry.
In late June 2020, a retrospective, cohort study involving over 4000 patients was reported on the association of ACE inhibitors or ARBs with COVID-19 diagnosis and mortality was reported (5). There were 895 patients taking ACE I or ARBs compared to 3585 non users of these medications. Taking into account the retrospective nature of the study, among 571 patients with COVID-19 and hypertension, their conclusion was that ACE I or ARB use among this cohort was not significantly associated with higher incidence of COVID-19 infection compared with those patients treated with other antihypertensive medications. Death or severe COVID-19 occurred in 31.9% of ACE or ARB users compared with 14.2% of non users by day 30. The authors suggest in the context of the COVID-19 pandemic, their findings do not support the discontinuation of ACE I or ARB that are clinically necessary.
More research is needed in terms of ACE2 and COVID-19 infection, from a virology, pathophysiology, and potential antiviral intervention perspective, as well as further risk/benefit assessments for hypertensive patients taking ACE I and ARBs.
References
-
1.
Joint Statement from AHA, AAC, and HFSA concerning the cessation of ACE inhibitors and/or ARBs in the context of the COVID-19 Pandemic. https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19. Last accessed 06/22/20
-
2.
Metha N, Kallra A, Nowacki AS, et al. Association of use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. Published online 5 May 2020. doi:10.1001/jamacarido.2020.1855.
-
3.
Curman G. Renin-Angiotensin Aldosterone inhibitors and susceptibility to and severity of COVID-19. JAMA 19 June 2020. Doi 10.1001/jama.2020.11401 https://jamanetwork.com. Last accessed 06/21/20
-
4.
Sukumaran V, Tsuchimochi H, Tatsumi E, Shirai M, et al. Azilsartan ameliorates diabetic cardiomyopathy in young db/db mice through the modulation of ACE2/ANG107/Mas receptor cascade. Biochem Pharmacol 2017;144:90 – 9. doi:10.1016/j.bcp.2017.07.022.
-
5.
Fosbol EL, Butt JH, Ostergaard L, Andersson C, et al. Association of angiotensin converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA Published online 19 June 2020. doi:10.1001/jama.2020.11301. Last accessed 21 June 2020.
Other interventions
In the course of ICU management of the severely ill patient a variety of interventions have been attempted, especially as pertains to COVID-19. These include mechanical ventilation, and extracorporeal membrane oxygenation – both of which are familiar to most clinicians.
In addition to the currently described, as well as ongoing research into options available to clinicians in the management of COVID-19, the use of hyperbaric oxygen has gained interest, and so we will provide a short discussion of research into this modality for severe pulmonary illness.
Hyperbaric oxygen therapy (HBOT)
Owing to the significant number of severely ill patients progressing to intensive care units, some requiring mechanical ventilation, ECMO and other aggressive interventions, alternative therapeutic approaches to address COVID-19 associated hypoxia have been posited. These include the use of hyperbaric oxygen delivery (1, 2).
Hyperbaric oxygen therapy (HBOT) is an FDA approved treatment, although currently not for the specific treatment of COVID-19, but has been successfully used for a variety of clinical indications to deliver high concentration oxygen. One of the benefits of HBOT, it can deliver oxygen under high pressure making tissue uptake more efficient. Clinical experience has shown some positive benefit from HBOT for chronic wounds, late effects of radiation, reperfusion injuries, necrotizing fasciitis, compromised grafts/skin flaps, carbon monoxide poisoning and diving associated decompression illness (3, 4).
The rationale for HBOT to overcome the hypoxia that contributes to progressive clinical decline in severe and critically ill patients. The hyperimmune response from COVID-19 contributes a cascade of effects that may contribute to the pulmonary damage and subsequent hypoxia. It is postulated HBOT may also attenuate the impact of this infection-hyperimmune response. Owing to the powerful oxygen delivery that results from HBOT research is underway to evaluate a possible role in the treatment of COVID-19 associated pulmonary compromise.
Additional considerations and potential benefits postulated (5) include the notion hyperbaric oxygen therapy (HBOT) - exposing patients to 100% oxygen under increased atmospheric pressure up to 2.4 atm could improve outcomes from COVID-19 not unlike benefit derived with other infections treated this way. Benefit may maximize when administered early.
Some data from human and animal models suggest as soon as a reduction of arterial oxygen concentration is noted. Referencing animal research, it has demonstrated early HBOT improved outcomes from sepsis. It is suggested this is the result of a reduction in the inflammatory response, catalyzed by infection (6). HBOT delivers oxygen at elevated partial pressure, facilitating tissue penetration rapidly, and at higher concentrations; more effective than hemoglobin oxygen delivery. To be sure, mechanical ventilation remains a mainstay of treatment for hypoxic COVID-19 patients in critical care. But both mechanic ventilation and HBOT can elevate the levels of arterial O2. It has been suggested that HBOT adds another dimension; the increased concentration of O2 delivered to tissues by HBOT administered at 2.4 atm provides acts as a signal for cells, which may induce two transcription factors, that enhance the immune system. These are Nrf-2 which stimulates the production of cell defense, some of which are involved in the oxidative stress response, and heat shock transcription factor 1 (HSTF 1) which can catalyze production of anti-inflammatory proteins (7). Moreover the increased level of oxygen by HBOT may help preserve cellular metabolism and organ function.
Results of a recent small study using HBOT seem to corroborate the potential clinical benefit to hypoxic COVID – 19 patients. The study demonstrated clinical improvement without the need to advance patients to mechanical ventilation (1, 8).
To date there remain a not insignificant proportion of COVID-19 patients who progress to severe disease. As the authors of this study rightly state older persons and individuals with severe comorbidities are at increased risk of developing severe pulmonary decline, often requiring mechanical ventilation (1, 8). Not surprisingly, once intubated, the risk of mortality increases significantly, in spite of the emergence of multiple pharmacologic agents and the use of combination therapies, including hydroxychloroquine-azithromycin, remdesevir, toclizumab, and others which have shown some clinical benefit – either reducing the impact of the infection, interrupting COVID-19s life-cycle or attenuating the cytokine storm effect. Rescue approaches, and interventions that may prevent, at least in some cases, the progression from severe disease to mechanical ventilation was a rationale for their study.
Caveats to the study (8) - enrollment was small (n=5). All patients had tachypnea and low oxygen saturation despite receiving high FiO2. HBOT was added with the objective to prevent a need for mechanical ventilation. Using a standard dive intervention protocol (2.0 ATA for 90 minutes) was utilized. Patients received between one and six treatments in one of two dedicated hyperbaric chambers. Their results -all patients recovered without need for mechanical ventilation; HBOT use led to oxygen saturation increase, resolution of tachypnea, and decline in inflammatory markers. They note achieving a decrease in oxygen requirement below an FiO2 of 50% took between one and six HBOT sessions, with an average of five HBOT treatments per patient. Their results are similar to a case series reported from China (8).
In their conclusion, the authors rightly remind once on the ventilator, COVID-19 patients are difficult to wean and not surprisingly the risk of mortality rises sharply. One approach that may have positive benefit, but requires additional study is HBOT. They postulate the hyperoxygenation state afforded by HBOT, breathing oxygen under pressure may reverse the hypoxia caused by the SARS-CoV-2 virus, and offer another hypothesis concerning anti-inflammatory and potential viricidal properties of HBOT making this intervention worth considering in COVID-19 patients (8).
While HBOT is an interesting potential intervention for consideration in the COVID-19 hypoxic patient, it is not FDA specifically approved for this indication, there remain unanswered questions. For example the potential risks remain to be fully elucidated as there have been only small studies to date (1, 5, 8, 9). What are the recommended dose schedules, time to administer, and under what conditions in COVID-19 illness? Moreover there is the issue of logistics. Not every health care facility or region has access to hyperbaric oxygen therapy. Transporting critically ill patients to an HBOT chamber, or where to locate one in proximity to an ICU, as well as the small number of patients that can be accommodated in HBOT at one time, will require more research. Of note, NYU is in the process of commencing an HBOT in COVID-19 study, and other research into this continues.
References
-
1.
Thibodeau K, Speyrer M, Raza A, Yaakov R, Serena TE, et al. Hyperbaric Oxygen Therapy in Preventing Mechanical Ventilation in COVID-19 Patients: A Retrospective Case Series J Wound Care 2020 May 1;29(Sup5a):S4-S8. doi:10.12968/jowc.2020.29.Sup5a.S4.
-
2.
Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323(11):1061. https://doi.org/10.1001/jama.2020.1585.
-
3.
Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective study. Lancet 2020; 395(10229):1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3. Last accessed 07/29/20.
-
4.
Undersea & Hyperbaric Medical Society. Indications for hyperbaric oxygen therapy. https://tinyurl.com/y8edrhyg.
-
5.
DeMaio A, Hightower LE. COVID-19, acute respiratory distress syndrome (ARDS), and hyperbaric oxygen therapy (HBOT): what is the link? Cell Stress and Chaperones https://doi.org/10.1007/s12192-020-01121-0. Last accessed 06/15/20
-
6.
Halbach JL, Prieto JM, Wang AW, Hawisher D, et al. Early hyperbaric oxygen therapy improves survival in a model of severe sepsis. Am J Physiol Regul Integr Comp Physiol 2019;317:R160–R168. https://doi.org/10.1152/ajpregu.00083.2019.
-
7.
Godman CA, Chheda KP, Hightower LE, Perdrizet G, et al. Hyperbaric oxygen induces a cytoprotective and angiogenic response in human microvascular endothelial cells. Cell Stress Chaperones 2010;15:431–442. https://doi.org/10.1007/s12192-009-0159-0.
-
8.
Zhong Xiaoling TX, Tang Yanchao, Chen Ruiyong. Effect of hyperbaric oxygen therapy on hypoxia in patients with severe new coronavirus pneumonia: first report. Chinese Journal of Marine Medicine and Hyperbaric Medicine, 2020
-
9.
Plafki C, Peters P, Almeling M et al. Complications and side effects of hyperbaric oxygen therapy. Aviat Space Environ Med 2000; 71(2):119–124
ADDENDUM – October 2020 Antibody Therapy
Numerous therapeutics continue to be studied, many of which are starting to be resulted. Due to size constraints and scope of journal, not all emerging data can be added to this edition. But when promising results emerge before publication, it is important to present the updates.
Monoclonal and polyclonal antibodies have been studied for a variety of disease processes, as noted earlier, including various infections. Protective antibodies can be transferred in serum, historically derived from convalescent serum, but with advances in technological capabilities, recombinant human antibodies can be utilized.
Recently it was reported POTUS received REGN-COV2, which includes a combination of two neutralizing antibodies (REGN10987+REGN10933); each one targets non-overlapping epitopes on the SARS-CoV-2 spike protein. Early animal and human trial results have shown clinical promise.
As with any cocktail of therapeutics, assigning benefit and risk with each component requires careful analysis.
REGN-COV2 (Combination REGN10987+REGN10933)
Study Design
Trial designed to evaluate anti-viral activity with REGN-COV2, and identify patient groups most likely to benefit from treatment. Patients were randomized in a 1:1:1 fashion to receive a one-time infusion of 8 grams of REGN-COV2 (high dose), 2.4 grams of REGN-COV2 (low dose) or placebo. All patients in the trial must have laboratory-confirmed COVID-19, being treated in an outpatient setting. Prior to treatment, patients were categorized by serology testing to determine if antiviral antibodies were generated or not; . Seronegatives (no measurable antiviral antibodies) or seropositives (measurable antiviral antibodies). Approximately 45% of patients were seropositive, 41% were seronegative and 14% were categorized as "other" due to unclear or unknown serology status.
Primary endpoint - reduction in viral load through Day 7 in seronegative patients. Results - REGN-COV2 rapidly reduced mean time-weighted-average change from baseline nasopharyngeal (NP) viral load through Day 7 in the seronegative group was a 0.60 log10 copies/mL greater reduction (p=0.03) in patients treated with high dose, and a 0.51 log10 copies/mL greater reduction (p=0.06) in patients treated with low dose, compared to placebo.
Infusion reactions were seen in 4 patients (2 on placebo and 2 on REGN-COV2). Serious adverse events occurred in 2 placebo patients, 1 low dose patient and no high dose patients. There were no deaths in the trial
REGN 2 contains two virus-neutralizing antibodies that bind non-competitively to the critical receptor binding domain of the virus's spike protein, potentially reducing the potential for mutant viruses to adapt/evade treatment and protects against spike variants that have arisen in the human population.
Preclinical studies also have shown that REGN-COV2 reduced the amount of virus and associated damage in the lungs of non-human primates.
In addition to this trial in non-hospitalized patients, REGN-COV2 is currently being studied in more advanced clinical trial for the treatment of COVID-19 in hospitalized patients, referred to as Phase 3 open-label RECOVERY trial of hospitalized patients in the UK and a Phase 3 trial for the prevention of COVID-19 in household contacts of infected individuals. Recruitment in all 4 trials is ongoing.
LY - CoV 555/ LY-CoV016 Combination
Recently data from a proof of concept study utilizing neutralizing antibody LY - CoV 555 in the COVID-19 outpatient setting have been reported.
The BLAZE 1 Study
Interim data from a randomized, double – blind, placebo-controlled Phase 2 study designed to assess LY-CoV555 and LY-CoV016 in the treatment of symptomatic COVID-19 in an outpatient setting. It is designed to enroll 800 patients. Both interventions are antibodies specifically directed against COVID-19; each binds to a different epitope in the SARS-CoV-2 spike region. Eligibility included mild to moderate symptoms, positive SARS-CoV-1 tested (sample less than or equal to 3 days of enrollment). Endpoints include 1. Change from baseline to Day 11 in COVID-10 viral load, percent of patients who progress to COVID-19 related emergency department (ED), hospitalization, or death from baseline through Day 29, and safety. Participants were enrolled in one of four trial arms – placebo, intervention at 700 mg, 2800 mg, and 7000 mg.
LY-CoV555 is a neutralizing IgG1 monoclonal antibody (mAb) directed against the spike protein of SARS-CoV-2, to block viral attachment and entry into human cells.
Results - The primary outpoint was viral load change from baseline at day 11 was met for one of three doses (2800 mg), with consistent effects of viral reduction seen at earlier time points. Rate of hospitalizations and emergency room (ER) visits was 1.7% for LY-CoV555 versus 6 percent for placebo, a 72% risk reduction. Those hospitalized tended to have underlying risk factors, age or BMI for example. No patients (placebo or intervention arms) progressed to mechanical ventilation or died. There were no serious adverse events considered to be drug related. Although not a primary endpoint, nevertheless, clinical improvement in symptoms occurred in the treatment arm compared to placebo. A question of viral RNA resistance variants in placebo, and treatment was raised, but the number of cases from placebo and intervention data are too small to make any generalizations. No serious adverse events were associated with the therapeutic was noted. The researchers remind this is in a limited population.
CAVEATs – The results from both types of antibody combinations seem promising, and suggest the potential for another intervention for an important subgroup of persons infected with COVID-19, especially in the outpatient setting. But the research is ongoing, and remains to be fully published in the peer review literature. In addition to these two, other human COVID-19 antibody research is ongoing, and emerging data pending. Further research, and thorough analysis, as well as identifying optimal patient populations, with review of safety data and other clinical issues need to be considered.