Background
In June 2012 a new CoV (Fig. 1 )1 was isolated from a patient who died from severe pneumonia and multi-organ failure in Saudi Arabia2, 3. This newly identified respiratory viral illness was caused by a novel coronavirus, which was initially designated as human betacoronavirus2, 3, 4, 5, but was eventually named Middle East Respiratory Syndrome Coronavirus (MERS CoV).
Fig. 1.
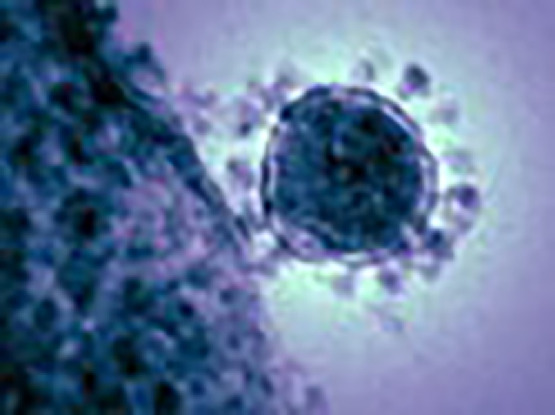
MERS CoV1
The National Institute of Allergy and Infectious Diseases (NIAID), this highly-magnified, digitally-colorized transmission electron microscopic (TEM) image reveals ultrastructural details exhibited by a single, spherical-shaped Middle East Respiratory Syndrome Coronavirus (MERS-CoV) virion.
Reminiscent of, and worth considering as a caution for greater vigilance towards emerging pathogens, the suddenness that SARS CoV emerged as a new cause of severe pulmonary illness, has been replicated in this new aggressive respiratory illness MERS CoV. Unlike SARS CoV, it has not caused the thousands of cases over a short period of time. But it also differs from SARS CoV in that it carries a much higher case fatality rate, and causes more severe illness6, 7.
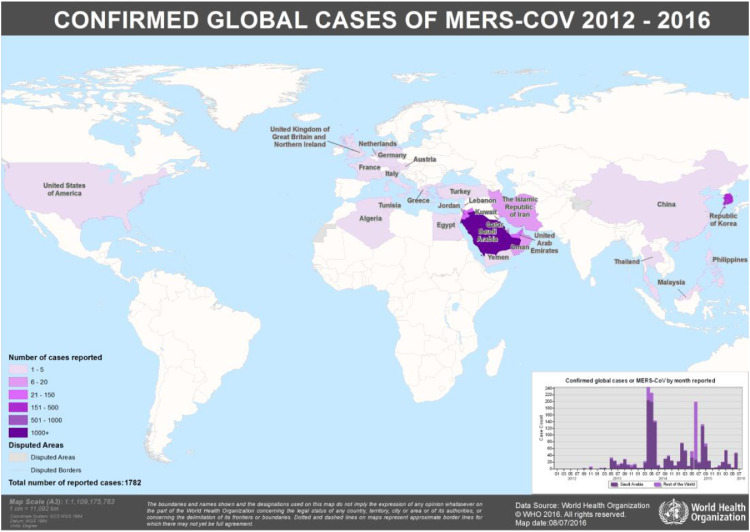
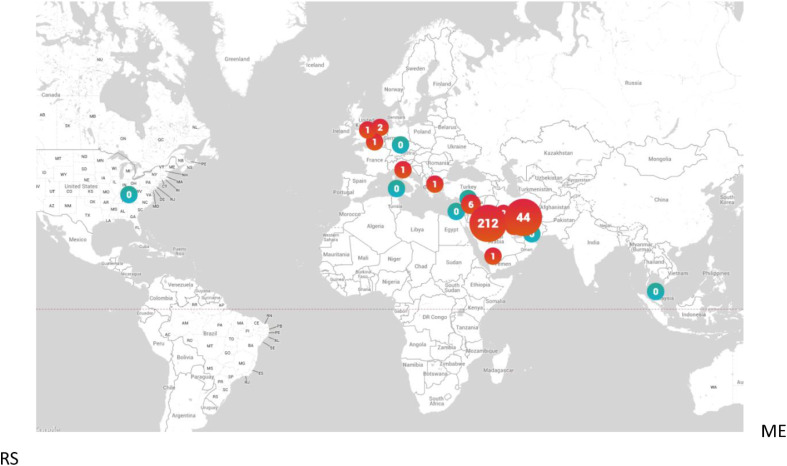
Unlike SARS which seems to have gone quiescent, new MERS cases have been reported well after the initial outbreak, including December 20196. Since September 2012 there have been cases reported in 27 countries across 4 continents, with most human cases occurring in Saudi Arabia (Map 1 and 2 )6. While the greatest spike in cases occurred near 2014, new cases to date continue to be reported6.
Map. 1.
Map 2.
http://cdn1.vox-cdn.com/assets/4499449/Screen_Shot_2014-05-23_at_10.42.47_AM.png
Consider by the end of 12/19 the number of MERS cases 2492, with 858 deaths, resulting in a case fatality rate of 34.43%6. Earlier data from World Health Organization (WHO) as of 12/05/16 there have been 1917 laboratory confirmed cases of MERS CoV since 09/12, reported from 27 countries (Maps 1 and 2), resulting in 677 deaths, yielding a significant case fatality rate (~35%)6; a fatality rate for a coronavirus that is significantly greater than that associated with SARS CoV Delta (~9%), at least among confirmed cases6, 7, 8. These data demonstrate MERS continues to cause illness, though thankfully not at pandemic rates in the same manner as COVID-19.
As with other CoV, including SARS CoV and COVID-19, the exact epidemiology, including the magnitude of mild or asymptomatic illness, remains unknown. That said, among those presenting with MERS, males over 60 yrs of age seem to be at higher risk of severe disease symptoms and death among those infected. The risk associated with age seems consistent with COVID-19 findings as well.
MERS–Virology
CoV belongs to lineage C of betacoronaviruses. It is an enveloped, single stranded, positive sense RNA virus. Structural proteins include spike (S), envelope (E), nucleocapsid (N, and membrane (M) protein9. MERS CoV attaches to the host cell functional receptor human dipeptidyl peptidase 4 (hDDP4). The most immunogenic of the four structural proteins is the S protein, which is the virus’ only surface glycoprotein – it mediates viral attachment and fusion via the hDDP4. Since this receptor is widely found in endothelial and subendothelial tissues in lungs, the gastrointestinal tract (GIT), and kidneys, this may at least in part explain the associated multisystem organ failure associated with severe cases.
Of note for health care providers, according to information from a June 2016 outbreak in Riyadh, Saudi Arabia, 24 contacts tested positive for MERS CoV from an index case who presented in critical condition to the hospital where it was confirmed as another case10. Of the 24, there were 20 healthcare workers that came into contact with the patient. Human to human transmission is well documented, mostly nosocomial outbreaks, but propagated transmission seems to be limited. Healthcare associated clusters of MERS CoV are associated with most reported cases10, 12. The estimated Ro or reproductive rate is estimated at 0.77, 13, 14, although in health care settings it is estimated at >1; still low when compared with measles, a highly contagious viral illness with Ro 12–1815. That notwithstanding, the risk of human to human transmission for MERS CoV or other infections cannot be understated. Not surprisingly, diagnosis has been delayed in certain regions that suffer from overcrowding and poor infection control measures. MERS CoV is still considered a risk for epidemics in Saudi Arabia.
MERS CoV is a zoonotic virus that has entered the human population through contact with infected dromedary camels in the Arabian Peninsula. Camel to human transmission is known16, 17, 18, 19, 20, 21, 22. Research suggests MERS CoV originated in bats before camels which may be the intermediate host. Closely related CoV have been found in bats from other regions23, 24. For example, a novel CoV found in a vesper bat from South Africa is very similar to MERS CoV, differing by only one amino acid. Studies suggest dromedary camels are the primary source for human infection. Nearly 67% of human cases are male, and the median age among reported cases is 527. The majority of cases are of Middle East origin, even though cases have been reported from outside the region, where they likely are seeking care, or are returning from travels. A CoV similar to the MERS CoV cases has been found in Camels from Egypt, Oman, Qatar, and Saudi Arabia. Direct contact with saliva of infected camels, or consuming milk or meat from same are considered a risk factor. One study noted the seroprevalence of MERS CoV is several times higher in people with regular exposure to camels compared with the general population17, 18, 19 although there are cases w/out contact with sick animals or products derived from them.
Clinical
MERS CoV can cause a spectrum of illness ranging from mild upper respiratory symptoms to rapidly progressive lower respiratory infection/pneumonia (1–8,16,25–30) (Table 1 )31b. Fever, cough, and breathing difficulties accompany pneumonia that can progress to acute respiratory distress syndrome (ARDS), multi-system organ failure, and death25, 26, 27, 28, 29, 30, 31 31b. Many patients present with non-pulmonary symptoms such as headache, myalgia, vomiting, and diarrhea. MERS CoV progresses more rapidly to pulmonary failure, as well as acute kidney injury (AKI) than SARS CoV. Patients with underlying comorbidities are associated with more advanced, complicated disease and death, than essentially healthy patients.
Table 1.
| Feature | SARS | MERS | COVID-19 |
|---|---|---|---|
| CLINICAL SIGN OR SYMPTOM | |||
| Fever or chills | Yes | Yes | Yes |
| Dyspnea | Yes | Yes | |
| Malaise | Yes | Yes | |
| Myalgia | Yes | Yes | |
| Headache | Yes | Yes | Yes |
| Cough | Dry | Dry or productive | Dry (productive w/progressive illness) |
| Diarrhea | Yes | Yes | +/- |
| Nausea or vomiting | Yes | Yes | Less common |
| Sore throat | Yes | Uncommon | Less common/but possible |
| Arthralgia | Yes | Uncommon | Less common/but possible |
| IMAGING FINDINGS | |||
| Acute phase | |||
| Initial imaging | |||
| Normal | 15–20% of patients | 17% of patients | 15–20% of patients |
| Abnormalities | |||
| Common | Peripheral multifocal airspace opacities (GGO, consolidation or both) on chest XRay lung and CT scans | Diffuse findings similar to SARS | Diffuse findings similar to SARS and MERS; may be, more diffuse early, or more rapidly progressive. B/L involvement to be expected |
| Rare | Pneumothorax | Pneumothorax | Pneumothorax |
| Not seen | Cavitation, lymphadenopathy | Cavitation, lymphadenopathy | Cavitation, lymphadenopathy |
| Appearance as | Unilateral, focal (50%); Multifocal (40%); diffuse (10%) Bilateral, multifocal CXR | Bilateral, multifocal basal airspace on or CT (80%), isolated unilateral (20%) | Bilateral, multifocal, as well as basal airspace are common findings. Of note, a </=15% may present with normal CXR |
| Follow-up imaging appearance | Unilateral, focal (25%); Progressive (most common, can be unilateral and multi-focal or bilateral with multi-focal consolidation) | Extensive into upper lobes or perihilar areas, pleural effusion (33%), interlobular septal thickening (26%). | Persistent or progressive airspace opacities |
| Indications of poor prognosis | Bilateral (like ARDS), four or more lung zones,progressive involvement after 12 d | Greater involvement of the lungs, pleural effusion, pneumothorax | Consolidation vs ground glass opacities (GGO) |
| SARS | MERS | COVID-19 | |
| Chronic phase | Data still being reviewed | ||
| Transient reticular opacities (e) | Yes | Yes | |
| Air trapping | Common (usually persistent) | ||
| Fibrosis | Rare | One-third of patients | Data still being reviewed |
Acronyms: GGO = ground-glass opacity, ARDS = acute respiratory distress syndrome. aOver a period of weeks or months.
MERS CoV rapidly triggers cellular damage, and induces a proinflammatory response in cells25.
The most common signs and symptoms associated with MERS CoV at presentation are fever, cough, and shortness of breath. Typically presenting symptoms include fever, cough, chills, dyspnea, myalgia, abdominal pain, nausea, vomiting, and diarrhea.
Laboratory
Abnormalities associated with MERS CoV include thrombocytopenia, lymphopenia, leukopenia, elevated serum lactate dehydrogenase (LDH), elevated aspartate aminotransferase (AST), elevated alanine aminotransferase (ALT), along with abnormal renal function tests5, 29, 30. Superinfection from viral or bacterial causes has been found in some patients and may complicate the course of the disease. Severe cases of MERS may require intensive care and mechanical ventilation, with a relatively large number of patients progressing to respiratory or renal failure.
A retrospective radiology review of CT findings in laboratory confirmed cases of MERS CoV noted that cough, fever, and dyspnea were the most common presenting symptoms. Three of the seven subjects died31, 31b.
The high case fatality rate associated with MERS CoV has sparked interest in the role of virus on the immune system. One area of interest is the potential for cytokine storm, which is also found in COVID-19 patients, and currently being studied extensively7, 32, 33.
Some studies have shown MERS CoV is associated with an attenuated interferon (IFN) response, and does not induce inflammatory cytokines7, 32, 33. However a study by Lau demonstrated delayed Proinflammatory cytokine induction by MERS CoV in Calu 3 cells, while there was a lack of production of antiviral cytokines TNF alpha, IFN beta, and IP 105. TNF alpha is noted as an important acute phase pyrogen that inhibits viral replication. IFN beta can induce antiviral response via upregulation of host factors. By attenuating TNF alpha and IFN beta MERS CoV in comparison to SARS CoV may more readily evade innate antiviral host immunity. The possibility that MERS CoV attenuates innate immunity while inducing an inflammatory response in human lung epithelial cells also exists. A macaque model for MERS CoV revealed the virus caused localized to widespread pneumonia in all infection animals. MERS CoV was noted by Lau et al to increase IL 8. In RSV, higher levels of IL 8 seems to correlate with increased disease severity.
According to the CDC and WHO data, the median incubation period for secondary cases associated with limited human-to-human transmission is approximately 5 days (range 2-14 days)6, 8, which, according to WHO data, is similar to COVID-19, as will be discussed later. Of note, in MERS-CoV patients, the median time from illness onset to hospitalization is approximately 4 days. In critically ill patients, the median time from onset to intensive care unit (ICU) admission is approximately 5 days, and median time from onset to death is approximately 12 days. In one series of 12 ICU patients, the median duration of mechanical ventilation was 16 days, and median ICU length of stay was 30 days, with 58% mortality at 90 days.
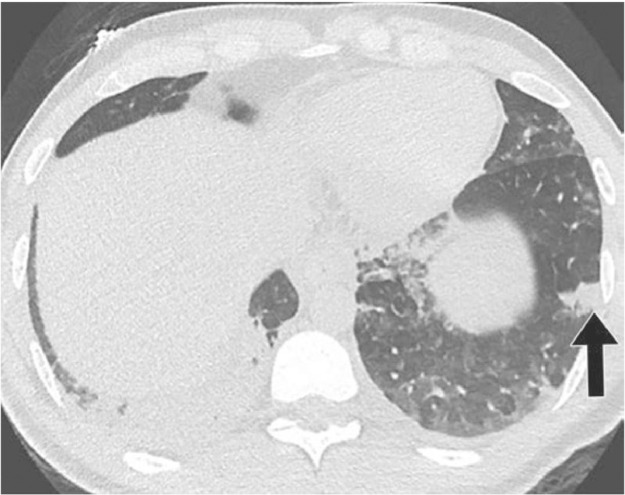
Chest XRays should be obtained early in the course of progressive pulmonary illness (Fig. 2 )31. Radiographic findings may include unilateral or bilateral patchy densities or opacities, interstitial infiltrates, consolidation, and pleural effusions. Rapid progression to acute respiratory failure, acute respiratory distress syndrome (ARDS), refractory hypoxemia, and extrapulmonary complications (acute kidney injury requiring renal replacement therapy, hypotension requiring vasopressors, hepatic inflammation, septic shock) has been reported.31b
Fig. 2.
Lung CT MERS CoV31
27-year-old man with Middle East respiratory syndrome Patient was a smoker who was healthy otherwise. CT was performed 8 days after admission, and 20 days after onset of symptoms. Patient was eventually discharged. Lower lung CT image show large right lower lobe and small focal left lower lobe subpleural consolidations.
Clinical testing
Key to appropriate testing is a thorough history and physical. It is important to consider multisystem function as well as pulmonary status in patients with severe respiratory illness, including suspected MERS CoV, especially those returning from regions where aggressive pathogens are noted. Involving infectious disease and pulmonologist specialists early on, as well as good critical care management are essential.
As noted earlier, laboratory findings at admission may include leukopenia, lymphopenia, thrombocytopenia, and elevated lactate dehydrogenase levels (LDH). Renal and hepatic function tests, at least for baseline information are worth obtaining. In the Ajlan study31, all seven patients had lymphopenia. Elevated creatinine developed after admission in all patients. AST also were elevated in all patients.
Of note, co-infection with other respiratory viruses and a few cases of co-infection with community-acquired bacteria at admission has been reported in MERS CoV patients. Not surprisingly, nosocomial bacterial and fungal infections have also been reported in mechanically-ventilated patients. According to clinical experience reported to CDC, MERS-CoV virus can be detected with higher viral load and longer duration in the lower respiratory tract compared to the upper respiratory tract, and has been detected in feces, serum, and urine. However, very limited data are available on the duration of respiratory and extrapulmonary MERS-CoV shedding.
Radiographic studies
As mentioned earlier, aggressive testing, isolation, and management are critical in treating highly pathogenic coronaviruses, especially MERS, which among patients who present for medical care, has a high case fatality rate, extrapulmonary involvement in some cases, and rapid deterioration. This includes early radiographic studies7b.
According to the Aljan radiology study31, Chest XRay findings were most notable for airspace and interstitial opacities with MERS CoV. Radiographic findings such as airspace opacities are nonspecific, whereas the interstitial changes were described as reticular or reticulonodular. Total lung opacification and thickening of the bronchovascular markings have been reported as well. Previous chest CT findings with MERS31b have been reported as bilateral patchy or extensive opacities5, 7b. Not unexpected, imaging that had features consistent with acute respiratory distress syndrome were typically identified in sicker patients, and can be found in COVID-19 patients as well (Table 1)31b.
In Aljan31 MERS CoV the most common findings on CT were shows that airspace opacities on CT are common in patients hospitalized with MERS-CoV infection. Airspace opacities are suggestive of an organizing pneumonia pattern. It was also described in H1N1 influenza A viral infections. In most of our patients, ground-glass opacities were more extensive than consolidation. Septal thickening and pleural effusions also were demonstrated. Importantly, tree-in-bud pattern, cavitation, and lymph node enlargement were not seen in our cohort.
Chest XRays and CT Scanning (Fig. 2) are recommended depending upon the presentation, and clinical course. In the Aljan study31 the most common CT finding in hospitalized patients with MERS – CoV infection reveals predominantly subpleural and basilar airspace changes, with more ground glass opacities than consolidation. It is important to note this is a small study. Nevertheless this pattern is consistent with organizing pneumonia. Patients recently returning from the Middle East, presenting with significant respiratory illness, with CT findings of peribronchial region abnormalities, organizing pneumonia, should be considered for MERS CoV infection, and if possible, queried about international travel and occupational exposures.
Laboratory testing/Case definitions8, 34, 35
Case definitions
The CDC provides case definitions. The reader is requested to review periodically this site for updates as the MERS CoV situation can change8, 34. Although emergency testing can be possible for suspected asymptomatic cases, the CDC guidelines for sending samples for laboratory confirmation are predicated upon a combination of clinical and epidemiological factors to identify Persons Under Investigation (PUI) – Table 2. The other two categories per CDC are:
Table 2.
Persons under investigation PUI34
| CLINICAL FEATURES EPIDEMIOLOGICAL RISKS | ||
|---|---|---|
|
Severe illness Fever1and pneumonia or acute respiratory distress syndrome (based on clinical or radiological evidence) |
and | A history of travel from countries in or near the Arabian Peninsula2 within 14 days before symptom onset, or close contact3 with a symptomatic traveler who developed fever1 and acute respiratory illness (not necessarily pneumonia) within 14 days after traveling from countries in or near the Arabian Peninsula2. |
| – or – | ||
| A member of a cluster of patients with severe acute respiratory illness (e.g., fever1 and pneumonia requiring hospitalization) of unknown etiology in which MERS-CoV is being evaluated, in consultation with state and local health departments in the US. | ||
|
Milder illness Fever1and symptoms of respiratory illness (not necessarily pneumonia; e.g., cough, shortness of breath) |
and | A history of being in a healthcare facility (as a patient, worker, or visitor) within 14 days before symptom onset in a country or territory in or near the Arabian Peninsula2 in which recent healthcare-associated cases of MERS have been identified. |
| Fever1or symptoms of respiratory illness (not necessarily pneumonia; e.g., cough, shortness of breath) | and | Close contact3 with a confirmed MERS case while the case was ill. |
Fever may not be present in some patients, such as those who are very young, elderly, immunosuppressed, or taking certain medications. Clinical judgement should be used to guide testing of patients in such situations.
Countries considered in the Arabian Peninsula and neighboring include: Bahrain; Iraq; Iran; Israel, the West Bank, and Gaza; Jordan; Kuwait; Lebanon; Oman; Qatar; Saudi Arabia; Syria; the United Arab Emirates (UAE); and Yemen.
Close contact is defined as a) being within approximately 6 feet (2 meters), or within the room or care area, of a confirmed MERS case for a prolonged period of time (such as caring for, living with, visiting, or sharing a healthcare waiting area or room with, a confirmed MERS case) while not wearing recommended personal protective equipment or PPE (e.g., gowns, gloves, NIOSH-certified disposable N95 respirator, eye protection); or b) having direct contact with infectious secretions of a confirmed MERS case (e.g., being coughed on) while not wearing recommended personal protective equipment. See CDC's Interim Infection Prevention and Control Recommendations for Hospitalized Patients with MERS (https://www.cdc.gov/coronavirus/mers/infection-prevention-control.html). Data to inform the definition of close contact are limited; considerations when assessing close contact include the duration of exposure (e.g., longer exposure time likely increases exposure risk) and the clinical symptoms of the person with MERS (e.g., coughing likely increases exposure risk). Special consideration should be given to those exposed in healthcare settings. For detailed information regarding healthcare personnel (HCP) please review CDC Interim U.S. Guidance for Monitoring and Movement of Persons with Potential Middle East Respiratory Syndrome (MERS-CoV) Exposure (https://www.cdc.gov/coronavirus/mers/hcp/monitoring-movement-guidance.html). Transient interactions, such as walking by a person with MERS, are not thought to constitute an exposure; however, final determination should be made in consultation with public health authorities.
Confirmed case
A confirmed case is a person with laboratory confirmation of MERS-CoV infection. Confirmatory laboratory testing requires a positive PCR on at least two specific genomic targets or a single positive target with sequencing on a second.
Laboratory confirmation of MERS CoV can be obtained by real time polymerase chain reaction (RT PCR)
Probable Case
A probable case is a PUI with absent or inconclusive laboratory results for MERS-CoV infection who is a close contact3 of a laboratory-confirmed MERS-CoV case. Examples of laboratory results that may be considered inconclusive include a positive test on a single PCR target, a positive test with an assay that has limited performance data available, or a negative test on an inadequate specimen.
MERS CoV Testing
NB The reader is advised to check for updated testing procedures with the CDC35.
Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Patients Under Investigation (PUIs) for Middle East Respiratory Syndrome Coronavirus (MERS-CoV) – Version 2.1
This is an updated version of the interim guidance document issued by the Centers for Disease Control and Prevention (CDC) January 2014 based on input from public health partners, healthcare providers, professional organizations, and others. CDC will continue to update the document as necessary to incorporate new information that increases our understanding of MERS-CoV.
Updates:
Minor changes were made to clarify specimen type and collection procedures.
-
(1)
Emphasized the recommendation to collect all 3 specimen types (lower respiratory, upper respiratory, and serum) if possible and not just one or two of the three specimen types for testing using the CDC MERS rRT-PCR assay
-
(2)
Deleted the recommendation to collect a stool specimen for MERS-CoV testing
-
(3)
Provided additional information for collection and processing serum specimens
Before collecting and handling specimens for Middle East Respiratory Syndrome Coronavirus (MERS-CoV) testing, determine whether the person meets the current definition for a “patient under investigation” (PUI) for MERS-CoV infection prepared by the Centers for Disease Control and Prevention (CDC). See case definitions ( https://www.cdc.gov/coronavirus/mers/case-def.html ).
Specimen type and priority
To date, little is known about pathogenic potential and transmission dynamics of MERS-CoV. To increase the likelihood of detecting infection, CDC recommends collecting multiple specimens from different sites at different times after symptom onset, if possible.
Points to consider when determining which specimen types to collect from a patient under investigation for MERS include:
-
•
The number of days between specimen collection and symptom onset
-
•
Symptoms at the time of specimen collection
Additional points to consider:
-
•
Maintain proper infection control when collecting specimens
-
•
Use approved collection methods and equipment when collecting specimens
-
•
Handle, store, and ship specimens following appropriate protocols
Collection of all three specimen types (not just one or two of the three), lower respiratory, upper respiratory and serum specimens for testing using the CDC MERS rRT-PCR assay is recommended.
-
•
Lower respiratory specimens are preferred
-
•Also collecting the following are strongly recommended depending upon the length of time between symptom onset and specimen collection:
-
○Nasopharyngeal and oropharyngeal (NP/OP) specimens
-
○Serum, are strongly recommended depending upon the length of time between symptom onset and specimen collection.
-
○
Of note - Respiratory specimens should be collected as soon as possible after symptoms begin – ideally within 7 days. However, if more than a week has passed since symptom onset and the patient is still symptomatic, respiratory samples should still be collected, especially lower respiratory specimens since respiratory viruses can still be detected by rRT-PCR.
For example – If symptom onset for a PUI with respiratory symptoms was less than 14 days ago, a single serum specimen, an NP/OP specimen and lower respiratory specimen should be collected for CDC MERS rRT-PCR testing.
-
1
If symptom onset for a PUI with an ongoing respiratory tract infection, especially lower, was 14 or more days ago, a single serum specimen for serologic testing in addition to a lower respiratory specimen and an NP/OP specimen are recommended.
-
2
if symptom onset for a PUI with an ongoing respiratory tract infection, especially lower, was 14 or more days ago, a single serum specimen for serologic testing in addition to a lower respiratory specimen and an NP/OP specimen are recommended.
General guidelines
For short periods (≤ 72 hours), most specimens should be held at 2-8°C rather than frozen. For delays exceeding 72 hours, freeze specimens at -70°C as soon as possible after collection (with exceptions noted below). Label each specimen container with the patient's ID number, specimen type and the date the sample was collected.
Respiratory specimens
Lower respiratory tract
Broncheoalveolar lavage, tracheal aspirate, pleural fluid
Collect 2-3 mL into a sterile, leak-proof, screw-cap sputum collection cup or sterile dry container. Refrigerate specimen at 2-8°C up to 72 hours; if exceeding 72 hours, freeze at -70°C and ship on dry ice.
Sputum
Have the patient rinse the mouth with water and then expectorate deep cough sputum directly into a sterile, leak-proof, screw-cap sputum collection cup or sterile dry container. Refrigerate specimen at 2-8°C up to 72 hours; if exceeding 72 hours, freeze at -70°C and ship on dry ice.
Upper respiratory tract
Nasopharyngeal swab AND oropharyngeal swab (NP/OP swab)
Use only synthetic fiber swabs with plastic shafts. Do not use calcium alginate swabs or swabs with wooden shafts, as they may contain substances that inactivate some viruses and inhibit PCR testing. Place swabs immediately into sterile tubes containing 2-3 ml of viral transport media. NP/OP specimens can be combined, placing both swabs in the same vial. Refrigerate specimen at 2-8°C up to 72 hours; if exceeding 72 hours, freeze at -70°C and ship on dry ice.
Nasopharyngeal swab - Insert a swab into the nostril parallel to the palate. Leave the swab in place for a few seconds to absorb secretions. Swab both nasopharyngeal areas.
Oropharyngeal swab (e.g., throat swab) - Swab the posterior pharynx, avoiding the tongue.
Nasopharyngeal wash/aspirate or nasal aspirate
Collect 2-3 mL into a sterile, leak-proof, screw-cap sputum collection cup or sterile dry container. Refrigerate specimen at 2-8°C up to 72 hours; if exceeding 72 hours, freeze at -70°C and ship on dry ice.
Serum
Serum (for serologic testing)
For serum antibody testing: Because we do not want to delay detection of MERS infection and since the prevalence of MERS in the US is low, serologic testing on a single serum sample collected 14 or more days after symptom onset may be beneficial. This is in contrast to serologic testing for many other respiratory pathogens which require collection and testing of acute and convalescent serum specimens. Serologic testing is currently available at CDC upon request and approval. Please be aware that the MERS-CoV serologic test is for research/surveillance purposes and not for diagnostic purposes - it is a tool developed in response to the MERS-CoV outbreak. Contact CDC's Emergency Operations Center (EOC) (770-488-7100) for consultation and approval if serologic testing is being considered
Serum (for rRT-PCR testing)
For rRT-PCR testing (i.e., detection of the virus and not antibodies): A single serum specimen collected optimally during the first 10-12 days after symptom onset is recommended. Note: The kinetics of MERS-CoV are not well understood. Once additional data become available, these recommendations will be updated as needed.
Minimum serum volume needed:
The minimum amount of serum required for MERS-CoV testing (either serologic or rRT-PCR) is 200 µL. If both MERS-CoV serology and rRT-PCR tests are planned, the minimum amount of serum required is 400 µL (200 µL for each test). Serum separator tubes should be stored upright for at least 30 minutes, and then centrifuged at 1000–1300 relative centrifugal force (RCF) for 10 minutes before removing the serum and placing it in a separate sterile tube for shipping (such as a cryovial). Refrigerate the serum specimen at 2-8°C and ship on ice-pack; freezing and shipment of serum on dry ice is permissible.
Children and adults: Collect 1 tube (5-10 mL) of whole blood in a serum separator tube.
Infant: A minimum of 1 mL of whole blood is needed for testing pediatric patients. If possible, collect 1 mL in a serum separator tube.
III. Shipping
Specimens from suspected MERS cases must be packaged, shipped, and transported according to the current edition of the International Air Transport Association (IATA) Dangerous Goods Regulations. Shipments from outside of the United States may require an importation permit that can be obtained from CDC.
Specimens should be stored and shipped at the temperatures indicated above. If samples are unable to be shipped within 72 hours of collection, they should be stored at -70 °C and shipped on dry ice. When shipping frozen specimen from long distances or from international locations, it is best to use a combination of dry ice and frozen gel ice-packs. The gel ice-packs will remain frozen for a day or two after the dry ice has dissipated.
All specimens must be pre-packed to prevent breakage and spillage. Specimen containers should be sealed with Parafilm® and placed in ziplock bags. Place enough absorbent material to absorb the entire contents of the Secondary Container (containing Primary Container) and separate the Primary Containers (containing specimen) to prevent breakage. Send specimens with cold packs or other refrigerant blocks that are self-contained, not actual wet ice. This prevents leaking and the appearance of a spill. When large numbers of specimens are being shipped, they should be organized in a sequential manner in boxes with separate compartments for each specimen.
Additional useful and detailed information on packing, shipping, and transporting specimens can be found at Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Middle East Respiratory Syndrome Coronavirus (MERS-CoV) (https://www.cdc.gov/coronavirus/mers/guidelines-lab-biosafety.html).
CDC recommends against the following:
-
•
Do not place any dry ice in the "Primary Container" or "Secondary Container", foam envelopes, ziplock bags, cryovial boxes, or hermetically sealed containers.
-
•
Do not place Primary Containers sideways or upside down in ziplock bags.
-
•
Do not place any paperwork in the Secondary Containers or ziplock bags, so as not to damage the paperwork.
-
•
Do not use biohazard/autoclave bags to prepack your materials due to the inadequate seal of these bags.
For additional information, consultation, or the CDC shipping address, contact the CDC Emergency Operations Center (EOC) at 770-488-7100. Specimens should be shipped for overnight delivery - if Saturday delivery is planned, special arrangements must be made with the shipping company.
MERS TREATMENT OPTIONS
There are no commercially available or FDA approved therapeutics specifically designated as MERS CoV antivirals. As of 2020 there remain a variety of candidate therapeutics in development against COVID019 specifically, highly pathogenic coronaviruses such as SARS and MERS, and coronaviruses in general, including those that cause “common cold-like” symptoms, but none have emerged as fully capable of treating these viruses with the same consistency as neuraminidase antivirals have against influenza. And even in that context there are some clinical considerations, and limitations.
There continues to be ongoing research into repurposing antivirals that are approved for other clinical indications such as HIV, influenza, and other illnesses, as possible treatments – monotherapy or in combination, against COVID-19 and other coronaviruses. Brief updates are provided below.
COVID-19 has reenergized research into greater study of the pathogenicity of coronaviruses, and newly or better described characteristics of highly pathogenic coronaviruses has led to increased insights into additional antiviral and vaccine targets. Significant research into a wide array of therapeutic approaches is undergoing at unprecedented levels internationally. As will be seen in the COVID-19 Therapeutics Section of this article, multiple options are being clinically tested that warrant consideration for the clinician faced with treating COVID-19 patients.
The mainstay of therapy for MERS CoV remains supportive care, especially respiratory care, while managing circulatory, renal, hepatic and neurological function, as well as protecting against secondary infections. Although attempted in both the SARS CoV and early MERS CoV outbreaks, immune based interventions – interferon – resulting in equivocal outcomes. Non human primate studies using IFN a2b and Ribavirin against MERS CoV demonstrated improved outcomes but it is worth noting treatment was initiated soon after viral infection initiated; this is unlikely going to be the case with human infection, where a delay in presentation to health care, or delayed diagnosis will likely preclude the same rapidity of treatment that occurred in the study6, 7, 8, 32, 33.
Therapeutic Interventions Attempted
The role for interferon in MERS CoV remains unclear; clinical trials are needed to better characterize successful strategies. But the limited number of cases make such studies difficult.
In addition to various interferon treatment protocols, Ribavirin – a potent nucleoside analog – has been utilized against RNA viruses with varying degrees of success7, 32, 33. Unfortunately Ribavirin presents a risk for adverse effects including hemolytic anemia. Interferon is not without side effect risk either.
The early use of corticosteroids in SARS CoV infected patients resulted in increased viral load, critical care/intensive care unit admission, and death7, 33.
Other approaches to CoV include neutralizing antibodies from convalescent plasma or hyperimmune globulin; these may hold some promise in reducing the CFR33, 36, 37, 38. Convalescent plasma was shown to decrease mortality in SARS CoV patients if provided to patients within 14 days of illness36. However in order for this to be effective, rapid diagnosis of patients, and survivors is required in order to obtain, and utilize these interventions. Towards that end, a network for convalescent plasma is being assembled, allowing safety, effectiveness and logistic feasibility testing.37. That said, to date no host derived interventions have been shown to consistently confer significant benefit to severely ill MERS CoV patients via controlled study.
While to date there are no antivirals currently licensed for use against MERS CoV, some candidate drugs are in development.
Two categories of candidates showed early promise. An adenine analogue that can be incorporated into viral RNA, which can disrupt virus replication, and has shown in vitro activity against MERS CoV, as well as benefit against Ebola in non human primates (NHPs). Also there is a nucleoside analogue for treatment of filoviruses, CoV, and other RNA viruses. Researchers are working on other molecular approaches to treat CoV7, 39, 40, 41, 42.
Ribavirin is a guanine, oral nucleoside analogue, which inhibits viral RNA-dependent RNA polymerase. In trials exploring the use of Ribavirin against MERS, it was often in combination with other therapeutics, including interferon. Evidence revealed it did not confer significant clinical benefit on outcomes or viral clearance33, 41, 42.
It has shown some in vitro activity against SARS, where high concentrations/high doses were required to inhibit viral replication (1.2 g to 2.4 g PO every 8 hours), and combination therapy via intravenous or enteral administration33, 42, 43. Studies on the use of Ribavirin as a treatment for SARS were either inconclusive in terms of clinical benefit, or suggested possible harm referable to adverse events, which included hepatic and hematologic deleterious effects33, 42, 43.
Ribaviran as a potent nucleoside analogue has been, and continues to be used with varying degrees of success against RNA viruses, but there is the potential for adverse side effects including hemolytic anemia, metabolic derangements. Interferon can also elicit adverse effects, although they have demonstrated value against viral infection44, 45, 46, 47.
Another repurposing of antivirals involved Lopinavir-ritonavir combination therapy trialed against MERS. The medication is an oral protease inhibitor48, 49. Early success with treating SARS in 2003, using lopinavir/ritonavir and ribavirin was suggested33b,48, 48. Mortality and need for intensive respiratory support was noted33b,48, 48. In an animal study involving marmosets, lopinavir-ritonavir or interferon beta – 1b reduced viral load and improved lung pathology48, 49. Combination antiviral therapy for patients hospitalized with severe influenza was noted in some cases to confer greater results, for those with high viral loads at presentation50, 51.
It is worth noting that studies have shown both SARS coronavirus and MERS coronavirus their viral loads peak at ~7 – 10 days after symptoms begin compared to COVID-19 coronavirus where viral loads seem to peak at the time of clinical presentation 33, 50, 51, 52, 53. This adds another level of clinical challenge in terms of managing this new, highly pathogenic coronavirus, and underscores the importance of time sensitivity in the administration of potentially lifesaving medications.
Another promising approach includes monoclonal antibodies (mAbs). mAbs have already demonstrated benefit against certain cancers and autoimmune disorder management7, 38. To date the only pathogen for which there is a licensed mAb is respiratory syncytial virus (RSV).
Prevention
WHO recommends standard precautions with all patients, especially those displaying early signs of respiratory illness, as the diagnosis – whether common cold, or MERS CoV or RSV or pertusis or other pathogen related infection. Also droplet precautions should be added to standard precautions when providing care to such patients, including those with acute respiratory illness. Although eye protection is recommended for probable or confirmed cases of MERS CoV, and airborne precautions for aerosol generating procedures, this would seem prudent even in cases clearly not MERS CoV or SARS CoV.
From an infection control perspective, among the known human coronaviruses they are capable of surviving on environmental surfaces for up to 3 hours11. The SARS and MERS experiences, as well as previous HCoV, underscore the threat of transmissibility; coronaviruses may be transmitted from person-to-person by droplets, hand contamination, fomites, and small particle aerosols.
Underscoring the risk of nosocomial infection, in 2015 there was an outbreak in the Republic of Korea involving 186 cases from 17 health care settings. It was the result of a single imported case. Human to human transmission to family contacts, healthcare workers and patients sharing the same wards. Of note, environmental contamination, and the potential for MERS CoV to survive on surfaces may have contributed to the outbreak. Other recent outbreaks in Jordan and the National Guard Hospital in Riyadh have occurred involving 16 and 100 cases respectively.
Not unlike the potential for other pathogens to spread, overcrowding in health care and residential facilities can facilitate transmission for MERS CoV, as with other respiratory contagions. As such, lessons learned from Saudi Arabia, Korea and elsewhere can serve to catalyze efforts to implement enhanced infection control in our clinical practice settings.
Avoiding camels, especially dromedary camels, farms, as well as not consuming raw milk, urine, or meat are also worth considering. Camels infected with MERS CoV may not appear ill. If contact with animals, especially dromedary camels cannot be avoided, strict hand washing is recommended. Personal protection, including face/eye if working with camels or other animals.
Patients who must travel to the region are encouraged to present to a health care facility especially if fever and respiratory symptoms develop. According to a WHO update, multiple cases from the United Arab Emirates, Qatar, Oman, Kuwait, and Bahrain have links to dromedary camels. These patients were thought to be infected via contact with infected dromedaries or close primary cases (UAE). A good practice for anyone who works with potential contaminants – whether in the US or Middle East farm – remove work clothes that may carry materials from farms and animal contact (Poster 1)53.
To date few cases have occurred in Europe or the US. However with the emergence of medical tourism – cosmetic and advanced surgeries – patients who reside in the Middle East may bring with them diseases endemic to the region, including MERS CoV. The astute clinician will be aware of potential importation of illness.
The CDC provides a following travel warning for persons planning on visiting regions where MERS CoV remains (Poster 1). For more information: call 800-CDC-INFO (232-4636) or visit www.cdc.gov/travel
Vaccines
Vaccination is a critically important preventive measure, but to date no MERS CoV vaccine is available.
As of 2020 there are sporadic cases of MERS-CoV, which continues to cause illness since its emergence in 2012, and with a mortality rate estimated at greater than 30%. MERS still remains a potential outbreak threat, and is a dangerous disease causing pathogen. As with SARS CoV and other viral threats of public health concern, there is a great need for both prophylactic measures – vaccines, and more targeted therapies.
There is significant research effort underway internationally to develop an effective vaccine. As with the SARS CoV, there are a variety of approaches. One attempt which shows early animal efficacy is by a vaccine candidate consisting of chimeric virus-like particles (VLP) expressing the receptor binding domain (RBD) of MERS-CoV. The researchers have fused canine parvovirus (CPV) VP2 structural protein gene with the RBD of MERS-CoV; it self-assembles into chimeric, spherical VLP (sVLP). This sVLP retained certain parvovirus characteristics, such as the ability to agglutinate pig erythrocytes, and structural morphology similar to CPV virions. Immunization with sVLP induced RBD-specific humoral and cellular immune responses in mice. sVLP-specific antisera from these animals were able to prevent pseudotyped MERS-CoV entry into susceptible cells, with neutralizing antibody titers reaching 1: 320. Of note, Interferon (IFN) - IFN-γ, IL-4 and IL-2 secreting cells induced by the RBD were detected in the splenocytes of vaccinated mice by ELISpot. Mice given sVLP or an adjuvanted sVLP vaccine elicited T-helper 1 (Th1) and T-helper 2 (Th2) cell-mediated immunity. Although early stage, resarch sVLP displaying the RBD of MERS-CoV may become, or lead to an effective vaccine against MERS-CoV. Human research is needed55. Of note, several of these approaches will be applied to developing vaccines against COVID-19, which will be discussed in the next section (33c).
 |
For more information: call 800-CDC-INFO (232-4636) or visit www.cdc.gov/travelPoster 154
References
- 1.Image of Middle East Respiratory Syndrome (MERS) Coronavirus as isolated by The National Institute of Allergy and Infectious Diseases (NIAID)https://phil.cdc.gov/phil/details.aspLast accessed 03/12/17
- 2.Zaki AM, van Boheemen S, Bestebroer TM, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 3.Bermingham A. Severe respiratory illness caused by a novel coronavirus in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro Surveill. 2012;17:20290. [PubMed] [Google Scholar]
- 4.DeGroot RJ. Middle East respiratory syndrome coronavirus (MERS – CoV) announcement of the Cornoavirus Study Group. J Virol. 2013;87:7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clnical characteristics of 47 cases of Midle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Inf Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) MERS update and Global Summary. http://www.who.int/emergencies/mers-cov/en/
- 7.Modjarrad K. Treatment strategies for Middle East Respiratory Syndrome Coronavirus. J Virus Erad. 2016;2:1–4. doi: 10.1016/S2055-6640(20)30696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseiny M, Kooraki2 S, Gholamrezanezhad A, Reddy S, Myers L. Radiology Perspective Of Coronavirus Disease 2019 (COVID-19): Lessons From Severe Acute Respiratory Syndrome And Middle East Respiratory Syndrome AJR 2020 May (214) www.ajronline.org doi.org/10.2214/AJR.20.22969 Last accessed 06/11/20 World Health Organization website. Middle East respiratory syndrome coronavirus (MERS-CoV). www.who.int/emergencies/mers-cov/en/Accessed 4 February 2020 [DOI] [PubMed]
- 8.Centers for Disease Control and Prevention (CDC) Middle East Respiratory Syndrome (MERS) Coronavirushttps://www.cdc.gov/coronavirus/mers/index.htmlLast accessed 03/12/17
- 9.Chan JFW, Chan KH, Choi GKY, To KKW, et al. Differential cell line susceptibility to the emerging novel human betacoronavirus 2c EMC/2012: implications for disease pathogenesis and clinical manifestation. J Infect Dis. 2013;207:1743–1752. doi: 10.1093/infdis/jit123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization Middle East Respiratory Syndrome Coronavirus Emergency Preparedness Responsehttp://www.who.int/csr/don/21-june-2016-mers-saudi-arabia/en/Last accessed 03/12/17
- 11.Sizun J, Yu MW, Talbot PJ. Survival of human coronaviruses 229E and OC43 in suspension after drying on surfaces: a possible source of hospital-acquired infections. J Hosp Infect. 2000;46:55–60. doi: 10.1053/jhin.2000.0795. http://SARSReference.com/lit.php?id=11023724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omrani AS. Shalhoub S. Middle East Respiratory Syndrome Coronavirus (MERS CoV): what lessons can we learn? J Hosp Infec. 2015;91(3):188–196. doi: 10.1016/j.jhin.2015.08.002. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poletto C, Pelat C, Levy-Bruhl D, et al. Assessment of the Middle East respiratory syndrome coronavirus (MERS–CoV) epidemic in the Middle East and risk of international spread using a novel maximum likelihood analysis approach. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.23.20824. [DOI] [PubMed] [Google Scholar]
- 14.Majumder MS, Riveres C, Lofgren E, Fisman D. Estimation of MERS – coronavirus reproductive number and case fatality rate for the Spring 2014 Saud Arabia outbreak insights from publicly available data. PloS Curr. 2014;6 doi: 10.1371/currents.outbreaks.98d2f8f3382d84f390736cd5f5fe133c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michigan Center for Public Health Preparedness, epidemiology of Ro and pathogen transmission. https://practice.sph.umich.edu/micphp/epicentral/basic_reproduc_rate.phpLast accessed 02/13/17
- 16.Aleanizy FS, Mohmed N, Alqahtani FY, Hadi Mohamed RAE. Outbreak of Middle East Respiratory Syndrome coronavirus in Saudia Arabia: a retrospective study. BMC Infectious Diseases. 2017;17:23. doi: 10.1186/s12879-016-2137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alagaili AN, Briese T, Mishra N, et al. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. M Bio. 2014 doi: 10.1128/mBio.00884-14. 5e00884-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deem SL, Fevre EM, Kinnaird M, et al. Serological evidence of MERS CoV antibodies in dromedary camesl (camelus dromedaries) in Laikipia County, Keny. PloS One20 [DOI] [PMC free article] [PubMed]
- 19.Raj VS, Faraq EA, Reusken CB, et al. Isolation of MERS coronavirus from a dromedary camel, Qatar, 2014. Emerg Infect Dis. 2014;20:1339–1342. doi: 10.3201/eid2008.140663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alraddadi BM, Watson JT, Amlarashi A, et al. Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans, Saudi Arabia. Emerg Infect Dis. 2016;22:49–55. doi: 10.3201/eid2201.151340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kayali G, Peiris M. A more detailed picture of the epidemiology of Middle East respiratory syndrome coronavirus. Lancet Infecct Dis. 2015;15:495–497. doi: 10.1016/S1473-3099(15)70128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller MA, Meyer B, Corman VM, Al Masri M, et al. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide cross sectional serological study. Lancet Infect Dis. 2015;15:629. doi: 10.1016/S1473-3099(15)00029-8. [DOI] [PubMed] [Google Scholar]
- 23.Woo PC, Lau SK, Lam CS, Lau CC, et al. Disocvery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruess as the gene source of alphacoronavirus and betacoronavirus and avian cornnaviruses as the gene source of gammacoronavirus and deltacornavirus. J Virol. 2012;86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hon CC, Lam TY, Shi ZL, Drummond AJ, et al. Evidence of the recombinant origin of a bat severe acute respiratory synddrome OSARS) like coronavirus and its implications on the direct ancestor of SARS coronavirus. J Virol. 2008;82:1819–1826. doi: 10.1128/JVI.01926-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau SKP, Lau CCY, Chan KH, Li CPY, et al. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle Easst respiratory syndrome coronavirus: implications for pathogenesis and treatment. J Gen Virol. 2013;94:2679–2690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 26.Mackay IM, Arden KE. Middle East Respiratory Syndrome: An emerging coronavirus infection tracked by the crowd. Virus Res. 2015;202:60–88. doi: 10.1016/j.virusres.2015.01.021. doi:10.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almaghirabi RS, Omrani AS. Middle East Respiratory Syndrome Coronavirus (MERS CoV) Infection. British J of Hosp Med. 2017;78(10):10. doi: 10.12968/hmed.2017.78.1.23. [DOI] [PubMed] [Google Scholar]
- 28.Memish ZA, Zumla AI, Al-Hakeem RF, Al Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 29.Assiri A, McGeer A, Perl TM, et al. Hospital outbreak of Middlle East Respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdel – Moneim AS. Middle East respiratory syndrome coronavirus (MERS CoV): evidence and speculation. Arch Virol (Epub 2014 Feb 11). [DOI] [PMC free article] [PubMed]
- 31.Ajlan AM Ahyad RA, Jamjoom LG, Alharthy A, Maddani TAMiddle East Respiratory Syndrome Coronavirus (MERS-CoV) Infection: Chest CT Findings AJR2014;203:782-787 [DOI] [PubMed]; Ye Q, Wang B, Mao J.The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J InfectApril 13; 17:20. 10.1016/j.jinf.2020.03.037Last accessed 05/30/20
- Hosseiny M, Kooraki S, Gholamrezanezhad A, Reddy S, Myers L, Hosseiny M, et al. Radiology perspective of coronavirus disease 2019 (COVID-19): lessons from severe acute respiratory syndrome and middle east respiratory syndrome. AJR Am J Roentgenol. 2020;214(5):1078–1082. doi: 10.2214/AJR.20.22969. [DOI] [PubMed] [Google Scholar]
- 32.Falzarano D, deWit E, Rasmussen AL, et al. Treatment with interferon-alpha2b and Ribavirin improves outcome in MERS CoV infected rhesus macaques. Nat Med. 2013;19:1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shalhoub S, Farahat F, Al – Jiffri A, et al. IFN alpha 2a or IFN beta 1 in combination with Ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129–2132. doi: 10.1093/jac/dkv085. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hung I, Lung K, Tso E, Liu R, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open label randomized, phase 2 trial. Lancet. 2020;(395) doi: 10.1016/S0140-6736(20)31042-4. www.thelancet.com 20 May. Last accessed 06/01/20. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19); A review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. Https://jamanetwork.com Last accessed 06/01/20. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control (CDC)MERS Interim Case Definitionshttps://www.cdc.gov/coronavirus/mers/case-def.htmlLast accessed 03/12/17
- 35.Centers for Disease Control (CDC)MERS Clinical Laboratory Testing Recommendationshttps://www.cdc.gov/coronavirus/mers/lab/lab-testing.htmlLast accessed 03/12/17
- 36.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of vonvalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arabi Y, Balkhy H, Hajeer AH, et al. Vol. 4. Springerplus; 2015. p. 709. (Feasibility, Safety, Clinical and Laboratory Effects of Convalescent Plasma Therapy for Patients with Middle East Respiratory Syndrome Coronavirus Infection: a Study Protocol). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du L, Zhao G, Yang Y, et al. A conformation dependent neutralizing monoclonal antibody specifically targeting receptor binding domain in Middle East respiratory syndrome coronavirus spike protein. J Virol. 2014;88:7045–7053. doi: 10.1128/JVI.00433-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warren T, Jordan R, Lo M, et al. Nucloeotide prodrug GS-5734 is a broad spectrum filovirus inhibitor that provides complete therapeutic protection against the development of Ebola virus disease (EVD) in infected non-human primates ID Week 2015 (Oct 7-11), San Diego, CA. https://idsa.confex.com/idsa/2015/webprogram/Paper54208.html
- 40.Bennett R.BioCryst announces Nature publication demonstrating efficacy of BCX4430 in a non-human primate model of filovirus infecton. 2014. http://globenewswire.com/news-release/2014/03/03/614909/10070784/en/biocryst-announcesnature-publication-demonstrating-efficacy-of-bcx4430-in-a-non-human-promate-model-of-filovirus-infection.html
- 41.Morra ME, Van Thanh L, Kamel MG, et al. Clinical outcomes of current medical approaches for Middle East respiratory syndrome: a systematic review and meta-aanalysis. Rev Med Virol. 2018;28(3):e1977. doi: 10.1002/rmv.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arabi YM, Shalhoub S, Mandourah Y, et al. Ribavirin and interferon therapy for critically ill patients with MERS: a multicenter observational study. Clin Infect DisPublished online 06/25/19. Doi:10.1093/cid/ciz544 [DOI] [PMC free article] [PubMed]
- 43.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PloS Med. 2006;3(9):e343. doi: 10.10371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Modjarrad K. Treatment strategies for Middle East respiratory syndrome coronavirus. J Virus Eradication. 2020;2:1–4. doi: 10.1016/S2055-6640(20)30696-8. 2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Falzarano D, deWit E, Rasmussen AL, et al. Treatment with interferon-alpha2b and Ribavirin improves outcome in MERS CoV infected rhesus macaques. Nat Med. 2013;19:1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shalhoub S, Farahat F, Al–Jiffri A, et al. IFN alpha 2a or IFN beta 1 in combination with Ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70:2129–2132. doi: 10.1093/jac/dkv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gross AE, Bryson ML. Oral Ribavirin for the treatment of noninfluenza respiratory viral infections: a systematic review. Ann Pharmacother. 2015;49:1125–1135. doi: 10.1177/1060028015597449. [DOI] [PubMed] [Google Scholar]
- 48.Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan JF, Yao Y, Yeung ML, et al. Treatment withlopinavir/ritonavir or interferon B1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunning J, Baillie JK, Cao B, Hayden FG. Antiviral combinations for severe influenza. Lancet Infect Dis. 2014;14:1259–1270. doi: 10.1016/S1473-3099(14)70821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hung IFN, To KKW, Cchan JFW, et al. Efficacy of clarithromycin-naproxen-oseltamivir combination in the treatment of patients hospitalized for influenza A (H3N2) infection: an open-label randomized, controlled, phase IIb/III trial. Chest. 2017;151:1069–1080. doi: 10.1016/j.chest.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 52.To KK-W, Tsang OT-Y, Leung W-S, et al. Temporal profiles of viral load inposterior oropharyngeal saliva sample and serum antibody responses during infection by SARS CoV-2: an observational cohort study. Lancet Infect Dis. 2020;(20) doi: 10.1016/S1473-3099. published online March 2330196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng VC, Tang BS, Wu AK, Chu CM, Yeun KY. Medical treatment of viral pneumonmia including SARS in immunocompetent adult. J Infect. 2004;49:262–273. doi: 10.1016/j.jinf.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention (CDC) MERS Travel Advisory Posterhttps://wwwnc.cdc.gov/travel/page/infographic-mers-poster
- 55.Wang C, Zheng X, Gai W, Wong G, et al. Novel chimeric viruslike particles vaccine displaying MERS-CoV receptor-binding domain induce specific humoral and cellular immune response in mice. Antiviral Res. 2017;140:55–61. doi: 10.1016/j.antiviral.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]