Abstract
Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS) represent a significant cause of morbidity and mortality in critically ill hospitalized patients. Emerging evidence suggest that the expression levels of P53 in the lungs are associated with the supportive effects of heat shock protein 90 inhibitors and growth hormone releasing hormone antagonists in the endothelium. In the current study, we employed an in vivo model of intratracheal administration of lipopolysaccharides (LPS)-induced ALI to investigate the role of P53 in counteracting LPS-induced lung inflammatory responses. In wild type mice, LPS induced the expression of IL-1α, IL-1β, and TNFα in the lungs, increased bronchoalveolar lavage fluid protein concentration, and activated cofilin. Remarkably; those responses were more potent in P53 knockout mice, suggesting the crucial role of P53 in orchestrating rigorous endothelial defenses against inflammatory stimuli. The present study supports previous endeavors on the protective role of P53 against lung inflammatory disease, and enrich our knowledge on the development of medical countermeasures against ARDS.
Keywords: Inflammation, Acute lung injury, Acute respiratory distress syndrome, Unfolded protein response
Highlights
-
•
P53 deactivates cofilin.
-
•
P53 suppresses pro-inflammatory cytokines.
-
•
P53 may serve as a target for opposing ARDS.
-
•
Mice that do not express P53 are more susceptible to inflammation.
1. Introduction
The lungs are exposed to a variety of direct (i.e. pathogen, pollutants, allergens) and indirect (i.e. sepsis, shock, pancreatitis) stimuli. The progression of severe inflammatory cascades due to those challenges may deteriorate the integrity of the alveolar-capillary membrane. The disruption of the endothelial and epithelial barriers due to those effects may result to ALI, and in more progressive cases to the lethal ARDS (Auriemma et al., 2020). The unacceptable mortality rates due to this respiratory disorder (32–45%) reflect the lack of efficient medical countermeasures against ARDS. Hence, laborious efforts aim to elucidate the molecular mechanisms involved in the regulation of lung endothelial permeability to oppose ARDS (Maca et al., 2017).
The endothelium defender P53 orchestrates various molecular mechanisms, so to oppose detrimental stimuli and maintain the cellular integrity and stability (Uddin and Barabutis, 2019). It was initially considered to function exclusively as an oncogene (Rotter, 1983), but it was later revealed that this transcription factor is destined to protect the integrity of the genome (Barabutis et al., 2018a). The defensive role of P53 is not only limited to protection against cancers. It also mediates robust anti-inflammatory activities in the vasculature, which in turn result in enhanced endothelial barrier function (Barabutis, 2020a). The reciprocal regulation of P53 and NF-kB indicates the importance of the former in maintaining physiological homeostasis and adaptation to different environments (Liu et al., 2009).
The generation of reactive oxygen species (ROS) due to inflammation compromises inter-endothelial junctions, contributing to lung hyperpermeability responses (Mittal et al., 2014). P53 has been reported to inhibit ROS production in the lungs (Akhter et al., 2020) and brain (Akhter et al., 2019a). Those anti-oxidant activities were supported by the P53-mediated apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APE1/Ref1 reduction) (Uddin et al., 2019a). Moreover, it was suggested that P53 orchestrates the actin cytoskeleton, by inhibiting the formation of the filamentous actin due to RhoA activation (Barabutis, 2020b; Barabutis et al., 2018b), and by inducing the Rac1-triggered cofilin deactivation (Barabutis et al., 2018b).
Heat shock protein 90 (Hsp90) is a molecular chaperone in charge of the maturation and folding of several client proteins; including inflammatory kinases and transcription factors (Schopf et al., 2017; Barabutis and Siejka, 2020). Hsp90 inhibitors have been shown to protect against the LPS-induced lung endothelial barrier dysfunction (Thangjam et al., 2016; Barabutis, 2020c), and therefore, may serve as a possible therapeutic approach in ARDS. The upregulation of P53 due to Hsp90 inhibition contributed towards the protective role of those compounds in the inflamed lungs (Barabutis et al., 2015, 2019,bib_Barabutis_et_al_2019,bib_Barabutis_et_al_2015). Interestingly, growth hormone-releasing hormone (GHRH) antagonists have also been reported to exert anti-inflammatory activities in normal and cancer lungs partially due to P53 induction (Barabutis, 2020d; Uddin et al., 2019b; Barabutis et al., 2020).
The purpose of the present study is to further our understanding of the protective role of P53 against lung endothelial barrier hyperpermeability in an in vivo model of ALI. We evaluated the LPS-induced augmentation of different inflammatory mediators and markers, including IL1α, IL1β, TNFα, and activated cofilin in P53 knock out (KO) mice as well as in wild type mice. Moreover, we measured the bronchoalveolar lavage fluid (BALF) protein concentration. Our findings support the anti-inflammatory role of P53 an in vivo model of ALI, since mice that did not globally express P53 appear to be more susceptible to LPS compared to the wild type mice.
2. Materials and methods
2.1. Reagents
RIPA was used as a cell lysis buffer (AAJ63306-AP), anti-mouse (95017–554) and anti-rabbit (95017–556) IgG HRP-linked antibodies and nitrocellulose membranes (10063–173) were purchased from VWR (Radnor, PA). Furthermore, specific antibodies for IL1-α (50794S), IL1-β (12703S), TNF-α (3707S), phospho-cofilin (3313S), Cofilin (3318S) were purchased from Cell Signaling Technology (Danvers, MA). Sigma-Aldrich (St Louis, MO) supplied the β-actin antibody (A5441).
2.2. Animals
Male C57BL/6 mice and male homozygous P53 knock out (KO) mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). Seven weeks old animals were used in our treatments. The mice were maintained under pathogen-free conditions in a 12:12 h light: dark cycle. The protocol of animal care and treatments was evaluated and approved by the University of Louisiana Monroe IACUC. All procedures complied with the principles of human-animal care, as adopted by the American Physiological Society.
2.3. In vivo treatments
Male C57BL/6 mice and male P53 KO mice received vehicle (saline) or LPS (1.6 mg/kg) via an intratracheal (i.t.) injection. The animals were sacrificed 24 h after treatment, and the pulmonary tissues were collected for tests.
2.4. Collection of BALF and total protein measurement
In order to assess the BALF measurements, BALF from mice was taken by instilling and withdrawing 1 ml of PBS by using a tracheal cannula. The total protein in BALF concentrations were estimated with the bicinchoninic acid protein assay kit (Thermo Scientific, Rockford, IL).
2.5. Western blot analysis
RIPA buffer was used to homogenize the tissues. The proteins were separated according to their molecular weight by electrophoresis onto 12% sodium dodecyl sulfate Tris-HCl gels. In order to transfer the proteins from the gels to the membranes, we employed a wet transfer technique. The membranes were exposed for at least 60 minutes at room temperature in a solution of 5% non-fat dry milk in Tris-buffered saline (TBS) – 0.1 % (v/v) Tween 20, so to cover the non-specific binding sites of the membranes. Then, those membranes were incubated overnight in a cold room with the corresponding primary antibodies (1:1000), and consequently to the appropriate secondary antibody. SuperSignal™ West Pico PLUS (PI34578) was the chemiluminescent substrate, and the light was captured in a ChemiDocTM Touch Imaging System from Bio-Rad (Hercules, CA, USA). The β-actin antibody (1:5000) was used as a loading control, when appropriate. All reagents were obtained from VWR (Radnor, PA, USA).
2.6. Densitometry and statistical analysis
The densitometry was performed with Image J, developed from NIH. Our data were expressed as mean values ± SEM (standard error of mean). A value of p < 0.05 was considered significant. GraphPad Prism 5.01, GraphPad (CA, USA) was used for our analysis. The letter n represents the number of animals.
3. Results
3.1. LPS produces a stronger induction of IL-1α, IL-1β, and TNFα in P53 KO mice compared to the wild type mice
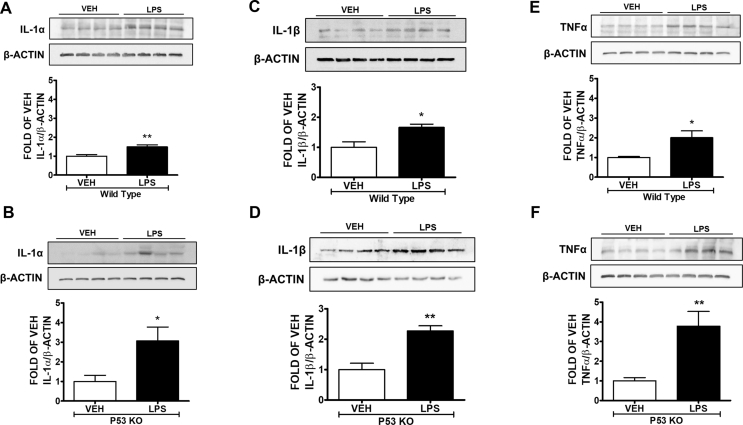
The pro-inflammatory IL-1α, IL-1β, and TNFα cytokines were increased due to LPS treatment in the lungs of the mice (Fig. 1A–F). Those effects were much stronger in the mice that did not express P53 (Fig. 1B, D, and F).
Fig. 1.
P53 KO mice are more susceptible to LPS-induced acute lung injury compared to the wild type mice. Wild type and P53 KO mice were treated with vehicle (saline) or LPS (1.6 mg/kg) via an intratracheal injection. Western Blot analysis of (1A) IL-1α and β-actin, (1C) IL-1β and β-actin, (1E) TNFα and β-actin in lung homogenates of wild type mice. The signal intensity of the bands was analyzed by densitometry. Protein levels were normalized to β actin. ∗P < 0.05, ∗∗P < 0.01 vs vehicle (VEH), n = 4. Means ± SEM. Western Blot analysis of (1B) IL-1α and β-actin, (1D) IL-1β and β-actin, (1F) TNFα and β-actin in lung homogenates of P53 KO mice. The signal intensity of the bands was analyzed by densitometry. Protein levels were normalized to β actin. ∗P < 0.05, ∗∗P < 0.01 vs vehicle (VEH), n = 4. Means ± SEM.
3.2. The LPS-induced BALF protein concentration is higher in P53 KO compared to the wild type mice
Wild type (C57BL/6) and P53 KO mice were challenged with intratracheal injection of vehicle (saline) or LPS (1.6 mg/kg) for 24 h. The BALF protein concentration in the P53 KO mice (Fig. 2B) was higher compared to the wild type mice (Fig. 2A) after LPS treatment.
Fig. 2.
Lack of P53 potentiates LPS-triggered lung inflammatory responses in vivo. Wild type and P53 KO mice were treated with vehicle (saline) or LPS (1.6 mg/kg) via an intratracheal injection. Measurements of total protein levels in the BALF of wild type (2A) and P53 KO mice (2B). ∗∗P < 0.01, ∗∗∗P < 0.001 vs vehicle (VEH), n = 4, Means ± SEM. Western Blot analysis of (2C) pCofilin and Cofilin in lung homogenates of wild type mice. The signal intensity of the bands was analyzed by densitometry. Protein levels of pCofilin were normalized to Cofilin. ∗P < 0.05 vs vehicle (VEH), n = 4. Means ± SEM. Western Blot analysis of (2D) pCofilin and Cofilin in lung homogenates of P53 KO mice. The signal intensity of the bands was analyzed by densitometry. Protein levels of pCofilin were normalized to Cofilin. ∗P < 0.05 vs vehicle (VEH), n = 4. Means ± SEM.
3.3. The expression levels of phospho cofilin in the LPS-treated P53 KO mice are lower compared to those of the LPS-treated wild type mice
In all cases LPS activated cofilin (Fig. 2C and D). However, in the P53 null mice those effects were more prominent. The inflamed mutant mice exerted higher levels of activated (non phospho) cofilin (Fig. 2D), as compared to the wild type mice.
4. Discussion
The molecular mechanisms mediating the effects of P53 in the lung vasculature are still not well understood. This endothelium defender regulates a wide variety of intracellular events to oppose the establishment of severe pathophysiologies, as manifested in the case of ARDS (Barabutis, 2020a). Furthermore, P53 has been recently associated with anti-inflammatory responses (Kubra et al., 2020a).
Recent evidence suggest that those protective effects in the lungs are due to a mild induction of the unfolded protein response (UPR) (Barabutis, 2019a, 2020e,bib_Barabutis_2020e,bib_Barabutis_2019a), and a direct association between P53 and UPR was recently established. Bovine cells exposed to brefeldin A, dithiothreitol, and thapsigargin (UPR inducers); expressed more P53 compared to the vehicle-treated cells. However, treatment with N-acetyl cysteine, kifunensine, and ATP-competitive IRE1α kinase-inhibiting RNase attenuator, all being UPR suppressors, resulted to P53 suppression (Akhter et al., 2019b).
P53 induction due to Tunicamycin revealed the negative regulation of APE1/Ref1 by this transcription factor (Uddin et al., 2019a). Indeed, P53 may hold the capacity to mediate the supportive effects of UPR in lung endothelial barrier function (Barabutis, 2019b); in the context of Hsp90 inhibition (Barabutis, 2020c; Kubra et al., 2020b) and the effects of GHRH antagonists in the vasculature (Uddin et al., 2019b; Barabutis et al., 2020). Hsp90 inhibition in the lungs was not associated to lethal effects, as it was indicated by cellular viability measurements (Uddin et al., 2020), partially due to the fact that the activated (inflamed) Hsp90 presents a higher affinity for Hsp90 inhibitors compared to normal cells (Barabutis, 2020c).
The present study supports the protective functions of P53 against the LPS-induced pulmonary inflammation. P53 mutants which do not express P53 exerted higher levels of BALF protein concentration compared to the wild type mice. We also evaluated the induction of the proinflammatory cytokines such as IL-1α, IL-1β and TNFα to conclude that the LPS-triggered inflammatory responses were more robust in the P53 KO compared to the wild type mice.
The phosphorylation of myosin light chain 2 (MLC2) in the mice lungs induces the actin-myosin interaction, weakens endothelial cell–cell adhesion, and causes vascular hyperpermeability (Shen et al., 2010). It was previously shown that P53 suppresses the LPS-induced activation of MLC2 both in vivo and in vitro (Barabutis et al., 2015, 2018b,bib_Barabutis_et_al_2018b,bib_Barabutis_et_al_2015). Cofilin is an actin-binding protein which promotes vascular hyperpermeability (Uddin et al., 2019b). LPS activates cofilin by dephosphorylation. The levels of activated cofilin in the inflamed wild type mice were lower than those in the lungs of the P53 null mice. Thus, those observations, in combination with previous studies, suggest that P53 induces the Rac1/pCofilin pathway in the lungs (Barabutis, 2020b; Barabutis et al., 2018b).
5. Conclusions
ARDS causes thousands of deaths worldwide. Our study shows for the first time that the intratracheal injection of LPS in mice that do not express P53 triggers more severe inflammatory responses compared to the wild type littermates. Thus, P53 modulation may serve as a promising therapeutic approach in ALI/ARDS.
Funding
Dr. Barabutis’ research is supported by the R&D, Research Competitiveness Subprogram (RCS) of the Louisiana Board of Regents through the Board of Regents Support Fund (LEQSF(2019-22)-RD-A-26) (Principal Investigator), and the National Institute of General Medical Sciences of the National Institutes of Health (5 P20 GM103424-15, 3 P20 GM103424-15S1).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Akhter M.S. P53-induced reduction of lipid peroxidation supports brain microvascular endothelium integrity. J. Pharmacol. Sci. 2019;141(1):83–85. doi: 10.1016/j.jphs.2019.09.008. [DOI] [PubMed] [Google Scholar]
- Akhter M.S., Uddin M.A., Barabutis N. Unfolded protein response regulates P53 expression in the pulmonary endothelium. J. Biochem. Mol. Toxicol. 2019;33(10) doi: 10.1002/jbt.22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter M.S., Uddin M.A., Barabutis N. P53 regulates the redox status of lung endothelial cells. Inflammation. 2020;43(2):686–691. doi: 10.1007/s10753-019-01150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auriemma C.L. Acute respiratory distress syndrome-attributable mortality in critically ill patients with sepsis. Intensive Care Med. 2020;46(6):1222–1231. doi: 10.1007/s00134-020-06010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N. Unfolded protein response in acute respiratory distress syndrome. Lung. 2019;197(6):827–828. doi: 10.1007/s00408-019-00279-4. [DOI] [PubMed] [Google Scholar]
- Barabutis N. Unfolded Protein Response supports endothelial barrier function. Biochimie. 2019;165:206–209. doi: 10.1016/j.biochi.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N. P53 in lung vascular barrier dysfunction. Vasc. Bio. 2020;2(1):E1–e2. doi: 10.1530/vb-20-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N. 2020. P53 in RhoA Regulation. Cytoskeleton (Hoboken) [DOI] [PubMed] [Google Scholar]
- Barabutis N. Heat shock protein 90 inhibition in the inflamed lungs. Cell Stress Chaperones. 2020;25(2):195–197. doi: 10.1007/s12192-020-01069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N. A glimpse at growth hormone-releasing hormone cosmos. Clin. Exp. Pharmacol. Physiol. 2020 doi: 10.1111/1440-1681.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N. Unfolded protein response in lung health and disease. Front. Med. 2020 doi: 10.3389/fmed.2020.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N., Siejka A. The highly interrelated GHRH, p53, and Hsp90 universe. Cell Biol. Int. 2020 doi: 10.1002/cbin.11356. [DOI] [PubMed] [Google Scholar]
- Barabutis N. p53 protects against LPS-induced lung endothelial barrier dysfunction. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;308(8):L776–L787. doi: 10.1152/ajplung.00334.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N., Schally A.V., Siejka A. P53, GHRH, inflammation and cancer. EBioMedicine. 2018;37:557–562. doi: 10.1016/j.ebiom.2018.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N. Wild-type p53 enhances endothelial barrier function by mediating RAC1 signalling and RhoA inhibition. J. Cell Mol. Med. 2018;22(3):1792–1804. doi: 10.1111/jcmm.13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N., Uddin M.A., Catravas J.D. Hsp90 inhibitors suppress P53 phosphorylation in LPS - induced endothelial inflammation. Cytokine. 2019;113:427–432. doi: 10.1016/j.cyto.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabutis N. GHRH antagonists protect against hydrogen peroxide-induced breakdown of brain microvascular endothelium integrity. Horm. Metab. Res. 2020;52(5):336–339. doi: 10.1055/a-1149-9347. [DOI] [PubMed] [Google Scholar]
- Kubra K.T. P53 versus inflammation: an update. Cell Cycle. 2020;19(2):160–162. doi: 10.1080/15384101.2019.1708575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubra K.T. Hsp90 inhibitors induce the unfolded protein response in bovine and mice lung cells. Cell. Signal. 2020;67:109500. doi: 10.1016/j.cellsig.2019.109500. [DOI] [PubMed] [Google Scholar]
- Liu G. p53 Attenuates lipopolysaccharide-induced NF-kappaB activation and acute lung injury. J. Immunol. 2009;182(8):5063–5071. doi: 10.4049/jimmunol.0803526. [DOI] [PubMed] [Google Scholar]
- Maca J. Past and present ARDS mortality rates: a systematic review. Respir. Care. 2017;62(1):113–122. doi: 10.4187/respcare.04716. [DOI] [PubMed] [Google Scholar]
- Mittal M. Reactive oxygen species in inflammation and tissue injury. Antioxidants Redox Signal. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter V. p53, a transformation-related cellular-encoded protein, can be used as a biochemical marker for the detection of primary mouse tumor cells. Proc. Natl. Acad. Sci. U. S. A. 1983;80(9):2613–2617. doi: 10.1073/pnas.80.9.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopf F.H., Biebl M.M., Buchner J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017;18(6):345–360. doi: 10.1038/nrm.2017.20. [DOI] [PubMed] [Google Scholar]
- Shen Q. Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc. Res. 2010;87(2):272–280. doi: 10.1093/cvr/cvq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangjam G.S. Hsp90 inhibition suppresses NF-kappaB transcriptional activation via Sirt-2 in human lung microvascular endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2016;310(10):L964–L974. doi: 10.1152/ajplung.00054.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M.A., Barabutis N. P53: the endothelium defender. J. Cell. Biochem. 2019;120(7):10952–10955. doi: 10.1002/jcb.28511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M.A. P53 supports endothelial barrier function via APE1/Ref1 suppression. Immunobiology. 2019;224(4):532–538. doi: 10.1016/j.imbio.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M.A. GHRH antagonists support lung endothelial barrier function. Tissue Barriers. 2019;7(4):1669989. doi: 10.1080/21688370.2019.1669989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M. Effects of heat shock protein 90 inhibition in the lungs. Med. Drug Discov. 2020;6 doi: 10.1016/j.medidd.2020.100046. [DOI] [PMC free article] [PubMed] [Google Scholar]