Abstract
Glutamine and glutamate are major bioenergy substrates for normal and cancer cell growth. Cancer cells need more biofuel than normal tissues for energy supply, anti-oxidation activity and biomass production. Genes related to metabolic chains in many cancers are somehow mutated, which makes cancer cells more glutamate dependent. Meanwhile, glutamate is an excitatory neurotransmitter for conducting signals through binding with different types of receptors in central neuron system. Interestingly, increasing evidences have shown involvement of glutamate signaling, guided through their receptors, in human malignancy. Dysregulation of glutamate transporters, such as excitatory amino acid transporter and cystine/glutamate antiporter system, also generates excessive extracellular glutamate, which in turn, activates glutamate receptors on cancer cells and results in malignant growth. These features make glutamate an attractive target for anti-cancer drug development with some glutamate targeted but blood brain barrier impermeable anti-psychosis drugs under consideration. We discussed the relevant progressions and drawbacks in this field herein.
Keywords: glutamate, cancers, metabolism, glutamate receptors, glutamate transporters
Introduction
Fast growing cancer cells have a high demand for catabolites to produce ATP, maintain a reduction-oxidation balance and generate biomass. After the early finding of a metabolic difference between cancer and normal tissue by Warburg and colleagues, people started to notice that metabolism of cancer is not only different from that of normal tissue but also among different type of tumorous cells[1–2]. Rather a common feature of cancer metabolism is that it needs a nutrient that is not only a decent donor of carbon and nitrogen for production of energy sugar, lipids, nucleotide and amine but also a precursor of an anti-oxidation by-product that can protect cancer cells from oxidative stress. Thus, glutamine appears to be a candidate. Glutamine is the most abundant amino acid precursor in circulation and the main vehicle for circulating ammonia in a nontoxic form[3]. In normal tissues and cancer cells, glutamine donates its amide (γ nitrogen) group firstly to become glutamic acid. Then carbon skeleton of glutamate can be incorporated into α-ketoacids for making ATP and fatty acids, while the nitrogen is used in the synthesis of purines and pyrimidines[4–5]. The glutamic acid is a precursor of ornithine, arginine and proline, and its skeleton can be used for synthesis of another non-essential amino acids through the Kreb's circle[4–5]. In addition, glutamate is a precursor of glutathione that is an antioxidant acting as a free radical scavenger and a detoxifying agent in cells[6–7]. It is known that reactive oxygen species (ROS like H2O2, O2•–, OH•,etc.) are significantly increased in cancer cells because of mitochondrial dysfunction and altered metabolism, which results in an accumulation of large amounts of oxidized protein, DNA, and lipids[6–7]. Therefore, as an adaptive response, cancer cells must harbor elevated levels of ROS-scavengers such as glutathione[6–7].
Glutamate receptors and transporters
Apart from the roles that glutamine plays in cancer cell metabolism, glutamic acid or its salt glutamate has been found to modulate cancer cell development, proliferation and metastasis through regulating cell signaling pathways[8–9]. Until recent decades, people have focused on studies of its function in the central nervous system (CNS) since glutamate has been found to be a key mediator of excitatory signals in the mammalian CNS[10–11]. It is known that neurons and non-neuron brain cells express different kinds of glutamate receptors that are classified into ionotropic (iGluR) and metabotropic glutamate receptors (mGluR) in the CNS[12]. The iGluRs are quaternary ligand-gate ion channels that allow cation influx upon glutamate binding, which are divided into 3 subfamilies: N-methyl-D-aspartate (NMDA) receptor, a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, and kainate (KA) receptor[13]. The mGluRs belong to the superfamily of G-protein coupled receptors, which are classified into three subgroups: group Ⅰ, Ⅱ and Ⅲ. The mGluR1 and mGluR5 belong to group I mGluRs, and they are coupled to Gq/11 G-protein. Upon glutamate binding, G protein activates PLCβ to cause hydrolytic cleavage of phosphatidylinositol-4,5-bisphosphate (PIP2) and then formation of inositol 1,4,5-triphosphate (IP3) and diacylglycerol. As a consequence, the secondary messenger leads to an increment of calcium release from endoplasmic reticulum stores and activation of protein kinase C, resulting in a downstream effector targeted stimulation. Group Ⅱ receptor consists of mGluR2 and mGluR3, and group Ⅲ comprises mGluR4, mGluR6, mGluR7 and mGluR8, all of which are coupled with Gi/oG-protein that negatively regulates adenylate cyclase[12].
In addition to these receptors, there are a group of glutamate transporters, termed as excitatory amino acid transporters (EAATs), which transport L-and D-aspartate along with glutamate from extracellular environment to the cells and maintain low level of extracellular glutamate. EAATs are a family of high-affinity Na+/K+-dependent transporters for glutamate and aspartate with five EAATs, the EAAT1–5 in this family, which are encoded by genes SLC1A3, SLC1A2, SLC1A1, SLC1A, and SLC1A7, respectively (https://en.wikipedia.org/wiki/Glutamate_transporter). EAAT1 and EAAT2 are predominantly expressed in astrocytes, especially within their processes surrounding glutamatergic synapses, where they are responsible for the immediate uptake of synaptic released glutamate. EAAT3 is expressed in neurons, but in the kidney and the intestinal mucosa as well[14–15]. EAAT4 is predominantly expressed in cerebellar Purkinje cells, and EAAT5 is expressed in rod photoreceptor and bipolar cells of the retina. Another transporter, termed as antiporter, is Na+-independent cystine/glutamate exchanger, e.g. system Xc− (SXC), which uptakes extracellular cysteine and exports intracellular glutamate outside of the cells. However in recent decades, increasing evidences have unveiled that specific glutamate receptors and/or glutamate transporters are also expressed in non-neurological tissues and even in immune system, which suggests that glutamate plays an important role in the regulation of physiological function in various peripheral organs[16]. Further, a large amount of data have shown that glutamine and glutamate has been extensively involved in development and transformation of tumors in both CNS and peripheral tissues[9,17]. This is the subject of our current review including the involvement of glutamine and glutamate in cancer development and their potential therapeutic significance.
Glutamine and glutamate in cancer metabolism
Altered energy metabolism is acknowledged as one of the emerging hallmarks of cancer. Initially, Warburg and co-workers showed that cancer tissues metabolize approximately 10-fold more glucose to lactate in a given time than normal tissues which led them to conclude that cellular respiration chain was damaged in cancer cells[1,18]. This proposal was argued for decades[1,18] and has been well accepted until recent years. Recently, people identified a number of mutated genes that encode enzymes functioning in cellular bioenergetics and biosynthesis metabolism in different cancer cells[19–21]. The fact that proliferating tumor cells are highly dependent on glutamine was firstly highlighted by Eagle in 1955[22], who discovered that the glutamine consumption rate in many cell lines exceeded the consumption of any other amino acid by 10-fold. Afterwards, Kovacevic and colleagues observed glutamine carbons was released by cells at the form of carbon dioxide, providing evidence that glutamine could serve as a combustible fuel[23]. Then, Reitzer and co-workers reported that Hela (derived from cervical cancer) cells predominantly consume glutamine as a biofuel[24]. These studies represented the earlier findings on substitution of glucose by glutamine in cancer bioenergetics metabolism. Of note, blood glutamine levels were increased in patients with advanced-stage cancers when serum compounds were being traced during cancer development[25]. Serum glutamate levels in prostate cancer patients were also correlated with Gleason score, which inferred the extent of progression of the prostate cancers[26]. An analysis of freshly frozen human samples also demonstrated that glutamate level is strikingly increased in chronic pancreatitis and pancreatic ductal adenocarcinoma tissues compared to that in normal pancreatic tissue[27]. Glutamate release was also found to be high in breast cancers, which makes it a new biomarker for breast cancer diagnosis[28].
For better understanding the glutamate associated metabolism, the major reactions involved are listed as the following[29]: Aspartate aminotransferase reaction: Glutamate + oxaloacetate ↔ α-ketoglutarate + aspartate; Glutamate dehydrogenase reaction: α-ketoglutarate + NH3 +NADH + H+ ↔ glutamate + NAD+; Glutamic acid decarboxylase reaction: Glutamate → GABA + CO2; Glutaminase reaction: Glutamine + H2O → glutamate + NH4+; GABA transaminase reaction: GABA + α-ketoglutarate ↔ glutamate + succinic semialdehyde; Glutamine synthetase reaction: Glutamate + NH3 + ATP ↔ glutamine + ADP + Pi; γ-glutamylcysteine synthetase reaction: Glutamate + L-cysteine + ATP → γ-glutamylcysteine + ADP + Pi; N-acetylated-α-linked-amino dipeptidase reaction: N-acetylaspartatylglutamate ↔ N-acetylaspartate + glutamate; Alanine aminotransferase reaction: Glutamate + pyruvate ↔ alanine + α-ketoglutarate; Ornithine aminotransferase reaction: Ornithine + α-ketoglutarate ↔ glutamate + glutamic acid semialdehyde; Branched-chain amino acid aminotransferase reaction: α-ketoisocaproate + glutamate ↔ leucine + α-ketoglutarate. Those are combined in Fig. 1.
1. Glutamine and glutamate metabolic reactions and key enzymes in cells.
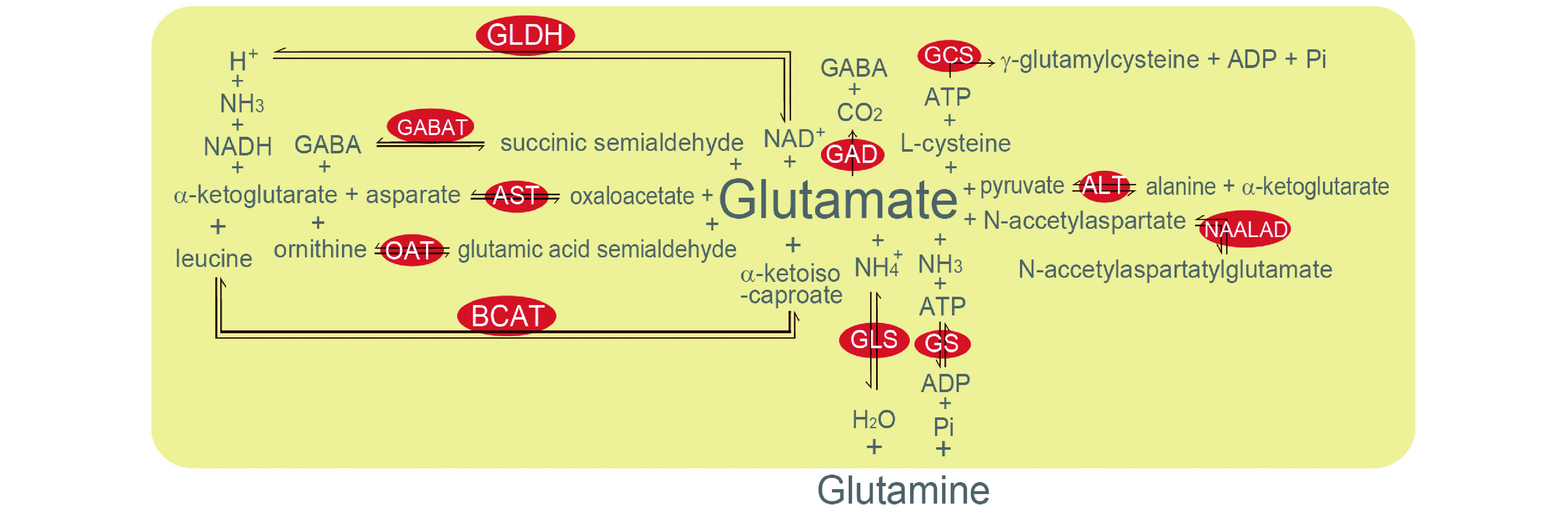
ALT: alanine aminotransferase; AST: aspartate aminotransferase; BCAT: branched-chain amino acid aminotransferase; GABAT: GABA transaminase; GAD: glutamic acid decarboxylase; GCS: γ-glutamylcysteine synthetase; GLDH: glutamate dehydrogenase; GLS: glutaminase; GS: glutamine synthetase; NAALAD: N-acetylated-α-linked-amino dipeptidase; OAT: ornithine aminotransferase.
The dependence of cancer cells on glutamine metabolism has made it an attractive anticancer therapeutic target[2,30–31]. Many glutamine mimetic antimetabolites (including acivicin, DON and azaserine) were developed and intended to become anti-cancer medicine before 1990s[5]. However, these substances deprive glutamine from both tumor and normal cells, which made them more toxic than therapeutic[31]. The pre-clinic trial for those substances generally failed, and then clinical development stopped. One exception was phenylacetate that can conjugate with glutamine in the blood plasma and form phenylacetylglutamine[32]. Long-term administration of phenylacetate was shown to reduce plasma glutamine levels and was well-tolerated[31–33]. Development of phenylacetate to be an anti-cancer reagent was stepped into phase Ⅰ and Ⅱ clinical trials in 2000s[34]. However, no promising report followed. Meanwhile, the mechanism on "glutamine-addiction" has been studied in efforts to find any key steps in mutant cancer metabolic pathways that differ from that of normal cells in host[2,31]. The findings from those works enlightened some new interest in the development of glutamine/glutamate associated anti-cancer therapeutic approaches. Among them, allosteric inhibitors of (kidney-type) glutaminase[35] have shown promise in preclinical models of cancer, such as CB839 that has shown efficacy against triple-negative breast cancer and haematological malignancies in preclinical studies[36], and bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide that has been shown to block the growth of cancer cells in vitro and xenografts in vivo[37–38].
Glutamate receptors and cancers
Association of iGluRs with cancers
The iGluRs family includes NMDA, AMPA, and KA receptors in the CNS[13]. The NMDA receptors are composed of two GluN1 subunits and two GluN2 subunits or a combination of a GluN2 and a GluN3 subunit[13] and activated upon binding to the glutamate and glycine on the cell membrane[39]. Activation of NMDA receptors allows calcium flux into the cells, which is thought to be critical in synaptic plasticity but not engaged in depolarizing membrane potential and initiation of action potential[40]. In recent years, both iGlutRs and mGluRs have been found in the peripheral tissues outside of CNS, such as the kidney, lung, liver, heart, stomach, bone, and skin[9,16,41]. The roles they might play in tumor or cancer development are also broadly studied[9,42]. For example, NMDA receptor activation affects some cell-proliferation signaling activity, such as mTOR pathway that controls cell growth[43–44], either through a Ca2+ dependent regulation of p-ERK, which in turn modulates mTOR, or via inhibition of membrane cationic amino acid transporters[43–44]. This role of NMDA receptor in peripheral tissues may be an important contributor for human malignancies[45].
Reasonably in the CNS, most of glutamate receptors and transporters including all EAATs are involved in the development of different types of glioma or neuroblastoma. The presence or lack of GluR2 subunits of the AMPA receptor appears to be crucial for glioma cell invasive potential. Previous studies have shown that the majority of invasive gliomas are either lack of GluR2 expression[46] or significantly lower level of its expression. Clinically, it was observed that GluR2 expression was lacking in highly malignant glioblastomas like ependymomas or medulloblastomas (all WHO Ⅲ or Ⅳ), and a higher level of GluR2 was expressed in low-grade astrocytomas (WHO Ⅰ or Ⅱ)[47]. In addition, an RNA intervention study on a low-grade glioma cell line revealed that down-regulation of GluR2 expression induced a significant increment of cell proliferation[48]. Nonetheless, absence of GlutR2 in highly malignant pediatric glioblastomas had been verified clinically[47]. It is known that in neurons, GlutR2 negative AMPA receptor is calcium permeable but a GlutR2 composed one with a positive charged amino acid facing the channel is not permeable to divalent cation[49]. This is consistent with an observation that intracellular calcium oscillation plays a crucial role in growth and motility of a patient derived glioma cell line probably due to disassembly of focal adhesions triggered by this oscillation[50]; meanwhile, both PCR and Western blot test exhibited those cells lack of GluR2 in their AMPA receptors[46].
In recent decades, iGlutRs have been identified in many types of cancers outside the CNS where glutamate signaling stimulates tumor cell proliferation via iGlutR activation[42,51]. The mRNA of iGluR subunits has been detected upregulated in thyroid, lung and breast carcinomas, multiple myeloma, colon adenocarcinoma, and T cell leukemia[52], in larynx[53] and gastric cancer cells[54], as well as in osteosarcoma[55]. The iGluR subunit proteins are also found elevated in prostate[56], breast[57], and lung[58] cancers, as well as in melanoma[59] and hepatocellular carcinoma cell lines[60]. Accordingly, iGlutRs antagonists are applied in treatment of many kinds of cancers. For example, an NMDA receptor antagonist GYKI52466, showed anti-tumor effect in colon adenocarcinoma, astrocytoma, and breast and lung carcinoma cells[51]. NMDA receptor antagonists, memantine and MK-801, can reduce viability of the cells derived from a recurrent and drug-resistant small cell lung cancer[58]. An AMPA receptor antagonist CFM-2 is able to inhibit cancer cell growth by down-regulating the expression of survivin[61]. Talampanel is a newly developed non-competitive antagonist of the AMPA receptor that has shown some promising results for clinical use in cancer therapy[62–63]; encouragingly, a phase Ⅱ clinic trial study of talampanel combined with radiation therapy plus the DNA methylation agent temozolomide has been performed for treatment of newly diagnosed glioblastoma patients[62–63].
Association of mGluRs with cancers
Similarly, ample evidences have shown the involvement of mGluRs in cancer development. Group Ⅰ mGluRs are coupled to Gq/11 G-protein, and group Ⅱ and Ⅲ mGluRs are coupled to Gi/o G-protein. All mGluRs share a basic structure that includes a large extracellular N-terminus, a cysteine-rich domain, a seven-alpha-helical transmembrane domain and an intracellular C-terminal. The mGluRs function when two glutamate molecules bind the N-terminus and the cysteine-rich domains are dimerized[64]. In recent years, high-throughput cancer genome deep sequencing analyses have revealed that approximately 20% of all cancers harbor mutated G-protein coupled receptors, with mGluRs highly mutated in many types of cancers, and that specific mutations in Gαs, Gαq and Gα11 sites lead to constitutive and persistent aberrant signaling that may drive tumor progression[65].
The function of mGluRs in human malignancy was first discovered in CNS tumors. In normal brain glial cells, mGlu1, mGlu3, and mGlu5 receptors are found in astrocytes, mGlu2, mGlu3, and mGlu5 receptors are expressed in microglial cells, and mGlu1 and mGlu4 are highly expressed in oligodendrocytes[66–67]. In brain tumors, over expression of mGluR1, mGluR2, and mGluR6 are observed in malignant medulloblastomas, ependymomas, and glioblastomas[47]. Expression or predominant expression of different groups and/or subunits of the mGluRs in different glioma cell lines and patient derived tumor cells has been reviewed in super detail by a large research group recently[68]. Noteworthy, a comprehensive study of pediatric brain tumors showed mGluR1, mGluR2 and mGluR6 expression levels are elevated in malignant medulloblastomas, ependymomas and glioblastomas, compared to those in low-grade astrocytomas[47]. This over expression pattern is similar among tumor cells of different histological origins, indicating that a common glutamatergic signaling cascade plays a key role in these cancers and could have potentials to be a therapeutic target in pediatric brain tumors[47]. On the other hand, pharmacological blockade of mGlu3 receptors by its antagonist LY341495 guided differentiation of highly malignant cultured glioblastoma cells into astrocyte lineage[69]. Consistently, clinical data suggest that transcript levels of mGluR3 may be a potential predictor for survival of glioblastoma patients and that mGluR3 antagonists could be recommended as adjuvant drugs for treatment of glioblastoma[70]. In a clinical study of 87 glioblastoma cases, the group of patients with low tumoral mGluR3 mRNA levels showed a significantly higher survival rate than high tumoral mGluR3 mRNA cases. Five patients who survived longer than 36 months had tumoral mGluR3 mRNA expression far below normal range[70].
In addition to CNS neoplasms, mGluRs are expressed broadly in peripheral organs, such as the gastrointestinal tract, kidney, liver and immune cells, and they play a role of commanding cell proliferation, differentiation and transformation[64]. In non-neuronal cancers, mGluRs are found elevated in melanoma, breast, prostate, renal and colorectal cancers, and osteosarcoma as well[71–73]; and over expression or activation of them promotes these cancers' growth. In contrast, activation of group Ⅲ mGluR4 in bladder cancer has been reported to promote cell apoptosis and inhibit proliferation[74]. Notably, expression of mGluR1 in breast cancer has been broadly explored and mGluR1 is up-regulated in five triple-negative breast cancer cell lines compared with that in normal mammary epithelial cells[75]. Silencing mGluR1 results in reduction of tumor cell proliferation and progression, and induction of tumor cell apoptosis in vitro and in vivo[75]. Furthermore, over expression of mGluR1 in another breast cancer cell line, e.g., MCF10AT1 cells, was reported to significantly increase the proliferation and facilitate cell migration and invasion[76]. Expression of mGluR1 is a specific feature of melanoma since approximately 80% of melanoma tissue samples express mGluR1, whereas normal melanocytes are mGluR1 negative[77]. Using an inducible mouse model, the authors found that induction of mGluR1 expression led melanocytes growth into melanoma, while silencing mGluR1 resulted in tumor abrogation[77]. Another group also showed downregulation of mGluR1 by shRNA decreased cell viability of human melanoma cells in vitro and tumor growth in vivo in a xenograft model[78]. Contrarily, stimulation of mGluR1 resulted in activation of MAPK and PI3K-AKT pathways, which enhanced cell proliferation but prevented apoptosis[79]. Accordingly, mGluR1 has been targeted for development of anti-cancer agents especially in treatment of breast cancer and melanoma[75,80–81]. However, clinical mGluR antagonist is still lacking, although mGluR1 antagonist LY367385 and BAY36-7620 or a potential antagonist riluzole have exhibited some anti-cancer effects in vitro and in mice xenograft[75,80,82].
The mGluR2 and mGluR3 of Group Ⅱ receptors are coupled to Gαi/o and linked to the regulation of cyclic adenosine[64]. In malignant melanoma, mGluR3 was shown to be highly mutated and mutations in the seven transmembrane domain resulted in increased activation of MEK1/2 kinase with more aggressive migration and anchorage independence of melanoma cells in vitro and in vivo[83]. In a recent study from our lab, we unveiled that the mGluR3 was up-regulated in colon cancer cell lines in vitro and colon cancer tissues in vivo[84]. Further, inhibition of mGluR3 expression by shRNA or with antagonist LY341495 reduced anchorage dependent growth of colon cancer cells, and attenuated tumor growth in a xenograft model[84]. In the following study, we attempted to explore the possibility of using different mGluR3 antagonists and/or its signaling pathway inhibitor, combined with other anti-cancer reagents for the treatment of colon cancer and other malignancies[84].
Significantly, in a clinical study on a large cohort of renal cell carcinoma samples with none-cancer controls, the authors detected gene expression of mGluR3, mGluR4 and mGluR5 on cancers of all participated patients[85]. Analysis of different risk factors implied associations of mGluR3, mGluR4 and mGluR5 with tumorigenesis and overall survivals[85]. Specifically, the mGluR4 and mGluR5 gene expression showed an increasing trend in carcinogenetic tissues, compared to that in normal tissue; whereas mGluR3 gene was down regulated in renal cancer cells[85]. The increased expression of mGluR4 gene and decreased expression of mGluR3 gene were associated with poorer survival of patients with renal cell carcinoma. Although there was a slight trend towards higher mGluR5 expression levels in cancer tissues than in adjacent normal tissues, GRM5 expression was not associated with survival of patients.
EAATs and cancers
In the CNS, different glutamate transporters work together and maintain a low extracellular glutamate concentration at 0.3–1 μmol/L, ten to several ten thousands fold lower than intracellular concentration which, for example, is 3–10 mmol/L in neurons[86–87]. This gradient of glutamate is probably driven by the ionic gradients generated by ion-exchanging pumps such as Na+/K+-ATPase[86–87]. In general, glutamate is not able to diffuse across the cell membrane[14]. EAATs are also found in peripheral tissues; for instance, EAAT3, originally known as EAAC1, was reported to have mRNA expressed in the liver, the intestine, the kidney and the heart of rabbits[14]. Lately, a low level of EAAT1 mRNA was identified in the mouse liver, kidney and intestine, with relatively a little higher EAAT2 in the liver, much higher EAAT3 at intestine and highest EAAT3 in the kidney[88]. Moreover, broad expression of EAAT5 in the liver, kidney, intestine, heart, lung and skeletal muscle of rats has been identified at both mRNA and protein levels[89]. The similar data on humans is still lacking. Meanwhile cystine/glutamate exchanger SXC is known to express widely in many human tissues, including the brain, the pancreatic islets, and the stromal and immune cells, and involvement of SXC in human carcinogenesis has been documented[90–91]. The uptake of cystine is significant for cells to maintain the intracellular levels of glutathione especially for the tissues that generate high levels of oxidative stress as in tumors[91]. However, the more cystine that is taken up through SXC, the more intracellular glutamate will be exchanged into extracellular spaces, which may be toxic to excitable cells. Consistently, overexpression and/or over activity of SXC transporter play pivotal roles in the growth and invasion of glioma cells by markedly enhancing extracellular glutamate concentration[46,90].
Aberrant expression or activity of glutamate transporters is usually attributed to an imbalance of glutamate homeostasis in the CNS[92]. In brain tumors, EAAT2 is found down regulated in high-grade glial tumors compared with that in low-grade astrocytoma and normal brain[93]. Accordingly, EAAT2 expression is inversely correlated with tumor grade; re-expression of EAAT2 significantly prevents cell proliferation in several glioma cell lines[93]. In addition, lacking of EAAT1 is found in rat C6 glioma cell line, which increases releasing of glutamate to the medium[94]. In animal models, C6 clone cells release more glutamate and ensure more robust tumorigenesis comparing to cells that do not release glutamate[94]. In addition, SXC mutation is also involved in glioma development and migration[46,90–91]. Gliomas are aggressive cancers, whose growth is restricted by the bony cavity of the skull or spinal canal. Overexpression of SXC and/or deficiency of EAATs is able to help gliomas to overcome this physical limitation by secreting or gathering more glutamate, which kills neurons and vacates space for the growth of tumor cells[46,91]. Meanwhile, cystine uptake through this process sustains the synthesis of glutathione, and promotes glioma cells survival and growth[91]. In addition, choroid plexus tumors are rare neoplasms of neuroectodermal origin and their distinction from metastatic or secondary carcinomas is often difficult in adult cases[95]. A clinical study comparing patient primary tumor biopsies with metastatic samples found that EAAT1 expression in primary tumors was positively correlated to patient age but EAAT1 immunostaining in metastatic tumors was absent[96]. This finding indicates that EAAT1 immunohistochemistry may be useful in distinguishing original choroid plexus tumors from metastatic carcinomas.
Potential roles of EAATs and/or SXC have been suggested in some cancer types outside of the CNS, such as in pancreatic and gastric cancers, leukemias and lymphomas[15,91]. In a large clinical study of primary gastric cancers plus most established cancer cell lines, CD44-EAAT-2 gene fusions were detected in 1%–2% of primary tumors with zero expression in adjacent matched normal gastric tissues[97]. Further, silence of this fusion gene led to reduction of intracellular glutamate level and sensitized cells to chemotherapy[97]. The role of SXC in leukemias and lymphomas seems peculiar since these tumor cells are incapable of synthesizing cysteine, which makes cystine/cysteine taken up from the micro-environment become essential amino acid for the viability and progression of such cancers[98]. However, clinically available specific activator or inhibitor for EAATs and/or SXC, especially for those mutated up- or down-regulated expression in cancers is still lacking. The drugs that can block glutamate release, such as riluzole[99–100], has been used in therapeutic approach for many types of cancers.
Riluzole is a US FDA approved drug originally for treatment of amyotrophic lateral sclerosis and it has a low toxicity in normal condition. Riluzole has shown promising anti-tumor effect in glioma, melanoma, breast and prostate cancers, and osteosarcoma as well[72,80–81,101]. Combination of riluzole with the other glutamate antagonistic drugs, such as memantine or valproate, is reported to have better effects for glioma treatment[101–102].
Apart from it, a series of amino acid transporters, belonging to 4 distinct gene families: SLC1, SLC6, SLC7, and SLC38 have been reported to be involved in carcinogenesis of many different organs[103]. Bhutia and Ganapathy[103] has comprehensively reviewed the distribution of these transporters with their links to glutamate metabolism in different tissues, the involvement of their gene modification in carcinogenesis, and the potential roles of their gene and/or proteins therein.
Conclusions
As aforementioned, the metabolic phenotype of cells within and around cancers is heterogeneous and distinct from their normal counterparts[2,30]. In order to target the glutamate metabolic chain in cancer but not severely disturb their normal counterparts, further study to find a tumor specific step in a metabolic chain is indispensable. Various kinds of mutations may occur in the cancer metabolic network[19–21,104] and these mutations may create specific therapeutic target for cancer treatment. The involvement of glutamate receptor-guided cell signaling and/or transporters in cancer development are well documented (Fig. 2) but clinically available antagonists for treatment of cancer still remain unclear. Actually, a set of glutamatergic signaling inhibitors or glutamate transmembrane traveling blocker, like LY341495, Riluzole and so on, were developed for therapy of neuropsychiatric disorders. Paradoxically, many of those antagonists and/or inhibitors lack the ability to penetrate blood brain barriers, which makes them fairly problematic for treatment of neuropsychiatric disorders. However, this defect may make them a promising medicine for peripheral cancer treatment as there is no concern of the effects beyond the blood brain barrier. Notably, a metabolic imaging guided identification for "glutamine addicted" cancer cells has been suggested to distinguish glutamine dependent tumor cells from those less dependent ones. Since not all of cancers or all of cells in a diagnosed cancer are exactly glutamine dependent, more detailed identification for the extent of glutamine addiction is undoubtedly beneficial[105]. As 18fluoro-dexoyglucose could indicate glucose metabolic level, using UDP-linked nacetylglucosamine as positron could reflect the glutamine metabolic level in cancers or cancer cells[105]. As we discussed above, as a consequence of glutamine addition, glutamate metabolism might be elevated in some types of cancers, for example, enzalutamide-resistant prostate cancers and triple negative or heavily treated breast cancers[105]. However, there is still lack of evidence to show whether those types of cancers are more dependent on glutamate signaling for survival. But recent discoveries on glutamate signaling in brain as well as peripheral cancers shed light on the importance of glutamate in tumor development. Future research might help to build a clear connection between glutamate addiction and glutamate signaling in cancer. Consequently, anti-glutamate treatment for cancers would reach its maximum efficacy in cancers that are more glutamine dependent.
2. Glutamate signaling involved in tumorigenesis.
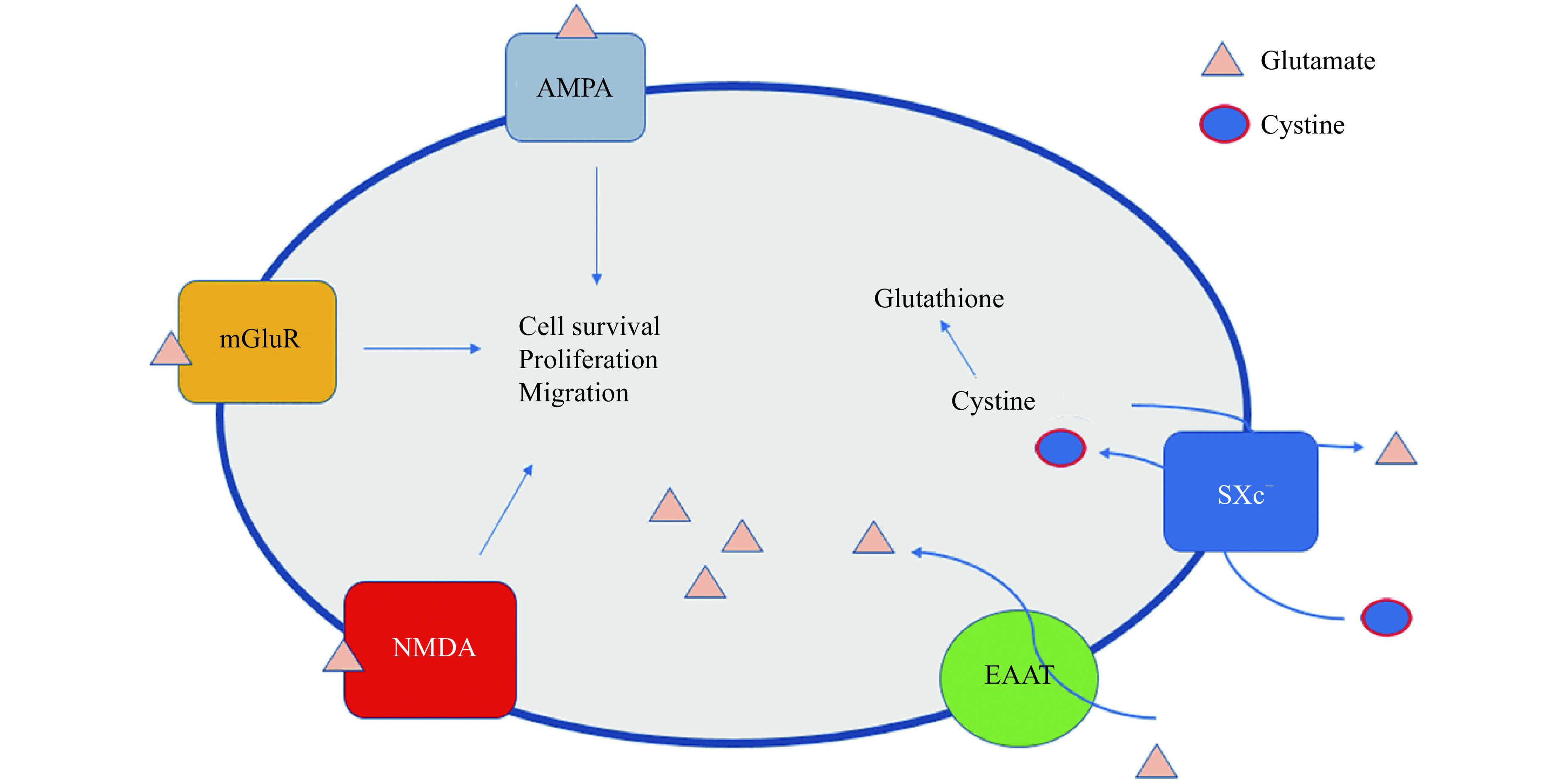
Extracellular glutamate binds to glutamate receptors (AMPA, mGluRs and NMDA) and activates signal pathways, such as MAPK, PI3K, to promote cell proliferation, survival and migration. Of note, EAATs transport extracellular glutamate into cells. Contrarily, Xc--glutamate antiporter exporting intracellular glutamate and importing extracellular cystine. Anabolism of cystine, L-glutamic acid and glycine produces glutathione that is a critical anti-oxidative element in cancer cells. AMPA: α-amino-3-hydroxy-5-methylisoxazole-4-proprionate; mGluR: metabotropic glutamate receptors; NMDA: N-methyl-D-aspartate; EAAT: excitatory amino acid transporters; SXc−: system Xc−.
Acknowledgments
This work was supported by NIH/NCI R01CA140988-01 to JW and partially supported by Chinese Scholar Council to HY.
Contributor Information
Haowei Yi, Email: roy250250@gmail.com.
Jing Wang, Email: jjwang@unmc.edu.
References
- 1.Koppenol WH, Bounds PL, Dang CV Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Outschoorn UE, Peiris-Pagés M, Pestell RG, et al Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14(1):11–31. doi: 10.1038/nrclinonc.2016.60. [DOI] [PubMed] [Google Scholar]
- 3.Medina MA, Sánchez-Jiménez F, Márquez J, et al Relevance of glutamine metabolism to tumor cell growth. Mol Cell Biochem. 1992;113(1):1–15. doi: 10.1007/BF00230880. [DOI] [PubMed] [Google Scholar]
- 4.Newsholme P, Procopio J, Lima MMR, et al Glutamine and glutamate—their central role in cell metabolism and function. Cell Biochem Funct. 2003;21(1):1–9. doi: 10.1002/cbf.1003. [DOI] [PubMed] [Google Scholar]
- 5.Ahluwalia GS, Grem JL, Zhang H, et al Metabolism and action of amino acid analog anti-cancer agents. Pharmacol Ther. 1990;46(2):243–271. doi: 10.1016/0163-7258(90)90094-I. [DOI] [PubMed] [Google Scholar]
- 6.Diehn M, Cho RW, Lobo NA, et al Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458(7239):780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bansal A, Simon MC Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol. 2018;217(7):2291–2298. doi: 10.1083/jcb.201804161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luksch H, Uckermann O, Stepulak A, et al Silencing of selected glutamate receptor subunits modulates cancer growth. Anticancer Res. 2011;31(10):3181–3192. [PubMed] [Google Scholar]
- 9.Stepulak A, Rola R, Polberg K, et al Glutamate and its receptors in cancer. J Neural Transm (Vienna) 2014;121(8):933–944. doi: 10.1007/s00702-014-1182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis DR, Phillis JW, Watkins JC Chemical excitation of spinal neurones. Nature. 1959;183(4661):611–612. doi: 10.1038/183611a0. [DOI] [PubMed] [Google Scholar]
- 11.Storm-Mathisen J, Leknes AK, Bore AT, et al First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature. 1983;301(5900):517–520. doi: 10.1038/301517a0. [DOI] [PubMed] [Google Scholar]
- 12.Willard SS, Koochekpour S Glutamate, glutamate receptors, and downstream signaling pathways. Int J Biol Sci. 2013;9(9):948–959. doi: 10.7150/ijbs.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traynelis SF, Wollmuth LP, McBain CJ, et al Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanai Y, Hediger MA Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360(6403):467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- 15.Lewerenz J, Hewett SJ, Huang Y, et al The cystine/glutamate antiporter system xc- in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal. 2013;18(5):522–555. doi: 10.1089/ars.2011.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du J, Li XH, Li YJ Glutamate in peripheral organs: biology and pharmacology. Eur J Pharmacol. 2016;784:42–48. doi: 10.1016/j.ejphar.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Yu JY, Li L, Huang CS SU6668 inhibits the proliferation and motility of colorectal cancer cells by inducing cycle arrest, and promotes their apoptosis. Transl Cancer Res. 2017;6(5):910–919. doi: 10.21037/tcr.2017.08.38. [DOI] [Google Scholar]
- 18.Vander Heiden MG, Cantley LC, Thompson CB Understanding the warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parsons DW, Jones S, Zhang XS, et al An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullen AR, Wheaton WW, Jin ES, et al Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2011;481(7381):385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao HX, Khalimonchuk O, Schraders M, et al SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma . Science. 2009;325(5944):1139–1142. doi: 10.1126/science.1175689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eagle H Nutrition needs of mammalian cells in tissue culture. Science. 1955;122(3168):501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- 23.Kovačević Z The pathway of glutamine and glutamate oxidation in isolated mitochondria from mammalian cells. Biochem J. 1971;125(3):757–763. doi: 10.1042/bj1250757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reitzer LJ, Wice BM, Kennell D Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254(8):2669–2676. [PubMed] [Google Scholar]
- 25.Lai HS, Lee JC, Lee PH, et al Plasma free amino acid profile in cancer patients. Semin Cancer Biol. 2005;15(4):267–276. doi: 10.1016/j.semcancer.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Koochekpour S, Majumdar S, Azabdaftari G, et al Serum glutamate levels correlate with Gleason score and glutamate blockade decreases proliferation, migration, and invasion and induces apoptosis in prostate cancer cells. Clin Cancer Res. 2012;18(21):5888–5901. doi: 10.1158/1078-0432.CCR-12-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herner A, Sauliunaite D, Michalski CW, et al Glutamate increases pancreatic cancer cell invasion and migration via AMPA receptor activation and Kras‐MAPK signaling . Int J Cancer. 2011;129(10):2349–2359. doi: 10.1002/ijc.25898. [DOI] [PubMed] [Google Scholar]
- 28.Budczies J, Pfitzner BM, Györffy B, et al Glutamate enrichment as new diagnostic opportunity in breast cancer. Int J Cancer. 2015;136(7):1619–1628. doi: 10.1002/ijc.29152. [DOI] [PubMed] [Google Scholar]
- 29.Shen J. Glutamate[M]//Stagg C, Rothman D. Magnetic Resonance Spectroscopy. San Diego: Academic Press, 2014: 111–121.
- 30.Wise DR, Thompson CB Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35(8):427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altman BJ, Stine ZE, Dang CV From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16(10):619–634. doi: 10.1038/nrc.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James MO, Smith RL, Williams RT, et al The conjugation of phenylacetic acid in man, sub-human primates and some non-primate species. Proc Roy Soc B: Biol Sci. 1972;182(1066):25–35. doi: 10.1098/rspb.1972.0064. [DOI] [PubMed] [Google Scholar]
- 33.Thibault A, Samid D, Cooper MR, et al Phase I study of phenylacetate administered twice daily to patients with cancer. Cancer. 1995;75(12):2932–2938. doi: 10.1002/1097-0142(19950615)75:12<2932::AID-CNCR2820751221>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 34.Chang SM, Kuhn JG, Robins HI, et al Phase II study of phenylacetate in patients with recurrent malignant glioma: a North American Brain Tumor Consortium report. J Clin Oncol. 1999;17(3):984–990. doi: 10.1200/JCO.1999.17.3.984. [DOI] [PubMed] [Google Scholar]
- 35.Curthoys NP, Watford M Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr. 1995;15:133–159. doi: 10.1146/annurev.nu.15.070195.001025. [DOI] [PubMed] [Google Scholar]
- 36.Gross MI, Demo SD, Dennison JB, et al Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther. 2014;13(4):890–901. doi: 10.1158/1535-7163.MCT-13-0870. [DOI] [PubMed] [Google Scholar]
- 37.Robinson MM, McBryant SJ, Tsukamoto T, et al Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES) Biochem J. 2007;406(3):407–414. doi: 10.1042/BJ20070039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacque N, Ronchetti AM, Larrue C, et al Targeting glutaminolysis has antileukemic activity in acute myeloid leukemia and synergizes with BCL-2 inhibition. Blood. 2015;126(11):1346–1356. doi: 10.1182/blood-2015-01-621870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furukawa H, Singh SK, Mancusso R, et al Subunit arrangement and function in NMDA receptors. Nature. 2005;438(7065):185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- 40.Paoletti P, Neyton J NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7(1):39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Skerry TM, Genever PG Glutamate signalling in non-neuronal tissues. Trends Pharmacol Sci. 2001;22(4):174–181. doi: 10.1016/S0165-6147(00)01642-4. [DOI] [PubMed] [Google Scholar]
- 42.Ribeiro MP, Custodio JBA, Santos AE Ionotropic glutamate receptor antagonists and cancer therapy: time to think out of the box? Cancer Chemother Pharmacol. 2017;79(2):219–225. doi: 10.1007/s00280-016-3129-0. [DOI] [PubMed] [Google Scholar]
- 43.Huang YF, Kang BN, Tian J, et al The cationic amino acid transporters CAT1 and CAT3 mediate NMDA receptor activation-dependent changes in elaboration of neuronal processes via the mammalian target of rapamycin mTOR pathway . J Neurosci. 2007;27(3):449–458. doi: 10.1523/JNEUROSCI.4489-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burket JA, Benson AD, Tang AH, et al NMDA receptor activation regulates sociability by its effect on mTOR signaling activity. Prog Neuropsychopharmacol Biol Psychiatry. 2015;60:60–65. doi: 10.1016/j.pnpbp.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deutsch SI, Tang AH, Burket JA, et al NMDA receptors on the surface of cancer cells: target for chemotherapy? Biomed Pharmacother. 2014;68(4):493–496. doi: 10.1016/j.biopha.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyons SA, Chung WJ, Weaver AK, et al Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res. 2007;67(19):9463–9471. doi: 10.1158/0008-5472.CAN-07-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brocke KS, Staufner C, Luksch H, et al Glutamate receptors in pediatric tumors of the central nervous system. Cancer Biol Ther. 2010;9(6):455–468. doi: 10.4161/cbt.9.6.10898. [DOI] [PubMed] [Google Scholar]
- 48.Beretta F, Bassani S, Binda E, et al The GluR2 subunit inhibits proliferation by inactivating Src-MAPK signalling and induces apoptosis by means of caspase 3/6-dependent activation in glioma cells. Eur J Neurosci. 2009;30(1):25–34. doi: 10.1111/j.1460-9568.2009.06804.x. [DOI] [PubMed] [Google Scholar]
- 49.Jonas P, Racca C, Sakmann B, et al Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression . Neuron. 1994;12(6):1281–1289. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 50.Uhm JH, Gladson CL, Rao JS The role of integrins in the malignant phenotype of gliomas. Front Biosci. 1999;4:D188–D199. doi: 10.2741/A421. [DOI] [PubMed] [Google Scholar]
- 51.Rzeski W, Turski L, Ikonomidou C Glutamate antagonists limit tumor growth. Proc Natl Acad Sci USA. 2001;98(11):6372–6377. doi: 10.1073/pnas.091113598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stepulak A, Luksch H, Gebhardt C, et al Expression of glutamate receptor subunits in human cancers. Histochem Cell Biol. 2009;132(4):435–445. doi: 10.1007/s00418-009-0613-1. [DOI] [PubMed] [Google Scholar]
- 53.Stepulak A, Luksch H, Uckermann O, et al Glutamate receptors in laryngeal cancer cells. Anticancer Res. 2011;31(2):565–573. [PubMed] [Google Scholar]
- 54.Watanabe K, Kanno T, Oshima T, et al The NMDA receptor NR2A subunit regulates proliferation of MKN45 human gastric cancer cells. Biochem Biophys Res Commun. 2008;367(2):487–490. doi: 10.1016/j.bbrc.2007.12.167. [DOI] [PubMed] [Google Scholar]
- 55.Kalariti N, Lembessis P, Koutsilieris M Characterization of the glutametergic system in MG-63 osteoblast-like osteosarcoma cells. Anticancer Res. 2004;24(6):3923–3929. [PubMed] [Google Scholar]
- 56.Abdul M, Hoosein N N-methyl-D-aspartate receptor in human prostate cancer. J Membr Biol. 2005;205(3):125–128. doi: 10.1007/s00232-005-0777-0. [DOI] [PubMed] [Google Scholar]
- 57.North WG, Gao GH, Memoli VA, et al Breast cancer expresses functional NMDA receptors. Breast Cancer Res Treat. 2010;122(2):307–314. doi: 10.1007/s10549-009-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.North WG, Gao GH, Jensen A, et al NMDA receptors are expressed by small-cell lung cancer and are potential targets for effective treatment. Clin Pharmacol. 2010;2:31–40. doi: 10.2147/CPAA.S6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song ZQ, He CD, Liu J, et al Blocking glutamate-mediated signalling inhibits human melanoma growth and migration. Exp Dermatol. 2012;21(12):926–931. doi: 10.1111/exd.12048. [DOI] [PubMed] [Google Scholar]
- 60.Yamaguchi F, Hirata Y, Akram H, et al FOXO/TXNIP pathway is involved in the suppression of hepatocellular carcinoma growth by glutamate antagonist MK-801. BMC Cancer. 2013;13:468. doi: 10.1186/1471-2407-13-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruiz DS, Luksch H, Sifringer M, et al AMPA receptor antagonist CFM-2 decreases survivin expression in cancer cells. Anticancer Agents Med Chem. 2018;18(4):591–596. doi: 10.2174/1871520618666180228123406. [DOI] [PubMed] [Google Scholar]
- 62.Grossman SA, Ye XB, Chamberlain M, et al Talampanel with standard radiation and temozolomide in patients with newly diagnosed glioblastoma: a multicenter phase II trial. J Clin Oncol. 2009;27(25):4155–4161. doi: 10.1200/JCO.2008.21.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iwamoto FM, Kreisl TN, Kim L, et al Phase 2 trial of talampanel, a glutamate receptor inhibitor, for adults with recurrent malignant gliomas. Cancer. 2010;116(7):1776–1782. doi: 10.1002/cncr.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Julio-Pieper M, Flor PJ, Dinan TG, et al Exciting times beyond the brain: metabotropic glutamate receptors in peripheral and non-neural tissues. Pharmacol Rev. 2011;63(1):35–58. doi: 10.1124/pr.110.004036. [DOI] [PubMed] [Google Scholar]
- 65.Nieto Gutierrez A, McDonald PH GPCRs: emerging anti-cancer drug targets. Cell Signal. 2018;41:65–74. doi: 10.1016/j.cellsig.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 66.Ribeiro FM, Vieira LB, Pires RGW, et al Metabotropic glutamate receptors and neurodegenerative diseases. Pharmacol Res. 2017;115:179–191. doi: 10.1016/j.phrs.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 67.Spampinato SF, Copani A, Nicoletti F, et al Metabotropic glutamate receptors in glial cells: a new potential target for neuroprotection? Front Mol Neurosci. 2018;11:414. doi: 10.3389/fnmol.2018.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pereira MSL, Klamt F, Thomé CC, et al Metabotropic glutamate receptors as a new therapeutic target for malignant gliomas. Oncotarget. 2017;8(13):22279–22298. doi: 10.18632/oncotarget.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ciceroni C, Arcella A, Mosillo P, et al Type-3 metabotropic glutamate receptors negatively modulate bone morphogenetic protein receptor signaling and support the tumourigenic potential of glioma-initiating cells. Neuropharmacology. 2008;55(4):568–576. doi: 10.1016/j.neuropharm.2008.06.064. [DOI] [PubMed] [Google Scholar]
- 70.Ciceroni C, Bonelli M, Mastrantoni E, et al Type-3 metabotropic glutamate receptors regulate chemoresistance in glioma stem cells, and their levels are inversely related to survival in patients with malignant gliomas. Cell Death Differ. 2013;20(3):396–407. doi: 10.1038/cdd.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang HJ, Yoo BC, Lim SB, et al Metabotropic glutamate receptor 4 expression in colorectal carcinoma and its prognostic significance. Clin Cancer Res. 2005;11(9):3288–3295. doi: 10.1158/1078-0432.CCR-04-1912. [DOI] [PubMed] [Google Scholar]
- 72.Liao S, Ruiz Y, Gulzar H, et al Osteosarcoma cell proliferation and survival requires mGluR5 receptor activity and is blocked by Riluzole. PLoS One. 2017;12(2):e0171256. doi: 10.1371/journal.pone.0171256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shourideh M, Azabdaftari G, Attwood K, et al. GRM1 is an androgen-regulated gene and its expression correlates with prostate cancer progression in pre-clinical models[J]. Clin Cancer Res, 2016.
- 74.Zhang ZC, Liu YF, Wang K, et al Activation of type 4 metabotropic glutamate receptor promotes cell apoptosis and inhibits proliferation in bladder cancer. J Cell Physiol. 2019;234(3):2741–2755. doi: 10.1002/jcp.27089. [DOI] [PubMed] [Google Scholar]
- 75.Speyer CL, Smith JS, Banda M, et al Metabotropic glutamate receptor-1: a potential therapeutic target for the treatment of breast cancer. Breast Cancer Res Treat. 2012;132(2):565–573. doi: 10.1007/s10549-011-1624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Banda M, Speyer CL, Semma SN, et al Metabotropic glutamate receptor-1 contributes to progression in triple negative breast cancer. PLoS One. 2014;9(1):e81126. doi: 10.1371/journal.pone.0081126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohtani Y, Harada T, Funasaka Y, et al Metabotropic glutamate receptor subtype-1 is essential for in vivo growth of melanoma . Oncogene. 2008;27(57):7162–7170. doi: 10.1038/onc.2008.329. [DOI] [PubMed] [Google Scholar]
- 78.Gelb T, Pshenichkin S, Rodriguez OC, et al Metabotropic glutamate receptor 1 acts as a dependence receptor creating a requirement for glutamate to sustain the viability and growth of human melanomas. Oncogene. 2015;34(21):2711–2720. doi: 10.1038/onc.2014.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu LJ, Wall BA, Wangari-Talbot J, et al Metabotropic glutamate receptors in cancer. Neuropharmacology. 2017;115:193–202. doi: 10.1016/j.neuropharm.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dolfi SC, Medina DJ, Kareddula A, et al Riluzole exerts distinct antitumor effects from a metabotropic glutamate receptor 1-specific inhibitor on breast cancer cells. Oncotarget. 2017;8(27):44639–44653. doi: 10.18632/oncotarget.17961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mehnert JM, Silk AW, Lee JH, et al A phase II trial of riluzole, an antagonist of metabotropic glutamate receptor 1 (GRM1) signaling, in patients with advanced melanoma. Pigment Cell Melanoma Res. 2018;31(4):534–540. doi: 10.1111/pcmr.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Namkoong J, Shin SS, Lee HJ, et al Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer Res. 2007;67(5):2298–2305. doi: 10.1158/0008-5472.CAN-06-3665. [DOI] [PubMed] [Google Scholar]
- 83.Prickett TD, Wei XM, Cardenas-Navia I, et al Exon capture analysis of G protein-coupled receptors identifies activating mutations in GRM3 in melanoma . Nat Genet. 2011;43(11):1119–1126. doi: 10.1038/ng.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yi H, Geng L, Black A, et al The miR-487b-3p/GRM3/TGFβ signaling axis is an important regulator of colon cancer tumorigenesis. Oncogene. 2017;36(24):3477–3489. doi: 10.1038/onc.2016.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang CY, Hsueh YM, Chen LC, et al Clinical significance of glutamate metabotropic receptors in renal cell carcinoma risk and survival. Cancer Med. 2018;7(12):6104–6111. doi: 10.1002/cam4.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robert SM, Sontheimer H Glutamate transporters in the biology of malignant gliomas. Cell Mol Life Sci. 2014;71(10):1839–1854. doi: 10.1007/s00018-013-1521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Danbolt, N C Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/S0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 88.Hu QX, Ottestad-Hansen S, Holmseth S, et al Expression of glutamate transporters in mouse liver, kidney, and intestine. J Histochem Cytochem. 2018;66(3):189–202. doi: 10.1369/0022155417749828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee A, Anderson AR, Stevens M, et al Excitatory amino acid transporter 5 is widely expressed in peripheral tissues. Eur J Histochem. 2013;57(1):e11. doi: 10.4081/ejh.2013.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bridges RJ, Natale NR, Patel SA System xc− cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS . Br J Pharmacol. 2012;165(1):20–34. doi: 10.1111/j.1476-5381.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lo M, Wang YZ, Gout PW The xc− cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases . J Cell Physiol. 2008;215(3):593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- 92.Takahashi M, Billups B, Rossi D, et al The role of glutamate transporters in glutamate homeostasis in the brain. J Exp Biol. 1997;200(2):401–409. doi: 10.1242/jeb.200.2.401. [DOI] [PubMed] [Google Scholar]
- 93.De Groot JF, Liu TJ, Fuller G, et al The excitatory amino acid transporter-2 induces apoptosis and decreases glioma growth in vitro and in vivo . Cancer Res. 2005;65(5):1934–1940. doi: 10.1158/0008-5472.CAN-04-3626. [DOI] [PubMed] [Google Scholar]
- 94.Takano T, Lin JH, Arcuino G, et al Glutamate release promotes growth of malignant gliomas. Nat Med. 2001;7(9):1010–1015. doi: 10.1038/nm0901-1010. [DOI] [PubMed] [Google Scholar]
- 95.Gyure KA, Morrison AL Cytokeratin 7 and 20 expression in choroid plexus tumors: utility in differentiating these neoplasms from metastatic carcinomas. Mod Pathol. 2000;13(6):638–643. doi: 10.1038/modpathol.3880111. [DOI] [PubMed] [Google Scholar]
- 96.Beschorner R, Schittenhelm J, Schimmel H, et al Choroid plexus tumors differ from metastatic carcinomas by expression of the excitatory amino acid transporter-1. Hum Pathol. 2006;37(7):854–860. doi: 10.1016/j.humpath.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 97.Tao J, Deng NT, Ramnarayanan K, et al CD44-SLC1A2 gene fusions in gastric cancer . Sci Transl Med. 2011;3(77):77ra30. doi: 10.1126/scitranslmed.3001423. [DOI] [PubMed] [Google Scholar]
- 98.Angelini G, Gardella S, Ardy M, et al Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci USA. 2002;99(3):1491–1496. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chéramy A, Barbeito L, Godeheu G, et al Riluzole inhibits the release of glutamate in the caudate nucleus of the cat in vivo . Neurosci Lett. 1992;147(2):209–212. doi: 10.1016/0304-3940(92)90597-Z. [DOI] [PubMed] [Google Scholar]
- 100.Jehle T, Bauer J, Blauth E, et al Effects of riluzole on electrically evoked neurotransmitter release. Br J Pharmacol. 2000;130(6):1227–1234. doi: 10.1038/sj.bjp.0703424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sachkova A, Sperling S, Mielke D, et al Combined applications of repurposed drugs and their detrimental effects on glioblastoma cells. Anticancer Res. 2019;39(1):207–214. doi: 10.21873/anticanres.13099. [DOI] [PubMed] [Google Scholar]
- 102.Yohay K, Tyler B, Weaver KD, et al Efficacy of local polymer-based and systemic delivery of the anti-glutamatergic agents riluzole and memantine in rat glioma models. J Neurosurg. 2014;120(4):854–863. doi: 10.3171/2013.12.JNS13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhutia YD, Ganapathy V Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim Biophys Acta. 2016;1863(10):2531–2539. doi: 10.1016/j.bbamcr.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Astuti D, Latif F, Dallol A, et al Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69(1):49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rajagopalan KN, DeBerardinis RJ Role of glutamine in cancer: therapeutic and imaging implications. J Nucl Med. 2011;52(7):1005–1008. doi: 10.2967/jnumed.110.084244. [DOI] [PMC free article] [PubMed] [Google Scholar]


