Abstract
The range of the invasive alien hornet, Vespa velutina nigrithorax, has been expanding since its introduction to Korea in 2003. Here, we compare the aggressive behaviors and body size of V. velutina nigrithorax with five native hornet species to identify the interspecific hierarchies that influence the rate of spread of this species. Aggressive behaviors were classified into 11 categories, and each interaction was scored as a win, loss, or tie. We found that V. velutina was superior to V. simillima in fights that V. velutina won and showed a high incidence of threatening behavior. V. mandarinia outperformed V. velutina in fights that V. mandarinia won and grappling behavior was common. V. analis was superior to V. velutina in fights that V. analis won and showed a high degree of threatening behavior. V. crabro was superior to V. velutina in fights that V. crabro won and showed a high rate of threatening behavior. V. dybowskii was superior to V. velutina in fights that V. dybowskii won and showed a high incidence of threatening and grappling behaviors. The body size of V. velutina was greater than V. simillima (although not statistically significant) and smaller than all other Vespa species. Therefore, according to this study, the low interspecific hierarchies of V. velutina seem to be a major cause of the slower spread in Korea than in Europe. However, over time, its density has gradually increased within the forest, where it seems to be overcoming its disadvantages and expanding its range, possibly because the large colonies and good flying abilities of this species help it secure food.
Introduction
In recent years, invasive alien species (IAS) have spread widely due to rapid climate change and global trade, resulting in a global biodiversity reduction, and economic and ecological impacts [1–3]. Indeed, IAS contribute to millions of dollars in economic losses per year [4], with negative impacts from insects and arthropods costing 70 billion US dollars annually [5]. In South Korea, the social and agricultural impacts are also gradually increasing due to invasions by black widow spiders (Latrodectus hesperus), spotted lanternfly (Lycorma delicatula), frosted moth-bug (Metcalfa pruinosa), leaf-footed bug (Leptoglossus gonagra) and black planthopper (Ricania speculum). In particular, social insects such as yellow-legged hornets (Vespa velutina), red imported fire ants (Solenopsis invicta), and argentine ant (Linepithema humile), which are particularly damaging due to the large number of individuals and their toxicities, are recent introductions to Korea and pose a significant social threat [6–13].
Many IAS spread rapidly and broadly after the successful invasion of new environments through resource and habitat competition with native species [14–17]. In particular, there are many cases of social insect invasions worldwide, such as in ants, wasps, and bees, and if the invasion is successful, it can have serious ecological and economic impacts, with population sizes ranging from hundreds to tens of thousands of individuals [18].
Vespa velutina nigrithorax, originating in southern China, has spread throughout South Korea since its first invasion in 2003, where it was introduced through trade ships [8, 19–21]. After invading Tsushima Island in Japan in 2012, V. velutina invaded Kyushu in mainland Japan in 2015 [22–24]. In Europe, this species spread to France, Spain, Portugal, Italy, Germany, Belgium, Switzerland and the UK by 2016 after the first invasion in France in 2004 [25–27]. V. velutina has a severe economic impact on beekeepers by foraging large quantities of honeybees in apiaries, removing approximately 30% of honeybee colonies [28].
In addition, V. velutina is a poisonous insect that has a public health impact. In Korea, there are more than 100,000 cases of removal of social wasps’ nests per year, and V. velutina’s nest removal rate is the highest among Vespa species. The average number of injuries caused by social wasps is about 15,000, and there have been about 10 deaths. In particular, due to the high density in urban areas, the damage caused by them is likely to be high [19, 20, 29]. In fact, two deaths have occurred in France since the invasion of V. velutina, and two deaths have been reported in Korea. At present, the impacts of V. velutina may not be noticeable [30], but the actual impacts are expected to be more because the extent of the impacts that this alien species causes may not be fully appreciated [28, 31–33].
This species also causes ecosystem disturbance through competition and interference with other Vespa species in nature [34–36]. Therefore, V. velutina shows the comprehensive impact of IAS. In Korea, V. velutina was designated as an Ecological Disturbance Organism (Ministry of Environment Notice 2019–185) on 26 July 2019 under the provisions of Article 23 of the Act on the Conservation and Use of Biodiversity (http://www.law.go.kr/LSW/admRulLsInfoP.do?chrClsCd=&admRulSeq=2100000180728, access date November 14, 2019).
Obtaining adequate nutrition is the most important factor for the primary survival and range expansion of V. velutina colonies. In general, social wasp prey differs between adult and larval stages. Larvae require protein for growth, and obtain this by being fed by adults, which hunt. Adults, on the contrary, consume carbohydrates in order to obtain energy due to their higher levels of activity. Therefore, in nature, adults eat oak sap, flower honey, and nectar [37]. In particular, oak sap comes from various butterflies and flies, as well as medium and large beetles such as dynastid beetles, stag beetles and weevils [38–40]. Some insects eat sap with other insects around the sap, but hornets and large beetles compete for limited sap resources [37, 38]. This competition also occurs among several Vespa species, where V. mandarinia is predominantly high hierarchy in regard to Japanese oak sap, followed by V. crabro, V. analis, and V. simillima [37]. The results of competition between these species, therefore, are very helpful in identifying the species’ ecological niche in the ecosystem.
Several factors determine the rate of spread of an invasive species. In addition to anthropogenic controls [1, 41, 42] and natural enemies [43, 44], the ecological hierarchy obtained through competition among similar species makes an important contribution to the likelihood and speed of range extension.
Therefore, in this study, we analyzed the interspecific hierarchies of V. velutina among Korean Vespa species by measuring aggressive behavior to secure food sources among native hornet species and V. velutina, in order to understand competitive ability, which is the main factor determining the successful spread of V. velutina. In addition, we analyzed the correlation between fighting ability and size by measuring the body size of each species.
Materials and methods
Study species and experiment sites
We planned to test for aggressive behaviors between V. velutina and nine native Korean Vespa species, but because of their different distributions, it was impossible to observe the behavior of all nine species in one place. In particular, Vespa simillima xanthoptera, Vespa binghami, and Vespa crabro crabroniformis have very limited distributions, making them difficult to compare [45]. Therefore, experimental sites at which many Vespa species, including V. velutina, are present were selected by referring to various studies such as those of Choi et al. [45] and Choi and Kwon [46]. As a result, we conducted the experiment in the Piagol Valley of Jirisan National Park, where seven Vespa species (V. velutina, V. simillima, V. crabro, V. dybowskii, V. mandarinia, V. analis, and V. ducalis) occur.
First, we randomly chose three experimental sites in Piagol Valley (Site A: N35 ° 13'37.96 "E127 ° 35'48.95", 174m; Site B: N35 ° 15'17.53 "E127 ° 35'58.24", 417m; Site C: N35 ° 15'42.43 "E127 ° 35'3.57", 398m, Since this experiment was conducted on a private site outside the boundaries of a Jirisan National park, it was conducted with the personal permission of the landlord. Therefore, there is no documented permit). Vespa species were captured using hornet traps in the apiaries near each site, and identified. As a result, four species (V. velutina, V. simillima, V. mandarinia, and V. crabro) were captured in the apiary near site A, six species (V. velutina, V. simillima, V. dybowskii, V. mandarinia, V. analis, and V. crabro) were captured in the apiary near site B, and five species were captured in the apiary near site C (V. velutina, V. simillima, V. mandarinia, V. analis, and V. crabro). Therefore, site B was selected as the experimental site, as it had the highest species diversity. V. ducalis was also seen flying near site B but did not appear in traps or the test site. Therefore, the behavioral experiment was conducted between V. velutina and five native hornet species.
Behavioral observation experiment
The behavioral observation experiment apparatus was set up as follows: a table (1m in height) was installed on a flat plain in a forest where hornets were present, 2–3 sheets of toilet paper were placed on top of the table, and an attractant was poured onto these, following the methods described by Choi et al. [19]. In addition, to increase hornet attraction, a 500-ml nebulizer was filled with attractant, and the liquid was sprayed for approximately 10–20 minutes before observations began. The attractant was composed of 1: 1: 1 brown sugar water, vinegar, and ethanol, which mimics oak sap. It is the most commonly used substance for attracting and capturing Vespinae species in hornet traps in Korea, and it has very little attractiveness bias for a particular species [19, 47].
This experiment was conducted only with workers, excluding gynes and males, to get rid of the differences in attack behaviors due to caste differences. Therefore, we conducted our study over a total of four days from August 12–13 and August 17–18, 2017, before the gynes and males came out in mid-September. The experiment was conducted at 8–10 am and 5–7 pm because workers tend to avoid outdoor activities when daytime temperatures exceed 35 degrees. The experiment was conducted eight times in total.
Behavioral description, intensity scores, winning percentages, and aggressive behavior trends
As there were no previous behavioral descriptions of the aggressive behavior of hornets, we prepared the first description based on Jang et al. [48] and Goyens et al. [49], by analyzing images from our experiment. Images of hornet behavior were taken by recording hornets with a camcorder (Digital Camcorder V 2000, Sunwoo Tech. Crop. Goyangsi, Korea), placed 50 cm in front of the experiment table.
Aggressive behavioral interactions were classified as wins, losses, and ties. Losses was given 0 points, and ties given 1 point. For wins, threatening behaviors were given 2 points, grappling behaviors were given 3–4 points, and killing was given 5 points. These intensity scores are summarized in Table 1. Winning percentages were calculated, showing the percentage of wins, losses, and ties. In addition, the tendency towards aggressive behavior between the two species was expressed in the radar chart of the number of each behavior. The score for each behavior is derived from Table 1 and Fig 2.
Table 1. Descriptions of hornet aggression behaviors observed and their corresponding scores.
| Behavior | Description | Intensity score | ||||
|---|---|---|---|---|---|---|
| Win | Loss | Tie | Win | Lose | Tie | |
| Threatening | Rushing opponent | Falling back or escaping | Confrontation and separation | 2 | 0 | 1 |
| Lifting antennae and front legs and shaking wings, (Fig 1B) | Falling back or escaping | Confrontation | 2 | 0 | 1 | |
| Opening mandible, (Fig 1F) | Falling back or escaping | Confrontation | 2 | 0 | 1 | |
| Threateningly flying over opponent | Escaping | Disregard or confrontation | 2 | 0 | 1 | |
| Chasing opponent | Escaping | Confrontation and separation | 2 | 0 | 1 | |
| Grappling | Pushing or fighting while flying | Falling back or escaping | Confrontation and separation | 3 | 0 | 1 |
| Banging or pushing with head | Falling back or escaping | Confrontation and separation | 3 | 0 | 1 | |
| Chasing and grabbing, (Fig 1E) | Being thrown off | Confrontation and separation | 3 | 0 | 1 | |
| Forcing down and throwing opponent, (Fig 1D) | Being thrown off | Confrontation and separation | 3 | 0 | 1 | |
| Getting opponent and biting or stinging, (Fig 1A) | Being bitten or stung | Confrontation and separation | 4 | 0 | 1 | |
| Killing | Killing opponent, (Fig 1C) | Being killed | Failure to kill opponent or both sides dying | 5 | 0 | 1 |
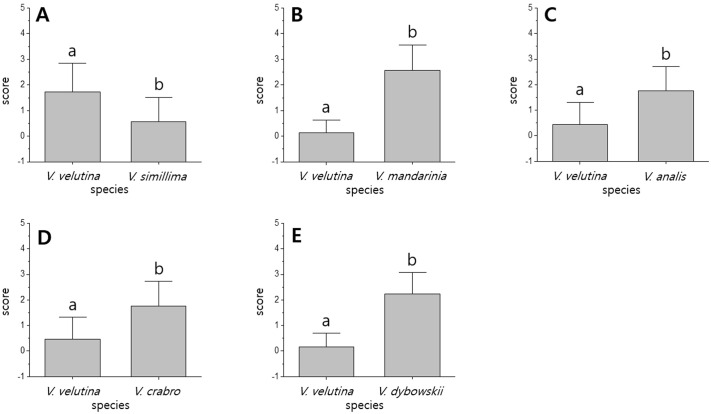
Fig 2. Aggressiveness scores between an invasive alien hornet, V. velutina, and five native Korean hornet species.
Scores are displayed as mean values ± SD. A: V. velutina (score 1.73±1.11) vs Vespa simillima (score 0.57±0.94), t(304) = 9.89, P < 0.001; B: V. velutina (score 0.13±0.48) vs Vespa mandarinia (score 2.58±0.98), t(206) = -22.75, P < 0.001; C: V. velutina (score 0.45±0.86) vs Vespa analis (score 1.76±0.96), t(132) = -8.38, P < 0.001; D: V. velutina (score 0.47±0.86) vs Vespa crabro (score 1.76±0.97), t(184) = -9.62, P < 0.001; E: V. velutina (score 0.16±0.54) vs Vespa dybowskii (score 2.24±0.83), t(262) = -24.22, P < 0.001. See S1 Table.
Morphological measurements
To measure the body size of the six species, we captured them using an insect net following the behavioral observation experiment and stored them in 95% alcohol. Both winners and losers were included in the sampling. If the test subjects were missed, the remaining opponents were excluded from the measurement. Captured samples were dried at room temperature (20°C—25°C) for one week in the laboratory and then pinned. The lengths (mm) of the specimens were obtained by measuring only the head width and thorax length through a stereoscopic microscope (Olympus optical, JP SZ61, Olympus Korea, Seoul, Korea), as the hornet's abdomen was elastic and could affect its body size. Body size of the five native species was measured by selecting 30 individuals from the samples collected during the experiment. In addition, V. velutina was selected from the individuals who fought the five native species, and a total of 30 individuals were measured.
Statistical analysis
We performed an independent t-test on the intensity scores of V. velutina and the five native hornets to verify the significance of any differences in aggression between species.
In addition, the size differences among the six hornets, including V. velutina were analyzed using a one-way analysis of variance (ANOVA) with a post hoc Tukey’s honest significant difference (HSD) test. All analyses were performed using IBM SPSS Statistics software version 23.0 (IBM, USA).
Results
Description of aggressive behavior
Aggressive behaviors between V. velutina and native hornets were classified into three categories: threatening, grappling, and killing. The full list of all behaviors within these categories are listed in Table 1.
First, threatening occurred when the two individuals maintained a distance from each other and ate the attractant, and as the distance between them became closer, they threatened their opponents without making direct contact. The following behaviors were considered threatening: an individual moving forward, lifting its antennae and front legs, shaking its wings, or opening its mandible (Fig 1B and 1F). In these cases, the individual was considered to have won when the opponent fell back or ran away. An individual flying above its opponent, or chasing the opponent, were also considered threatening behaviors. In these cases, the individual was considered to have won when the opponent ran away. Interactions were considered a tie when opponents threatened and fell behind each other.
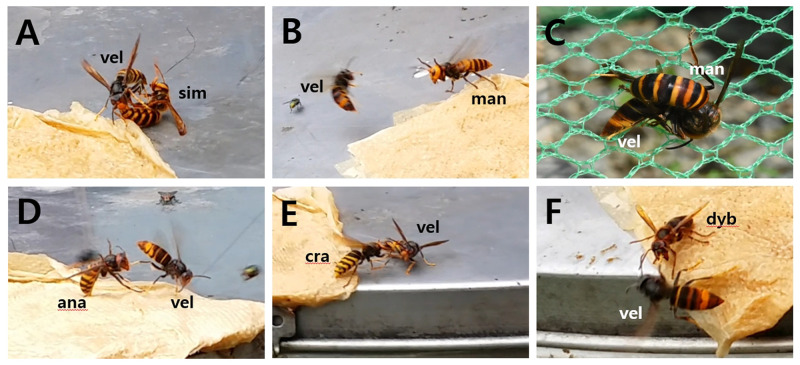
Fig 1. Aggressive behavior between an invasive alien hornet, Vespa velutina, and five native Korean hornet species (Vespa simillima, Vespa mandarinia, Vespa analis, Vespa crabro, and Vespa dybowskii).
A: biting, V. velutina bites V. simillima using its mandibles, B: lifting antennae and shaking wings, V. mandarinia threatens V. velutina by raising its antenna and shaking its wings as V. velutina approaches, C: killing, V. mandarinia hunts V. velutina, D: forcing down and throwing, V. analis throws V. velutina, E: banging or pushing with head, V. crabro pushes V. velutina with its head, F: open mandible, V. dybowskii threatens V. velutina with its mandibles. Species codes: vel: V. velutina, sim: V. simillima, man: V. mandarinia, ana: V. analis, cra: V. crabro, dyb: V. dybowskii.
Grappling occurred between two individuals if the distance between them became very narrow or touched. They flew into the air, either fighting or chasing each other and throwing their opponent. Pushing their opponent’s head, hitting, throwing, climbing on top of, biting, or attacking their opponent with a stinger were all considered grappling behaviors (Fig 1A, 1D and 1E). In these interactions, the individual who fell back, ran away, fell, or was bitten was considered to have lost. If the individuals fall back behind each other while facing each other, this was a tie.
Finally, killing occurred when an overwhelmingly strong individual fought with an opponent, and killed it (Fig 1C). If predation failed or both individuals died, this was a tie.
Aggressive intensity, winning percentages, and aggressive behavior trends
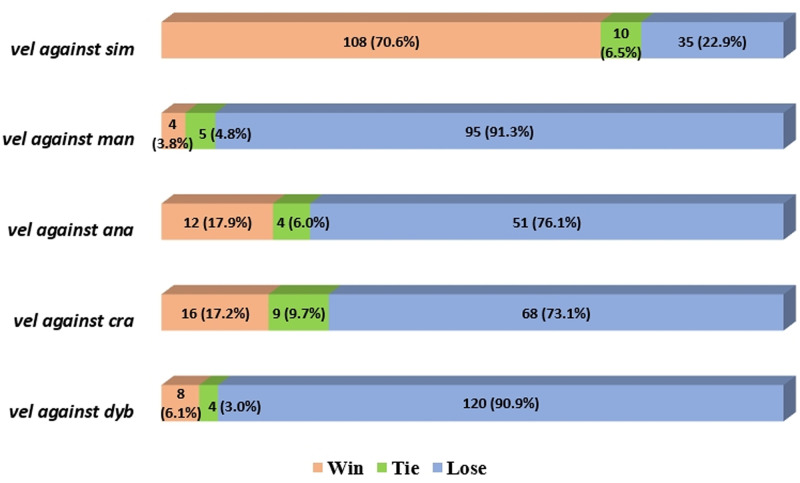
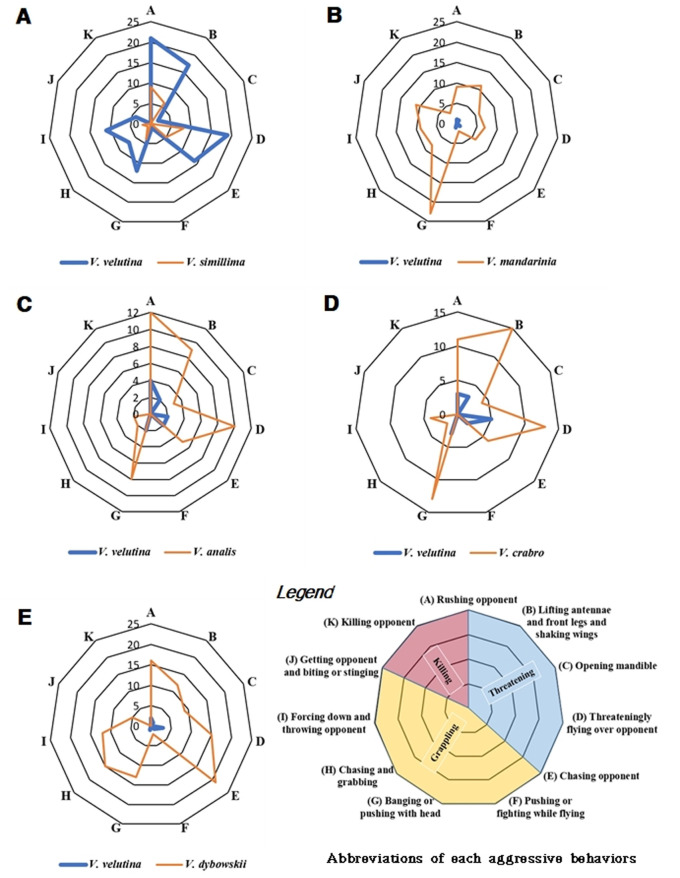
In V. velutina vs. V. simillima, V. velutina was superior to V. simillima in 153 fights (t(304) = 9.89, P < 0.001, Fig 2A), and won 71% of the encounters (Fig 3). V. velutina had a high rate of threatening behavior, such as rushing the opponent, and lifting the antennae and front legs and shaking the wings. V. simillima, however, rushed the opponent more often than V. velutina (Fig 4A).
Fig 3. Winning percentages between an invasive alien hornet, V. velutina, and five native Korean hornet species.
All wins and losses are based on V. velutina. Species codes: vel: V. velutina, sim: V. simillima, man: V. mandarinia, ana: V. analis, cra: V. crabro, dyb: V. dybowskii.
Fig 4. Trends of aggressive behaviors between an invasive alien hornet, V. velutina and five native Korean hornet species.
The aggressiveness score of behaviors increases clockwise and the score of each behavior is the sum of the intensity score.
In V. velutina vs. V. mandarinia, V. mandarinia outperformed V. velutina in a total of 104 fights (t(206) = -22.75, P < 0.001, Fig 2B) and won 91% of the encounters (Fig 3). V. mandarinia displayed a high rate of grappling behavior, such as banging or pushing with the head (Fig 4B). V. mandarinia also preyed upon V. velutina, although rarely.
In V. velutina vs. V. analis, V. analis was superior to V. velutina in 67 fights (t(132) = -8.38, P < 0.001, Fig 2C) and won 76% of the encounters (Fig 3). Concerning threatening behavior, V. analis displayed threat most often by rushing the opponent, and this species’ second favorite ploy was to fly threateningly over the adversary. In contrast, V. velutina made a weak show of rushing the opponent (Fig 4C).
In contests between V. velutina and V. crabro, V. crabro was superior to V. velutina in 93 fights (t(184) = -9.62, P < 0.001, Fig 2D) and won 73% of the encounters (Fig 3). Lifting antennae and front legs and shaking wings were the most common threatening behaviors in V. crabro, and banging or pushing with the head were the most common grappling behaviors. In contrast, V. velutina displayed minor aggressive behavior such as threateningly flying over the opponent (Fig 4D).
Finally, in encounters between V. velutina and V. dybowskii, V. dybowskii was superior to V. velutina in a total of 132 fights (t(262) = -24.22, P < 0.001, Fig 2E) and won 91% of the contests (Fig 3). The most common threatening behavior employed by V. dybowskii was chasing the opponent, and the second most common action was rushing the opponent; chasing and grabbing were the most common grappling behaviors for this species. V. velutina’s counterattack action was to fly threateningly over the opponent but not vigorously or often (Fig 4E).
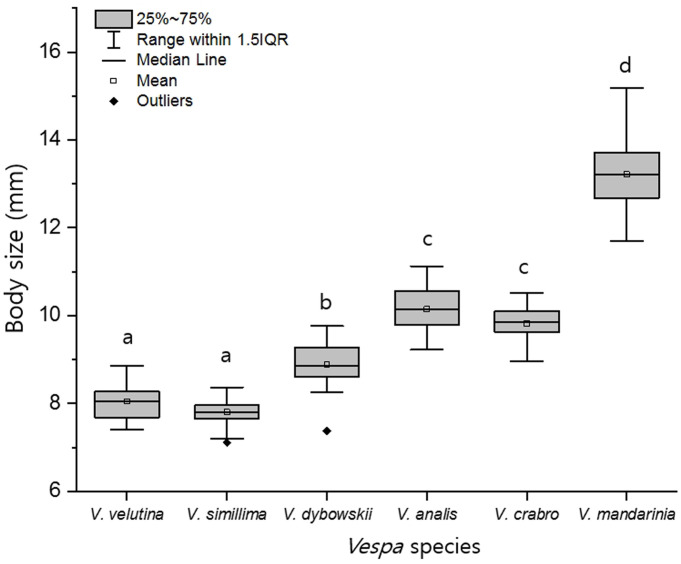
Body size
Numerically, the body size of V. velutina was 8.04 ± 0.41 mm, which was slightly larger than V. simillima 7.80 ± 0.29 mm. V. dybowskii was 8.88 ± 0.49 mm, V. analis was 10.14 ± 0.51 mm, V. crabro was 9.82 ± 0.4 mm, and V. mandarinia was 13.21 ± 0.83 mm. Thus, except for V. simillima, all species were larger than V. velutina. There were significant differences in body size among Vespa species (F(5, 174) = 434.9, P <0.001), being the differences significant between all the species except for V. simillima and V. velutina, and V. analis and V. crabro (Fig 5).
Fig 5. Body size differences between an invasive alien hornet, V. velutina and five native Korean hornet species.
F(5, 174) = 434.9, P < 0.001.
Discussion
Spread effect of V. velutina on interspecific competition in Korea
The invasion of IAS generally results in interspecific competition for resources with similar native species [50]. If the native species are strong, invasive species tend to avoid competition temporally or spatially, but if the native species are weak or similar, exploitation or competition may interfere [29, 51], resulting in interspecific displacement [50].
In Europe, where V. velutina was introduced in 2004, there were already two native hornet species, V. crabro and V. orientalis, so that the two species were in close competition because of their similar ecological niche, due overlaps in food preference, nesting sites, and life history [34, 35]. However, in Korea, where there are nine native hornets, V. velutina clearly differ in their ecological niche. V. mandarinia is the most aggressive among hornets, with the highest hierarchy, nesting in the ground, and mainly foraging on honeybees and large beetles [37]. V. crabro nests in the ground, under trees, and V. analis nests under grasses and leaves, hunting small and medium-sized insects such as bees, flies, and moths [37]. V. dybowskii nests in tree trunks and often display social parasitism [37]. V. velutina, however, nests at the tops of trees, with a lifespan of about one month longer and a higher rate of honeybee foraging [28, 52]. V. simillima are phylogenetically closest to V. velutina [53] and have the most ecological overlap because of similarities in life history, nesting, and food preference. Therefore, V. simillima may be most severely affected by V. velutina [19]. According to the results of this study, V. velutina is less aggressive than the other four species except V. simillima, indicating that the ecological niche is low. Therefore, V. velutina avoids competition from four species with strong ecological niche when nesting, as it nests in a place with low competition. Indeed, after the invasion of V. velutina in Busan, Korea, there was a decrease in V. similima [19]. Therefore, after V. velutina's invasion of Korea, its distribution and spread seem to be affected by its ecological niche. From Busan, the point of first invasion of V. velutina, it spread northwards into cities such as Ulsan, Gimhae, and Changwon, where the road network is directly connected, and the east and south coasts [19, 45, 54]. Since there are nine native hornets within Korean forest habitats, V. velutina would have had considerable difficulty invading deep forests and crossing large forests and mountains [54].
This ecological niche is often determined by differences in fighting ability, which is largely influenced by body size. In many animals, large body size is likely to corelate with physical fighting ability, which can lead to intraspecific and interspecific resource competition. This is termed resource-holding potential (RHP) [55, 56]. For example, in various insects, larger individuals in the intraspecific have higher aggressiveness and higher RHP than small ones, indicating that they are competitive [48, 49, 57, 58]. Large invasive species have been shown to spread more easily in nature with competitive advantages at higher RHP than smaller native species [59]. Therefore, in this study, as V. velutina is a similar size to or larger than V. simillima, but smaller in size than the other four native hornets, its RHP seems to be relatively low, as shown in the aggressiveness results of this study.
Therefore, V. velutina appears to avoid direct competition with native hornets, inhabiting urban centers with relatively low-density hornet populations, or on mountain edges adjacent to cities. According to Choi et al. [19], in Busan, the occurrence rate of V. velutina was overwhelmingly higher in cities than in forests.
Meanwhile, the low interspecific hierarchy of V. velutina may have influenced their initial spread. In general, the initial incubation period is determined according to the presence or absence of competing species after the invasion of alien species, and after a certain period of time, when the alien species have a firm ecological niche, the population expands and spreads rapidly [60]. Thus, in Europe where the competition was V. crabro at the beginning of the invasion [34, 35], the annual spread rate was 60–80 km/year [61, 62]. In Korea, however, the rate of spread is slower than that of Europe at 10–20 km/year, because 63% of the land is forested, and nine Vespa species already inhabit forests [19, 63, 64]. The forest landscape of Korea and presence of many competing hornet species had the effect of prolonging the incubation period. Thus, for V. velutina, one of the main reasons why it could survive without natural extinction, despite its low ecological niche, seems to be its choice of habitat in cities with low competition among hornets during incubation period.
V. velutina has ended its initial incubation period, and the rate of spread has increased rapidly from 2008–2010 [64], culminating in its distribution throughout South Korea as of 2018 [20]. Of course, this spread may have been affected by human factors, such as human activity or transport [61], but competition among hornets seems to be the main reason.
However, despite its low aggression and small size, V. velutina population density tended to increase not only in urban areas but also in forested areas over time, and this trend is present in most parts of southern-central Korea [46].
Many invasive alien social insects, such as ants, hornets, yellowjackets, and paper wasps, have large colonies, high polygyny rates, or invasive generalist insect predators, which often lead to dominant distribution and species displacement with successful invasion. This is because alien species with larger colonies than native species are much easier to feed [16, 17, 65–69]. Although V. velutina has a lower ecological niche than native hornets, the colonies are two to four times larger [19, 70], which is an overwhelming number of individuals that can obtain food resources in nature. In addition, V. velutina has a good flight ability compared to other hornets [71, 72] and hunts for food quickly and safely through hawking [46, 73], making it very efficient. Altering the concentration and composition of alarm pheromones in areas it has invaded also creates an effective defense strategy against potential predators [74, 75]. Therefore, in the early stages of the invasion, it spread to urban areas, competing with native hornets, but due to its large colonies and high foraging ability, they seemed to overcome their disadvantages and gradually increase their abundance even in the forest.
Interspecific hierarchies of Korean Vespa species
In this study, the interspecific hierarchies of Korean Vespa species was determined in part through the aggression behavior of six Vespa species, but the remaining four Korean species were not tested [45]. However, of these four species, V. crabro crabroniformis is a subspecies of V. crabro (the V. crabro used in this experiment is V. crabro flavofasciata), and V. simillima xanthoptera is a subspecies of V. simillima (the V. simillima used in this experiment was V. simillima simillima). These are very similar to the two species we observed, so we expect there to be little difference in ecological characteristics between them. In particular, V. simillima xanthoptera only inhabits Jeju Island in Korea, and has no geographical overlap with the mainland species. V. binghami was first reported in Korea in 2011 [76], and there is a lack of ecological information on this species, which requires further study. Finally, V. ducalis was not addressed in this study, but Yoshimoto and Nishida [39] showed it was less aggressive than V. crabro and V. analis, and Matsuura [77] showed it was less aggressive than V. simillima. It is therefore likely to be the least aggressive among the Vespa species.
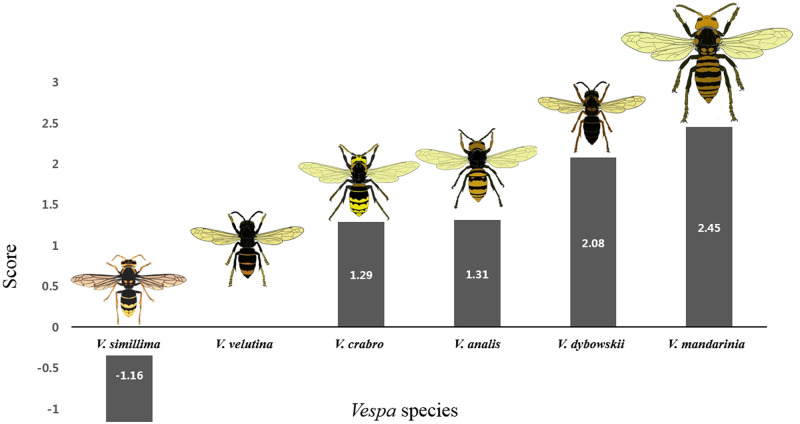
As a result, according to Matsuura and Yamane [37], the order of hierarchies Vespa species in Japan is V. mandarinia > V. crabro > V. analis > V. simillima.
However, in this study, our results suggested the order for Korean Vespa species should be V. mandarinia > V. dybowskii > V. analis > V. crabro > V. velutina > V. simillima (Fig 6). Although the order of hierarchies V. analis and V. crabro changed in comparison with the order in Matsuura and Yamane [37], the sequence is similar. If V. ducalis were to be added here, we would expect the order to be V. mandarinia > V. dybowskii > V. analis > V. crabro flavofasciata (or V. v. crabroniformis) > V. velutina > V. simillima (or V. s. xanthoptera) > V. ducalis.
Fig 6. Interspecific hierarchies of an invasive alien hornet V. velutina and five native Korean hornet species inferred by aggressiveness scores.
Supporting information
(DOCX)
Acknowledgments
This study is a part of MB Choi’s Ph. D study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Arim M, Abades SR, Neill PE, Lima M, Marquet PA. Spread dynamics of invasive species. Proc Natl Acad Sci U S A. 2006;103: 374–378. 10.1073/pnas.0504272102 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hulme PE. Trade, transport and trouble: managing invasive species pathways in an era of globalization. J Appl Ecol. 2009;46: 10–18. 10.1111/j.1365-2664.2008.01600.x [DOI] [Google Scholar]
- 3.Ziska LH, Blumenthal DM, Runion GB, Hunt ER Jr, Diaz-Soltero H. Invasive species and climate change: an agronomic perspective. Clim Change. 2011;105: 13–42. 10.1007/s10584-010-9879-5 [DOI] [Google Scholar]
- 4.Pyšek P, Richardson DM. Invasive species, environmental change and management, and health. Annu Rev Environ Resour. 2010;35: 25–55. 10.1146/annurev-environ-033009-095548 [DOI] [Google Scholar]
- 5.Bradshaw CJA, Leroy B, Bellard C, Roiz D, Albert C, Fournier A, et al. Massive yet grossly underestimated global costs of invasive insects. Nat Commun. 2016;7: 12986 10.1038/ncomms12986 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi MB, Lee SY, Yoo JS, Jun J, Kwon O. First record of the western black widow spider Latrodectus hesperus Chamberlin & Ivie, 1935 (Araneae: Theridiidae) in South Korea. Entomol Res. 2019a;49: 141–146. 10.1111/1748-5967.12350 [DOI] [Google Scholar]
- 7.Sung S, Kwon YS, Lee DK, Cho Y. Predicting the Potential Distribution of an Invasive Species, Solenopsis invicta Buren (Hymenoptera: Formicidae), under Climate Change using Species Distribution Models. Entomol Res. 2018;48: 505–513. 10.1111/1748-5967.12325 [DOI] [Google Scholar]
- 8.Kim JK, Choi MB, Moon TY. Occurrence of Vespa velutina Lepeletier from Korea, and a revised key for Korean Vespa species (Hymenoptera: Vespidae). Entomol Res. 2006;36: 112–115. 10.1111/j.1748-5967.2006.00018.x [DOI] [Google Scholar]
- 9.Lee HS, Kim DE, Lyu DP. Discovery of the Invasive Argentine ant, Linepithema humile (Mayr) (Hymenoptera: Formicidae: Dolichoderinae) in Korea. Korean J Appl Entomol. 2020;59(1): 71–72. [Google Scholar]
- 10.Han JM, Kim H, Lim EJ, Lee S, Kwon YJ, Cho S. Lycorma delicatula (Hemiptera: Auchenorrhyncha: Fulgoridae: Aphaeninae) finally, but suddenly arrived in Korea. Entomol Res. 2008;38(4): 281–286. [Google Scholar]
- 11.Kim Y, Kim M, Hong KJ, Lee S. Outbreak of an exotic flatid, Metcalfa pruinosa (Say) (Hemiptera: Flatidae), in the capital region of Korea. J Asia-Pac Entomol. 2011;14: 473–478. [Google Scholar]
- 12.Choi DS, Kim DI, Ko SJ, Kang BR, Lee KS, Park JD, et al. Occurrence ecology of Ricania sp. (Hemiptera: Ricaniidae) and selection of environmental friendly agricultural materials for control. Korean J Appl Entomol. 2012;51(2): 141–148. [Google Scholar]
- 13.Lee H, Kim M, Bae YS, Kim DE. New occurrence of the invasive alien leaf-footed bug Leptoglossus gonagra (Hemiptera: Coreidae) in South Korea. Entomol Res. 2020;50: 14–22. 10.1111/1748-5967.12402 [DOI] [Google Scholar]
- 14.Mooney HA, Drake JA. Ecology of biological invasions of North America and Hawaii. Springer-Verlag, New York, USA; 1986. [Google Scholar]
- 15.Kareiva P. Developing a predictive ecology for non-indigenous species and ecological invasions. Ecology. 1996;77: 1651–1652. 10.2307/2265766 [DOI] [Google Scholar]
- 16.Holway DA. Competitive mechanisms underlying the displacement of native ants by the invasive argentine ant. Ecology. 1999;80: 238–251. 10.1890/0012-9658(1999)080[0238:CMUTDO]2.0.CO;2 [DOI] [Google Scholar]
- 17.Carpintero S, Reyes-López J. The role of competitive dominance in the invasive ability of the Argentine ant (Linepithema humile). Biol Invas. 2008;10: 25–35. 10.1007/s10530-007-9103-3 [DOI] [Google Scholar]
- 18.Moller H. Lessons for invasion theory from social insects. Biol Conserv. 1996;78: 125–142. 10.1016/0006-3207(96)00022-5 [DOI] [Google Scholar]
- 19.Choi MB, Martin SJ, Lee JW. Distribution, spread, and impact of the invasive hornet Vespa velutina in South Korea. J Asia-Pac Entomol. 2012b;15: 473–477. 10.1016/j.aspen.2011.11.004 [DOI] [Google Scholar]
- 20.Choi MB, Kim TG, Kwon O. Recent trends in wasp nest removal and Hymenoptera stings in South Korea. J Med Entomol. 2019b;56: 254–260. 10.1093/jme/tjy144 . [DOI] [PubMed] [Google Scholar]
- 21.Do Y, Kim JB, Shim JH, Choi MB. Quantitative analysis of research topics and public concern on Vespa velutina as invasive species in Asian and European countries. Entomol Res. 2019;49: 456–461. 10.1111/1748-5967.12390 [DOI] [Google Scholar]
- 22.Sakai Y, Takahashi J. Discovery of a worker of Vespa velutina (Hymenoptera: Vespidae) from Tsushima Island, Japan. Kontyu. 2014;17: 32–36. [Google Scholar]
- 23.Ueno T. Establishment of the invasive hornet Vespa velutina (Hymenoptera: Vespidae) in Japan. Int J Chem Environ Biol Sci. 2014;2(4): 220–222. [Google Scholar]
- 24.Minoshima YN, Yamane S, Ueno T. An invasive alien hornet, Vespa velutina nigrithorax du Buysson (Hymenoptera: Vespidae), found in Kitakyushu, Kyushu island: A first record of the species from mainland Japan. Jpn J Syst Entomol. 2015;21(2): 259–261. [Google Scholar]
- 25.Haxaire J, Bouguet J-P, Tamisier J-P. Vespa velutina Lepeletire, 1836, une redoutable novueauté pour la faune de France (Hymenoptera, Vespidae). Bull Soc entomol Fr. 2006;111: 194. [Google Scholar]
- 26.Arca M, Mougel F, Guillemaud T, Dupas S, Rome Q, Perrard A, et al. Reconstructing the invasion and the demographic history of the yellow-legged hornet, Vespa velutina, in Europe. Biol Invas. 2015;17: 2357–2371. 10.1007/s10530-015-0880-9 [DOI] [Google Scholar]
- 27.Budge GE, Hodgetts J, Jones EP, Ostoja-Starzewski JC, Hall J, Tomkies V, et al. The invasion, provenance and diversity of Vespa velutina Lepeletier (Hymenoptera: Vespidae) in Great Britain. PLOS ONE. 2017;12(9): e0185172 10.1371/journal.pone.0185172 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monceau K, Bonnard O, Thiéry D. Vespa velutina: a new invasive predator of honeybees in Europe. J Pest Sci. 2014;87: 1–16. 10.1007/s10340-013-0537-3 [DOI] [Google Scholar]
- 29.Choi MB, Kim JK, Lee JW. Increase trend of social Hymenoptera (wasps and honeybees) in urban areas, inferred from moving-out case by 119 rescue services in Seoul of South Korea. Entomol Res. 2012a;42: 308–319. 10.1111/j.1748-5967.2012.00472.x [DOI] [Google Scholar]
- 30.de Haro L, Labadie M, Chanseau P, Cabot C, Blanc-Brisset I, Penouil F, National Coordination Committee for Toxicovigilance. Medical consequences of the Asian black hornet (Vespa velutina) invasion in Southwestern France. Toxicon. 2010;55: 650–652. 10.1016/j.toxicon.2009.08.005 [DOI] [PubMed] [Google Scholar]
- 31.Park JJ, Jung C. Risk prediction of the distribution of invasive hornet, Vespa velutina nigrithorax in Korea using CLIMEX model. J Apic. 2016;31(4): 293–303. [Google Scholar]
- 32.Lioy S, Manino A, Porporato M, Laurino D, Romano A, Capello M, et al. Establishing surveillance areas for tackling the invasion of Vespa velutina in outbreaks and over the border of its expanding range. NeoBiota. 2019;46: 51–69. [Google Scholar]
- 33.Monceau K, Thiery D. Vespa velutina nest distribution at a local scale: An 8-year survey of the invasive honeybee predator. Insect Sci. 2017;24(4): 663–674. 10.1111/1744-7917.12331 [DOI] [PubMed] [Google Scholar]
- 34.Cini A, Cappa F, Petrocelli I, Pepiciello I, Bortolotti L, Cervo R. Competition between the native and the introduced hornets Vespa crabro and Vespa velutina: a comparison of potentially relevant life-history traits. Ecol Entomol. 2018;43: 351–362. 10.1111/een.12507 [DOI] [Google Scholar]
- 35.Monceau K, Maher N, Bonnard O, Thiery D. Evaluation of competition between a native and an invasive hornet species: do seasonal phenologies overlap? Bull Entomol Res. 2015;105: 462–469. 10.1017/S0007485315000280 . [DOI] [PubMed] [Google Scholar]
- 36.Yamasaki K, Takahashi R, Harada R, Matsuo Y, Nakamura M, Takahashi JI. Reproductive interference by alien hornet Vespa velutina threatens the native populations of Vespa simillima in Japan. Naturwissenschaften. 2019;106: 15 10.1007/s00114-019-1609-x . [DOI] [PubMed] [Google Scholar]
- 37.Matsuura M, Yamane S. Biology of Vespine Wasps. Springer-Verlag, Berlin, Germany; 1990. [Google Scholar]
- 38.Yoshimoto J, Kakutani T, Nishida T. Influence of resource abundance on the structure of the insect community attracted to fermented tree sap. Ecol Res. 2005;20(4): 405–414. 10.1007/s11284-005-0054-9 [DOI] [Google Scholar]
- 39.Yoshimoto J, Nishida T. Factors affecting behavioral interactions among sap-attracted insects. Annals of the Entomological Society of America. 2009;102(2): 201–209. 10.1603/008.102.0203 [DOI] [Google Scholar]
- 40.Yoshimoto J. Interspecific variation in competitor avoidance and foraging success in sap-attracted insects. Eur J Entomol. 2009;106: 529–533. 10.14411/eje.2009.066 [DOI] [Google Scholar]
- 41.Simberloff D, Martin JL, Genovesi P, Maris V, Wardle DA, Aronson J, et al. Impacts of biological invasions: what’s what and the way forward. Trends in Eco & Evol. 2013;28(1): 58–66. [DOI] [PubMed] [Google Scholar]
- 42.Carrasco LR, Baker R, MacLeod A, Knight JD, Mumford D. Optimal and robust control of invasive alien species spreading in homogeneous landscapes. J R Soc Interface. 2010;7: 529–540. 10.1098/rsif.2009.0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toepfer S, Kuhlmann U. Survey for natural enemies of the invasive alien chrysomelid, Diabrotica virgifera virgifera, in Central Europe. BioControl. 2004;49: 385–395. [Google Scholar]
- 44.Villemant C, Zuccon D, Rome Q, Muller F, Poinar GO, Justine JL. Can parasites halt the invader? Mermithid nematodes parasitizing the yellow-legged Asian hornet in France. PeerJ. 2015;3: e947 10.7717/peerj.947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi MB, Kim JK, Lee JW. Checklist and distribution of Korean Vespidae revisited. Korean J Appl Entomol. 2013;52: 85–91. 10.5656/KSAE.2013.02.1.072 [DOI] [Google Scholar]
- 46.Choi MB, Kwon O. Occurrence of Hymenoptera (wasps and bees) and their foraging in the southwestern part of Jirisan National Park, South Korea. J Ecol Environ. 2015;38(3): 367–374. 10.5141/ecoenv.2015.038 [DOI] [Google Scholar]
- 47.Chio MB, Park BA, Lee JW. The Species Diversity and Distribution of Vespidae in Southeast Region (Sangdong-eup, Gimsatgat-myeon, Jungdong-myeon) of Yeongwol-gun, Gangwon-do, Korea. J Korean Nat. 2012;5(4): 305–310. [Google Scholar]
- 48.Jang Y, Gerhardt HC, Choe JC. A comparative study of aggressiveness in eastern North American field cricket species (genus Gryllus). Behav Ecol Sociobiol. 2008;62: 1397–1407. 10.1007/s00265-008-0568-6 [DOI] [Google Scholar]
- 49.Goyens J, Dirckx J, Aerts P. Stag beetle battle behavior and its associated anatomical adaptations. J Insect Behav. 2015;28: 227–244. 10.1007/s10905-015-9495-3 [DOI] [Google Scholar]
- 50.Reitz SR, Trumble JT. Competitive displacement among insects and arachnids. Annu Rev Entomol. 2002;47: 435–465. 10.1146/annurev.ento.47.091201.145227 . [DOI] [PubMed] [Google Scholar]
- 51.Ronconi RA, Burger AE. Foraging space as a limited resource: inter- and intra-specific competition among sympatric pursuit-diving seabirds. Can J Zool. 2011;89: 356–368. 10.1139/z11-006 [DOI] [Google Scholar]
- 52.Perrard A, Haxaire J, Rortais A, Villemant C. Observations on the colony activity of the Asian hornet Vespa velutina Lepeletire 1836 (Hymenoptera: Vespidae: Vespinae) in France. Ann Soc Entomol Fr. 2009;45: 119–127. 10.1080/00379271.2009.10697595 [DOI] [Google Scholar]
- 53.Perrard A, Pickett KM, Villemant C, Kojima J, Carpenter J. Phylogeny of hornets: a total evidence approach (Hymenoptera, Vespidae, Vespinae, Vespa). J Hymenoptera Res. 2013;32: 1–15. 10.3897/jhr.32.4685 [DOI] [Google Scholar]
- 54.Park JJ, Jung C. Risk prediction of the distribution of invasive hornet, Vespa velutina nigrithorax in Korea using CLIMEX model. J Apic. 2016;31(4): 293–303. [Google Scholar]
- 55.Parker GA. Assessment strategy and the evolution of fighting behaviour. J Theor Biol. 1974;47: 223–243. 10.1016/0022-5193(74)90111-8 . [DOI] [PubMed] [Google Scholar]
- 56.Parker GA, Stuart RA. Animal behavior as a strategy optimizer evolution of resource assessment strategies and optimal emigration thresholds. Am Nat. 1976;110: 1055–1076. 10.1086/283126 [DOI] [Google Scholar]
- 57.Songvorawit N, Butcher BA, Chaisuekul C. Resource holding potential and the outcome of aggressive interactions between paired male Aegus chelifer chelifer (Coleoptera: Lucanidae) stag beetles. J Insect Behav. 2018;31: 347–360. 10.1007/s10905-018-9683-z [DOI] [Google Scholar]
- 58.Briffa M. Decisions during fights in the house cricket, Acheta domesticus: mutual or self assessment of energy, weapons and size? Anim Behav. 2008;75: 1053–1062. 10.1016/j.anbehav.2007.08.016 [DOI] [Google Scholar]
- 59.Sanches FHC, Miyai CA, Costa TM, Christofoletti RA, Volpato GL, Barreto RE. Aggressiveness overcomes body-size effects in fights staged between invasive and native fish species with overlapping niches. PLOS ONE. 2012;7(1): e29746 10.1371/journal.pone.0029746 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruiz GM, Carlton JT. Invasive species Vectors and management strategies. Island Press; Washington. USA; 2003. [Google Scholar]
- 61.Robinet C, Suppo C, Darrouzet E. Rapid spread of the invasive yellow-legged hornet in France: the role of human-mediated dispersal and the effects of control measures. J Appl Ecol. 2017;54: 205–215. 10.1111/1365-2664.12724 [DOI] [Google Scholar]
- 62.Rome Q, Muller FJ, Touret-Alby A, Darrouzet E, Perrard A, Villemant C. Caste differentiation and seasonal changes in Vespa velutina (Hym.: Vespidae) colonies in its introduced range. J Appl Entomol. 2015;139: 771–782. 10.1111/jen.12210 [DOI] [Google Scholar]
- 63.Korea Forest Service. Forest basic statistics (2015). Korea Forest Service, Daejeon, Korea; 2016.
- 64.Jung C. Spatial expansion of an invasive hornet, Vespa velutina nigrithorax (Hymenoptera: Vespidae) in Korea. Korean J Apic. 2012;27: 7–93. [Google Scholar]
- 65.Crowder DW, Snyder WE. Eating their way to the top? Mechanisms underlying the success of invasive insect generalist predators. Biol Invas. 2010;12: 2857–2876. 10.1007/s10530-010-9733-8 [DOI] [Google Scholar]
- 66.Wittman SE, O'Dowd DJ, Green PT. Carbohydrate supply drives colony size, aggression, and impacts of an invasive ant. Ecosphere. 2018;9: e02403 10.1002/ecs2.2403 [DOI] [Google Scholar]
- 67.Walters AC, Mackay DA. Importance of large colony size for successful invasion by Argentine ants (Hymenoptera: Formicidae): Evidence for biotic resistance by native ants. Austral Ecol. 2005;30: 395–406. 10.1111/j.1442-9993.2005.01481.x [DOI] [Google Scholar]
- 68.Boulay R, Arnan X, Cerda X, Retana J. The ecological benefits of large colony size may promote polygyny in ants. J Evol Biol. 2014;27: 2856–2863. 10.1111/jeb.12515 . [DOI] [PubMed] [Google Scholar]
- 69.Beggs JR, Brockerhoff EG, Corley JC, Kenis M, Masciocchi M, Muller F, et al. Ecological effects and management of invasive alien Vespidae. BioControl. 2011;56: 505–526. 10.1007/s10526-011-9389-z [DOI] [Google Scholar]
- 70.Jung C, Kang MS, Kim DW, Lee HS. Vespid wasps (Hymenoptera) occurring around apiaries in Andong, Korea. I. Taxonomy and life history. Korean J Apic. 2007;22: 53–62. [Google Scholar]
- 71.Sauvard D, Imbault V, Darrouzet É. Flight capacities of yellow-legged hornet (Vespa velutina nigrithorax, Hymenoptera: Vespidae) workers from an invasive population in Europe. PLOS ONE. 2018;13(6): e0198597 10.1371/journal.pone.0198597 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poidatz J, Monceau K, Bonnard O, Thiéry D. Activity rhythm and action range of workers of the invasive hornet predator of honeybees Vespa velutina, measured by radio frequency identification tags. Ecol Evol. 2018;8: 7588–7598. 10.1002/ece3.4182 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tan K, Radloff SE, Li JJ, Hepburn HR, Yang MX, Zhang LJ, et al. Bee-hawking by the wasp, Vespa velutina, on the honeybees Apis cerana and A. mellifera. Naturwissenschaften. 2007;94: 469–472. 10.1007/s00114-006-0210-2 . [DOI] [PubMed] [Google Scholar]
- 74.Thiéry D, Bonnard O, Riquier L, de Revel G, Monceau K. An alarm pheromone in the venom gland of Vespa velutina: evidence revisited from the European invasive population. Entomol Gen. 2018;38(2): 145–156. [Google Scholar]
- 75.Cheng Y, Wen P, Dong S, Tan K, Nieh JC. Poison and alarm: The Asian hornet Vespa velutina uses sting venom volatiles as alarm pheromone. J Exp Biol. 2017;220: 645–651. 10.1242/jeb.148783 [DOI] [PubMed] [Google Scholar]
- 76.Kim JK, Kim IK. Discovery of Vespa binghami (Vespidae: Hymenoptera) in Korea. Anim Syst Evol Divers. 2011;27: 105–107. 10.5635/KJSZ.2011.27.1.105 [DOI] [Google Scholar]
- 77.Matsuura M. Comparative biology of the five Japanese species of the genus Vespa (Hymenoptera, Vespidae). Bull Fac Agric. Mie University. 1984;69: 1–131. [Google Scholar]