Summary
TH17 is a newly identified pathogenic memory CD4+ T-cell lineage with potent agonist effects in some murine experimental autoimmunity models, however, its role in tumor immunology is still unclear. Clinical experience with interleukin (IL)-2 and ipilumumab [anti-cytotoxic T-lymphocyte antigen 4 (CTLA4) antibody], particularly in treating immunogenic malignancies such as melanoma and renal cell carcinoma (RCC), has suggested an association between a variety of autoimmune phenomena and tumor regression. To investigate this issue in patients with RCC, we isolated T-cell clones from peripheral blood that released IL-17 on stimulation with their autologous RCC tumor line. Clones were generated from 1 patient before any systemic treatment by in vitro stimulation with dendritic cells, autologous tumor, and IL-2 in the presence of anti-CTLA4 antibody. That patient subsequently received treatment with ipilimumab and showed both objective tumor regression and immune-mediated colitis. Limiting dilution cloning of his tumordendritic cell-stimulated T cells produced the 3G8D CD4+ clone, which secreted both IL-17 and interferon-g when cocultured with the autologous RCC line (transduced with class II transactivation molecule to induce major histocompatibility complex-class II expression). Broader analysis of its cytokine secretion profile showed large amounts of IL-8 when cocultured with RCC, but not when triggered with phorbol 12-myristate 13-acetate and ionomycin. This led to the discovery that IL-8 was being produced by the RCC cells in response to T-cell–derived IL-17. This effect of exogenous IL-17 on IL-8 release from tumor was seen in 5 of 8 RCCs, but not in other tumors tested. Preliminary data on the frequency of IL-17–secreting T cells in the lymphocytes infiltrating RCCs suggest that there may be a positive correlation between this frequency and IL-8 production by nonlymphoid cells as determined by quantitative reverse transcription-polymerase chain reaction. This report extends the known bidirectional interactions between immune cells and malignant cells in the tumor microenvironment that can shape and modulate the host immune response to cancer.
Keywords: renal cell carcinoma, IL-17, IL-8, microenvironment
TH17 is a recently identified CD4+ T-cell lineage, which has been shown to have a key exacerbating role in various murine experimental autoimmunity models such as experimental autoimmune encephalomyelitis and collagen-induced arthritis (CIA).1 In these models, experimental autoimmune encephalomyelitis and CIA did not occur in mice deficient in the p19 or p40 subunits of interleukin (IL)-23, and were exacerbated in mice deficient in p35 of IL-12.2,3 IL-23 is central to the development of the TH17 lineage and IL-12 commits TH0 cells to the TH1 lineage, which diverts them from TH17 differentiation. There was a positive correlation between the availability of IL-23 and the development of IL-17–producing effector CD4+ T cells.4 More directly, generation of CIA was impaired in IL-17 deficient mice,5 and neutralization of IL-17 decreased disease severity.6 In humans, there were reports showing the infiltration of IL-17–producing T cells in various inflammatory conditions such as rheumatoid arthritis,7,8 inflammatory bowel disease,9–11 and psoriasis vulgaris.12 A recent publication of particular interest is a murine model using the adoptive transfer of T-cell receptor (TCR) transgenic CD4+ cells to treat the B16 melanoma which showed that CD4 cells directed toward the TH17 lineage were more effective in inducing tumor regression and also induced autoimmunity by attacking antigenpositive cells in the eye.13
However, in humans, the biologic significance of IL-17–producing T cells in autoimmunity and tumor immunology is still unclear. Many publications have described associations between immunotherapy-induced cancer regression with IL-2 or ipilimumab and a variety of immune-mediated adverse events such as colitis, hypophysitis, thyroiditis, and vitiligo.14–16 However, the mechanisms responsible for these phenomena are largely unknown. Human renal cell carcinoma (RCC) is known to be a cytokine-secreting tumor producing IL-6, granulocyte-macrophage colony-stimulating factor, and other cytokines. Yet again, the factors, which control these biologic activities and their clinical or immunologic significance often remain unclear. In this report, we chose to screen for T cells in patients with RCC, which produced IL-17 in response to autologous tumor stimulation. T-cell clones were established from the peripheral blood mononuclear cell (PBMC) of an RCC patient in whom tumor regression and autoimmune-toxicities were observed during treatment with anti-CTLA4 antibody. Characterization of this lymphocyte-tumor interaction identified what seems to be a bidirectional interaction demonstrated in multiple renal cancer lines, which produces cytokines from both T cells and cancer cells, and may shape immune cell infiltration and function in tumors.
MATERIALS AND METHODS
Tumor Cell Lines
Melanoma and renal cancer cell lines from primary surgical specimens were established as described.17 Briefly, surgically resected tumors were enzymatically digested with 0.1% collagenase type IV, 0.01% hyaluronidase type V, and 30U/mL deoxyribonuclease type IV (Sigma Chemical Co, St Louis, MO) in RPMI 1640 (Life Technologies, Gaithersburg, MD) at room temperature for overnight. After the filtration and separation by density gradient, 1107 cells were cultured in T75-cm2 flasks in Dulbecco Modified Eagle medium (DMEM; Life Technologies), including 10% fetal bovine serum (FBS; Life Technologies), 100U/mL penicillin, 100μg/mL streptomycin, 50μg/mL gentamicin, and 0.5μg/mL amphotricin B (Fungizone; Biofluids, Camarillo, CA). Renal cancer lines were supplemented additionally with 10% tryptose phosphate (Sigma), 10μg/mL insulin, 5.5μg/mL transferring, and 6.7ng/mL selenium (Life Technologies). Tumors were passaged at approximately 80% to 90% confluence and used when free of fibroblasts and proliferating beyond passage 10%. To introduce human leukocyte antigen (HLA)-DRβ3*01 into non-HLA-DRβ3*01–expressing tumor lines, the HLA-DRβ3*01 gene was cloned by reverse transcription-polymerase chain reaction (RT-PCR) from an Epstein-Barr virus (EBV) B-cell line derived from the patient who generated the T-cell clones. The gene was sequenced and subcloned into the retroviral vector pQCXIN (Clontech, Mountain View, CA). Vesicular stomatitis virus G protein—pseudotyped retrovirus was prepared by transiently transfecting the 293 GP cell line as described.18 Non–HLA-DRβ3*01 tumor cell lines stably transfected with the class II transactivation molecule (CIITA) were infected with filtered cell-free viral supernatant in the presence of 8μg/mL polybrene and subsequently maintained in selection with 1mg/mL G418 (Invitrogen, Carlsbad, CA). EBV transformed B cells (EBV-B cells) were established as follows: 107 PBMCs were added to an upright T25 flask with 5mL of supernatant from the EBV-producing cell line B95.9 and cyclosporine A (0.5μg/mL; Sandoz, Princeton, NJ). Established lines were obtained approximately 3 to 4 weeks after infection. All other cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD).
Antibodies
For antibody blocking experiments, W6/32 (anti-HLA class I), and HB55 (anti-HLA DR) were kind gifts from Dr Paul Robbins (National Cancer Institute). Anti–IL-17A (eBio 64CAP17) and isotype control antibodies were purchased from eBioscience (San Diego, CA). For T-cell clone analysis, CD3-fluorescein isothiocyanate (FITC), CD4-phycoerythrin (PE), and isotype controls including immunoglobulin G1 and immunoglobulin G2a were purchased from BD pharmingen (San Jose, CA). Anti-interferon (IFN)- γ (clone B27)-Allophycocyanin and isotype control antibody were purchased from BD pharmingen (San Jose). Anti–IL-17A (clone eBio64DEC17)-PE and isotype control were purchased from eBioscience (San Diego).
Establishment of T-cell Clones
PBMCs were obtained by apheresis from a patient with metastatic RCC before any systemic therapy. CD14+ cells were isolated from patient PBMC using CD14 microbeads according to product instructions (Miltenyi Biotec, Auburn, CA). Monocyte-derived dendritic cells (DC) were generated by plating CD14+ cells in 6-well plates at concentrations of 5105/mL in RPMI 1640 (Life Technologies), supplemented with 10% human serum (HS; Valley Biomedical, Winchester, VA), IL-4 (1000U/mL; Peprotech, Rocky Hill, NJ), and granulocyte-macrophage colony-stimulating factor (1000U/mL; Peprotech) for 6 days. Day 6 DC were harvested by gentle agitation and resuspended in RPMI 1640+10% HS at a concentration of 5104/mL, and 50μL/well was then cocultured overnight in U-bottom plates with an equivalent number and volume of apoptotic autologous tumor cells, induced by ultraviolet B irradiation (312mm; Spectroline, Westbury, NY) for 5 minutes (256mJ/cm2).7 On day 7, T cells were isolated by negative selection using a pan-T-cell isolation kit (Miltenyi). Enriched T cells were then added to the DC-tumor coculture at a concentration of 2.5105/mL (100mL/well) in RPMI 1640 with 10% HS, IL-2 (120IU/mL; Novartis, East Hanover, NJ) and CD40L (500ng/mL; Immunex, Seattle, WA). Half of the cultures also contained anti-CTLA4 antibody (ipilimumab) at 0.1mg/mL and this was replenished with each change of media. IL-2 was replenished every 3 to 4 days as indicated by growth and visual pH monitoring. On day 7, T cells were restimulated once by transferring them to a second identically prepared DC-tumor coculture. Two weeks after restimulation, reactivity of each well of 96-well plate cultures was tested by IL-17A and IFN-g production when cocultured with autologous tumor cells transduced with CIITA or autologous RCC/ CIITA preincubated with the major histocompatibility complex (MHC) class I and class II blocking antibodies W6/32 and HB55 (10μg/mL). Criteria for a positive microwell were an IL-17A concentration of at least 100pg/mL and twice the value of the coculture with autologous RCC/CIITA with or without W6/32 and HB55.
T-cell clones were derived from positive microwell cultures by limiting dilution as described previously with some modifications.19 In brief, 1, 3, or 10 T cells/well were cultured with 5104 irradiated PBMCs (3000 to 4000cGy) from 3 allogeneic donors in round-bottom 96-well plates. Cells were cultured in RPMI 1640+10% HS+IL-2 (300IU/mL) +anti-CD3 (30ng/mL OKT-3, Ortho-McNeil Phamaceuticals, Raritan, NJ). Growing clones were selected visually, and half of each limiting dilution culture was tested for recognition of autologous RCC/CIITA tumor cells 14 days after stimulation. Tumor reactive T-cell clones were further expanded by placing the remainder in a T25 flask with 2.5107 irradiated PBMCs from 3 allogenic donors in 25mL of RPMI 1640+10% HS+OKT3 (30ng/mL). IL-2 (300IU/mL) was added the next day. Fresh RPMI 1640+10% HS+IL-2 (300IU/mL) were replenished on day 5. Reactivity of the expanded cells was tested on day 12 by IFN-γ and IL-17A production.
Clonality of expanded T cells was verified by TCRβ-chain V region (TRBV) analysis based on the 5′RACE method.20 Briefly, a primer that was complementary to the TCRb-chain C region, 5′-CTCTTGACCATGGCCATC-3′ was used with the SMART RACE cDNA Amplification kit (BD bioscience, Palo Alto, CA) to amplify the TRBV region sequences. Amplified products were subcloned into pCR4-TOPO vector (Invitrogen, Carlsbad, CA) and sequenced.
Flow Cytometric Analysis
Cells were resuspended in staining buffer (phosphatebuffered saline containing 3% FBS) and stained with fluorochrome-conjugated antibodies specific for human antigens. Stained cells were subsequently washed in staining buffer twice and briefly stained with propidium iodide for nonviable cell exclusion before analyzing in a FACSCaliber (Becton Dickinson, San Jose, CA). Intracellular staining of T-cell clones and cryo-preserved enzymatically-dispersed fresh RCC tissues to detect IFN-γ and IL-17A production were performed by using Cytofix/Cytoperm Kit (eBioscience) according to product instruction. Before fixation and staining, T-cell clones were stimulated with phorbol 12-myristate 13-acetate (PMA) (10 ng/mL) in combination with ionomycin (2.2 mmol/mL) for 4 hours, in the presence of blefeldin A (Golgiplug; BD Bioscience). Single cell suspensions of cryo-preserved tumor digests were thawed and stimulated by PMA/ionomycin in RPMI 1640 containing 10% FBS and 30 U/mL deoxyribonuclease type IV for 4 hour, in the presence of blefeldin A.
T-cell Recognition Assays and IL-8 Release Assays
Cultured tumor lines (5 to 10 104 cells/well) were used as target cells. Cloned T cells at 5 104 cells/well were added to tumor in a final volume of 200 μL and incubated for 20 hours. Supernatant was assayed by enzyme-linked immunosorbent assay for IFN-γ (Endogen, Woburn, MA) and IL-17A (eBioscience). For IL-8 release assay from RCC cell lines stimulated with T-cell clones or exogenous IL-17A, tumor cell lines (5 104/well) were plated in flatbottom 96-well plates and cultured in Dulbecco Modified Eagle medium containing 10% FBS, 10% on day 0. Next day, recombinant human IL-17A (50 ng/mL; eBioscience) or T cells (5 104/well) were added. In some experiment, anti–IL-17A (eBioscience) was added at the concentration of 10 μg/mL. After the 24-hour incubation, culture supernatants were collected, and IL-8 concentration were measured by enzyme-linked immunosorbent assay (Endogen, Woburn, MA).
Quantitative RT-PCR
Fresh tumor digests were thawed, and cells separated into CD3+ and CD3 populations using CD3-FITC and FITC microbeads (Miltenyi Biotec.) according to manufacture’s instructions. In brief, 107 single cell suspensions were suspended in 100 mL of cold MACS buffer (phosphatebuffered saline +0.5% bovine serum albumin +2mM ethylenediaminetetra-acetate) and incubated with 10 μL of FITC-conjugated anti-CD3 antibody on ice for 10 minutes. Then labeled cells were washed with 2 mL of cold MACS buffer twice and resuspended in 100 μL of cold MACS buffer. Cells were incubated with 10 μL of FITC microbeads on ice for 15 minutes. After washing, cells were loaded onto a preequilibrated MS column attached to a magnet (Miltenyi Biotec). Flow-through was collected as the CD3 population. After washing the column for 3 times with MACS buffer, the column was separated from the magnet. MACS buffer (3 mL) was then added to the column, and CD3+ cells were expelled from the column with a plunger. Total RNA from CD3 and CD3+ population of fresh tumor digests were prepared by using RNeasy mini kit (Qiagen, Valencia, CA). First-strand cDNA was synthesized by Superscript Preamplification Systems (Life Technologies Inc) using 1 mg of total RNA from either CD3 and CD3+ population of fresh tumor digests.
Quantitative analysis of IL-8 mRNA expression was performed using the ABI prism 7700 Sequence Detection Systems (Perkin-Elmer, Foster City, CA) as described.21 Thermal cycler parameters were: 2 minutes at 501C, 10 minutes at 95°C, and 40 cycles of denaturation at 95°C for 15 seconds and annealing/extension at 60°C for 1 minute. Samples were normalized for the number of copies of β-actin mRNA. For IL-8 mRNA expression analysis, TaqMan gene expression assays (Hs00174103_m1; Applied Biosystems, Foster City, CA) were used. The primers and probes for β-actin were: forward primer, 5′-GGCACCCA GCACAATGAAG-3′; reverse primer, 5′-GCCGATCCAC ACGGAGTACT-3′; and TaqMan probe, 5′−6FAM-TCAA GATCATTGCTCCTCCTGAGCGC-TAMRA-3′.
RESULTS
Clinical History
A 55-year-old man presented with a 7cm right renal primary clear cell cancer with biopsy-documented metastases to the pleura and lungs. He under went nephrectomy and pleurodesis. He was considered a poor candidate for IL-2 owing to obesity and poor respiratory function and received anti-CTLA4 antibody (ipilimumab; Medarex Corporation, Princeton, NJ) at 3mg/kg every 3 weeks for 3 doses. He then developed diarrhea and colonoscopy with biopsies showed acute and chronic colitis. Ipilimumab was stopped and the patient was treated with several weeks of high-dose dexamethasone and gastrointestinal symptoms resolved. Computed tomography scans at 6 and 12 weeks after starting ipilimumab showed a near-complete resolution of all metastatic disease. This was sustained for approximately a year until a new brain metastasis was found. He subsequently showed progression at other noncentral nervous system sites and was referred for other treatment. PBMCs were procured by pheresis before beginning ipilimumab therapy and a tumor line was established from his primary tumor.
CD4+ IL-17A Producing RCC-reactive T Cells Generated From PBMC
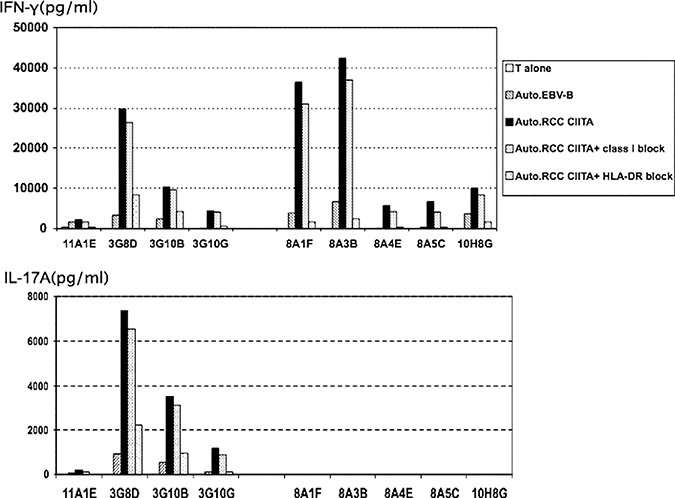
A CD3-enriched population of the patient’s PBMC was twice stimulated in vitro with his DC and autologous tumor, in the presence of CD40L and IL-2 and with or without anti-CTLA4 antibody. Individual wells were screened for bulk activity and reactive wells were cloned by limiting dilution. Cytokine release assays for activity compared reactivity against autologous RCC/CIITA and autologous RCC/CIITA preblocked with W6/32 (antipan HLA class I) and HB55 (anti-HLA DR). Of 96 wells generated without anti-CTLA4, 9 wells were positive for release of both IL-17A and IFN-γ, 0 for only IL-17A, and 87 for only IFN-γ. Of 96 microcultures generated with anti-CTLA4 antibody, 1 well was positive for release of both IL-17A and IFN-γ, 0 for only IL-17A, and 95 for only IFN-γ. In this and other experiments, the addition of anti-CTLA4 antibody in culture medium did not seem to have a significant impact on the induction of tumor reactive T cells. Two representative wells that secreted IFN-γ alone and 2 that secreted IFN-γ and IL-17A were cloned by limiting dilution with cloning efficiencies of approximately 10%. Four out of 80 clones derived from IL-17A and IFN-γ–producing microcultures produced IL-17A and IFN-γ on stimulation with autologous RCC/CIITA. Five clones were obtained from IFN-γ–secreting bulk wells and all still secreted IFN-γ and not IL-17A after cloning. The clones were expanded for further characterization. All were CD4+ and blocked by anti–HLA-DR antibody but not anti-MHC class I antibody (Fig. 1). In the control experiment, anti–HLA-DR antibody did not affect the recognition of 624mel (HLA-A*0201+ MART-1+melanoma cell line) by JKF6 (HLA-A*0201 restricted, MART-1 recognizing CD8+ T-cell clone), confirming that blocking was MHC class II specific (data not shown). Reactivities of these clones to autologous EBV-B cells were minimal. One IL-17 plus IFN-g–producing T-cell clone (3G8D) and 1 T-cell clone that made only IFN-g (8A1F) were expanded for further characterization.
FIGURE 1.
Cytokine secretion of representative T-cell clones is shown. Autologous Epstein-Barr virus (EBV) transformed B cells or renal cell carcinoma (RCC)/class II transactivation molecule (CIITA) cells were plated in a 96-well plate, and some blocked with anti–HLA-class I or anti–HLA-DR antibodies. An equal number of clonal T cells was added and incubated overnight. Supernatant was harvested and tested for IFN-g secretion (upper panel) and interleukin (IL)-17A (lower panel). 11A1E, 3G8D, 3G10B, and 3G10G are IL-17A–producing clones derived from autologous RCC/CIITA reactive microcultures with IL-17A production. 8A1F, 8A3B, 8A4E, 8A5C, and 10H8G are control clones derived from autologous RCC/CIITA reactive bulk microcultures which produced IFN-g but not IL-17A. All clones show greater cytokine release to autologous RCC/CIITA than autologous EBV transformed B cells and are blocked by anti–HLA-DR.
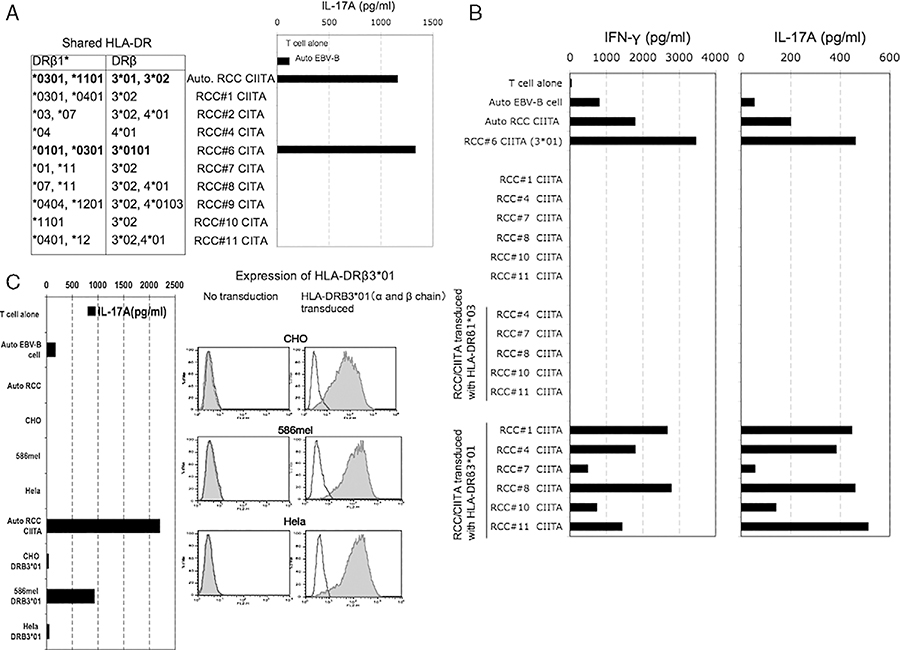
3G8D Recognizes Multiple RCC Lines in the Context of HLA-DRβ3*01
Clonality of 3G8D and 8A1F were confirmed by TRBV analysis based on 5′RACE method (data not shown). The 3G8D clone recognized 1 of 9 allogeneic RCC/CIITA lines and that line shared expression of both HLA-DRβ1*03 and HLA-DRβ3*01 with the autologous RCC/CIITA (Fig. 2A). To further determine the restriction element of 3G8D, allogeneic RCC/CIITA lacking these 2 alleles were retrovirally transduced with separate vectors expressing 1 of these 2 alleles and tested for recognition by 3G8D. The 3G8D clone recognized multiple allogeneic RCC/CIITA transduced with HLA-DRβ3*01 but not with HLA-DRβ1*03 (Fig. 2B). Reactivity of 3G8D against non-RCC cell lines was also tested. CHO, 586mel, and Hela were transduced with HLA-DRβ3*01, and recognition by 3G8D was checked. 3G8D produced IL-17A on stimulation with 586mel transduced with HLA-DRβ3*01, but did not produce significant IL-17A after stimulation with CHO transduced with HLA-DRβ3*01 or Hela transduced with HLA-DRβ3*01 (Fig. 2C). Thus, 3G8D recognizes an antigen expressed by multiple RCCs and 586mel in the context of HLA-DRβ3*01.
FIGURE 2.
A, Clone 3G8D recognized renal cell carcinoma (RCC)/class II transactivation molecules (CIITAs) expressing HLA-DRB3*01 and 1*03. The matched HLA-DR genotype of allogeneic RCC/CIITA tumors to autologous RCC/CIITA was shown in the table. B, Clone 3G8D recognized RCC/CIITAs transduced with HLA-DRB3*01. Allogeneic RCC/CIITA tumors that were not recognized by clone 3G8D and does not express HLA-DRB3*01 and 1*03 were transduced with either HLA-DRB3*01 or 1*03 retroviral supernatants and tested for the recognition by 3G8D. C, Clone 3G8D recognized 586mel transduced with HLA-DRB3*01, but not CHO and Hela transduced with HLA-DRB3*01, despite of similar HLA-DRB3*01 expression level. 586mel, CHO, and Hela that do not express HLA-DR molecule were transduced with a and b chain of HLA-DRB3*01, and tested for the recognition by 3G8D. Expression level of HLA-DRB3*01 on native tumors and transfectants were detected by anti–HLA-DR monoclonal antibody.
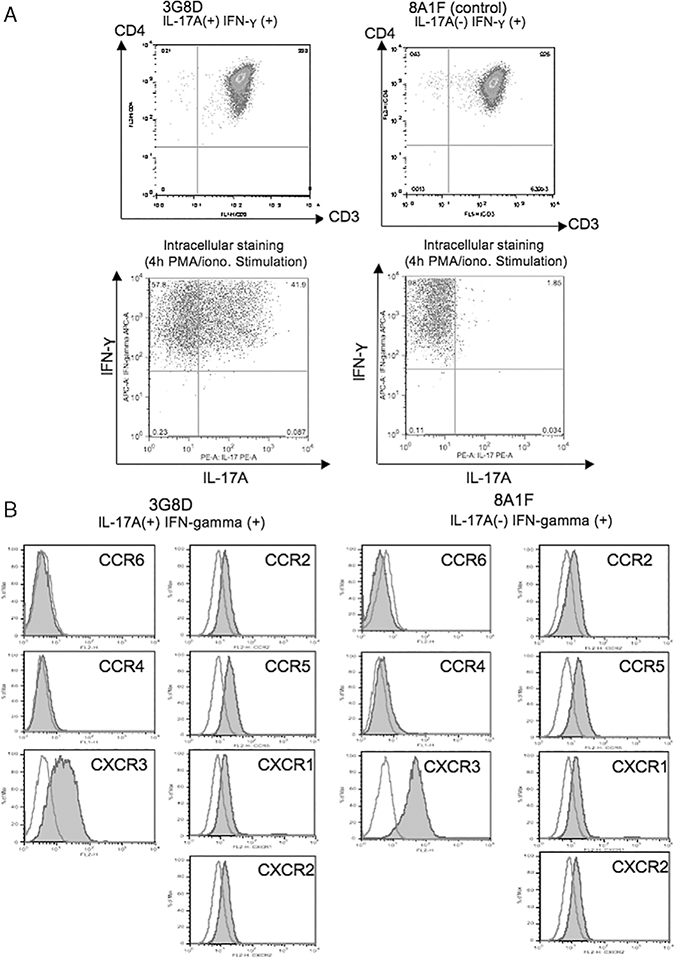
Phenotype and Cytokine Secretion of 3G8D
To confirm IL-17 production by 3G8D, intracellular cytokine staining for IL-17A and IFN-γ were performed. 3G8D and control clone 8A1F were stimulated with PMA and ionomycin for 4 hours in the presence of brefeldin A. After fixation and permeabilization, cells were phenotyped with fluorescent antibodies. After 4-hour stimulation with PMA and ionomycin, approximately 40% of the 3G8D population made both IFN-γ and IL-17A, refuting that these cytokines are being produced by separate, nonclonal populations. On the other hand, 8A1F made IFN-γ but did not make IL-17A (Fig. 3A). Recent reports show high expression of CCR6 and CCR4 on human TH17 cells in PBMC,9,22 however, this was not true for 3G8D (Fig. 3B).
FIGURE 3.
A, 3G8D is a CD4+ T-cell clone (TRBV29–1*01) that produces both interleukin (IL)-17A and IFN-γ. 3G8D and 8A1F were stimulated with PMA and ionomycin in the presence of blefeldin A. Four hours after the stimulation, intracellular cytokine staining for IL-17A and IFN-γ was performed. B, Staining for CCR chemokine receptors were performed. 3G8D does not express CCR6 and CCR4.
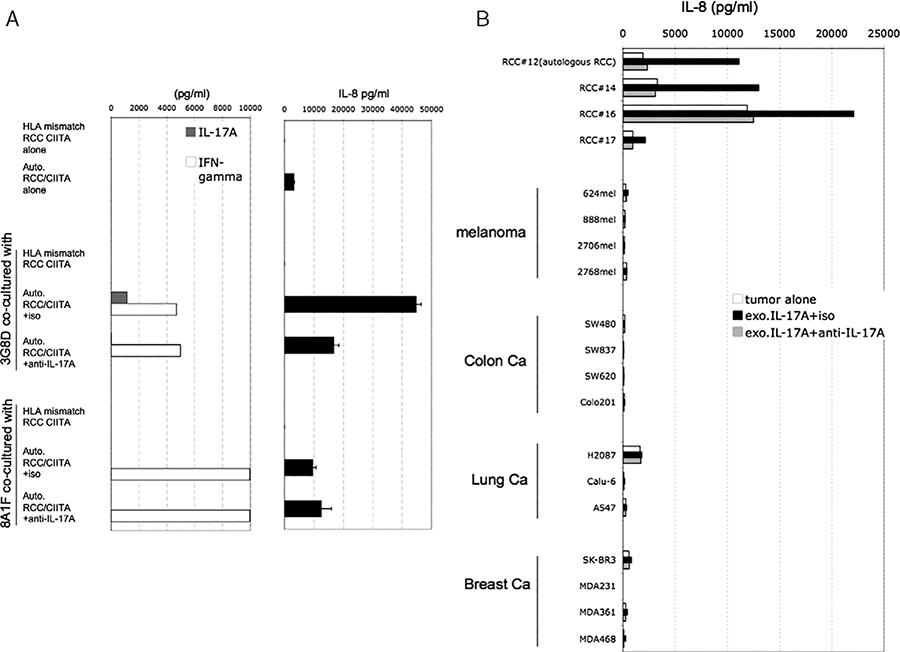
3G8D Increases Release of IL-8 From RCC Mediated by IL-17
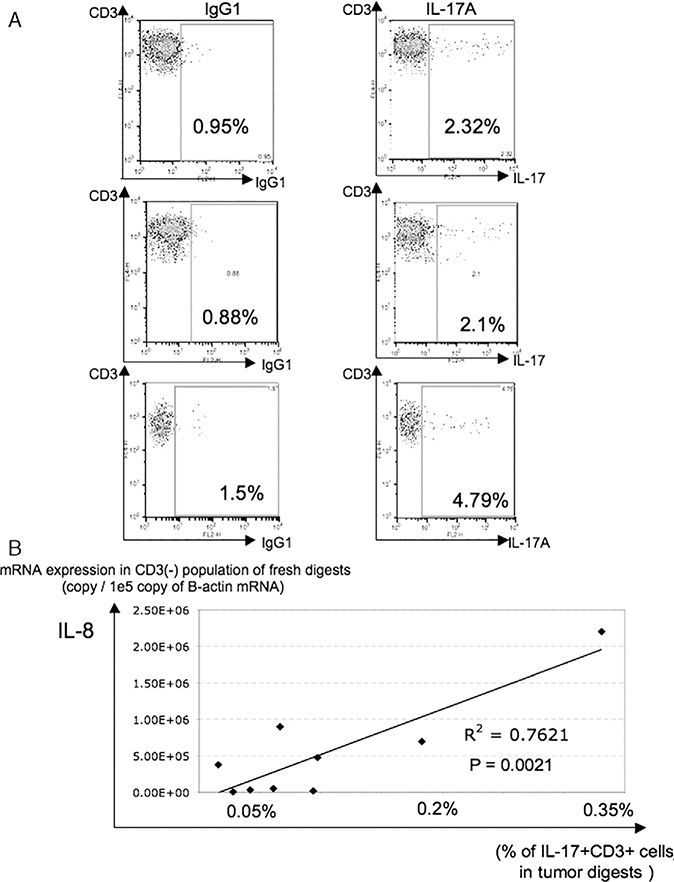
Coculture of 3G8D with RCC lines greatly increased levels of IL-8 in the supernatant, but positive control stimulation of 3G8D alone with PMA and ionomycin did not. There are published reports showing that IL-8 is released from various epithelial and endothelial cells by exogenous IL-17 stimulation, however, there are no reports showing this effect with RCC lines. We tested whether IL-17 released by 3G8D could be inducing IL-8 release from autologous RCC/CIITA (Fig. 4A). After 24-hour coculture, increased IL-8 release from tumor cells was observed only in the presence of IL-17 released from T cells, and this IL-8 release were significantly and reproducibly blocked by addition of anti–IL-17 antibody (Fig. 4A). Next, we checked the impact of exogenous IL-17 on IL-8 release from RCC and non-RCC tumor cell lines (Fig. 4B). In 5/8 tested RCC cell lines, exogenous IL-17 increased IL-8 release, and this was completely blocked by addition of anti–IL-17 blocking antibody. On the other hand, in other tumors, IL-17 did not show any impact on IL-8 release. These results may suggest that this bidirectional T-cell tumor interaction may be characteristic of, and somewhat specific for, renal cancer. Therefore, to investigate the in vivo frequency of IL-17–producing T cells and its impact on local IL-8 production, we analyzed fresh RCC tumor digests. First, the frequency of IL-17–producing T cells in RCC-infiltrating lymphocytes was assessed by intracellular staining for IL-17 after stimulation with PMA and ionomycin. Cryopreserved, enzymatically digested RCC tumors were stimulated by PMA and ionomycin for 4 hours in the presence of blefeldin A immediately after thawing and stained. In tested samples, 2% to 5% of CD3+ T cells in tumor digests were producing IL-17 (Fig. 5A). Next, we checked the relationship between the percentage of IL-17–producing T cells in tumor and local IL-8 production from non-T cells (primarily tumor and stromal cells). Thawed fresh tumor digests were divided into CD3+ and CD3 populations by magnetic bead separation and total RNA isolated from the CD3 population. The mRNA expression level of IL-8 in the CD3 population was quantified by quantitative RT-PCR. Figure 5B shows the relationship between the IL-8 mRNA expression level in the CD3 population from tumor specimens by quantitative RT-PCR and the percentage of IL-17–producing CD3+ cells quantified by IL-17 intracellular staining. In a limited analysis of 9 specimens, a statistically significant association was seen between IL-17–producing T cells and IL-8 mRNA levels in non-T cells (P=0.0021).
FIGURE 4.
A, 3G8D increases interleukin (IL)-8 release from autologous renal cell carcinoma (RCC)/class II transactivation molecules (CIITA) depending on IL-17A. 3G8D and 8A1F were cocultured with autologous RCC/CIITA in the presence of anti–IL-17A monoclonal antibody or isotype control, or with HLA-DRB3*01 negative RCC/CIITA control for 24 hours. Concentration of IL-17A, IFN-γ, and IL-8 in the culture supernatants was measured by enzyme-linked immunosorbent assay (ELISA). B, Exogenous IL-17A increases IL-8 release from RCC cell lines. RCCs were treated with recombinant IL-17A for 24 hours, in the presence of anti–IL-17A monoclonal antibody or an isotype control antibody (iso). Concentration of IL-8 in the culture supernatants were measured by ELISA.
FIGURE 5.
A, Interleukin (IL)-17A–producing T cells were detected in some renal cell carcinoma (RCC) tumor digests. Cryo-preserved enzymatically dispersed RCC tumor samples were thawed, and immediately stimulated by PMA and ionomycin for 4 hours in the presence of brefeldin A. After anti-CD3 staining, preparations were fixed, permeabilized, and stained with anti–IL-17A monoclonal antibody or a immunoglobulin G1 (IgG1) isotype control antibody. Lymphocyte gating by forward and side scatter was used before performing the analysis shown. B, Relationship of IL-8 production from CD3 cells in fresh single cell preparations of human RCC specimens and the percentage of infiltrating IL-17A–producing T cells. Cryo-preserved RCC tumor digests were thawed, and IL-17A intracellular staining performed as described in A. The CD3 population in tumor digests was isolated by negative selection on magnetic microbeads. Quantitative polymerase chain reaction for IL-8 mRNA was performed using total RNA isolated from this population. IL-8 mRNA expression level is described as the IL-8 copy number/105 copies of b-actin mRNA, and the % of IL-17A+CD3+ cells in tumor digests was expressed as the % of all cells in thawed preparation and calculated using % of IL-17A+CD3+ cells, % of CD3+ gated population, and % of lymphocyte gated population.
DISCUSSION
Recently, a new memory CD4+ T-cell lineage characterized by IL-17 production has been identified and designated as TH17 cells.23 In some murine experimental models, TH17 cells are suggested to mediate autoimmune encephalitis and CIA.1 Also in humans, there have been reports presenting circumstantial evidence of a relationship between autoimmune disease and infiltration of IL-17–producing T cells.7–11 However, the role of TH17 cells in human tumor immunology is not clear and to our knowledge, there have been no reports characterizing or analyzing human tumor-reactive, IL-17–producing T cells. At least 1 murine tumor model suggests that such cells could be potent mediators of tumor rejection.13 Therefore, in this study, we have attempted to generate and clone RCC-reactive CD4+ T-cell clones that produce IL-17 from the PBMC of an RCC patient in whom tumor regression and autoimmune toxicities were observed during treatment with anti-CTLA4 antibody. Two classes of tumor reactive cells were seen; CD4 cells making both IFN-g and IL-17 and CD4 cells making only INF-g, but none of our cultures made only IL-17. One of each of these types of clones was further expanded and characterized. The clonality of 3G8D (which produced both IL-17 and IFN-γ) was confirmed by TRBV analysis and production of IL-17A was also confirmed by intracellular staining for IL-17A. Its phenotype, CCR6, CCR4, CXCR3+, differs from that reported by Acosta-Rodriguez et al22 for TH17 cells isolated from normal PBMC where CCR6 and CCR4 were associated with IL-17 production and the CCR6+ CXCR3+ population also secreted IFN-g. Annunziato et al9 also reported the presence of CCR6+ T cells producing IL-17 in the gut of patient with Crohn disease, by establishing CD4+ T-cell clones but their reactivity is unknown. Those that produced both IL-17 and IFN-γ on stimulation with anti-CD3 or PMA-ionomycin were designated as TH17/ TH1 cells. Although clone 3G8D seems to be functionally similar to a TH17/TH1 cell and is CXCR3+, it expresses neither CCR6 nor CCR4, and releases both IL-17 and IFN-γ after recognizing an antigen shared by the autologous and several allogeneic RCC lines in the context of HLA-DRβ3*01.
The role of TH17 cells in cancer, autoimmunity, and inflammation would in large part depend on the local consequences of the release of IL-17. IL-17 has been reported to mediate the release of other proinflammatory cytokines and chemokines,24 particularly from pulmonary epithelium and smooth muscle. A major observation of this study was the fact that IL-17 dramatically increased IL-8 secretion from RCC lines. Although some other tumor lines are reported to increase IL-8 secretion in response to IL-17, this requires days of stimulation and levels are much lower than seen here.25 In addition, the secretion of IL-17 by T cells in response to RCC recognition establishes a potential physiologic source for IL-17 that may have relevance to the in vivo tumor microenvironment. It is of interest that this potential crosstalk was only demonstrable for RCC and not other tumors we tested. Three of 4 RCC lines secreted high levels of IL-8 in response to exogenous IL-17, but not a variety of melanoma, lung cancer, colon cancer, and breast cancer lines. Finally, in a preliminary analysis using fresh RCC tumor specimens, there was a suggestion of a relationship between the frequency of infiltrating IL-17+CD3+ T cells in tumor digests, and IL-8 mRNA expression level in the non–T-cell population of these tumor digests. Given the known chemotactic activity of IL-8 for lymphocytes,26,27 these initial data would support the hypothesis that tumor-reactive T cells secreting IL-17 could contribute to the local accumulation of T lymphocytes through the elaboration of IL-8 by renal cancer cells. This is unlikely to be the sole determinant of lymphocyte infiltration into tumor, so further study of a much larger sample of specimens will be needed to investigate this more rigorously.
In summary, we have generated RCC-reactive CD4+ T-cell clones that produce IL-17 and extensively characterized one of these clones, the first such report of such a reactivity. Efforts to determine the exact antigen being recognized could lead to a better molecular understand of the immune response to one of the few human cancers that can be cured with systemic immunotherapy.28 The finding that T-cell–secreted IL-17 induces the elaboration of a cytokine, IL-8, from tumor cells which has both chemoattractant and angiogenic activities reveals the complex interplay that may be occurring in the human tumor microenvironment.
REFERENCES
- 1.Harrington LE, Mangan PR, Weaver CT. Expanding theeffector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349–356. [DOI] [PubMed] [Google Scholar]
- 2.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather thaninterleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. [DOI] [PubMed] [Google Scholar]
- 3.Murphy CA, Langrish CL, Chen Y, et al. Divergent proand antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198: 1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aggarwal S, Ghilardi N, Xie MH, et al. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278: 1910–1914. [DOI] [PubMed] [Google Scholar]
- 5.Nakae S, Nambu A, Sudo K, et al. Suppression of immuneinduction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. [DOI] [PubMed] [Google Scholar]
- 6.Lubberts E, Koenders MI, Oppers-Walgreen B, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–659. [DOI] [PubMed] [Google Scholar]
- 7.Ziolkowska M, Koc A, Luszczykiewicz G, et al. High levels ofIL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol. 2000;164:2832–2838. [DOI] [PubMed] [Google Scholar]
- 8.Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovialfluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103: 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic andfunctional features of human Th17 cells. J Exp Med. 2007; 204:1849–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujino S, Andoh A, Bamba S, et al. Increased expression ofinterleukin 17 in inflammatory bowel disease. Gut. 2003;52: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saruta M, Yu QT, Avanesyan A, et al. Phenotype and effectorfunction of CC chemokine receptor 9-expressing lymphocytes in small intestinal Crohn’s disease. J Immunol. 2007;178: 3293–3300. [DOI] [PubMed] [Google Scholar]
- 12.Teunissen MB, Koomen CW, de Waal Malefyt R, et al. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–649. [DOI] [PubMed] [Google Scholar]
- 13.Muranski P, Boni A, Antony PA, et al. Tumor-specific Th17polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis inpatients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24: 2283–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkins MB, Mier JW, Parkinson DR, et al. Hypothyroidismafter treatment with interleukin-2 and lymphokine-activated killer cells. N Engl J Med. 1988;318:1557–1563. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg SA, White DE. Vitiligo in patients with melanoma: normal tissue antigens can be targets for cancer immunotherapy. J Immunother Emphasis Tumor Immunol. 1996;19: 81–84. [PubMed] [Google Scholar]
- 17.Wang QJ, Hanada K, Perry-Lalley D, et al. Generating renalcancer-reactive T cells using dendritic cells (DCs) to present autologous tumor. J Immunother. 2005;28:551–559. [DOI] [PubMed] [Google Scholar]
- 18.Wang RF, Wang X, Johnston SL, et al. Development of aretrovirus-based complementary DNA expression system for the cloning of tumor antigens. Cancer Res. 1998;58:3519–3525. [PubMed] [Google Scholar]
- 19.Riddell SR, Greenberg PD. The use of anti-CD3 and antiCD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128:189–201. [DOI] [PubMed] [Google Scholar]
- 20.Robbins PF, Dudley ME, Wunderlich J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fink L, Seeger W, Ermert L, et al. Real-time quantitativeRT-PCR after laser-assisted cell picking. Nat Med. 1998;4: 1329–1333. [DOI] [PubMed] [Google Scholar]
- 22.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surfacephenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8: 639–646. [DOI] [PubMed] [Google Scholar]
- 23.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. [DOI] [PubMed] [Google Scholar]
- 24.Witowski J, Ksiazek K, Jorres A. Interleukin-17: a mediator of inflammatory responses. Cell Mol Life Sci. 2004;61:567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kehlen A, Thiele K, Riemann D, et al. Interleukin-17 stimulates the expression of IkappaB alpha mRNA and the secretion of IL-6 and IL-8 in glioblastoma cell lines. J Neuroimmunol. 1999;101:1–6. [DOI] [PubMed] [Google Scholar]
- 26.Hess C, Means TK, Autissier P, et al. IL-8 responsivenessdefines a subset of CD8 T cells poised to kill. Blood. 2004;104: 3463–3471. [DOI] [PubMed] [Google Scholar]
- 27.Gasser O, Missiou A, Eken C, et al. Human CD8+ T cellsstore CXCR1 in a distinct intracellular compartment and upregulate it rapidly to the cell surface upon activation. Blood. 2005;106:3718–3724. [DOI] [PubMed] [Google Scholar]
- 28.Klapper JA, Downey SG, Smith FO, et al. High-doseinterleukin-2 for the treatment of metastatic renal cell carcinoma: a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]