Abstract
The objective of this study was to evaluate the impact of the tuberculosis (TB) mobile teams on treatment outcomes in Riyadh Region by comparing patients who received treatment under mobile teams and those who did not, from 2013 to 2015. This was a retrospective descriptive study using National TB Control and Prevention Program data from 2013 to 2015 from Riyadh, Kingdom of Saudi Arabia. Descriptive analyses were used to summarize characteristics of TB case-patients served by mobile teams and those who were not served. The χ2 test measured the significant differences between mobile-served and non-mobile-served case-patients. Exposure was whether or not the TB case-patient was under the care of the mobile team; the outcome of interest was whether or not treatment was successful, defined as treatment completed and cured. We found that the ratio of treatment success among mobile team case-patients was 1.28 greater than among those not served by mobile teams. The χ2 test showed a statistically significant finding (probability ratio = 1.28; 95% confidence interval = 1.21–1.35, p < 0.01). Mobile teams increased the treatment success rate to 92%, compared to 71.77% among those not served by mobile teams. This study shows that community mobilization of mobile teams is an effective strategy to enhance TB treatment, reduced mortality and loss to follow-up and improve TB treatment outcomes.
Keywords: Directly observed therapy, Mobile teams, Saudi Arabia, Tuberculosis
1. Introduction
Despite improvement in diagnosis, treatment, and prevention of tuberculosis (TB), it remains a significant public health concern globally and is considered a re-emerging infectious disease. The 2016 Global Tuberculosis Report showed that almost one-third of the global population was infected with TB, with around 10.4 million new cases. There were 480,000 new cases of multi-drug resistant TB (MDRTB) and an additional 100,000 new cases with rifampicin-resistant TB. There were 1.4 million deaths resulting from TB and an additional 400,000 TB-related deaths among people living with human immunodeficiency virus (HIV). Unfortunately, from 2014 to 2015, the drop in TB incidence was only 1.5% globally [1].
One of the targets of the Sustainable Development Goals for 2030, implemented by the United Nations in 2015, is to end the global TB epidemic. The World Health Assembly, in 2014, approved the World Health Organization (WHO) End TB Strategy (2014), which demands by 2030, a 90% reduction in TB deaths; an 80% reduction in the TB incidence rate (as compared with 2015); and that no TB-affected household faces catastrophic costs [1]. To meet the first milestones of the End TB Strategy, huge efforts are needed to fast track the decline in incidence to a 4–5% annual decline by 2020 [1].
Such efforts are needed in the Kingdom of Saudi Arabia (KSA), which is the third largest country in terms of land area in the Middle East, representing the vast majority of the Arabian Peninsula [2], and is a low-to-middle TB burden country according 2015 reports by the WHO, with an incidence rate of 12 per 100,000 [3]. According to the KSA Ministry of Health (MoH) statistics, overall TB incidence showed an uprising trend from 1992 onwards, with a peak in 1999 (12.2 cases per 100,000 population), which then started to drop only slightly [4]. The total number of new cases of pulmonary TB in 2015 was 2505, with an incidence rate of 7.95 cases per 100,000 population. The total number of cases of nonpulmonary TB reached 841, with an incidence rate of 2.67 per 100,000 population [5]. KSA has experienced vast economic expansion that has resulted in improvement of social and health services. This has led to an influx of large numbers of foreign workers (currently there are 12,185,284 non-Saudi nationals [6]), which is a potential population health risk for KSA, given that international travel, immigration, and movement of populations can facilitate the spread of TB [7]. In fact, 56% of reported TB cases have been found among non-Saudis, in comparison to 44% for Saudi nationals [5].
The KSA MoH implemented a National TB Control and Prevention Program (NTCPP) that worked for >30 years in response to the world plan to eliminate TB [8]. Based on the WHO End TB Strategy, the NTCPP has developed ambitious targets: to raise the cure rate of smear-positive TB to >85%; to detect >70% of the estimated TB cases; and to decrease the incidence rate of pulmonary smear-positive TB in the country to 1 per 100,000 population. Also, NTCPP adopted the Directly Observed Therapy short course (DOTs) program in 1999, which is the standard WHO TB treatment strategy. After many years of dealing with different TB treatment method, DOTs strategy has emerged as the best solution to many problems, especially nonadherence, and has been proven effective in several parts of the world. DOTs strategy reduced the treatment failure rate from 17.6% to 6.2% in a large Chinese study [9]. Furthermore, primary and acquired TB drug resistance have been found to reduce as a result of DOTs strategy implementation, from 13% and 14% to 6.7% and 2.1%, respectively, according to Weis et al. [10]. Moreover, in the long run, DOTs strategy is considered to be more cost-effective in comparison with other TB treatment and control methods [11].
Due to the efforts of NTCPP in 2015, TB treatment coverage was 87%. However, the treatment success rate of new and relapsed cases was 62%, which remains below the international target set by the WHO (85%) [1]. Nonadherence and drug interruption are major barriers towards TB control in KSA [12]. Research shows that the most important aspect in TB treatment success is patient adherence with the prescribed drugs. It is unclear whether the patients take the prescribed medication even if they visit clinics regularly. As a result of noncompliance and drug interactions, drug resistance and relapse occur [13]. However, improved control efforts and widespread implementation of DOTs by NTCPP has not led to the expected fall in TB trends [14]. In response to this, NTCPP has added mobile teams in Riyadh and Jizan cities, aiming to decrease the default rates and improve patient outcomes through community outreach. This addition is expected to lower the incidence of the disease. Therefore, based on such results, the expansion of mobile teams to cover the whole country is currently under consideration by NTCPP [15,16].
In Riyadh City, which was the focus of the present study, there are currently 20 mobile teams distributed according to population density. Every mobile team is fully equipped and consists of a physician, nurse, heath inspector, and driver. The main objective of the mobile team is to ensure adherence to all aspects of the DOTs therapy strategy when treating TB patients in a holistic care approach. Mobile teams focus on treatment and sputum follow-up of the positive cases at 2 months, 4 months, and 6 months (treatment monitoring) in addition to contact tracing. They also play an important role regarding the diagnosis of TB suspects in their facilities and the nearby ones (in their area), and send the samples to the laboratory for confirmation. Furthermore, they visit the private hospitals in their area looking for suspect cases. Every TB patient is eligible to receive service by mobile teams except for those patients who require hospitalization [16].
The aim of this study was to evaluate the impact of the TB Mobile Teams on TB treatment outcomes in Riyadh Region by comparing patients who received treatment by mobile teams and those who did not, from 2013 to 2015. The findings will help NTCPP in the decision on whether to expand mobile teams to cover the whole country to help with TB control and move forward towards elimination of the disease.
2. Materials and methods
2.1. Data source
TB is one of many notifiable diseases in KSA. According to the MoH Department of Infectious Diseases, healthcare facilities should report new TB cases monthly to NTCPP [17]. These reports include demographical, clinical, and epidemiological data. NTCPP then publishes the data after deep analysis via the Annual Statistical Health Report [5].
2.2. Study design
This was a retrospective study using the NTCPP data from 2013 to 2015. Mobile TB teams have been implemented in two cities only (Riyadh and Jizan) [16]. Riyadh is the capital, with the highest population density (6.195 million) [18] and therefore this study used NTCPP data from Riyadh to compare TB case-patients served by mobile teams and those not served. The study started in August 2016 and ended in March 2017.
2.3. Study variables
There were 10 variables included in the study: (1) year (3 subvariables: 2013, 2014, and 2015); (2) team (2 subvariables: mobile and nonmobile); (3) age (continuous variable); (4) sex (2 subvariables: male and female); (5) nationality (2 subvariables: Saudi and non-Saudi); (6) patient type (2 subvariables: new and relapse); (7) TB site of infection (3 subvariables: pulmonary, extrapulmonary, and both); (8) acid-fast bacillus (AFB) test result (3 subvariables: positive, negative, and not done); (9) HIV test result (3 subvariables: positive, negative, and not done); and (10) treatment outcome (6 subvariables: complete treatment, cured, failed, died, not evaluated yet, and lost to follow-up)
2.4. Ethics
This study was based on secondary data without any personal identifiers; it did not meet the category of human subject research and was reviewed by Emory University Institutional Review Board.
2.5. Statistical analyses
Descriptive analyses were used to summarize characteristics of TB case-patients served by mobile teams and those not served. χ2 tests measured the significant differences between mobile and nonmobile groups. Exposure was whether or not the TB case-patient was served by the mobile team; the outcome was whether or not treatment was successful. According to the United Nations [19] developmental goals, “treatment success rates are calculated from the data as the proportion of new smear-positive TB cases registered under DOTS in a given year that successfully completed treatment, whether with (‘cured’) or without (‘treatment completed’) bacteriologic evidence of success” [19]. SAS (SAS Institute Inc., Cary, NC, USA) was the platform used to perform all analyses. All p values were two-tailed. A p value <0.05 was considered significant. Rates were compared using probability of success ratios and 95% confidence intervals.
3. Results
From 2013 to 2015, there were 1600 TB patients in Riyadh Region registered in the NTCPP database with treatment outcomes recorded (Table 1). Nine hundred and thirty-four (58.38%) patients were served by mobile teams, while 666 (41.63%) were not served. Overall, patients ranged from 7 months to 101 years old, with a mean age of 36.4 years. There were 1145 (71.56%) male case-patients and 455 (28.44%) female case-patients. Also, 651 (40.69%) were Saudi case-patients, while the majority, 949 (59.31%), was non-Saudi. Only 67 (4.19%) were relapsed TB case-patients (received treatment previously and confirmed cured but had redeveloped smear-positive pulmonary TB) [20], while 1533 (95.81%) were new TB patients. Pulmonary TB was the most prominent TB site, with 1113 (69.56%), extrapulmonary with 447 (27.94%), and the combination of pulmonary and extrapulmonary with 40 (2.50%). Additionally, 938 (58.63%) had AFB sputum-smear-positive results at the start of treatment, 478 (29.88%) had a negative result; and the smear was not done for 184 (11.50%) patients. The majority (1058; 66.13%) of case-patients were HIV negative; only 20 (1.25%) were tested positive and 519 (32.44%) were not tested.
Table 1.
Characteristics of TB case-patients served by mobile teams and not served by mobile teams, Riyadh Region, 2013–2015.
| TB case-patient profile | All case-patients (n = 1600) | Mobile-team served (n = 934) | Non-mobile-team served (n = 666) | ||||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | ||
| Sex | Male | 1145 | (71.56) | 674 | (72.16) | 471 | (70.72) |
| Female | 455 | (28.44) | 260 | (27.84) | 195 | (29.28) | |
| Age | Median (IQR) | 31 (25–46) | 30 (25–42) | 34 (26–53) | |||
| Nationality | Saudi | 651 | (40.69) | 268 | (28.69) | 383 | (57.51) |
| Non-Saudi | 949 | (59.31) | 666 | (71.31) | 283 | (42.49) | |
| Case-patient type | New | 1533 | (95.81) | 897 | (96.04) | 636 | (95.5) |
| Relapse | 67 | (4.91) | 37 | (3.96) | 30 | (4.5) | |
| TB site | Pulmonary | 1113 | (69.56) | 673 | (72.06) | 440 | (66.07) |
| Extrapulmonary | 447 | (27.94) | 237 | (25.37) | 210 | (31.53) | |
| Both | 40 | (2.50) | 24 | (2.57) | 16 | (2.40) | |
| AFB result | Positive | 938 | (58.63) | 609 | (65.20) | 329 | (49.4) |
| Negative | 478 | (29.88) | 295 | (31.58) | 183 | (27.48) | |
| Not done | 184 | (11.50) | 30 | (3.21) | 154 | (23.12) | |
| HIV status | Positive | 20 | (1.25) | 9 | (0.96) | 11 | (1.65) |
| Negative | 1058 | (66.13) | 755 | (80.84) | 303 | (45.50) | |
| Not done | 519 | (32.44) | 167 | (17.88) | 352 | (52.85) | |
| Treatment outcome | Successa | 1338 | (83.62) | 860 | (92.08) | 478 | (71.77) |
| Completed | 690 | (43.13) | 359 | (38.44) | 331 | (49.7) | |
| Cured | 648 | (40.5) | 501 | (53.64) | 147 | (22.07) | |
| Died | 73 | (4.56) | 11 | (1.18) | 62 | (9.31) | |
| Failed | 19 | (1.19) | 8 | (0.86) | 11 | (17.19) | |
| Lost to follow-up | 82 | (5.13) | 18 | (1.93) | 64 | (9.61) | |
| Not evaluated yet | 88 | (5.50) | 37 | (3.96) | 51 | (7.66) | |
AFB = acid-fast bacillus; HIV = human immunodeficiency virus; IQR = interquartile range; TB = tuberculosis.
Treatment success = completed + cured.
We found 684 (40.5%) among all patients were cured based on at least three negative consecutive sputum smear tests. However, 690 (43.13%) patients were classified as having completed the treatment, so, overall treatment success rate (completed + cured patients) was 83.63% (n = 1338). Treatment failure was 1.19% (n = 19), which included TB patients who, “while on treatment, remained smear-positive; or once more became smear-positive at the fifth month or later during the course of treatment, or those who were initially smear-negative before starting treatment and became smear-positive after the second month of treatment” [20]. Furthermore, 73 (4.56%) patients died, 82 (5.13%) patients were lost to follow-up, and 88 (5.50%) patients have not yet been evaluated by healthcare professionals.
A descriptive analysis of variables by mobile versus nonmobile teams showed that both mobile and nonmobile teams had almost the same distribution of patient sex (Table 1). Mean age for mobile-team-served case-patients was 34 years, compared with 40 years for non-mobile-team-served case-patients. A total of 71.31% of mobile-team-served case-patients were non-Saudi; in contrast, the majority of non-mobile-team-served case-patients were Saudi (57.51%). New pulmonary TB was the main type among patients in both arms. The AFB sputum smear test was not done in 23.12% of non-mobile-team-served case-patients, which was high compared to that of mobile-team-served case-patients, as only 2.75% did not undergo the test. Moreover, 52.85% of non-mobile-team-served case-patients did not have HIV test results, while only 3.21% of mobile-team-served case-patients did not undergo the test.
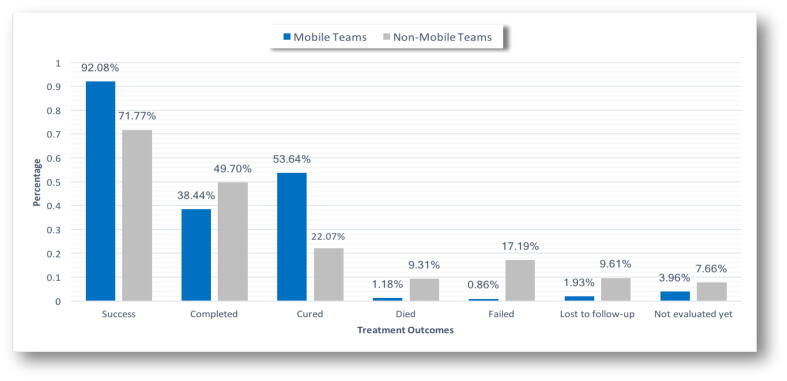
The percentage of treatment outcomes by teams showed that, in comparison to non-mobile-team-served case-patients, mobile-team-served case-patients increase the rate of treatment success to 92.08% (Fig. 1). Moreover, the mobile teams helped reduce mortality, treatment failure, and loss to follow-up rates by 1.18%, 0.68%, and 1.93%, respectively.
Fig. 1.
Treatment outcomes between mobile-served TB case-patients and non-mobile-served TB case-patients, Riyadh Region, 2013–2015. TB = tuberculosis.
The ratio of treatment success among mobile team patients was 1.28 higher than that among non-mobile teams (Table 2). The χ2 test showed statistical significance (ratio = 1.28; 95% confidence interval = 1.21–1.35, p < 0.01). Possible confounders were considered (e.g., nationality, patient type, TB site, AFB results, and HIV status) (Table 3). A statistically significant impact was observed in most levels after stratification with precise confidence intervals and p values <0.01.
Table 2.
Probability ratio of treatment success between mobile-team-served TB case-patients compared to non-mobile-team-served TB case-patients, Riyadh Region, 2013–2015.
| Mobile teams vs. non-mobile teams | |||
|---|---|---|---|
| Ratio | 95% CI | p | |
| Treatment success | 1.28 | 1.21–1.35 | < 0.001 |
CI = confidence interval; TB = tuberculosis.
Table 3.
Probability ratio of treatment success between mobile-team-served TB case-patients compared to non-mobile-team-served TB case-patients, stratified by possible confounders
| Variable | Mobile-team-served vs. non-mobile-team-served TB case-patients | |||
|---|---|---|---|---|
| Ratio | 95% CI | p | ||
| Nationality | Saudi | 1.27 | 1.18–1.36 | <0.001 |
| Non-Saudi | 1.29 | 1.19–1.39 | <0.001 | |
| Patient type | New | 1.29 | 1.22–1.36 | <0.001 |
| Relapse | 1.08 | 0.86–1.34 | 0.6995 | |
| TB site | Pulmonary | 1.36 | 1.27–1.46 | <0.001 |
| Extrapulmonary | 1.17 | 1.08–1.25 | <0.001 | |
| Both | 1.03 | 0.67–1.56 | >0.999 | |
| AFB result | Positive | 1.35 | 1.25–1.46 | <0.001 |
| Negative | 1.29 | 1.17–1.42 | <0.001 | |
| Not done | 1.12 | 0.96–1.28 | 0.2160 | |
| HIV status | Positive | 1.43 | 0.74–2.71 | 0.5449 |
| Negative | 1.25 | 1.16–1.34 | <0.001 | |
| Not done | 1.24 | 1.13–1.36 | <0.001 | |
AFB = acid-fast bacillus; CI = confidence interval; HIV = human immunodeficiency virus; TB = tuberculosis.
4. Discussion
It is clear from the findings of this study that mobile team service of TB case-patients had a positive and significant impact on TB treatment outcomes, and increased treatment success to 92%. In fact, the mortality rate among mobile-team-served case-patients was only 1.18%, in comparison to 9.31% among non-mobile-team-served case-patients during 2013–2015. This potentially was because TB case-patients under mobile team service were 1.28 times more likely to experience treatment success than those not under mobile team service. In fact, the failure of treatment was only 0.86% among mobile-team-served case-patients, compared to a significantly higher rate of 17.19% among non-mobile-team-served case-patients. This success in treatment of the mobile TB teams can be explained by their goal, which is to guarantee the implementation of one of DOTs recommendations: to ensure that case-patients take the prescribed drugs under direct supervision of a healthcare professional. With the use of mobile teams, healthcare workers directly follow up on drug adherence. The use of mobile teams resulted in a lowering of the loss-to-follow-up rate, which was reduced to 1.93%, compared to 9.61% among non-mobile-team-served case-patients.
Treatment success rates where high among mobile-team-served case-patients, and the completion of therapy rates among mobile-team-served case-patients was lower than among non-mobile-team-served case-patients: 38.44% and 49.70%, respectively. This was either because the case-patients could not produce sputum to confirm being cured, or they a negative smear results from the beginning of the course. However, the cure and treatment success rates were higher among mobile-team-served case-patients; 53.64% and 92.08%, respectively. This is in comparison to non-mobile-team-served case-patients whose rates were 22.07% and 71.77%, respectively. To prevent future TB drug resistance and relapse, higher cure and treatment success rates should be obtained rather than completion rates.
This statistically significant impact of mobile teams was noticed in both Saudi and non-Saudi case-patients; new TB case-patients; case-patients with different sites of TB infection (pulmonary or extrapulmonary); case-patients with different AFB smear test results (positive and negative); case-patients with negative HIV status; and those who did not do the HIV test. Results showed that relapsed case-patients; case-patients with infection in both TB sites (pulmonary + extrapulmonary); case-patients who did not have AFB sputum smear test results; and case-patients with positive HIV test results experienced a positive impact. The results did not reach statistical significance.
Although mobile teams were successful in diagnosing TB by AFB sputum smear, we noticed that 31.58% of case-patients tested negative. This finding could be explained by the WHO definition of a smear-negative TB case-patient, which is one with “symptoms suggestive of TB, with at least two sputum specimens which were negative for AFB by microscopy, and with chest radiographic abnormalities consistent with active pulmonary TB” [20]. Also, 3.21% of case-patients who did not undergo the AFB test used other diagnostic tests such as Xpert (Xpert MTB/RIF by Cepheid, California, United States), which initiates TB treatment.
The strength of this study was that it was the first to measure the impact of TB mobile teams in KSA. Also, it included the most updated data, obtained from the NTCPP surveillance data; therefore, it is a useful tool for policy makers in the MoH Department of Public Health to decide whether or not to expand the use of TB mobile teams to include more cities in the near future.
One of the limitations of this study was that mobile teams were implemented by NTCPP in only two cities in KSA: Riyadh and Jizan. Furthermore, this study was limited to data from Riyadh as data from Jizan were unavailable at the time of this study. This study could be stronger if Jizan City data were included in the analysis to determine whether the positive impact of mobile teams was affected by geographical area of implementation. Another limitation was that data did not show the geographical distribution of the case-patients in Riyadh City nor their socioeconomic status, which may play a role in treatment success. Also, around 519 TB case-patients included in this study did not undergo an HIV test, which also may have affected the results. Finally, the data did not show any information about TB drug resistance. MDRTB is an emerging public health threat and it would be useful to gather data regarding the effectiveness of mobile teams (following the DOTs strategy) in reducing drug resistance.
Conclusion and recommendations
TB control in KSA currently faces many challenges, including the large influx of foreign workers, illegal immigrants, and religious visitors who come from high-TB-burdened countries. Furthermore, the global emergence of MDRTB and the TB/HIV syndemic make TB control difficult. This study showed that mobile TB teams can have a positive impact on TB control. We recommend that NTCPP ensures that mobile teams cover the entire KSA, and that staff are fully equipped and trained periodically, especially in TB program management and case-load distribution, and that continuous evaluation and monitoring occur. This will result in ensuring a sustainable program. Along with the use of mobile teams that adhere to DOTs, other TB control strategies should be used as well, such as providing education, holistic case management, and combination of TB drugs, incentives, and enablers. A combination of DOTs and these strategies can reduce acquired drug resistance, treatment failure, and relapse [21].
In conclusion, with the synergy of the TB, MDRTB, and HIV epidemics, the need to increase treatment success rates and to decrease failure and relapse rates is essential [22]. This study provides important data on the efficacy of using mobile teams to improve TB outcomes in Riyadh Region, KSA. Results prove that community mobilization of these teams is an effective strategy to mitigate TB treatment failure, mortality, loss to follow-up rates, and improve TB treatment outcomes. Further study of TB mobile teams in Jizan will be useful to compare results. These results prove the need to implement a full-scale TB mobile team system throughout KSA, especially in high TB endemic regions such as Makkah [4], along with ongoing monitoring and evaluation of the mobile teams by the NTCPP.
Acknowledgments
I would like to express my deepest appreciation to my mentor, supervisor, and thesis advisor, Dr Scott McNabb, for his kind support, in-depth knowledge, and skills generously shared with me; Dr Kenneth Castro for his encouragement and insightful comments; Dr Jose Binongo, for his help with the statistical analysis of my thesis; the Director of Infection Prevention and Control at the Saudi MoH; Dr Abdullah Assiri, for his endless support whenever needed; the Acting Director of the NTCPP at the MoH; Dr Abdulhameed Kashkary, for his patience, motivation, enthusiasm, and assistance in data collection; last but not least, Dr Heba Kamal, Director of Riyadh Sector Tuberculosis Control Program.
Footnotes
Peer review under responsibility of Ministry of Health, Saudi Arabia.
Conflicts of interest
None.
References
- [1].World Health Organization . Global tuberculosis report. Geneva: WHO; 2016. [Google Scholar]
- [2].Al-Hajoj S, Varghese B. Tuberculosis in Saudi Arabia: the journey across time. J Infect Dev Countries. 2015;9:222–31. doi: 10.3855/jidc.5296. [DOI] [PubMed] [Google Scholar]
- [3].World Health Organization . Tuberculosis country profiles. Geneva: WHO; 2016. [Google Scholar]
- [4].Al-Orainey I, Alhedaithy MA, Alanazi AR, Barry MA, Almajid FM. Tuberculosis incidence trends in Saudi Arabia over 20 years. Ann Thorac Med. 2013;8:148–52. doi: 10.4103/1817-1737.114303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ministry of Health, Kingdom of Saudi Arabia . MoH; 2015. Statistical yearbook 1436. [Google Scholar]
- [6].General Authority for Statistics, Kingdom of Saudi Arabia . GASTAT; 2016. Population estimates. [Google Scholar]
- [7].Al-Bishri J, Masoodi I, Adnan M, Tariq M, Abdullah H, Abdulgoni T, et al. Population dynamics and tuberculosis: a cross sectional study of an overlooked disease in Saudi Arabia. Ger Med Sci. 2014;12 doi: 10.3205/000187. Doc02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Memish ZA, Bamgboye EA, Abuljadayel N, Smadi H, Abouzeid MS, Al Hakeem RF. Incidence of and risk factors associated with pulmonary and extra-pulmonary tuberculosis in Saudi Arabia (2010–2011) PLoS One. 2014;9:e95654. doi: 10.1371/journal.pone.0095654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].MMWR primary multi-drug resistant tuberculosis 1999 Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm4830a3.htm; 1999 [accessed 16.03.07].
- [10].Weis SE, Slocum PC, Blais FX, King B, Nunn M, Matney GB, et al. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. N Engl J Med. 1994;330:1179–84. doi: 10.1056/nejm199404283301702. [DOI] [PubMed] [Google Scholar]
- [11].Burman WJ, Dalton CB, Cohn DL, Butler JR, Reves RR. A cost-effectiveness analysis of directly observed therapy vs self-administered therapy for treatment of tuberculosis. Chest. 1997;112:63–70. doi: 10.1378/chest.112.1.63. [DOI] [PubMed] [Google Scholar]
- [12].Al-Hajjaj MS. The outcome of tuberculosis treatment after implementation of the national tuberculosis control and prevention program in Saudi Arabia. Ann Saudi Med. 2000;20:125–8. doi: 10.5144/0256-4947.2000.125. [DOI] [PubMed] [Google Scholar]
- [13].Menzies R, Rocher I, Vissandjee B. Factors associated with compliance in treatment of tuberculosis. Tuber Lung Dis. 1993;74:32–7. doi: 10.1016/0962-8479(93)90066-7. [DOI] [PubMed] [Google Scholar]
- [14].Dowdy DW, Chaisson RE. The persistence of tuberculosis in the age of DOTS: reassessing the effect of case detection. Bull World Health Org. 2009;87:296–304. doi: 10.2471/blt.08.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].WHO-EMRO. EMROPUB_2015_SaudiArabia 2015 Available from: http://www.emro.who.int/entity/statistics/statistics.html; 2015 [accessed 07.02.07].
- [16].National Tuberculosis Control and Prevention Program (NTCPP), M.O.H., Saudi Arabia . Mobile Teams Guidlines. 2012. [hard book reference published by Ministry of Health, Saudi Arabia]. [Google Scholar]
- [17].Mushkhes AAE. Infectious disease surviellance guidelines for health care professionals. Saudi Arabia: MOH, Department of Infectious Diseases; 2007. [hard book reference published by Ministry of Health, Saudi Arabia] [Google Scholar]
- [18].Central Intelligence Agency The world factbook. 2015.
- [19].Division UNS Treatment success rates under DOTs. 2015.
- [20].World Health Organization . Geneva: WHO; 2018. Tuberculosis handbook. WHO/TB/98.253. [Google Scholar]
- [21].Centers for Disease Control and Prevention . Tuberculosis fact sheet. CDC; 2016. [Google Scholar]
- [22].Bronner LE, Podewils LJ, Peters A, Somnath P, Nshuti L, van der Walt M, et al. Impact of community tracer teams on treatment outcomes among tuberculosis patients in South Africa. BMC Public Health. 2012;7(12):621. doi: 10.1186/1471-2458-12-621. [DOI] [PMC free article] [PubMed] [Google Scholar]