Abstract
A surprising feature of the SARS-CoV-2 pandemic to date is the low burdens reported in sub-Saharan Africa (SSA) countries relative to other global regions. Potential explanations (e.g., warmer environments1, younger populations2-4) have yet to be framed within a comprehensive analysis accounting for factors that may offset the effects of climate and demography. Here, we synthesize factors hypothesized to shape the pace of this pandemic and its burden as it moves across SSA, encompassing demographic, comorbidity, climatic, healthcare and intervention capacity, and human mobility dimensions of risk. We find large scale diversity in probable drivers, such that outcomes are likely to be highly variable among SSA countries. While simulation shows that extensive climatic variation among SSA population centers has little effect on early outbreak trajectories, heterogeneity in connectivity is likely to play a large role in shaping the pace of viral spread. The prolonged, asynchronous outbreaks expected in weakly connected settings may result in extended stress to health systems. In addition, the observed variability in comorbidities and access to care will likely modulate the severity of infection: We show that even small shifts in the infection fatality ratio towards younger ages, which are likely in high risk settings, can eliminate the protective effect of younger populations. We highlight countries with elevated risk of ‘slow pace’, high burden outbreaks. Empirical data on the spatial extent of outbreaks within SSA countries, their patterns in severity over age, and the relationship between epidemic pace and health system disruptions are urgently needed to guide efforts to mitigate the high burden scenarios explored here.
The trajectory of the SARS-CoV-2 pandemic in lower latitude, lower income countries including in Sub-Saharan Africa (SSA) remains uncertain. To date, reported case counts and mortality in SSA have lagged behind other geographic regions: all SSA countries, with the exception of South Africa, reported less than 27,000 total cases as of June 2020 5 (Table S1) - totals far less than observed in Asia, Europe, and the Americas 5,6. However, recent increases in reported cases in many SSA countries make it unclear whether the relatively few reported cases to date indicate a reduced epidemic potential or rather an initial delay relative to other regions.
Correlation between surveillance capacity and case counts 7 obscure early trends in SSA (Figure S1). Experience from locations in which the pandemic has progressed more rapidly provides a basis of knowledge to assess the relative risk of populations in SSA and identify those at greatest risk. For example, individuals in lower socio-economic settings have been disproportionately affected in high latitude countries,8,9 indicating poverty as an important determinant of risk. Widespread disruptions to routine health services have been reported 10-12 and are likely to be an important contributor to the burden of the pandemic in SSA 13. The role of other factors from demography 2-4 to health system context 14 and intervention timing 15,16 is also increasingly well-characterized.
Factors expected to increase and decrease SARS-CoV-2 risk in SSA
Anticipating the trajectory of ongoing outbreaks in SSA requires considering variability in known drivers, and how they may interact to increase or decrease risk across populations in SSA and relative to non-SSA settings (Figure 1). For example, while most countries in SSA have ‘young’ populations, suggesting a decreased burden (since SARS-CoV-2 morbidity and mortality increase with age 2-4), prevalent infectious and non-communicable comorbidities may counterbalance this demographic ‘advantage’ 14,17-19. Similarly, SSA countries have health systems that vary greatly in their infrastructure, and dense, resource-limited urban populations may have fewer options for social distancing 20. Yet, decentralized, community-based health systems that benefit from recent experience with epidemic response (e.g., to Ebola 21,22) can be mobilized. Climate is frequently invoked as a potential mitigating factor for warmer and wetter settings 1, including SSA, but climate varies greatly between population centers in SSA and large susceptible populations may counteract any climate forcing during initial phases of the epidemic 23. Connectivity, at international and subnational scales, also varies greatly 24,25 and the time interval between viral introductions and the onset of interventions such as lockdowns will modulate the trajectory 7. Finally, burdens of malnutrition, infectious diseases, and many other underlying health conditions are higher in SSA (Table S2), and their interactions with SARS-CoV-2 are, as of yet, poorly understood.
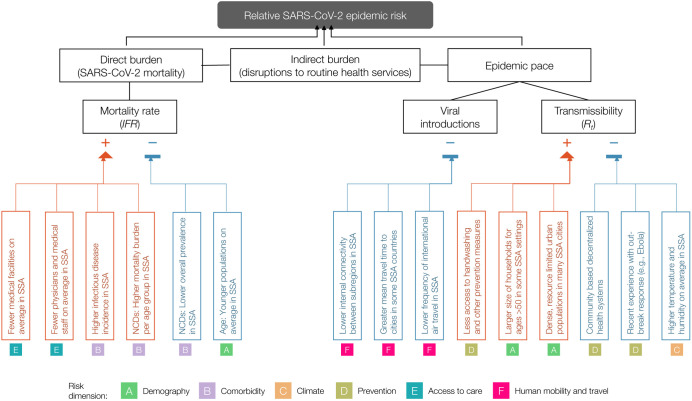
Figure 1 ∣. Hypothesized modulators of relative SARS-CoV-2 epidemic risk in sub-Saharan Africa.
Factors hypothesized to increase (red) or decrease (blue) mortality burden or epidemic pace within sub-Saharan Africa, relative to global averages, are grouped in six categories or dimensions of risk (A-F). In this framework, epidemic pace is determined by person to person transmissibility (which can be defined as the time-varying effective reproductive number, Rt) and introduction and geographic spread of the virus via human mobility.
SARS-CoV-2 mortality (determined by the infection fatality ratio, IFR) is modulated by demography, comorbidities (e.g., non-communicable diseases (NCDs)), and access to care. Overall burden is a function of direct burden and indirect effects due to, for example, disruptions in health services such as vaccination and infectious disease control. Table S2 contains details and the references used as a basis to draw the hypothesized modulating pathways.
The highly variable social and health contexts of countries in SSA will drive location-specific variation in the magnitude of the burden, the time-course of the outbreak, and options for mitigation. Here, we synthesize the range of factors hypothesized to modulate the potential outcomes of SARS-CoV-2 outbreaks in SSA settings by leveraging existing data sources and integrating novel SARS-CoV-2 relevant mobility and climate-transmission models. Data on direct measures and indirect indicators of risk factors were sourced from publicly available databases including from the WHO, World Bank, UNPOP, DHS, GBD, and WorldPop, and newly generated data sets (see Table S3 for details). We organize our assessment around two aspects that will shape national outcomes and response priorities in the event of widespread outbreaks: i) the burden, or expected severity of the outcome of an infection, which emerges from age, comorbidities, and health systems functioning, and ii) the rate of spread within a geographic area, or pace of the pandemic.
We group factors that may drive the relative rates of these two features (mortality burden and pace of the outbreak) along six dimensions of risk: (A) Demographic and socio-economic parameters related to transmission and burden, (B) Comorbidities relevant to burden, (C) Climatic variables that may impact the magnitude and seasonality of transmission, (D) Capacity to deploy prevention measures to reduce transmission, (E) Accessibility and coverage of existing healthcare systems to reduce burden, and (F) Patterns of human mobility relevant to transmission (Table S2).
National and subnational variability in SSA
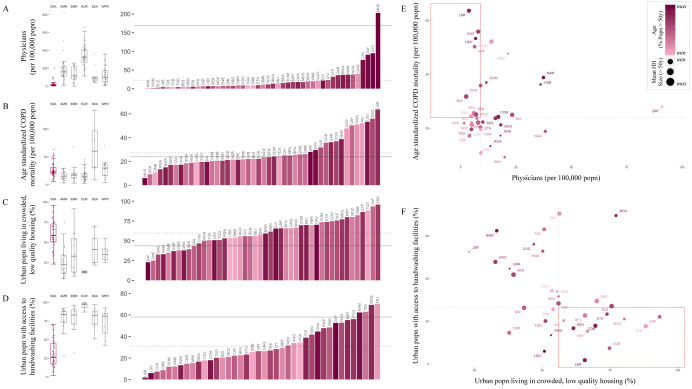
National scale variability in SSA among these dimensions of risk often exceeds ranges observed across the globe (Figure 2A-D). For example, estimates of access to basic handwashing (i.e., clean water and soap 26) among urban households in Mali, Madagascar, Tanzania, and Namibia (62-70%) exceed the global average (58%), but fall to less than 10% for Liberia, Lesotho, Congo DRC, and Guinea-Bissau (Figure 2D). Conversely, the range in the number of physicians is low in SSA, with all countries other than Mauritius below the global average (168.78 per 100,000 population) (Figure 2A). Yet, estimates are still heterogeneous within SSA, with, for example, Gabon estimated to have more than 4 times the physicians of neighboring Cameroon (36.11 and 8.98 per 100,000 population, respectively). This disparity is likely to interact with social contact rates among the elderly in determining exposure and clinical outcomes (e.g., for variation in household size see Figure 2E-F). Relative ranking across variables is also uneven among countries with the result that this diversity cannot be easily reduced (e.g., the first two principal components explain only 32.6%, and 13.1% of the total variance as shown in Figure S5), motivating a more holistic approach to projecting burden.
Figure 2 ∣. Variation among sub-Saharan African countries in select determinants of SARS-CoV-2 risk.
A-D: At right, SSA countries are ranked from least to greatest for each indicator; bar color shows population age structure (% of the population above age 50). Solid horizontal lines show the global mean value; dotted lines show the mean among SSA countries. At left, boxplots show median and interquartile range, grouped by geographic region, per WHO: sub-Saharan Africa (SSA); Americas Region (AMR); Eastern Mediterranean Region (EMR); Europe Region (EUR); Southeast Asia Region (SEA); Western Pacific Region (WPR).
E-F: Dot size shows mean household (HH) size for HHs with individuals over age 50; dashed lines show median value among SSA countries; quadrants of greatest risk are outlined in red (e.g., fewer physicians and greater age standardized Chronic Obstructive Pulmonary Disease (COPD) mortality). See Table S3, Figure S3, and the [SSA-SARS-CoV-2-tool] for full description and visualization of all variables.
Severity of infection outcome
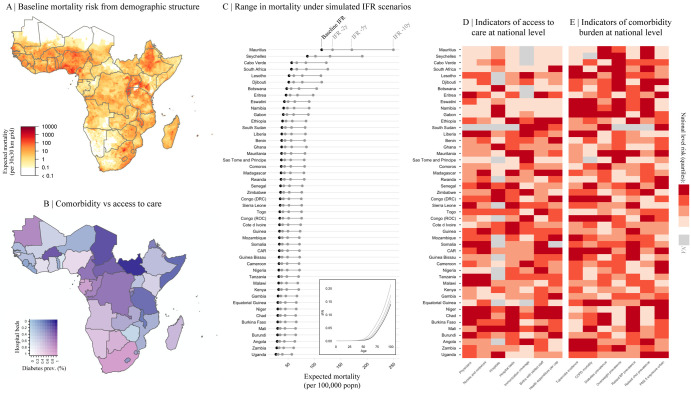
To first evaluate variation in the burden emerging from the severity of infection outcome, we consider how demography, comorbidity, and access to care might modulate the age profile of SARS-CoV-2 morbidity and mortality 2-4. Subnational variation in the distribution of high risk age groups indicates considerable variability, with higher burden expected in urban settings in SSA (Figure 3A), where density and thus transmission are likely higher 27.
Figure 3 ∣. Variation in expected burden for SARS-CoV-2 outbreaks in sub-Saharan Africa.
A: Expected mortality in a scenario where cumulative infection reaches 20% across age groups and the infection fatality ratio (IFR) curve is fit to existing age-stratified IFR estimates (see methods, Table S4). B: National level variation in comorbidity and access to care variables, for e.g., diabetes prevalence among adults and the number of hospital beds per 100,000 population for sub-Saharan African countries. C: The range in mortality per 100,000 population expected in scenarios where cumulative infection rate is 20% and IFR per age is the baseline (black) or shifted 2, 5, or 10 years younger (gray). Inset, the IFR by age curves for each scenario.
D-E: Select national level indicators; estimates of reduced access to care (e.g., fewer hospitals) or increased comorbidity burden (e.g., higher prevalence of raised blood pressure) shown with darker red for higher risk quartiles (see Figure S4 for all indicators). Countries missing data for an indicator (NA) are shown in gray. For comparison between countries, estimates are age-standardized where applicable (see Table S3 for details). See the [SSA-SARS-CoV-2-tool] for high resolution maps for each variable and scenario.
Comorbidities and access to clinical care also vary across SSA (e.g., for diabetes prevalence and hospital bed capacity see Figure 3B). In comparison to settings where previous SARS-CoV-2 infection fatality ratio (IFR) estimates have been reported, mortality due to noncommunicable diseases in SSA increases more rapidly with age (Figure S6). Consequently, we explore scenarios where the SARS-CoV-2 IFR increases more rapidly with age than the baseline expected from other settings. Small shifts (e.g., of 2-10 years) in the IFR profile result in large effects on expected mortality for a given level of infection. For example, Chad, Burkina Faso, and the Central African Republic, while among the youngest SSA countries, have a relatively high prevalence of diabetes and relatively low density of hospital beds. A five year shift younger in the IFR by age profile of SARS-CoV-2 in these settings would result in nearly a doubling of mortality, to a rate that would exceed the majority of other, ‘older’ SSA countries at the unshifted baseline (Figure 3C, see supplement for details of methods). Although there is greater access to care in older populations by some metrics (Figure 2A, correlation between age and the number of physicians per capita, r = 0.896, p < 0.001), access to clinical care is highly variable overall (Figure 3D) and maps poorly to indicators of comorbidity (Figure 3E). Empirical data are urgently needed to assess the extent to which the IFR-age-comorbidity associations observed elsewhere are applicable to SSA settings with reduced access to advanced care. Yet both surveillance and mortality registration 28 are frequently underresourced in SSA, complicating both evaluating and anticipating the burden of the pandemic, and underscoring the urgency of strengthening existing systems 22.
Pandemic pace
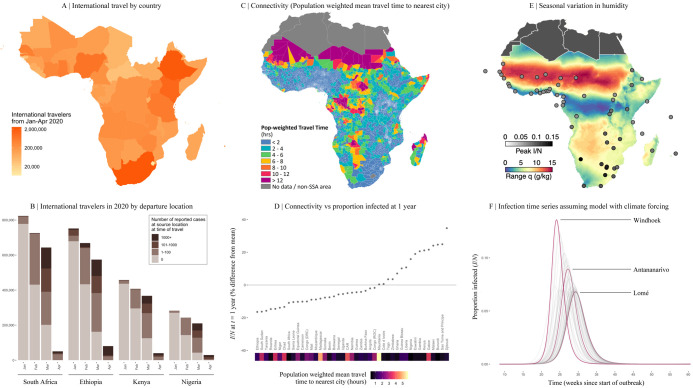
Next, we turn to the pace of the pandemic within each country. The frequency of viral introduction to each country, likely governed by international air travel in SSA 29, determines both the timing of the first infections and the number of initial infection clusters that can seed subsequent outbreaks. The relative importation risk among SSA cities and countries was assessed by compiling data from 108,894 flights arriving at 113 international airports in SSA from January to April 2020 (Figure 4A), stratified by the SARS-CoV-2 status at the departure location on the day of travel (Figure 4B). A small subset of SSA countries received a disproportionately large percentage (e.g., South Africa, Ethiopia, Kenya, Nigeria together contribute 47.9%) of the total travel from countries with confirmed SARS-CoV-2 infections, likely contributing to variation in the pace of the pandemic across settings 29,30.
Figure 4 ∣. Variation in connectivity and climate in sub-Saharan Africa and expected effects on SARS-CoV-2.
A: International travelers to sub-Saharan Africa (SSA) from January to April 2020, as inferred from the number of passenger seats on arriving aircraft. B: For the four countries with the most arrivals, the proportion of arrivals by month coming from countries with 0, 1-100, 101-1000, and 1000+ reported SARS-CoV-2 infections at the time of travel (see Table S5 for all others).
C: Connectivity within SSA countries as inferred from average population weighted mean travel time to the nearest urban area greater than 50,000 population.
D: Mean travel time at the national level and variation in the fraction of the population expected to be infected (I/N) in the first year from stochastic simulations (see methods). E: Climate variation across SSA as shown by seasonal range in specific humidity, q (g/kg) (max average q - min average q). Circles show peak proportion infected. F: The effect of local seasonality in SSA cities on outbreaks (I/N over time) in susceptible populations beginning in March 2020 (see methods).
Once local chains of infection are established, the rate of spread within countries will be shaped by efforts to reduce spread, such as handwashing (Figure 2D), population contact patterns including mobility and urban crowding 27 (e.g., Figure 2C), and potentially the effect of climatic variation 1. Where countries fall across this spectrum of pace will shape interactions with lockdowns and determine the length and severity of disruptions to routine health system functioning.
Subnational connectivity varies greatly across SSA, both between subregions of a country and between cities and their rural periphery (e.g., as indicated by travel time to the nearest city over 50,000 population, Figure 4C). As expected, in stochastic simulations using estimates of viral transmission parameters and mobility (assuming no variation in control efforts, see methods), a smaller cumulative proportion of the population is infected at a given time in countries with larger populations in less connected subregions (Figure 4D). At the national level, susceptibility declines more slowly and more unevenly in such settings (e.g., Ethiopia, South Sudan, Tanzania) due to a lower probability of introductions and re-introductions of the virus locally; an effect amplified by lockdowns. It remains unclear whether the more prolonged, asynchronous epidemics expected in these countries or the overlapping, concurrent epidemics expected in countries with higher connectivity (e.g. Malawi, Kenya, Burundi) will be a greater stress to health systems. Outbreak control efforts are likely to be further complicated during prolonged epidemics if they intersect with seasonal events such as temporal patterns in human mobility 31 or other infections (e.g., malaria).
Turning to climate, despite extreme variation among cities in SSA (Figure 4E), large epidemic peaks are expected in all cities (Figure 4F), even from models where transmission rate significantly declines in warmer, more humid settings. In the absence of interventions, with transmission rate modified by climate only, peak timing varies only by 4-6 weeks with peaks generally expected earlier in more southerly, colder, drier, cities (e.g., Windhoek and Maseru) and later in more humid, coastal cities (e.g., Bissau, Lomé, and Lagos). Apart from these slight shifts in timing, large susceptible populations overwhelm the effects of climate 23, and earlier suggestions that Africa’s generally more tropical environment may provide a protective effect1 are not supported by evidence.
Context-specific preparedness in SSA
Our synthesis emphasizes striking country to country variation in drivers of the pandemic in SSA (Figure 2), indicating variation in the burden (Figure 3) and pace (Figure 4) is to be expected even across low income settings. As small perturbations in the age profile of mortality could drastically change the national level burden in SSA (Figure 3), building expectations for the risk for each country requires monitoring for deviations in the pattern of morbidity and mortality over age. Transparent and timely communication of these context-specific risk patterns could help motivate population behavioral changes and guide existing networks of community case management.
Because the largest impacts of SARS-CoV-2 outbreaks may be through indirect effects on routine health provisioning, understanding how existing programs may be disrupted differently by acute versus longer outbreaks is crucial to planning resource allocation. For example, population immunity will decline proportionally with the length of disruptions to routine vaccination programs 31, resulting in more severe consequences in areas with prolonged epidemic time courses.
Others have suggested that this crisis presents an opportunity to unify and mobilize across existing health programs (e.g., for HIV, TB, Malaria, and other NCDs) 22. While this may be a powerful strategy in the context of acute, temporally confined crises, long term distraction and diversion of resources 32 may be harmful in settings with extended, asynchronous epidemics. A higher risk of infection among healthcare workers during epidemics 33,34 may amplify this risk.
Due to the lag relative to other geographic regions, many SSA settings retain the opportunity to prepare for and intervene in the earlier epidemic phases via context-specific deployment of both routine and pandemic related interventions. As evidenced by failures in locations where the epidemic progressed rapidly (e.g., USA), effective governance and management prior to reaching large case counts is likely to yield the largest rewards. Mauritius 35 and Rwanda 36, for example, have reported extremely low incidence thanks in part to a well-managed early response.
Conclusions
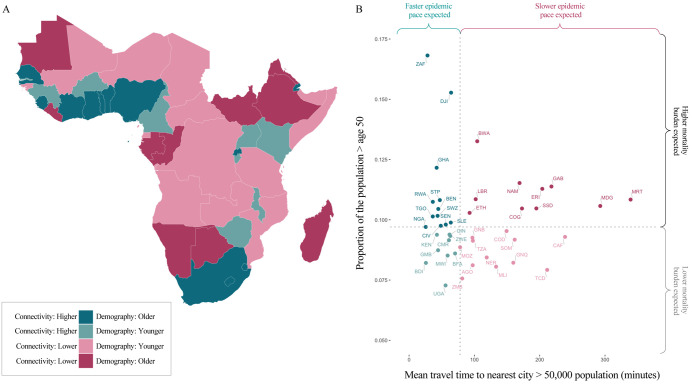
The burden and time-course of SARS-CoV-2 is expected to be highly variable across sub-Saharan Africa. As the outbreak continues to unfold, critically evaluating this mapping to better understand where countries lie in terms of their relative risk (e.g., see Figure 5) will require increased surveillance, and timely documentation of morbidity and mortality over age. Case counts are rising across SSA, but variability in testing regimes makes it difficult to compare observations to date with expectations in terms of pace (Figure S7). The potential to miss large clusters of cases (in contexts with weaker surveillance), combined with the potential that large areas remain unreached by the pandemic for longer (as a result of slower ‘pace’), indicate that immunological surveys are likely a powerful lens for understanding the landscape of population risk 37. When considering hopeful futures with the possibility of a SARS-CoV-2 vaccine, it is imperative that vaccine distribution be equitable, and in proportion with need. Understanding factors that both drive spatial variation in vulnerable populations and temporal variation in pandemic progression could help approach these goals in SSA.
Figure 5 ∣. Expected pace versus expected burden at the national level in SARS-CoV-2 outbreaks in sub-Saharan Africa.
Countries are colored by with respect to indicators of their expected epidemic pace (using as an example subnational connectivity in terms of travel time to nearest city) and potential burden (using as an example the proportion of the population over age 50).
A: In pink, countries with less connectivity (i.e., less synchronous outbreaks) relative to the median among SSA countries; in blue, countries with more connectivity; darker colors show countries with older populations (i.e., a greater proportion in higher risk age groups).
B: Dotted lines show the median; in the upper right, in dark pink, countries are highlighted due to their increased potential risk for an outbreak to be prolonged (see metapopulation model methods) and high burden (see burden estimation methods).
Supplementary Material
Acknowledgements
REB is supported by the Cooperative Institute for Modeling the Earth System (CIMES). AA acknowledges support from the NIH Medical Scientist Training Program 1T32GM136577. AJT is funded by the BMGF (OPP1182425, OPP1134076 and INV-002697). MB is funded by NWO Rubicon grant 019.192EN.017.
Footnotes
Online Content
Methods and additional figures are available in the supplementary materials. In addition, high resolution maps and further visualizations of the risk indicators and simulations studied here can be accessed online through an interactive tool: Link to SSA-SARS-CoV-2 online companion tool: https://labmetcalf.shinyapps.io/covid19-burden-africa/
Data Availability
All data have been deposited into a publicly available GitHub repository: Link to GitHub repository containing data and code: https://github.com/labmetcalf/SSA-SARS-CoV-2
Code Availability
All code has been deposited into the publicly available GitHub repository (same as above): Link to GitHub repository containing data and code: https://github.com/labmetcalf/SSA-SARS-CoV-2
Supplementary Information is available for this paper.
Data and materials availability
All materials are available in the online content
Competing interests
The authors declare no competing interests
References
- 1.Mecenas P., Bastos R., Vallinoto A. & Normando D. Effects of temperature and humidity on the spread of COVID-19: A systematic review. doi: 10.1101/2020.04.14.20064923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salje H. et al. Estimating the burden of SARS-CoV-2 in France. Science (2020) doi: 10.1126/science.abc3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rinaldi G. & Paradisi M. An empirical estimate of the infection fatality rate of COVID-19 from the first Italian outbreak. doi: 10.1101/2020.04.18.20070912 [DOI] [Google Scholar]
- 4.Verity R. et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect. Dis. 20, 669–677 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Africa CDC - COVID-19 Daily Updates. Africa: CDC; https://africacdc.org/covid-19/. [Google Scholar]
- 6.Mortality Analyses. Johns Hopkins Coronavirus Resource Center; https://coronavirus.jhu.edu/data/mortality. [Google Scholar]
- 7.Skrip L. A. et al. Seeding COVID-19 across sub-Saharan Africa: an analysis of reported importation events across 40 countries. doi: 10.1101/2020.04.01.20050203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng X. et al. Case fatality risk of novel coronavirus diseases 2019 in China. doi: 10.1101/2020.03.04.20031005 [DOI] [Google Scholar]
- 9.Collaborative, T. O. et al. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. doi: 10.1101/2020.05.06.20092999 [DOI] [Google Scholar]
- 10.COVID-19 significantly impacts health services for noncommunicable diseases. https://www.who.int/news-room/detail/01-06-2020-covid-19-significantly-impacts-health-services-for-noncommunicable-diseases.
- 11.Maintaining essential health services: operational guidance for the COVID-19 context. https://www.who.int/publications/i/item/covid-19-operational-guidance-for-maintaining-essential-health-services-during-an-outbreak.
- 12.Santoli J. M. Effects of the COVID-19 Pandemic on Routine Pediatric Vaccine Ordering and Administration — United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 69, (2020). [DOI] [PubMed] [Google Scholar]
- 13.Roberton T. et al. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: a modelling study. Lancet Glob Health 8, e901–e908 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker P. G. T. et al. The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science (2020) doi: 10.1126/science.abc0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pei S., Kandula S. & Shaman J. Differential Effects of Intervention Timing on COVID-19 Spread in the United States. medRxiv (2020) doi: 10.1101/2020.05.15.20103655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai S. et al. Assessing the effect of global travel and contact reductions to mitigate the COVID-19 pandemic and resurgence. doi: 10.1101/2020.06.17.20133843 [DOI] [Google Scholar]
- 17.Nepomuceno M. R. et al. Besides population age structure, health and other demographic factors can contribute to understanding the COVID-19 burden. Proceedings of the National Academy of Sciences of the United States of America vol. 117 13881–13883 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark A. et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health (2020) doi: 10.1016/S2214-109X(20)30264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghisolfi S. et al. Predicted COVID-19 fatality rates based on age, sex, comorbidities, and health system capacity. doi: 10.1101/2020.06.05.20123489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.[No title]. https://www.imperial.ac.uk/media/imperial-college/medicine/mrc-gida/2020-05-12-COVID19-Report-22.pdf.
- 21.Kapata N. et al. Is Africa prepared for tackling the COVID-19 (SARS-CoV-2) epidemic. Lessons from past outbreaks, ongoing pan-African public health efforts, and implications for the future. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases vol. 93 233–236 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nachega J. B. et al. From Easing Lockdowns to Scaling-Up Community-Based COVID-19 Screening, Testing, and Contact Tracing in Africa - Shared Approaches, Innovations, and Challenges to Minimize Morbidity and Mortality. Clin. Infect. Dis. (2020) doi: 10.1093/cid/ciaa695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker R. E., Yang W., Vecchi G. A., Metcalf C. J. E. & Grenfell B. T. Susceptible supply limits the role of climate in the early SARS-CoV-2 pandemic. Science (2020) doi: 10.1126/science.abc2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss D. J. et al. A global map of travel time to cities to assess inequalities in accessibility in 2015. Nature 553, 333–336 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Tatem A. J. WorldPop, open data for spatial demography. Scientific Data vol. 4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.[No title]. https://washdata.org/sites/default/files/documents/reports/2019-05/JMP-2018-core-questions-for-household-surveys.pdf.
- 27.Korevaar H. M. et al. Quantifying the impact of US state non-pharmaceutical interventions on COVID-19 transmission. doi: 10.1101/2020.06.30.20142877 [DOI] [Google Scholar]
- 28.Mikkelsen L. et al. A global assessment of civil registration and vital statistics systems: monitoring data quality and progress. Lancet 386, 1395–1406 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Gilbert M. et al. Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet 395, 871–877 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haider N. et al. Passengers’ destinations from China: low risk of Novel Coronavirus (2019-nCoV) transmission into Africa and South America. Epidemiol. Infect. 148, e41 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi S. et al. Reduced vaccination and the risk of measles and other childhood infections post-Ebola. Science vol. 347 1240–1242 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cash R. & Patel V. Has COVID-19 subverted global health? Lancet 395, 1687–1688 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams J. G. & Walls R. M. Supporting the Health Care Workforce During the COVID-19 Global Epidemic. JAMA (2020) doi: 10.1001/jama.2020.3972 [DOI] [PubMed] [Google Scholar]
- 34.Kilmarx P. H. et al. Ebola virus disease in health care workers--Sierra Leone, 2014. MMWR Morb. Mortal. Wkly. Rep. 63, 1168–1171 (2014). [PMC free article] [PubMed] [Google Scholar]
- 35.Covid 19 Mauritius Dashboard. Google Data Studio http://datastudio.google.com/reporting/510dbd29-25cd-4fbb-a47a-68effeda6cf5/page/0z6JB?feature=opengraph.
- 36.Rwanda: WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int.
- 37.Mina M. J. et al. A Global lmmunological Observatory to meet a time of pandemics. Elife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Population Prospects - Population Division - United Nations. https://population.un.org/wpp/Download/Standard/Population/.
- 39.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) — China, 2020. CCDCW 2, 113–122 (2020). [PMC free article] [PubMed] [Google Scholar]
- 40.Team, C. C.-19 R. et al. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) — United States, February 12–March 16, 2020. MMWR. Morbidity and Mortality Weekly Report vol. 69 343–346 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simonnet A. et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (2020) doi: 10.1002/oby.22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conticini E., Frediani B. & Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pollut. 261, 114465 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griffith G. et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. doi: 10.1101/2020.05.04.20090506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shankar A. H. Nutritional Modulation of Malaria Morbidity and Mortality. The Journal of Infectious Diseases vol. 182 S37–S53 (2000). [DOI] [PubMed] [Google Scholar]
- 45.The DHS Program - Quality information to plan, monitor and improve population, health, and nutrition programs. https://dhsprogram.com.
- 46.Chin T. et al. U.S. county-level characteristics to inform equitable COVID-19 response. medRxiv (2020) doi: 10.1101/2020.04.08.20058248 [DOI] [Google Scholar]
- 47.Hersbach H. et al. The ERA5 global reanalysis. Q.J.R. Meteorol. Soc. 64, 29 (2020). [Google Scholar]
- 48.Report 23 - State-level tracking of COVID-19 in the United States. Imperial College; London: http://www.imperial.ac.uk/medicine/departments/school-public-health/infectious-disease-epidemiology/mrc-global-infectious-disease-analysis/covid-19/report-23-united-states/. [Google Scholar]
- 49.Kissler S. M., Tedijanto C., Goldstein E., Grad Y. H. & Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science 368, 860–868 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiwani S. S. & Antiporta D. A. Inequalities in access to water and soap matter for the COVID-19 response in sub-Saharan Africa. Int. J. Equity Health 19, 82 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoler J., Jepson W. E. & Wutich A. Beyond handwashing: Water insecurity undermines COVID-19 response in developing areas. J. Glob. Health 10, 010355 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makoni M. Keeping COVID-19 at bay in Africa. Lancet Respir Med 8, 553–554 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tatem A. J. et al. Ranking of elimination feasibility between malaria-endemic countries. Lancet 376, 1579–1591 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Metcalf C. J. E. et al. Transport networks and inequities in vaccination: remoteness shapes measles vaccine coverage and prospects for elimination across Africa. Epidemiol. Infect. 143, 1457–1466 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adepoju P. Africa’s struggle with inadequate COVID-19 testing. The Lancet Microbe vol. 1 e12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pindolia D. K. et al. The demographics of human and malaria movement and migration patterns in East Africa. Malar. J. 12, 397 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qiu Y., Chen X. & Shi W. Impacts of social and economic factors on the transmission of coronavirus disease (COVID-19) in China. doi: 10.1101/2020.03.13.20035238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li H. et al. Age-Dependent Risks of Incidence and Mortality of COVID-19 in Hubei Province and Other Parts of China. Front. Med. 7, 190 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Global Burden of Disease Study 2017 (GBD 2017) Population Estimates 1950-2017 ∣ GHDx; http://ghdx.healthdata.org/record/ihme-data/gbd-2017-population-estimates-1950-2017. [Google Scholar]
- 60.Brauer M., Zhao J. T., Bennitt F. B. & Stanaway J. D. Global access to handwashing: implications for COVID-19 control in low-income countries. doi: 10.1101/2020.04.07.20057117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alegana V. A. et al. National and sub-national variation in patterns of febrile case management in sub-Saharan Africa. Nat. Commun. 9, 4994 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murthy S., Leligdowicz A. & Adhikari N. K. J. Intensive care unit capacity in low-income countries: a systematic review. PLoS One 10, e0116949 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Linard C., Gilbert M., Snow R. W., Noor A. M. & Tatem A. J. Population distribution, settlement patterns and accessibility across Africa in 2010. PLoS One 7, e31743 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.[No title]. https://dl.acm.org/doi/10.5555/1953048.2078195.
- 65.Jolliffe I. T. & Cadima J. Principal component analysis: a review and recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 374, 20150202 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meyerowitz-Katz G. & Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection-fatality rates. doi: 10.1101/2020.05.03.20089854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wood S. N. Generalized Additive Models: An Introduction with R, Second Edition. (CRC Press, 2017). [Google Scholar]
- 68.R: The R Project for Statistical Computing. https://www.R-project.org/.
- 69.Global Burden of Disease Study 2017 (GBD 2017) Population Estimates 1950–2017 ∣ GHDx; http://ghdx.healthdata.org/record/ihme-data/gbd-2017-population-estimates-1950-2017. [Google Scholar]
- 70.Dong E., Du H. & Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 20, 533–534 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bjornstad O. N., Finkenstadt B. F. & Grenfell B. T. Dynamics of Measles Epidemics: Estimating Scaling of Transmission Rates Using a Time Series SIR Model. Ecological Monographs vol. 72 169 (2002). [Google Scholar]
- 72.Shaman J., Pitzer V. E., Viboud C., Grenfell B. T. & Lipsitch M. Absolute humidity and the seasonal onset of influenza in the continental United States. PLoS Biol. 8, e1000316 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.