Abstract
Objectives:
Skeletal muscle weakness and an increase in fatigability independently contribute to age-related functional decline. The objective of this study was to examine the combined contribution of these deficiencies (i.e., torque capacity) to physical function, and then to assess the functional implications of progressive resistance training (PRT) mediated-torque capacity improvements in mobility-limited older adults.
Design:
Randomized controlled trial.
Setting:
Exercise laboratory on the Health Sciences campus of an urban university.
Participants:
Seventy mobility-limited (Short Physical Performance Battery (SPPB) ≤9) older adults (~79 yrs).
Intervention:
Progressive resistance training or home-based flexibility 3 days/week for 12 weeks.
Measurements:
Torque capacity was defined as the sum of peak torques from an isokinetic knee extension fatigue test. Relationships between torque capacity and performance-based and patient-reported functional measures before and after PRT were examined using partial correlations adjusted for age, sex, and body mass index.
Results:
Torque capacity explained (P<0.05) 10 and 28% of the variance in six-minute walk distance and stair climb time, respectively. PRT-mediated torque capacity improvements were paralleled by increases (P<0.05) in self-reported activity participation (+20%) and advanced lower extremity function (+7%), and associated (P<0.05) with a reduction in activity limitations (r=0.44) and an improved SPPB score (r=0.32).
Conclusion:
Skeletal muscle torque capacity, a composite of strength and fatigue, may be a proximal determinant of physical function in mobility-limited older individuals. To more closely replicate the musculoskeletal demands of real-life tasks, future studies are encouraged to consider the combined interaction of distinct skeletal muscle faculties to overall functional ability in older adults.
Keywords: Skeletal muscle, torque capacity, physical function, older adults, sarcopenia
Introduction
For the past 165 years, life expectancy in the United States has been steadily increasing such that babies born after the year 2000 are projected to live for more than 100 years (1). As a result, the oldest-old (>85 yrs) are the fastest growing segment of the world’s population (2). The prevalence of disability in these very-old adults is high, reaching 97% in centenarians (3), two-thirds of which can be attributed to ambulatory difficulty (e.g., walking or climbing a flight of stairs) (4). Age-related mobility limitations often stem from muscular impairments (5). Lower extremity muscular impairments in particular are proven to be a key instigator of the inability to perform daily tasks (e.g., housekeeping, food preparation, and grocery shopping) (6). Preserving musculoskeletal performance is an effective means to maintain functional independence (7, 8). To maximize the therapeutic potential of interventions seeking to preserve skeletal muscle health, previous reports have attempted to define the individual contribution of various musculoskeletal characteristics (e.g., size, strength, power, and fatigue) to overall physical function, and ultimately, healthy independent aging (9-11).
Skeletal muscle mass begins to decline around the fourth decade of life (1-2% per year) (12, 13) due to a loss of individual muscle fibers (14) and selective fast fiber atrophy (15). Aging muscle loss is paralleled by even more precipitous reductions in skeletal muscle strength and power (2-3% per year) (16-18) that are exacerbated by reduced activity levels (i.e., disuse atrophy) commonly seeing with aging (19). Pertinently, age-related muscle weakness is shown to hinder performance of routine daily tasks such as climbing a flight of stairs (20) or rising from a chair (21) that compromise functional independence and reduce quality of life in older adults. Distinct from skeletal muscle strength and power, muscle fatigue, defined as a decline in performance associated with activity (22) is also negatively affected by the aging process (23, 24), exaggerating age-related functional decline (25, 26). It is important to realize however that unlike laboratory assessments measuring skeletal muscle strength or fatigue in isolation, real-life tasks mandate a threshold level of performance in many skeletal muscle competencies.
Recently, using a novel isokinetic knee extensor fatigue test, Kent-Braun and colleagues summed the peak torques of 120 consecutive maximal contractions to generate a composite index incorporating both strength and fatigue (11). We have since labeled this measure as “skeletal muscle torque capacity”, and demonstrated it to be both reliable and sensitive to progressive resistance training (PRT) induced skeletal muscle adaptations in mobility-limited older adults (27). To define the summative contribution of deficits in muscle strength and fatigue to age-related functional decline, the present study examined relationships between torque capacity and physical function in a large cohort of mobility-limited older adults. We then assessed the functional implications of PRT-mediated torque capacity improvements to evaluate the therapeutic benefit of improving torque capacity. We hypothesized that torque capacity would align with both performance-based and patient-reported measures of physical function, and that linearity between these variables would be reflected by enhancements in functional capacity aligned with PRT-mediated torque capacity improvements (27).
Methods
Study Design
This study was a secondary analysis on data collected as part of a single-blind, parallel group, randomized controlled trial comparing the effects of a 12-week progressive resistance training (PRT) program to 12 weeks of home-based flexibility (FLEX) on lower extremity muscle torque capacity in mobility-limited older adults (27). The present study focused on the relationship between knee extensor muscle torque capacity and performance-based and patient-reported functional measures.
Participants
A detailed description of participant characteristics along with accompanying inclusion/exclusion criteria are described elsewhere (27). A total of 70 mobility-limited (SPPB ≤9) community-dwelling older adults (≥70 years) without a history of structured exercise participation (≤20 minutes/week of moderate-intensity physical activity) were randomized to either PRT or FLEX, 3 days/week for 12 weeks. Signed informed consent was obtained from all study participants and study procedures were approved by the Tufts University Health Sciences Institutional Review Board.
Physiological Measure (Independent Variable)
Muscle Torque Capacity. A novel knee extensor torque capacity test was performed with the non-dominant leg using the Biodex System 3 Isokinetic Dynamometer (BiodexMedical Systems, Shirley, NY) as previously described (11). Participants were instructed to perform 120 maximal effort knee extensions, one every two seconds, with the whole test lasting about four minutes. Knee extension velocity was set to 120°/s with passive knee flexion at 240°/s. To ensure adequate familiarization, each participant completed two baseline fatigue tests at least 7 but no more than 21 days apart. Our primary variable of interest was summed torque (i.e., torque capacity), a composite index incorporating both strength and fatigue, calculated by adding the peak torque for each of the 120 repetitions. Due to the marked similarity between baseline torque capacity tests, an average of these two values was used for analysis (27). Each of the participants performed the isokinetic fatigue protocol four times (familiarization, baseline, mid-study, and upon study completion) and it was well tolerated, without a single adverse event and only minor muscle soreness generally diminishing within an hour of test completion. To ensure adequate recovery, the isokinetic fatigue protocol and assessments of physical function were separated by at least 48 hours.
Physical Performance Measures (Dependent Variables)
Performance-based and patient-reported outcomes were collected at baseline, and upon study completion to evaluate both objective and subjective interpretation of the efficacy of the intervention to enhance physical function.
Performance Based Outcomes. Physical function was assessed at baseline and after 12-wk of PRT or FLEX using three lower-extremity performance measures with highly relevant clinical significance.
1) The Short Physical Performance Battery (SPPB) consists of three subtasks: standing balance, habitual walking, and repeated chair rise (28). Each subtask is scored 0-4, which accumulates to a summary score ranging from 0-12 that can be used to evaluate lower-extremity muscle function. Participants are asked to: 1) maintain their balance while standing in different positions (feet side-by-side, semi-tandem, and tandem), 2) walk 4m at their usual pace, 3) stand up from a chair 5 times without using their arms.
2) The six minute walk test (6MWT) is a submaximal exercise test to assess aerobic capacity and endurance (29). Using a 30-meter course, participants are asked to walk as far as they can in six minutes and the distance they cover is recorded in meters. If a participant feels it is too difficult they are allowed to take a rest or to quit, and the distance they covered prior to termination is recorded.
3) For the stair climb test, participants are asked to ascend a set of 10 stairs. They are then asked to ascend the stairs one step at a time as quickly as they can while remaining safe. Time is recorded. Participants are allowed to hold on to the handrails if they need, and study personnel are instructed to follow participants up the stairs to provide assistance if necessary.
Participant Reported Outcomes. To complement objective measures of physical function, we surveyed participants to better understand their subjective interpretation of the effects of the intervention. Questionnaires were given to the participants at baseline and after the 12-week intervention. Surveys were carefully chosen based on their functional relevance for older people and included:
1) The Late Life Function and Disability Index (LLFDI) (30) is an outcome assessment designed specifically for community-dwelling older adults. This instrument assesses and responds to meaningful changes in two distinct outcomes: function (32 items) - a person’s ability to do discrete actions or activities, and disability (16 items) - a person’s performance of socially defined tasks.
2) The Age-Related Muscle Loss Questionnaire (31) is a 14-item measure designed to evaluate the functional impact of sarcopenia and the accompanying loss of muscle strength either in clinical practice or clinical trials.
3) The Pittsburgh Fatigability scale (32) is a brief (10-item) questionnaire that asks participants to rate their levels of physical and mental fatigue while performing various tasks. This assessment is a valid and reliable measure to identify older adults at risk of mobility limitation in clinical or research settings.
Study Intervention
A detailed description of the study interventions (3 days/week for 12 weeks in both groups) has been reported previously (27). In brief, PRT sessions were preceded by a light warm-up (5-minute treadmill walking) and included leg press, seated row, leg extension, chest press and leg curl exercises. Participants initially performed 2 sets of 10 repetitions, progressing to 3 sets of 12 repetitions at approximately 80% of their one repetition maximum (1-RM) with 2-3 minutes between sets. 1RM was evaluated monthly (baseline, 4 and 8 weeks) for training progression purposes. Home-based flexibility consisted of four stretching exercises targeting the hamstrings, quadriceps, chest, and upper back, each of which was held for 30 seconds. Adherence was monitored by a monthly telephone call where adverse events, changes in medication, or issues with the flexibility program were discussed.
Statistical Analysis
Analyses were conducted using SAS v9.4 (Cary, NC). Linear regressions, adjusted for sex (stratification variable), were used to assess the between group treatment effect (change from baseline to 12-week evaluation). Partial correlations adjusted for age, sex, and BMI were used to assess the relationship between baseline and changes in muscle torque capacity with performance-based and patient-reported functional measures. Partial correlations adjusted for the same three variables were similarly used to determine if changes in performance-based or patient-reported functional outcomes were related to baseline muscle torque capacity. Hypothesis testing was conducted at the 0.05 level.
Results
Participant Characteristics
Screening and enrollment data for the 70 mobility-limited (Short Physical Performance Battery (SPPB) ≤9) older adults (~79 yrs; ~60% female) that participated in this study have been reported previously (27). Participant height (1.7 ± 0.1 m), weight (77.6 ± 14.0 kg), body mass index (28.2 ± 4.0 kg/m2), MMSE score (27.9 ± 1.9), and number of medications (3.5 ± 2.4) were similar between groups.
Progressive Resistance Training Improves Torque Capacity and Perceived Functional Status
At baseline, performance-based measures and patient-reported functional outcomes were similar between PRT and FLEX (Table 1). Improvements in muscle fatigability (summed torque) were greater with PRT than FLEX (~20% vs. ~5%, respectively, P<0.01) (27). Although changes in performance-based functional measures were similar between groups, PRT elicited greater (P<0.05) improvements than FLEX in some of the patient-reported functional outcomes, such as self-reported activity participation (+20%) and advanced lower extremity function (+7%).
Table 1.
Changes in Performance-based Measures and Patient-reported Outcomes
| PRT (n = 35) | FLEX (n = 35) | Between group Δ | |||
|---|---|---|---|---|---|
| Baseline | Δ | Δ | p-value* | ||
| Performance Based Measures | |||||
| SPPB (0-12) | 7.6 (1.4) | 1.6 (2.0) | 7.3 (1.7) | 1.9 (2.0) | 0.58 |
| Balance score | 2.6 (1.1) | 0.3 (1.4) | 2.5 (1.3) | 0.7 (13) | 0.23 |
| Chair stand time (s) | 16.0 (5.1) | −3.9 (5.3) | 16.7 (4.7) | −2.5 (3.4) | 0.23 |
| 4-meter walk time (s) | 5.4 (0.9) | −0.7 (0.8) | 5.3 (1.0) | −0.5 (0.9) | 0.34 |
| Stair Climb (s) | 6.8 (1.8) | −0.4 (1.1) | 6.9 (2.4) | −0.1 (1.8) | 0.39 |
| 6 Minute Walk Distance (m) | 375 (58.6) | 29.6 (44.7) | 377 (62.0) | 25.3 (59.6) | 0.75 |
| Patient Reported Outcomes | |||||
| Pittsburgh Fatigability Scale | |||||
| Activity Participation | 5.4 (1.9) | 1.1 (1.6) | 5.4 (2.0) | 0.2 (1.4) | 0.03 |
| Physical | 21.0 (9.0) | −2.5 (7.9) | 21.8 (9.8) | −2.1 (6.5) | 0.81 |
| Mental | 12.0 (10.5) | −1.6 (9.2) | 15.6 (10.1) | 0.7 (8.6) | 0.30 |
| Late Life Functioning and Disability Index | |||||
| Difficulty performing activities | 123 (18.6) | 4.3 (10.2) | 123 (22.0) | −0.03 (11.4) | 0.11 |
| Limited doing activities | 67.7 (9.6) | 3.9 (75) | 64.2 (11.2) | 2.2 (7.4) | 0.33 |
| Frequency of activities | 57.1 (7.0) | 2.3 (3.9) | 55.8 (7.7) | 0.7 (4.0) | 0.12 |
| Basic lower extremity raw score | 59.3 (8.0) | 1.3 (4.6) | 58.8 (9.4) | 0.5 (5.1) | 0.47 |
| Advanced lower extremity raw score | 33.3 (9.4) | 2.3 (5.0) | 34.6 (10.4) | −0.7 (5.0) | 0.02 |
| Age-Related Muscle Loss Questionnaire | 37.2 (27.8) | −10.0 (19.7) | 35.5 (28.0) | −3.3 (15.0) | 0.12 |
P-value calculated from a linear regression model adjusting for sex; Results are means (SD), unless otherwise stated. Changes calculated as 12-week minus baseline value. MMSE, Mini-Mental State Exam; SPPB, Short physical performance battery.
Torque Capacity is Associated with Performance-based Physical Function
Associations between baseline torque capacity and performance-based and patient-reported functional measures are provided in Table 2 and illustrated in Figure 1. Torque capacity was significantly correlated with performance-based functional measures (P<0.05), explaining 28 and 10% of the variance for stair climb time and six-minute walk distance (Figure 1 A and B, respectively). Although torque capacity was not significantly correlated with any other baseline performance-based or patient-reported functional assessment, relationships between torque capacity and SPPB score (p=0.05) and patient-reported advanced lower extremity score (p=0.07) neared statistical significance.
Table 2.
Baseline Correlations of Muscle Torque Capacity with Performance-based and Patient-reported Outcomes
| Measure | r | p-value |
|---|---|---|
| Performance Based Measures | ||
| Total SPPB Score | 0.24 | 0.05 |
| Balance score | 0.20 | 0.10 |
| Chair stand time | −0.16 | 0.22 |
| Walk time | −0.20 | 0.11 |
| Patient Reported Outcomes | ||
| Pittsburgh Fatigability Scale | ||
| Activity Participation | 0.17 | 0.19 |
| Mental | 0.07 | 0.61 |
| Physical | −0.14 | 0.28 |
| Late Life Function and Disability Instrument | ||
| Difficulty with activities | 0.21 | 0.10 |
| Limited doing activities | −0.03 | 0.79 |
| Frequency of activities | 0.10 | 0.44 |
| Basic Lower Extremity Score | 0.19 | 0.13 |
| Advanced Lower Extremity Score | 0.23 | 0.07 |
| Aging Muscle Loss Questionnaire | −0.18 | 0.15 |
Changes calculated as 12-week minus baseline value. Partial r-values adjusted for age, sex, and BMI. Muscle torque capacity defined as average summed torque from Baseline Visits 1 & 2. SPPB, Short Physical Performance Battery; 6MW, Six Minute Walk
Figure 1.
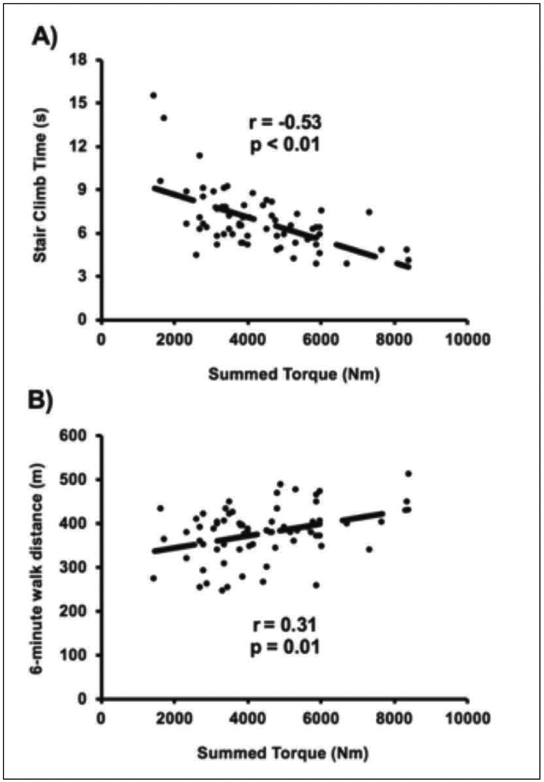
Relationship between baseline muscle torque capacity (summed torque, Nm) and (A) stair climb time (seconds) and (B) 6-minute walk distance (meters). r-values are partial correlation coefficients adjusted for age, sex, and BMI
Functional Improvements are Related to Baseline Torque Capacity
Relationships between baseline torque capacity and functional changes over the course of the intervention were queried to investigate the hypothesis that participants with the lowest baseline torque capacity would receive the greatest functional benefit (Table 3). Indeed, improvements in stair climb time and perceived muscle loss were related to baseline torque capacity (r2=0.15 and 0.08, respectively; P<0.05), with the most marked improvements occurring in participants exhibiting the lowest torque capacity at baseline.
Table 3.
Correlations Between Changes in Performance-Based and Patient-Reported Functional Outcomes and Muscle Torque Capacity
| Baseline Torque Capacity | Change in Torque Capacity | |||
|---|---|---|---|---|
| r | p-value | r | p-value | |
| Performance Based Measures | ||||
| Total SPPB Score | −0.13 | 0.34 | 0.32 | 0.01 |
| Balance Score | −0.20 | 0.12 | 0.33 | 0.01 |
| Chair stand time | 0.02 | 0.90 | −0.25 | 0.07 |
| Walk time | 0.11 | 0.41 | −0.16 | 0.21 |
| Stair climb time | 0.39 | <0.01 | −0.12 | 0.37 |
| 6MW distance | 0.02 | 0.90 | 0.08 | 0.54 |
| Patient Reported Outcomes | ||||
| Pittsburgh Fatigability Scale | ||||
| Activity Participation | 0.09 | 0.50 | 0.38 | <0.01 |
| Mental | −0.07 | 0.60 | −0.17 | 0.22 |
| Physical | 0.17 | 0.21 | −0.15 | 0.26 |
| Late Life Function and Disability Instrument | ||||
| Difficulty with activities | −0.13 | 0.31 | 0.20 | 0.13 |
| Limited doing activities | −0.13 | 0.33 | 0.44 | <0.01 |
| Frequency of activities | 0.02 | 0.89 | 0.32 | 0.01 |
| Basic lower extremity score | −0.19 | 0.15 | 0.19 | 0.15 |
| Advanced lower extremity | −0.19 | 0.15 | 0.24 | 0.07 |
| Aging Muscle Loss Questionnaire | 0.29 | 0.03 | −0.06 | 0.64 |
Changes calculated as 12-week minus baseline value. Partial r-values adjusted for age, sex, and BMI. Muscle torque capacity defined as average summed torque from Baseline Visits 1 & 2. SPPB, Short Physical Performance Battery; 6MW, Six Minute Walk.
Improved Torque Capacity is Associated with Function Enhancement
Torque capacity improvements were related to positive changes in SPPB score (r2=0.10, P<0.05; Table 3) and this finding appeared to be driven by related changes in balance (r2=0.11, P<0.05). Additionally, improvements in torque capacity were associated with patient-reported functional outcomes, explaining between 10-20% of perceived increases in activity participation (Table 3).
Discussion
The primary finding of this study is that skeletal muscle torque capacity, a measure capturing both strength and fatigue, is significantly associated with physical performance (stair climb time and 6-minute walk distance) in older adults with mild-to-moderate mobility limitations. This highlights the significance of coupled decrements in skeletal muscle strength and fatigue to age-related functional deficits, helping to establish skeletal muscle torque capacity as an influential determinant of physical function with advancing age. Pertinently, PRT-mediated torque capacity improvements (27) were paralleled by perceived gains in functional performance (increased activity participation and lower extremity function) and associated with improvements in SPPB score. Future studies seeking to optimize the way PRT is prescribed (e.g., number of repetitions, contraction velocity, etc.) to maximize torque capacity benefits in older adults and subsequently enhance down-stream functional outcomes are of interest.
While the independent contribution of reductions in skeletal muscle strength and increased fatigability to age-related functional decline are well-documented (9-11), the combination of these decrements is likely to have an even greater impact on functional status. Torque capacity values from mobility-limited older adults in the present investigation were roughly half of what has been reported in healthy young individuals (21-35 yrs) (11, 27), portending to age-related torque capacity disparities that are greater than what is seen with muscle strength (16-18) or fatigue alone (11, 26, 33). As many daily activities require prolonged periods of active muscle force production (e.g., walking, housework), maintaining muscle torque capacity with age appears to be a prominent factor for preserving functional independence and quality of life. Consistent with this notion, muscle torque capacity was strongly associated with physical function, explaining nearly 30% of the variance for stair climb time, a functional activity that is essential to maintaining independence (34). This magnitude of association is similar to the relationship between physical performance and skeletal muscle power (r2=0.12-0.35) (9), a critical determinant of physical function in older adults (35). Contrary to our hypothesis, muscle torque capacity was not significantly related to SPPB score, although this relationship neared statistical significance (P = 0.05). This may be explained by the relatively homogenous SPPB scores of older individuals in the present investigation (~80% of persons between 7-9), a likely product of the lower SPPB inclusion criteria (≤9) utilized in the present study, compared to previous reports (SPPB ≤11) that have observed relationships between muscle strength and power and SPPB score (9, 36). Regardless, associations between muscle torque capacity and meaningful performance measures such as six-minute walk distance and stair climb time highlight the adverse functional implications of combined reductions in strength and fatigue with advancing age.
Progressive resistance training (PRT) is a highly effective means to combat aging muscle size and strength deficits (15), but less is known about its ability to influence fatigue-related outcomes. Despite eliciting torque capacity improvements (27), PRT failed to improve a number of performance-based functional outcomes (e.g., SPPB, stair climb time, etc.). While This may be explained by the lack of consideration for improving functional outcomes by the study design (i.e., lack of statistical power), previous research in frail older adults (~78y) has suggested that initial frailty level may affect the impact of muscle strengthening on performance (37). Thus, the lack of performance-based functional improvement may be explained by the greater baseline functional capacity (~50% further 6MWT) in our study cohort compared with previous research showing associated improvements in muscle strength and physical function (37). Meanwhile, a comparable high-intensity PRT program similarly failed to inspire an appreciable change in performance-based functional measures (e.g., 6-minute walk, stair climb time, chair rise, etc.) in older (~65y) long-term stroke survivors (38). It is interesting to note that participants in both studies reported significant improvements in perceived functional status. This likely reflects patient-reported outcomes predicting a broader array of important health attributes and possibly being more sensitive to PRT-mediated improvements than our chosen performance-based metrics (39). In the present study, participants reported increased activity participation and lower-extremity function, highlighting the translational benefit of PRT-mediated torque capacity improvements, and the importance of collecting objective and subjective measures of functional status when evaluating the efficacy of interventional strategies with therapeutic aims. Seeing as torque capacity benefits seemed to be largely mediated by improvements in muscle strength (27), it would be interesting to determine if modalities seeking to optimize torque capacity by also improving fatigability would have an even greater functional impact that may be reflected by performance-based improvements on fatigue-related outcomes (e.g., 6MWT).
In the absence of an appreciable performance-based functional benefit from PRT compared to FLEX, it is worthy to note that SPPB score increased by ~25% in both groups. The significance of these improvements is highlighted by the fact that post-intervention nearly 50% of our participants achieved an SPPB score ≥10, thus exceeding the threshold for what is classically defined as being mobility-limited (7). Relevantly, collinear improvements in muscle torque capacity and SPPB score suggests that PRT-mediated torque capacity benefits may have contributed to observed functional improvements. Torque capacity and SPPB-related changes appeared to be driven by improvements in balance sub-score, which is shown to improve with resistance exercise in older adults (40). Interestingly, functional enhancements (i.e., stair climb time) were most pronounced in participants exhibiting the lowest baseline torque capacity, emphasizing the value of future research seeking to optimize interventions aimed at improving torque capacity as a means to enhance physical function in mobility-limited older adults.
Conclusion
In summary, these findings from a large cohort of well-characterized mobility-limited older adults identify combined strength and fatigue-related decrements, torque capacity, as an important determinant of meaningful performance-based and patient-reported functional measures. Importantly, improvements in torque capacity were aligned with enhancements in patient-reported functional status and associated with beneficial changes in performance-based functional outcomes. Future interventions seeking to improve physical performance in mobility-limited older adults are advised to consider the cumulative contribution of muscle strength and fatigability deficits to reduced functional capacity with advancing age.
Acknowledgements:
We thank our participants for their time and efforts that made this study possible.
Sources of support: Dr. Grosicki reports grants from Astellas Pharmaceuticals Inc, grants from U.S. Department of Agriculture (agreement No. 58-1950-4-003), grants from Boston Claude D Pepper Center (OAIC; 1P30AG031679), grants from National Center for Advancing Translational Sciences (Award Number UL1TR001064), National Institutes of Health, during the conduct of the study. Dr. Englund reports grants from Astellas Pharma Inc, during the conduct of the study. Ms. Price reports other from Tufts University/HNRCA, during the conduct of the study. Dr. Iwai reports personal fees from Astellas Pharma Inc., during the conduct of the study; personal fees from Astellas Pharma Inc., outside the submitted work. Dr. Kashiwa reports personal fees from Astellas Pharma Inc., during the conduct of the study; personal fees from Astellas Pharma Inc., outside the submitted work. Dr. Reid has nothing to disclose. Dr. Fielding reports grants from National Institutes of Health (National Institute on Aging), during the conduct of the study; grants, personal fees and other from Axcella Health, other from Inside Tracker, grants and personal fees from Biophytis, grants and personal fees from Astellas, personal fees from Cytokinetics, personal fees from Amazentis, grants and personal fees from Nestle’, personal fees from Glaxo Smith Kline, outside the submitted work. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. ClinicalTrials.gov Identifier: NCT03083275.
Footnotes
Sponsor’s Role: Astellas Pharma INC. approved of study procedures and helped to edit and revise the manuscript.
Ethical standard: This study was reviewed and approved by the Tufts University Health Sciences Institutional Review Board.
References
- 1.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009. October 3;374(9696):1196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hobbs F The Elderly Population. Washington, DC: United States Bureau of Census; 2000. [Google Scholar]
- 3.Berlau DJ, Corrada MM, Kawas C. The prevalence of disability in the oldest-old is high and continues to increase with age: findings from The 90+ Study. Int J Geriatr Psychiatry. 2009. November;24(11):1217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He W, Larsen L. Older americans with a disability: 2008–2012. In: Services USDoHaH, editor. Online2014. [Google Scholar]
- 5.Jette AM, Branch LG. Musculoskeletal impairment among the non-institutionalized aged. Int Rehabil Med. 1984;6(4):157–61. [DOI] [PubMed] [Google Scholar]
- 6.Jette AM, Branch LG, Berlin J. Musculoskeletal impairments and physical disablement among the aged. J Gerontol. 1990. November;45(6):M203–8. [DOI] [PubMed] [Google Scholar]
- 7.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000. April;55(4):M221–31. [DOI] [PubMed] [Google Scholar]
- 8.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995. March 2;332(9):556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bean JF, Kiely DK, Herman S, Leveille SG, Mizer K, Frontera WR, et al. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002. March;50(3):461–7. [DOI] [PubMed] [Google Scholar]
- 10.Reid KF, Naumova EN, Carabello RJ, Phillips EM, Fielding RA. Lower extremity muscle mass predicts functional performance in mobility-limited elders. The journal of nutrition, health & aging. 2008;12(7):493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kent-Braun JA, Callahan DM, Fay JL, Foulis SA, Buonaccorsi JP. Muscle weakness, fatigue, and torque variability: effects of age and mobility status. Muscle Nerve. 2014. February;49(2):209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid KF, Pasha E, Doros G, Clark DJ, Patten C, Phillips EM, et al. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: Influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol. 2014. January;114(1):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. The journals of gerontology Series A, Biological sciences and medical sciences. 2006. October;61(10):1059–64. [DOI] [PubMed] [Google Scholar]
- 14.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988. April;84(2-3):275–94. [DOI] [PubMed] [Google Scholar]
- 15.Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994. June 23;330(25):1769–75. [DOI] [PubMed] [Google Scholar]
- 16.Aniansson A, Hedberg M, Henning GB, Grimby G. Muscle morphology, enzymatic activity, and muscle strength in elderly men: a follow-up study. Muscle Nerve. 1986. September;9(7):585–91. [DOI] [PubMed] [Google Scholar]
- 17.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol (1985). 2000. April;88(4):1321–6. [DOI] [PubMed] [Google Scholar]
- 18.Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci. 1997. September;52(5):B267–76. [DOI] [PubMed] [Google Scholar]
- 19.Thompson LV. Skeletal muscle adaptations with age, inactivity, and therapeutic exercise. J Orthop Sports Phys Ther. 2002. February;32(2):44–57. [DOI] [PubMed] [Google Scholar]
- 20.Woolley S, Topp R, Khuder S, et al. Function: which factors predict ability in OA patients. Biomech in Ger. 1998(17):6–12. [Google Scholar]
- 21.Hughes MA, Myers BS, Schenkman ML. The role of strength in rising from a chair in the functionally impaired elderly. J Biomech. 1996. December;29(12):1509–13. [PubMed] [Google Scholar]
- 22.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008. January;88(1):287–332. [DOI] [PubMed] [Google Scholar]
- 23.Baudry S, Klass M, Pasquet B, Duchateau J. Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. Eur J Appl Physiol. 2007. July;100(5):515–25. [DOI] [PubMed] [Google Scholar]
- 24.Lindstrom B, Lexell J, Gerdle B, Downham D. Skeletal muscle fatigue and endurance in young and old men and women. J Gerontol A Biol Sci Med Sci. 1997. January;52(1):B59–66. [DOI] [PubMed] [Google Scholar]
- 25.Senefeld J, Yoon T, Hunter SK. Age differences in dynamic fatigability and variability of arm and leg muscles: Associations with physical function. Exp Gerontol. 2017. January;87(Pt A):74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Justice JN, Mani D, Pierpoint LA, Enoka RM. Fatigability of the dorsiflexors and associations among multiple domains of motor function in young and old adults. Exp Gerontol. 2014. July;55:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Englund DA, Price LL, Grosicki GJ, Iwai M, Kashiwa M, Liu C, et al. Progressive resistance training improves torque capacity and strength in mobility-limited older adults. J Gerontol A Biol Sci Med Sci. 2018. August 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994. March;49(2):M85–94. [DOI] [PubMed] [Google Scholar]
- 29.Enright PL. The six-minute walk test. Respir Care. 2003. August;48(8):783–5. [PubMed] [Google Scholar]
- 30.Sayers SP, Jette AM, Haley SM, Heeren TC, Guralnik JM, Fielding RA. Validation of the Late-Life Function and Disability Instrument. J Am Geriatr Soc. 2004. September;52(9):1554–9. [DOI] [PubMed] [Google Scholar]
- 31.Evans CJ, Chiou CF, Fitzgerald KA, Evans WJ, Ferrell BR, Dale W, et al. Development of a new patient-reported outcome measure in sarcopenia. J Am Med Dir Assoc. 2011. March;12(3):226–33. [DOI] [PubMed] [Google Scholar]
- 32.Glynn NW, Santanasto AJ, Simonsick EM, Boudreau RM, Beach SR, Schulz R, et al. The Pittsburgh Fatigability scale for older adults: development and validation. J Am Geriatr Soc. 2015. January;63(1):130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunter SK, Critchlow A, Enoka RM. Muscle endurance is greater for old men compared with strength-matched young men. J Appl Physiol (1985). 2005. September;99(3):890–7. [DOI] [PubMed] [Google Scholar]
- 34.Hinman MR, O’Connell JK, Dorr M, Hardin R, Tumlinson AB, Varner B. Functional predictors of stair-climbing speed in older adults. J Geriatr Phys Ther. 2014. Jan-Mar;37(1):1–6. [DOI] [PubMed] [Google Scholar]
- 35.Reid KF, Fielding RA. Skeletal muscle power: A critical determinant of physical functioning in older adults. Exercise and sport sciences reviews. 2012. January;40(1):4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? The journals of gerontology Series A, Biological sciences and medical sciences. 2003. August;58(8):728–33. [DOI] [PubMed] [Google Scholar]
- 37.Chandler JM, Duncan PW, Kochersberger G, Studenski S. Is lower extremity strength gain associated with improvement in physical performance and disability in frail, community-dwelling elders? Arch Phys Med Rehabil. 1998. January;79(1):24–30. [DOI] [PubMed] [Google Scholar]
- 38.Ouellette MM, LeBrasseur NK, Bean JF, Phillips E, Stein J, Frontera WR, et al. High-intensity resistance training improves muscle strength, self-reported function, and disability in long-term stroke survivors. Stroke. 2004. June;35(6):1404–9. [DOI] [PubMed] [Google Scholar]
- 39.Bean JF, Olveczky DD, Kiely DK, LaRose SI, Jette AM. Performance-based versus patient-reported physical function: what are the underlying predictors? Phys Ther. 2011. December;91(12):1804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orr R, de Vos NJ, Singh NA, Ross DA, Stavrinos TM, Fiatarone-Singh MA. Power training improves balance in healthy older adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2006. January;61(1):78–85. [DOI] [PubMed] [Google Scholar]


