Abstract
Introduction
Metformin, a common medication used in the treatment of Diabetes Mellitus is known to have anti-cancer effects. We hypothesized that the salutary effect of metformin on the survival of patients with stage I NSCLC is influenced by body mass index (BMI).
Methods
Patients undergoing lobectomy for stage I NSCLC without neoadjuvant therapy were included. Univariate and multivariate survival analyses to examine the association between metformin use and overall, disease specific and recurrence free survival (OS, DSS and RFS respectively) were performed, stratified by BMI (>25 and <25). Expression of immune checkpoints in patients on metformin and not was performed in a separate cohort of 205 patients with advanced disease.
Results
434 stage I patients (including 74 metformin users) were deemed eligible for analysis. Univariate and multivariate analysis revealed an association between metformin use and OS (HR=0.52; P=0.04) as well as DSS (HR=0.21; P=0.04) but not RFS (HR=0.67; P=0.33) in high-BMI patients only. In a separate cohort of 205 tumors of all stages (including 35 metformin users), downregulation of immune checkpoint gene expression (PDCD1, CTLA4, BTLA, CD27, LAG3 and ICOS) in metformin users was seen only in high-BMI patients, with upregulation of these genes seen in low-BMI patients with metformin use.
Conclusions
Metformin use may be associated with better OS and DSS only in high-BMI patients. This hypothesis is supported by gene expression data of immune checkpoint genes in metformin users using a separate cohort of advanced stage tumors. Further studies examining the interaction of BMI with metformin in NSCLC are worthwhile.
Keywords: Lung cancer, Metformin, Diabetes, survival
Introduction
Metformin (Dimethylbiguanide) is an oral biguanide extensively used in the treatment of type 2 Diabetes Mellitus. While the anti-diabetic effects of metformin have been known for decades, the anti-cancer effects of the drug are being studied only recently. These effects are thought to be mainly mediated via activation of the AMPK pathway [1]. Recently, more attention has been paid to metformin’s ability to influence the antitumor immune response. These have been focused on the role of T cell mediated antitumor responses [2]. Large database and smaller retrospective studies have demonstrated an association between metformin use and outcomes of lung cancer [3, 4]. However, the variable effect sizes seen in these studies suggest that other confounders and determinants of this association exist. Obesity, defined as body mass index greater than 30, has come to be recognized as one of the determinants of outcomes after cancer. Particularly, in lung cancer, analysis of large datasets have demonstrated a positive impact of obesity [5] - the “Obesity Paradox”. However, this observation is not universal and seems to depend on other clinical covariates [6, 7]. In this study, we sought to examine the impact of BMI on the salutary long term effects of metformin on patients undergoing lobectomy for stage I NSCLC. We chose this population to study the influence of metformin on tumor biology to avoid confounding by treatment response. In a cohort with early stage NSCLC treated by surgery alone, we demonstrate that the association of metformin with long term survival benefit is influenced by BMI. In a separate cohort of advanced stage tumors, we found that immune checkpoint genes are downregulated in patients treated with metformin, suggesting a reversal of T cell exhaustion.
Materials and Methods
Methodologic details are described in detail in supplementary file 1. Patients with stage I NSCLC undergoing lobectomy without neoadjuvant therapy were included. Data were obtained from institutional databases. Univariate and multivariate analyses were used to examine the association between metformin use and survival. Patient samples sent for molecular testing to guide therapy were used to generate an immune report card, as previously described [8]. Gene ranks were used to compare differential expression of immune checkpoint genes between groups of patients using t-tests.
Results
Survival analyses of stage I NSCLC patients
Of 756 patients undergoing resection in this time period, 474 had stage I disease. Excluding patients without adenocarcinoma or squamous cell carcinoma led to a final analytic population of 434. Of these, 35 patients had a diagnosis of diabetes but did not use metformin, 74 patients had a diagnosis of diabetes and used metformin and 325 patients were non-diabetic. Table 1 summarizes the patient characteristics of each subgroup. Pulmonary functions were similar in the three groups. Diabetic patients had a significantly higher BMI when compared to the non-diabetic patients. Of 434 patients, cause of death was unknown in 38; DSS was assessed in 396 patients.
Table 1.
Patient characteristics. DM= Patients with a diagnosis of Diabetes Mellitus not on metformin. FEV1 = Forced expiratory volume in first second. DLCO = Diffusion capacity of lung to carbon monoxide. Adeno = adenocarcinoma. SqCC = Squamous cell carcinoma. BMI = Body mass index. ASA = American Society for Anesthesiology score.
| Characteristic | Non-diabetic | DM | Metformin | P-Value |
|---|---|---|---|---|
| N | 325 | 35 | 74 | |
| Mean Age | 66 | 69 | 72 | <0.05 |
| Female (%) | 210 (65%) | 23(66%) | 37 (50%) | 0.06 |
| Race | ||||
| White | 295 (91%) | 30 (86%) | 64 (86%) | 0.13 |
| Black | 22 (7%) | 3 (9%) | 10(14%) | |
| Other | 8 (2%) | 2 (6%) | 0 (0%) | |
| FEV1 %pred (mean) | 82% | 83% | 83% | NS |
| DLCO%pred (mean) | 77% | 71% | 78% | NS |
| Tumor grade | ||||
| I/II | 222 (68%) | 21 (60%) | 51 (68%) | 0.59 |
| III/IV | 103 (32%) | 14 (40%) | 23 (32%) | |
| Smoking status | 0.27 | |||
| Current | 92 (28.3%) | 9 (25.7%) | 14 (18.9%) | |
| Former | 132 (40.6%) | 21 (60.0%) | 38 (51.4%) | |
| Never | 27 (8.3%) | 3 (8.6%) | 8 (10.8%) | |
| Quit | 49 (15.1%) | 0 (0%) | 9 (12.2%) | |
| Recent quit | 24 (7.4%) | 2(5.7%) | 5 (6.8%) | |
| Unknown | 1 (0.3%) | |||
| Histology | ||||
| Adeno | 243 (75%) | 23 (66%) | 47 (64%) | 0.1 |
| SqCC | 82 (25%) | 12 (34%) | 27 (36%) | |
| BMI (mean) | 26.9 | 30.3 | 31.4 | <0.05 |
| ASA | ||||
| 2 | 184 (57%) | 11 (31%) | 22 (30%) | <0.01 |
| 3 | 138 (42%) | 24 (69%) | 52 (70%) | |
| 4 | 3 (1%) | 0 (0%) | 0 (0%) | |
| BMI ≥25 | 209 (64%) | 29 (83%) | 66 (89%) | <0.01 |
| BMI ≥30 | 69 (21%) | 15 (43%) | 37 (50%) | <0.01 |
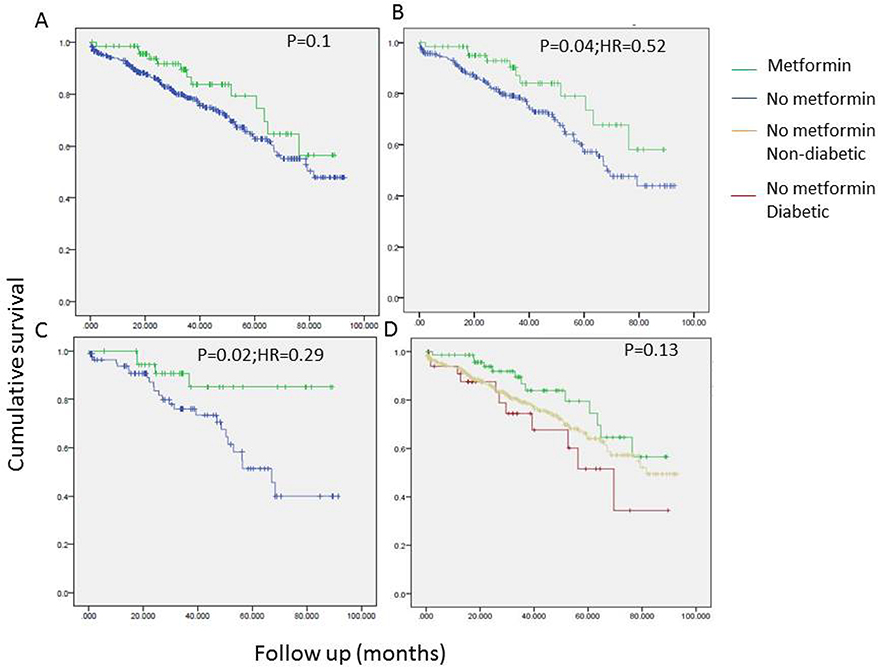
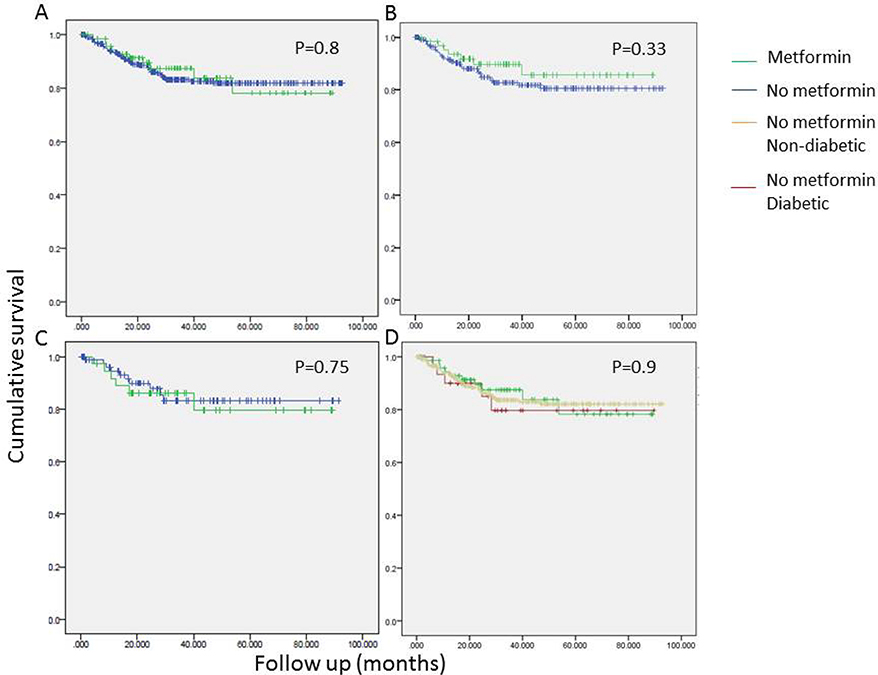
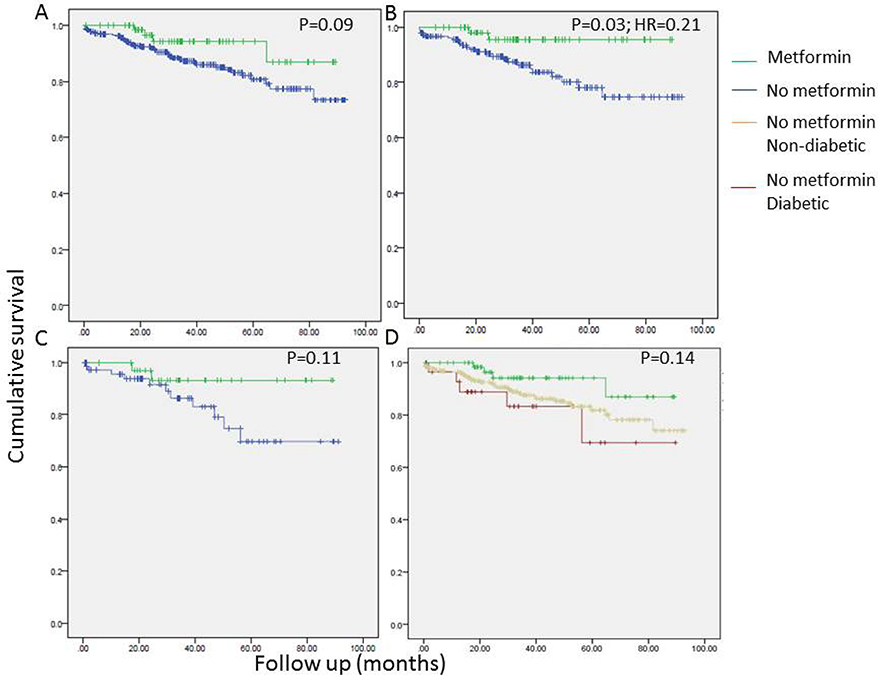
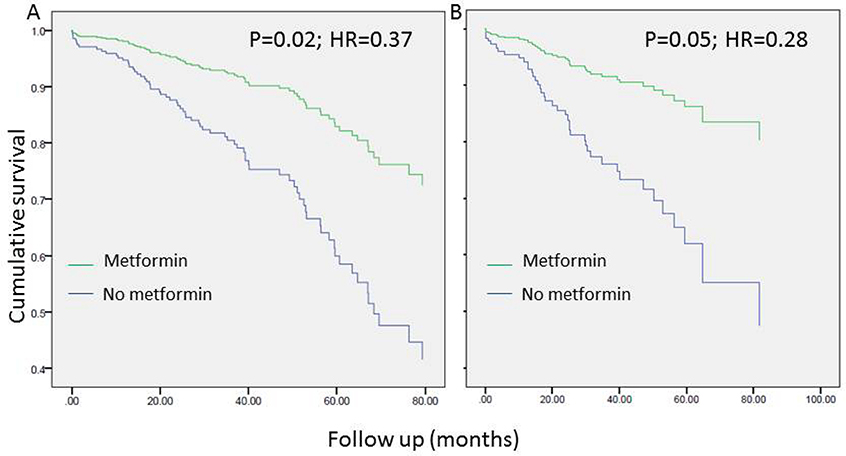
Univariate analyses revealed a tendency to better overall survival in metformin users. The association between metformin use and OS was statistically significant only in patients with a BMI > 25 and the strength of the association was higher in patients with BMI > 30 (Figure 1). Conversely, no such association between metformin use and recurrence free survival (RFS) was seen (Figure 2). As seen in Figure 3, improved DSS was associated with metformin use; this was statistically significant in high BMI patients. Multivariate Cox regression analysis was used to model OS, RFS and DSS using age, gender, race, pulmonary function tests, smoking status, ASA, diabetes, metformin use, grade, histology and BMI (categorized as high and low, using a cutoff of 25). Only age, gender, DLCO, ASA and metformin use remained significant predictors of overall survival. Figure 4A shows the survival curves for metformin users vs not in after adjusting for other variables. Similarly, for DSS, only gender, tumor grade, DLCO, ASA and metformin use were retained in the final model. Figure 4B shows covariate adjusted DSS curves for metformin users vs. not. Similar analyses performed to model RFS did not retain metformin use, diabetes or BMI in the final model, confirming associations seen in the univariate analyses (therefore, no covariate adjusted curve is shown).
Figure 1.
Univariate survival analyses examining the association of overall survival (OS) with metformin use in all stage I patients (N=434;A, D), in patients with BMI > 25 (B) and BMI > 30 (C).
Figure 2.
Univariate survival analyses examining the association of recurrence free survival (RFS) with metformin use in all stage I patients (N=434;A, D), in patients with BMI > 25 (B) and BMI > 30 (C).
Figure 3.
Univariate survival analyses examining the association of disease-specific survival (DSS) with metformin use in all stage I patients (N=434;A, D), in patients with BMI > 25 (B) and BMI > 30 (C).
Figure 4.
Survival curve depicting the association of overall survival (A) and disease- specific survival (B) with metformin use in all stage I patients (N=434) after adjusting for other statistically significant covariates in multivariable modeling.
Details of the covariates included in the final multivariable models are founds in supplementary Table 1.
Immune checkpoint gene expression results
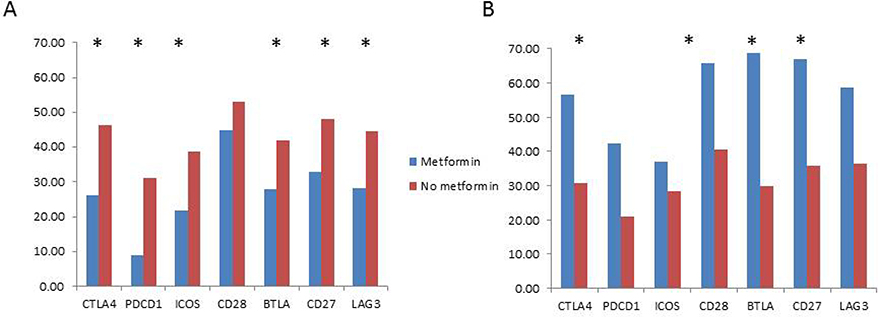
Other investigators have demonstrated that metformin modulates the anti-tumor immune response [2]. In addition, obesity itself is known to impact the immune system in cancer patients[9]. Therefore, we hypothesized that metformin and BMI may interact with respect to the expression of immune checkpoint genes in lung tumors. In order to test this hypothesis, the expression of immune checkpoint genes were examined in patients using metformin vs not, in a cohort of patients with advanced cancer (stage III / IV). These results were obtained according to methods described briefly above and in detail elsewhere [8, 10]. Of 242 patients with such an immune report card available, 236 patients had BMI within 60 days of the acquisition of the tissue sample. Exclusion of patients with neuroendocrine (including small cell) and sarcomatoid cancer histology and immunotherapy prior to tissue acquisition further decreased the cohort to 205 patient samples. Of these 205 patients, 35 were metformin users. 74 patients had a BMI less than 25; 7 of these patients were metformin users. 131 patients had a BMI greater than 25; 28 of these patients were metformin users. There were no differences in the age, sex, race and histology between metformin users and non-users. As shown in figure 5, only in patients with a high BMI, metformin use is associated with statistically significantly decreased expression of six out of 7 immune checkpoint genes. In the low BMI group, the trend was in the opposite direction with the four out of 7 immune checkpoint genes with statistically significant increased expression in patients on metformin.
Figure 5.
Comparison of immune checkpoint gene expression in tumors of patients with BMI > 25 (A) and BMI < 25 (B) in advanced stage patients (N=205) using metformin vs. not. * represents comparisons that are statistically significant.
Discussion
The incidence and mortality of several cancer types are enhanced among obese individuals [11]. Therefore it was indeed surprising that, in the present study, metformin, a widely used type 2 diabetes drug with reported anti-cancer properties, was associated with improved survival metrics among overweight lung cancer patients, and particularly those with a BMI greater than 30 (i.e. obese). This unexpected finding suggests that elements of obesity may work to sensitize patients to the mechanisms responsible for metformin’s anti-tumor effects that, until recently, had remained ill-defined. It is widely recognized that obesity induces a state of “meta-inflammation” typified by chronic cytokine production, widespread dysfunction of both innate and adaptive immune cells, and premature “immune aging” and upregulation of immune checkpoint molecules. Obese mice supported more aggressive tumor growth while harboring CD8+ T cells with surface markers (PD-1, LAG3, and Tim3) and gene expression profiles associated with exhaustion [12].
Curiously, despite these observations, clinical evidence suggests that obese patients tend to experience a greater benefit from immunotherapeutic interventions than their low BMI counterparts. [13]. A recent study by Wang et al. reported improved disease outcomes for human immunotherapy patients with a BMI>30 [12]. This study also implicated the hormone leptin, a known regulator of energy balance, to be at the heart of obesity-induced T cell exhaustion. Depriving T cells of leptin signaling undermined the exhausted T cell phenotype typical in obese mice and improved the efficacy of anti- PD1 immunotherapy. The implication of leptin as a mediator of obesity-associated immune dysfunction presents a potential underlying mechanism for the metformin- induced benefits observed in our present study. Metformin is a demonstrated activator of the energy sensor AMPK. This important enzyme has itself been suggested as a negative regulator of leptin sensitivity [14]. Thus it is possible that activators of AMPK (such as metformin) might be capable of therapeutically correcting leptin-driven T cell exhaustion in obese cancer patients. Notably, metformin was recently shown to have rejuvenating effects on T cells in the cancer setting. Treating mice with metformin was shown to mediate rejection of solid tumors by T cell-dependent means and enhance immune infiltration of tumors particularly by CD8+ T cells that were multifunctional and protected from both apoptosis and exhaustion. The authors of this report showed that this metformin-driven enhancement of CD8 function was AMPK-dependent [15], suggesting that the drug’s immunomodulatory action involves the activation of this energy sensor.
Limitations of the study include its retrospective nature, small sample size, lack of more granular data with respect to comorbidities and the inability to confirm the dose and duration of metformin use. The authors also recognize that the gene expression data is obtained from a different population than the ones with clinical data. While this is admittedly a sample of convenience, the results provide valuable insights into possible mechanisms deserving further investigation.
Our findings raise the possibility that metformin may improve the survival of lung cancer patients by reversing obesity induced T cell exhaustion and enhanced anti-tumor immunity. However, further study, using animal models as well as clinical data, are necessary to verify this hypothesis.
Supplementary Material
Acknowledgements
The authors thank Adrienne Groman, Department of Biostatistics at the Roswell Park Comprehensive Cancer Center for her assistance in verification of the analyses.
Funding sources
This work was supported by National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park Cancer Institute’s Pathology Network, Genomic, and Clinical Data Network Shared Resources.
Footnotes
Conflict of interest: SP, MN, PD, APS, CM, and STG are employees of OmniSeq, Inc. (Buffalo, NY) and hold restricted stock in OmniSeq, Inc. CM, and STG are employees of Roswell Park Comprehensive Cancer Center (Buffalo, NY), which is the majority shareholder of OmniSeq, Inc. SY, JB, CP, AP, GD and PE have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pernicova I and Korbonits M, Metformin--mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol, 2014. 10(3): p. 143–56. [DOI] [PubMed] [Google Scholar]
- 2.Eikawa S, et al. , Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A, 2015. 112(6): p. 1809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin JJ, et al. , Survival of patients with stage IV lung cancer with diabetes treated with metformin. Am J Respir Crit Care Med, 2015. 191(4): p. 448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhillon SS, et al. , Metformin and Not Diabetes Influences the Survival of Resected Early Stage NSCLC Patients. J Cancer Sci Ther, 2014. 6(7): p. 217222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang R, et al. , Obesity and weight loss at presentation of lung cancer are associated with opposite effects on survival. J Surg Res, 2011. 170(1): p. e75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preston SH and Stokes A, Obesity paradox: conditioning on disease enhances biases in estimating the mortality risks of obesity. Epidemiology, 2014. 25(3): p. 454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, et al. , Obesity Paradox in Lung Cancer Prognosis: Evolving Biological Insights and Clinical Implications. J Thorac Oncol, 2017. 12(10): p. 1478–1488. [DOI] [PubMed] [Google Scholar]
- 8.Paluch BE, et al. , Robust detection of immune transcripts in FFPE samples using targeted RNA sequencing. Oncotarget, 2017. 8(2): p. 3197–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canter RJ, et al. , Obesity as an immune-modifying factor in cancer immunotherapy. J Leukoc Biol, 2018. 104(3): p. 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conroy JM, et al. , Analytical Validation of a Next-Generation Sequencing Assay to Monitor Immune Responses in Solid Tumors. J Mol Diagn, 2018. 20(1): p. 95–109. [DOI] [PubMed] [Google Scholar]
- 11.Calle EE, et al. , Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med, 2003. 348(17): p. 1625–38. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, et al. , Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med, 2019. 25(1): p. 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richtig G, et al. , Body mass index may predict the response to ipilimumab in metastatic melanoma: An observational multi-centre study. PLoS One, 2018. 13(10): p. e0204729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su H, et al. , Glucose enhances leptin signaling through modulation of AMPK activity. PLoS One, 2012. 7(2): p. e31636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McFarland MS and Cripps R, Diabetes mellitus and increased risk of cancer: focus on metformin and the insulin analogs. Pharmacotherapy, 2010. 30(11): p. 1159–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.