Abstract
Pathogenic bacteria frequently acquire virulence traits via horizontal gene transfer, yet additional evolutionary innovations may be necessary to integrate newly acquired genes into existing regulatory pathways. The plant bacterial pathogen Pseudomonas syringae relies on a horizontally acquired type III secretion system (T3SS) to cause disease. T3SS-encoding genes are induced by plant-derived metabolites, yet how this regulation occurs, and how it evolved, is poorly understood. Here we report that the two-component system AauS-AauR and substrate-binding protein AatJ, proteins encoded by an acidic amino acid-transport (aat) and -utilization (aau) locus in P. syringae, directly regulate T3SS-encoding genes in response to host aspartate and glutamate signals. Mutants of P. syringae strain DC3000 lacking aauS, aauR or aatJ expressed lower levels of T3SS genes in response to aspartate and glutamate, and had decreased T3SS deployment and virulence during infection of Arabidopsis. We identified an AauR-binding motif (Rbm) upstream of genes encoding T3SS regulators HrpR and HrpS, and demonstrated that this Rbm is required for maximal T3SS deployment and virulence of DC3000. The Rbm upstream of hrpRS is conserved in all P. syringae strains with a canonical T3SS, suggesting AauR regulation of hrpRS is ancient. Consistent with a model of conserved function, an aauR deletion mutant of P. syringae strain B728a, a bean pathogen, had decreased T3SS expression and growth in host plants. Together, our data suggest that, upon acquisition of T3SS-encoding genes, a strain ancestral to P. syringae co-opted an existing AatJ-AauS-AauR pathway to regulate T3SS deployment in response to specific host metabolite signals.
Author summary
Compared to well-established models of plant immunity, little is known about how plant pathogenic microorganisms detect their hosts to deploy virulence factors. Here we identify a two-component system AauS-AauR in the plant pathogenic bacterium Pseudomonas syringae that links perception of specific plant-exuded amino acids directly to regulation of genes encoding a virulence-promoting type III secretion system. AauS-AauR also functions in amino acid transport and is present in non-pathogenic pseudomonads, indicating that P. syringae has repurposed this pathway for virulence. Comparative genomics suggests that all pathogenic P. syringae rely on AauS-AauR signaling for T3SS deployment during infection. Therefore, future efforts to inhibit this pathway may be an effective strategy to curb diseases caused by P. syringae across its broad host range.
Introduction
Outcomes of host-pathogen interactions are often determined by complex reciprocal signaling events that occur at the start of infection. Typically, a successful pathogen must recognize the presence of a host to begin deploying virulence factors, whereas to prevent infection, the host must recognize the invader and respond with effective defenses. Because defense and virulence factors are energetically costly to produce and can decrease fitness in the absence of interaction [1], many hosts and pathogens keep their respective defense and virulence programs in check prior to interaction, yet capable of rapid deployment. In this regard, plants have evolved receptors that constantly surveil the extracellular space for pathogen/microbe-associated molecular patterns (PAMPs/MAMPs) [2], and these receptors can trigger effective defenses when activated [3]. Many PAMPs and their respective plant receptors are known, and molecular details of PAMP-induced signaling are extensively studied [3]. In contrast, very little is known about how plant pathogens detect host signals to upregulate genes required for pathogenesis [4].
Pseudomonas syringae are Gram-negative bacteria that, as a species complex, can infect many plants including economically-important crops, and serve as model pathogens for studies of the molecular basis of pathogenesis and disease resistance [5]. A key virulence determinant of P. syringae is the type III secretion system (T3SS), a syringe-like apparatus that delivers effector proteins into host cells to suppress PAMP-activated defense signaling and other defense-associated cellular responses [6–8]. The T3SS must be produced de novo at the start of infection. A metabolomics analysis identified specific plant-exuded metabolites, including aspartate, citrate and 4-hydroxybenzoate, as potent inducers of T3SS-encoding genes in the model strain P. syringae pv. tomato DC3000 [9]. The importance of these metabolite signals for infection outcomes was demonstrated by Arabidopsis mapk phosphatase 1 (mkp1), a mutant that exudes lower amounts of these metabolites and consequently is more resistant to infection [9].
Proteins that regulate T3SS deployment by P. syringae include the alternative sigma factor HrpL that binds to conserved hrp box sequences within the promoters of T3SS-encoding genes to upregulate their expression [10]. In turn, the expression of hrpL is regulated by two NtrC-like enhancer binding proteins HrpR and HrpS that bind the hrpL promoter and recruit the sigma factor RpoN (σ54) [10–14]. It is not clear how the HrpR/HrpS-HrpL node, central to regulating type III secretion, is activated by host signals. HrpR and HrpS are expressed from a single operon that can be expressed under both T3SS-inducing and non-inducing conditions [10, 12, 13, 15]. Further, both HrpR and HrpS lack signal receiver domains commonly found in NtrC-like proteins, suggesting they are not directly regulated by intracellular signals [10, 11]. Proteins that regulate HrpR and HrpS transcriptionally and post-transcriptionally are known [15–17], yet are unlikely to be directly involved in perceiving T3SS-inducing host signals.
In this work we identified an ABC transporter-associated two-component system AauS-AauR and substrate-binding protein AatJ in P. syringae that directly connect perception of host-derived acidic amino acids to transcriptional regulation of T3SS-encoding genes. We show that AauR regulates hrpRS expression through binding to a conserved cis regulatory motif within the hrpRS promoter, and that this motif is conserved in essentially all pathogenic P. syringae. Because AauS-AauR and AatJ are present in all pseudomonads including non-pathogens, our data suggest that the transporter-associated functions of AatJ-AauS-AauR were co-opted for T3SS regulation early in P. syringae evolution and that this host signal-responsive signaling pathway has been maintained throughout subsequent evolution and host diversification of P. syringae.
Results
AauS-AauR and AatJ are required for maximal expression of T3SS-associated genes in DC3000
Multiple plant-exuded organic acids and amino acids are, in the presence of sugars, inducers of T3SS-encoding genes in P. syringae strain DC3000 [9]. To investigate the molecular basis of how these metabolites are perceived by P. syringae, we previously screened a population of 20,000 Tn5-mutagenized DC3000 colonies carrying an avrRpm1promoter:gfp reporter for decreased GFP fluorescence in response to fructose and aspartate [18]. Among mutants isolated by this screen, we identified four Tn5 insertions within the amino acid transport/ amino acid uptake (aat/aau) locus encoding a predicted ABC transporter (AatQMP) and two-component system (AauSR) (Fig 1A). Three of the Tn5 insertions were located within genes encoding the predicted sensor kinase (aauS) and response regulator (aauR), whereas the fourth Tn5 insertion was located within aatJ, a predicted periplasmic substrate-binding protein (SBP). To verify the role of these genes in avrRpm1 expression, we generated three deletion mutants that individually lack aauS, aauR, and aatJ, and tested these mutants for response to T3SS-inducing metabolites. Fructose alone is sufficient to induce T3SS gene expression [18–19], whereas aspartate acts synergistically with fructose to induce hyper-expression of T3SS-associated genes [9]. In response to fructose only, relative to each other and DC3000, each of the three deletion mutants had similar levels of expression of T3SS effector avrRpm1 based on GFP fluorescence from an avrRpm1promoter:gfp reporter plasmid (Fig 1B). In contrast, all three deletion strains showed a clear reduction in avrRpm1 expression when aspartate was also included (Fig 1B). We also generated a DC3000 mutant with both aauS and aauR deleted. Consistent with a model of AauS and AauR acting in the same signaling pathway, the level of avrRpm1 expression in the ΔaauSΔaauR double mutant was similar to avrRpm1 expression levels in ΔaauS and ΔaauR strains (Fig 1B).
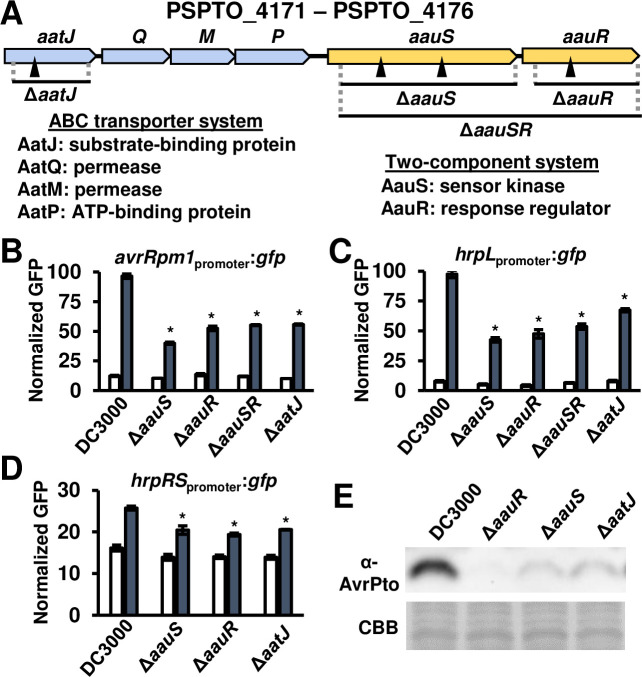
Fig 1. ABC transporter periplasmic substrate-binding protein AatJ and two-component system AauS-AauR are required for maximum expression of T3SS-associated genes in DC3000.
(A) Schematic of aat-aau locus in DC3000. Filled triangles indicate Tn5 insertion sites. (B-D) GFP fluorescence of DC3000, ΔaauS, ΔaauR, ΔaauSR, and ΔaatJ, strains carrying (B) avrRpm1promoter:gfp, (C) hrpLpromoter:gfp or (D) hrpRpromoter:gfp reporter plasmids. Bacteria were incubated in a minimal medium (MM) with 10 mM fructose (open bars) or 10 mM fructose and 200 μM aspartate (filled bars) for 6 hours (B and C) or 12 hours (D). Graphed are means ± SE of GFP fluorescence normalized to OD600 and background fluorescence from empty vector strains; n = 3. Data are representative of three independent experiments. Asterisks denote significant difference based on two-sample t-test comparison with DC3000 treated with fructose and aspartate, P < 0.05. (E) AvrPto levels in DC3000, ΔaauR, ΔaauS and ΔaatJ cultured in MM supplemented with 200 μM aspartate and 10 mM fructose for 4 hours. Upper panel is immunoblot detection of AvrPto in treated bacteria. Lower panel is Coomassie Brilliant Blue (CBB) staining of blot as a loading control.
To investigate if aauS, aauR and aatJ regulate T3SS signaling pathways upstream of avrRpm1, we measured the expression of genes for T3SS regulators HrpL and HrpR/S, using hrpLpromoter:gfp and hrpRSpromoter:gfp reporter constructs. Based on levels of GFP fluorescence, expression of both hrpL and hrpRS was significantly reduced in ΔaauS, ΔaauR, and ΔaatJ mutants in response to fructose and aspartate (Fig 1C and 1D). We also tested the response of these same mutants to glutamate, another potent T3SS-inducing metabolite, and observed a similar decrease in the expression of avrRpm1, hrpL and hrpRS (S1 Fig). Together, these data indicate that the aat/aau locus regulates T3SS signaling upstream of hrpRS expression in response to acidic amino acids. To confirm these GFP reporter results, we measured the levels of endogenous type III effector protein AvrPto in DC3000, ΔaauS, ΔaauR and ΔaatJ strains, and in response to treatment with fructose and aspartate. We observed reduced AvrPto levels in all three mutant strains relative to DC3000 (Fig 1E).
To assess if AauS-AauR broadly functions in the perception of T3SS-inducing metabolites [9], we incubated DC3000 and a ΔaauSΔaauR strain, both carrying a hrpLpromoter:gfp reporter plasmid, in minimal medium supplemented with or without fructose, or supplemented with fructose and citrate, 4-hydroxybenzoic acid (4-hba) or glutamate. Glutamate, in combination with fructose, elicited the highest levels of hrpL expression in DC3000 relative to all other metabolite treatments. Citrate and 4hba, in combination with fructose, also induced significantly higher levels of hrpL expression in DC3000 compared to fructose alone, albeit to lower levels compared to glutamate and fructose (S2 Fig). In response to glutamate, citrate or 4-hba, we measured significantly lower levels of hrpL expression in ΔaauSΔaauR strain compared to hrpL levels in DC3000. However, strikingly, glutamate-induced hrpL expression was attenuated by ~70% in the ΔaauSΔaauR strain relative to DC3000 (S2 Fig), whereas hrpL expression in ΔaauSΔaauR in response to either citrate or 4-hba was only decreased by ~20% relative to DC3000. No significant difference in hrpL expression between strains was measured in response to fructose alone (S2 Fig). These data indicate that, among metabolites tested, glutamate is the most potent in terms of its ability to induce hrpL, and that loss of AauS-AauR disproportionately effects the response to glutamate compared to other T3SS-inducing metabolites.
AauS-AauR and AatJ positively regulate T3SS deployment and virulence of DC3000 during infection of Arabidopsis
We next determined if AauS, AauR and AatJ regulate T3SS deployment by DC3000 during plant infection. DC3000, ΔaauS, ΔaauR and ΔaatJ strains were syringe-infiltrated individually into Arabidopsis leaves. Similar to decreased T3SS gene expression observed in vitro, we detected decreased AvrPto abundance in all tissues infected with these deletion strains relative to levels of AvrPto in DC3000-infected leaf tissue (Fig 2A). We also infiltrated DC3000 and ΔaauR hrpLpromoter:gfp reporter strains into Arabidopsis leaves, and measured significantly decreased hrpL expression in ΔaauR-infected tissue relative to DC3000-infected tissue (Fig 2B). No difference in the population of leaf bacteria was measured at this early time point (S3 Fig). At later time points post-infection, we measured significantly decreased populations of bacteria in Arabidopsis leaves infected with ΔaauS, ΔaauR and ΔaatJ mutants relative to DC3000 (Fig 2C, S3 Fig), as well as observed decreased disease symptoms in Arabidopsis leaves infected with the ΔaauR mutant (Fig 2D). No differences in growth of DC3000, ΔaauS, ΔaauR or ΔaatJ strains cultured in KB medium, LB medium, or a defined minimal medium were observed (S4 Fig, Fig 3D), indicating that decreased growth in leaf tissue is not due to a general fitness defect of these mutants. Together, these data show that aauS, aauR and aatJ are required in DC3000 virulence and T3SS deployment during infection of Arabidopsis.
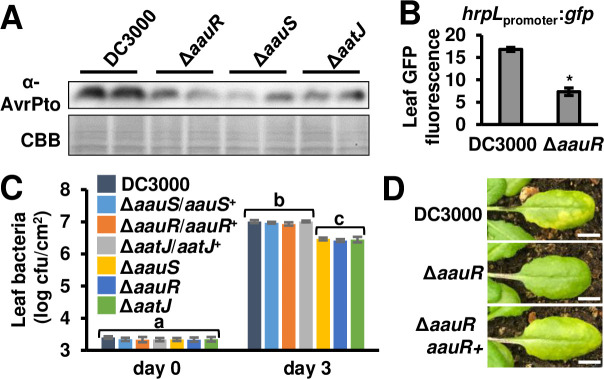
Fig 2. AatJ, AauS and AauR are required for maximal level of DC3000 virulence in Arabidopsis leaves.
(A) AvrPto levels in Arabidopsis leaves syringe-infiltrated with 1 x 108 cfu/mL of DC3000, ΔaauS, ΔaauR, ΔaauSR, or ΔaatJ strains. Upper panel is immunoblot detection of AvrPto in infected leaf tissue collected 6 hours post-infiltration. Lower panel is Coomassie Brilliant Blue (CBB) staining of blot as a loading control. (B) GFP fluorescence of Arabidopsis leaf tissue syringe-infiltrated with 5 x 108 cfu/mL of DC3000 or ΔaauR hrpLpromoter:gfp reporter strains. Graphed are means ± SE of GFP fluorescence from infected tissue 6 hours post-infection, n = 3. Asterisks denote significant difference between strains based on t-test, P < 0.001. (C) 1 x 106 cfu/mL of DC3000, ΔaauS, ΔaauR, ΔaatJ and respective complemented strains were syringe-infiltrated into Arabidopsis leaves. Leaf bacteria populations were enumerated on day 0 and day 3 by serial dilution plating of leaf extracts. Graphed are log-transformed means ± SE of bacteria colony-forming units (cfus) isolated from infected tissue, n = 6 for day 0 and n = 8 for day 3. Small-case letters denote significance groupings based on ANOVA with Bonferroni correction, P < 0.01. Data shown were pooled from two independent experiments. Results from additional replicated experiments are shown in S3 Fig. (D) Photograph of disease symptoms on leaves 3 days post-syringe infiltration with 1 x 106 cfu/mL of DC3000, ΔaauR or ΔaauR/aauR+. White line is 0.5 cm.
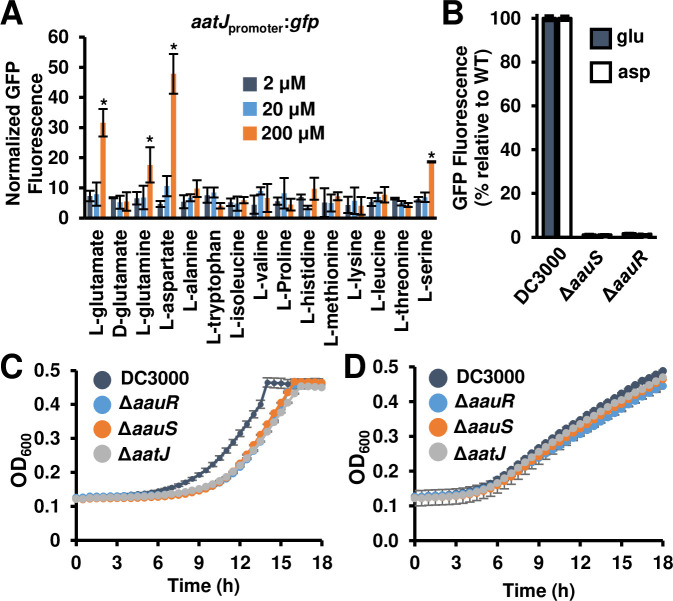
Fig 3. Genes within the aat/aau locus are required for glutamate-induced growth of DC3000.
(A) Expression of the aatJ promoter in response to specific amino acids. Graphed is GFP fluorescence from a DC3000 aatJpromoter:gfp reporter strain incubated for 24 hours in a minimal medium supplemented with 2, 20 or 200 μM of amino acids as indicated. Graphed are means ± SE of GFP fluorescence normalized to OD600 and background fluorescence from empty vector strains; n = 3. Asterisks denote significant difference based on t-test comparison to 2 μM treatment condition, P < 0.05. (B) GFP fluorescence of DC3000, ΔaauS, and ΔaauR aatJpromoter:gfp reporter strains incubated with 10 mM fructose and 200 μM glutamate or 200 μM aspartate. Graphed are means ± SE of GFP fluorescence normalized to OD600 and background fluorescence from empty vector strains; n = 3. (C-D) Growth of DC3000, ΔaatJ, ΔaauS, and ΔaauR strains in (C) M9 minimal medium containing 10 mM glutamate as the sole carbon and nitrogen resource, or (D) standard M9 minimal medium containing glucose and ammonium chloride. Cultures were grown for 24 hours at 28°C. Optical density at λ = 600 nm (OD600) of each well was measured at 30 min intervals. Graphed are means ± SD of OD600 readings, n = 3. Results in all panels are representative of 3 independent experiments.
AauS/-R activates expression of aatJQMP genes required for maximal uptake of acidic amino acids by DC3000
In the soil bacterium Pseudomonas putida KT2440, AauS and AauR regulate the expression of aatJQMP in response to extracellular glutamate and aspartate [20–21]. To investigate if aatJQMP expression in DC3000 is regulated in a similar manner, we fused the promoter of aatJ to a promoterless gfp and introduced a plasmid carrying this aatJpromoter:gfp reporter into DC3000. We then cultured this reporter strain in minimal medium amended with aspartate or glutamate. We also tested thirteen other amino acids for their ability to induce aatJ expression. Consistent with the reported function of aatJQMP in P. putida KT2440, the highest levels of induction of aatJ expression occurred in response to aspartate and glutamate, with little to no induction of aatJ expression by the remaining amino acids tested with the exception of weaker induction by serine and glutamine (Fig 3A). We also introduced the aatJpromoter:gfp reporter construct into ΔaauS and ΔaauR strains and measured essentially no expression of aatJ in these mutants when cultured in minimal medium containing glutamate or aspartate (Fig 3B). Together, these data show that aspartate and glutamate induce the expression of aatJQMP in DC3000 and this response requires both AauS and AauR.
Based on previous functional studies of the P. putida Aat ABC transporter [20] and our results above, we predicted that the transporter encoded by aatJQMP in DC3000 is necessary for uptake/utilization of acidic amino acids. To test this, we examined the growth rate of DC3000, ΔaauS, ΔaauR and ΔaatJ strains cultured in minimal medium M9 supplemented with glutamate as the sole N and C source. We measured a delay in growth of all three deletion mutants relative to DC3000 (Fig 3C). Growth of all three mutant strains could be restored by introduction of plasmids carrying respective wild type alleles of each mutated gene (S5 Fig). A similar delay in growth was not observed for ΔaauS, ΔaauR or ΔaatJ strains cultured in minimal medium supplemented with ammonium and glucose as N and C sources, respectively (Fig 3D). We conclude from these data that the aat/aau locus encodes for proteins that contribute to uptake of extracellular acidic amino acids by DC3000.
Transport and T3SS regulation functions of the aat/aau locus are genetically separable
AatQMP encodes a predicted ABC transporter, with AatM and AatQ forming a membrane-spanning channel, and AatP functioning as an ATP-hydrolyzing protein that provides energy for metabolite transport [20, 22]. To investigate the role of AatMQP transporter function in T3SS deployment, we deleted aatP from the DC3000 genome. Consistent with a loss of glutamate transport, the resulting ΔaatP mutant had delayed growth in M9 medium with glutamate as a sole N and C source (Fig 4A), but no general growth defect in King’s B medium (S6 Fig) or standard M9 amended with glucose and NH4Cl (Fig 4A). However, the ΔaatP mutant had DC3000 levels of avrRpm1 expression in response to fructose and aspartate (Fig 4B), as well as DC3000 levels of hrpL expression when infiltrated into Arabidopsis leaves (Fig 4C). We also did not observe any decrease in growth of ΔaatP mutant in Arabidopsis (Fig 4D). Similar phenotypes were observed for a ΔaatQΔaatM mutant (S7 Fig). These data provide genetic evidence that the Aat transporter is dispensable for AatJ-AauS-AauR regulation of T3SS induction and virulence but is nonetheless required for growth on glutamate.
Fig 4. Amino acid transport and T3SS-inducing functions of the aat/aau locus are genetically separable.
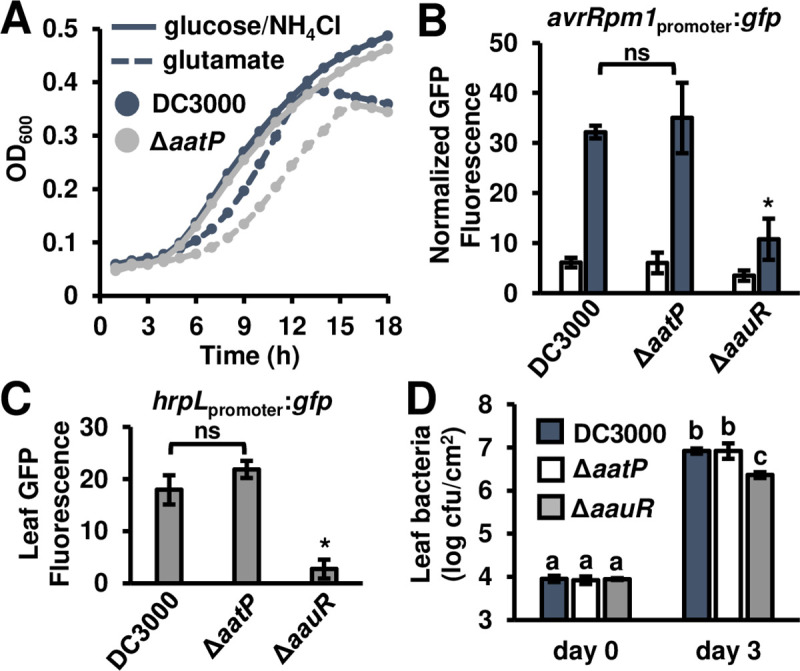
(A) Growth of DC3000 and ΔaatP strains in M9 minimal medium supplemented with 10 mM glutamate. Graphed are means of optical density at λ = 600 nm (OD600), n = 3. (B) GFP fluorescence from DC3000, ΔaatP, and ΔaauR avrRpm1promoter:gfp reporter strains cultured in minimal medium (MM) with 10 mM fructose (open bars) or 10 mM fructose and 200 μM glutamate (filled bars) for 6 hours. Graphed are means ± SE of GFP fluorescence normalized to OD600 and background fluorescence from empty vector strains; n = 4. Asterisk denotes significant difference based on t-test comparison with DC3000, P < 0.01; abbreviation ns is not significant, α = 0.05. (C) GFP fluorescence of Arabidopsis leaf tissue syringe-infiltrated with 1 x 108 cfu/mL of DC3000, ΔaatP or ΔaauR strains each carrying a hrpLpromoter:gfp reporter plasmid. Graphed are means ± SE of GFP fluorescence from infected tissue 6 hours post-infection, n = 4. Asterisk denotes significant difference based on t-test comparison with DC3000, P < 0.01; abbreviation ns is not significant, α = 0.05. (D) 1 x 106 cfu/mL of DC3000, ΔaatP or ΔaauR strains were syringe-infiltrated into Arabidopsis leaves. Leaf bacteria populations were enumerated on day 0 and day 3 by serial dilution plating of leaf extracts. Graphed are log-transformed means ± SE of bacteria from infected plants, n = 3 for day 0 and n = 4 for day 3. Small-case letters denote significance groupings based on pairwise t-tests, P < 0.05. Results in all panels are representative of 3 independent experiments.
An AauR binding motif upstream of hrpRS is required for maximal T3SS deployment and virulence of DC3000 during infection of Arabidopsis
In P. putida, AauR regulates the expression of aatJQMP by binding to an inverted DNA repeat motif “TTCGG-(N,4)-CCGAA” located upstream of aatJ [20]. This motif is also present within the promoter region of aatJ in DC3000 (Fig 5A). We searched for this AauR-binding motif (Rbm) within the DC3000 genome sequence ([23], Pseudomonas genome database), and discovered that an identical Rbm is also present within the intergenic region upstream of hrpRS (Fig 5A). To assess if this Rbm is required for hrpRS expression, we deleted a 20-bp region containing the Rbm from our hrpRSpromoter:gfp reporter plasmid and introduced this altered reporter plasmid into DC3000. We also used allelic exchange to delete the same Rbm-containing region upstream of hrpRS in the DC3000 chromosome, generating a ΔRbm mutant. In response to fructose and aspartate, DC3000 carrying the hrpRSpromoter(ΔRbm):gfp reporter had significantly decreased levels of GFP fluorescence compared to DC3000 carrying an unaltered hrpRSpromoter:gfp reporter (Fig 5B). Levels of GFP fluorescence from the hrpRSpromoter(ΔRbm):gfp reporter strain were similar to levels from a hrpRSpromoter:gfp ΔaauR strain, suggesting deletion of Rbm is sufficient to phenocopy loss of aauR. We also observed significantly decreased avrRpm1 and hrpL expression in the ΔRbm mutant under the same inducing conditions (Fig 5C and 5D).
Fig 5. An AauR binding motif within the hrpRS promoter is required for T3SS deployment and maximal virulence of DC3000 in Arabidopsis.
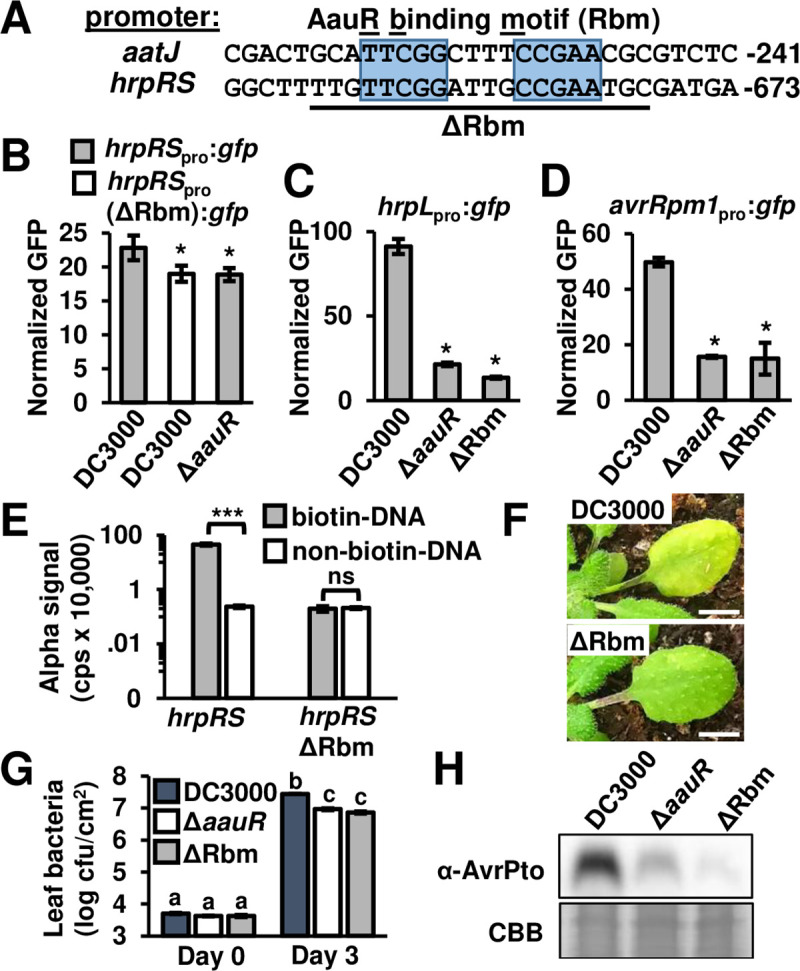
(A) Alignment of DC3000 aatJ and hrpRS promoter regions containing the AauR-binding motif (Rbm). Conserved palindromic repeats are highlighted in blue. Numbers are distances in base pairs of each Rbm from predicted translation start sites for each gene. The 20 bp region deleted in ΔRbm strain is underlined. (B-D) GFP fluorescence from bacteria cultured in minimal medium with 10 mM fructose and 200 uM aspartate for 6 hours. Graphed are means ± SE of GFP fluorescence normalized to OD600 and background fluorescence from empty vector strains; n = 3. (B) DC3000 carrying a hrpRSpromoter:gfp reporter or hrpRSpromoter(ΔRbm):gfp reporter lacking the predicted Rbm. A ΔaauR strain carrying hrpRSpromoter:gfp was included for comparison. (C) DC3000, ΔaauR and ΔRbm strains each carrying a hrpLpromoter:gfp reporter. (D) DC3000, ΔaauR and ΔRbm strains each carrying an avrRpm1promoter:gfp reporter. Asterisks in B-D denote significant difference between strains based on t-test, P < 0.05. (E) AlphaScreen assay of recombinant AauR protein binding to hrpRS promoter DNA with or without Rbm. Graphed are means ± SE of luminescence from assay wells, n = 4. Asterisks indicate P < 0.001 based on t-test, ns is not significant at α = 0.05. (F-H) 1 x 106 cfu/mL of DC3000 or ΔRbm were syringe-infiltrated into Arabidopsis leaves. (F) Photographs of disease symptoms taken 3 days post-infection. White bar is 0.5 cm. (G) Leaf bacteria populations enumerated on day 0 and day 3 by serial dilution plating of leaf extracts. Graphed are log-transformed means ± SE of bacteria colonies isolated from infected tissue, n = 3 for day 0 and n = 3 for day 3. Small-case letters denote significance groupings based on pairwise t-tests, P < 0.001. (H) AvrPto levels in Arabidopsis leaves syringe-infiltrated with 5 x 108 cfu/mL of DC3000 or ΔRbm. Upper panel is immunoblot detection of AvrPto in infected leaf tissue 6 hours post-infiltration. Lower panel is Coomassie Brilliant Blue (CB) staining of blot as a loading control. Results in panels B-H are representative of 3 independent experiments.
We next assessed if AauR can directly bind to Rbm upstream of hrpRS. We produced a recombinant AauR protein in E. coli and tested this protein for interaction with DNA fragments with Rbm-containing hrpRS and aatJ promoter sequences. We used the bead-based AlphaScreen technology to measure AauR binding to these DNA fragments, with luminescence generated by increased proximity of protein- and DNA-bound beads serving as readout for detecting protein-DNA interactions. Consistent with P. putida experiments, we detected binding of AauR to aatJ promoter DNA sequence (S8 Fig). We also detected significant interaction between AauR and hrpRS promoter DNA sequence relative to control assays containing non-biotinylated hrpRS promoter DNA (Fig 5E). No interaction was detected between AauR and hrpRS promoter DNA lacking the Rbm sequence (ΔRbm), confirming the specificity of the detected AauR-hrpRS interaction (Fig 5E). We conclude from these data that AauR can bind directly to the hrpRS promoter region containing Rbm.
Next, we infiltrated DC3000, ΔaauR and ΔRbm strains into Arabidopsis leaves to determine the requirement of Rbm for virulence. We observed that the ΔRbm strain caused decreased disease symptoms relative to DC3000 (Fig 5F). Levels of ΔRbm bacteria in infected tissue were significantly reduced compared to DC3000, yet indistinguishable from ΔaauR (Fig 5G). The abundance of AvrPto protein produced by the ΔRbm mutant was also lower than DC3000 in infected leaf tissues (Fig 5H). These results show that the AauR-binding site in the hrpRS promoter is required for maximal expression of T3SS-associated genes and for full virulence and bacterial growth of DC3000 in Arabidopsis.
Phylogenetics suggests AauS-AauR regulation of hrpRS is highly conserved amongst all P. syringae with a canonical T3SS
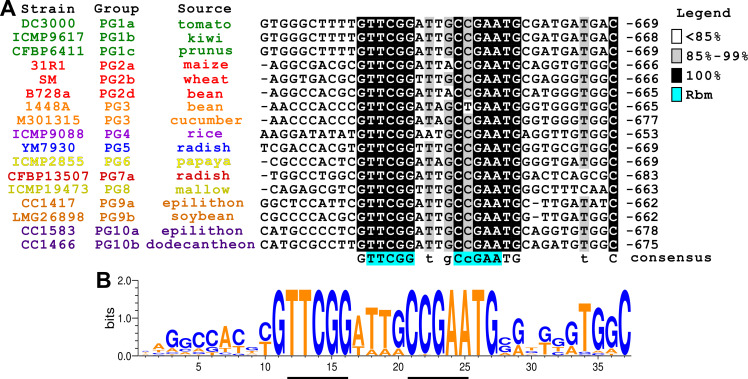
We hypothesized that Rbm might be variable or even absent in some strains as a consequence of adaptation of P. syringae to particular plant hosts or host micro-environments. To investigate, we characterized 38 genome-sequenced isolates of P. syringae representing nine phylogroups with strains that carry a canonical T3SS tripartite pathogenicity island (T-PAI) [24]. We first confirmed the reported phylogroup associations of each representative strain by generating a citrate synthase (cts)—based phylogeny that included 800 additional P. syringae cts sequences from genomes available at NCBI [25]. We then extracted and aligned nucleotide sequences between hrpH and hrpR from genome sequences of all 38 isolates (S9 Fig). This alignment showed that the Rbm motif (TTCGG-(N,4)-CCGAA) is absolutely conserved (Fig 6A and 6B, S9 Fig), with the exception of a single nucleotide polymorphism in the Rbm sequence of P. syringae pv. phaseolicola strain 1448a. Guanine and thymine nucleotides that immediately flank the Rbm are also highly conserved, as well as an “ATTG”-rich signature within the spacer sequence between inverted repeats (Fig 6B). Sequences farther upstream and downstream of the Rbm are less conserved (Fig 6A, S9 Fig). Nevertheless, these regions are more conserved within phylogroups than between phylogroups (Fig 6A, S9 Fig), suggesting vertical inheritance of regions flanking the Rbm. We further investigated inheritance of the hrpR region by aligning the hrpR open reading frames, along with the intergenic region between hrpH and hrpR, from all 38 isolates examined. Phylogenies based on these alignments showed strong intra-phylogroup cohesion (S10 Fig, S11 Fig). Together, these data do not support our hypothesis of positive selection of Rbm between isolates. Rather, they suggest negative selection has occurred to maintain the Rbm within the context of a hrpRS promoter region that has been vertically inherited in all T-PAI-carrying isolates.
Fig 6. An AauR binding motif upstream of hrpRS is highly conserved in all P. syringae phylogroups that possess a canonical type III secretion system.
(A) Alignment of hrpRS promoter sequences from 17 strains chosen to represent 9 phylogroups (PG) of P. syringae that carry a canonical T3SS. Strains are color coded according to phylogroup. Numbers correspond to nucleotide position relative to predicted hrpR translation start sites. Letters below alignment are consensus sequence of nucleotides based on >90% identity. Highlighted in blue is AauR-binding motif (Rbm) from aatJ promoter. (B) Sequence logo generated from multiple sequence alignments of hrpRS promoter region containing Rbm from 38 P. syringae isolates. Relative sizes and heights of the letters indicate their frequency in the sequences and information content, respectively. AT and GC content is highlighted by orange and blue coloring, respectively.
AauR is required for maximal T3SS deployment and virulence of bean pathogen P. syringae pv. syringae B728a
The aat/aau locus is conserved among pseudomonads [20, 21]. A phylogenetic analysis of orthologous aatJ and AauS sequences from diverse P. syringae isolates confirmed vertical inheritance of this locus (S12 Fig, S13 Fig). Because aat/aau genes and the Rbm upstream of hrpRS are both conserved, we predicted that all P. syringae use the AatJ-AauS-AauR signaling pathway to regulate T3SS-encoding genes in response to host signals. To test this prediction, we deleted the orthologous aauR gene in P. syringae pv. syringae B728a, a pathogen that causes brown spot of bean and belongs to a phylogroup distinct from that of DC3000 [24–26]. Similar to DC3000 ΔaauR, B728a ΔaauR showed delayed growth in M9 medium containing glutamate as the sole N and C source (Fig 7A), but not in standard M9 or LB medium (S14 Fig), indicating that AauR is required for the uptake of acidic amino acids in B728a. We then introduced the hrpLpromoter:gfp reporter plasmid into B728a and B728a ΔaauR strains. With the B728a reporter strain, we detected a significant increase in hrpL expression in response to fructose and aspartate relative to fructose only, indicating that aspartate is also an inducer of T3SS genes in B728a (Fig 7B). Consistent with our prediction of conserved AauS-AauR function, hrpL expression was significantly decreased in B728a ΔaauR cultured with fructose and aspartate (Fig 7B). We also syringe-infiltrated B728a and B728a ΔaauR into leaves of Phaseolus vulgaris (common bean), and measured significantly reduced bacterial populations (Fig 7C) in leaves infected with B728a ΔaauR. We conclude from these data that AauR is required for aspartate-induced expression of T3SS genes in B728a, and is required for maximal growth of B728a during infection of host bean plants.
Fig 7. AauR is required for glutamate-dependent growth, T3SS deployment and virulence of the bean pathogen P. syringae pv. syringae B278a.
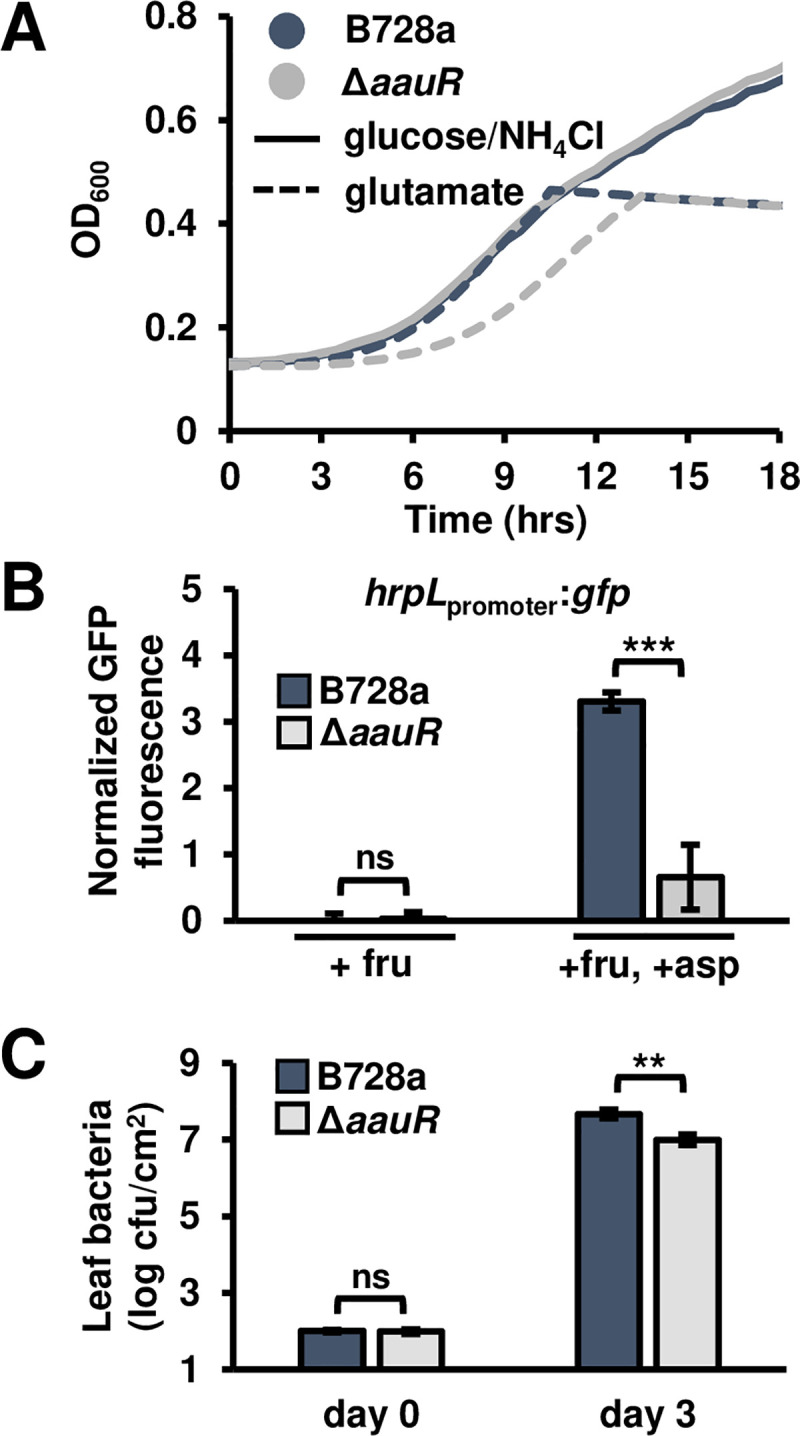
(A) Growth of wild type B728a and B728a ΔaauR in M9 minimal medium supplemented with 10 mM glutamate as the sole carbon and nitrogen source. Cultures were inoculated at an optical density at λ = 600 nm (OD600) of 0.1 and grown for 24 hours at 28°C within a 96-well plate. Graphed are means ± SD of OD600 readings taken at 30 min intervals, n = 3. (B) GFP fluorescence of B728a and B728a ΔaauR strains carrying hrpLpromoter:gfp reporter plasmids. Bacteria were incubated in a minimal medium (MM) with 10 mM fructose (fru) or 10 mM fructose and 200 μM aspartate (asp) for 6 hours. Graphed are means ± SE of GFP fluorescence normalized to OD600 and background fluorescence from empty vector strains; n = 3. (C) Leaves of 14-day-old bean plants were syringe-infiltrated with 1 x 104 cfu/mL of B728a. Bacterial populations in infected leaves 0 and 3 days post-infection. Graphed are log-transformed means ± SE of colony-forming units (cfus) in infected leaf tissue, n = 4. Data in all panels are representative of results from three independent experiments. Asterisks denote significant differences based on t-test. ** is P < 0.05, *** is P < 0.01 and ns is not significant at α = 0.05.
Discussion
The evolutionary transition of bacteria from non-pathogenic to pathogenic often involves the horizontal acquisition of genes that confer novel virulence traits [27]. However, acquiring virulence-promoting genes is likely often only a “foothold” moment, as additional genomic changes must occur to optimize expression and reduce genetic conflicts to maximize fitness gains and reduce fitness costs [28]. Previous phylogenetic analyses revealed that the canonical T3SS-encoding pathogenicity island was gained early in P. syringae evolution [29–30]; yet how T3SS-encoding genes within the island became integrated into existing gene regulatory networks, and how these genes became responsive to host signals, was not known. In this study we present evidence that AauS-AauR and AatJ, proteins involved in regulating the housekeeping task of acidic amino acid uptake in both non-pathogenic and pathogenic pseudomonads, were co-opted to regulate T3SS deployment in P. syringae strain DC3000 (S15 Fig). Our phylogenetic analyses indicate that co-option of AatJ-AauS-AauR signaling, through acquisition of an Rbm upstream of hrpRS, occurred early in P. syringae evolution and was maintained throughout subsequent diversification of the species. The placement of an Rbm upstream of hrpRS may have occurred through recombination events that captured and re-purposed existing Rbm elements within the genome, or by de novo assembly of an Rbm through the accumulation of random mutations [31]. Alternatively, AauS-AauR regulation of hrpRS may pre-date P. syringae speciation, with an Rbm already present within the hrpRS promoter upon integration of the T3SS pathogenicity island into the ancestral P. syringae genome.
Co-option of environment-responsive signaling pathways for virulence has occurred in other pathogenic bacteria. In Salmonella enterica, the Mg2+-responsive two-component system PhoQ-PhoR regulates the expression of multiple virulence-associated genes, including T3SS-associated genes within the SPI-2 pathogenicity island, as well as numerous non-virulence genes scattered throughout the genome [32]. The widespread presence and conserved functions of PhoQ-PhoR in both pathogenic and non-pathogenic Gram-negative bacteria suggest it was likely present in an ancestral strain of Salmonella and adapted for virulence [33]. In the gall-forming plant pathogen Agrobacterium tumefaciens, virulence (vir) genes are regulated by VirA-VirG, a two-component system activated by plant-derived phenolic compounds [4]. Interestingly, activation of VirA is enhanced by host-derived sugars through the action of Chromosomal virulence gene E (ChvE), a periplasm-localized SBP with broad specificity for binding aldose monosaccharides [34–36]. Similar to the dual functions of AatJ in virulence and transport, ChvE also functions as an SBP for MmsAB-AraD, a sugar-specific ABC transporter that is dispensable for Agrobacterium virulence [36]. The striking parallels between AatJ and ChvE indicate that two unrelated plant pathogens have similarly co-opted the metabolite-binding capabilities of SBPs, suggesting this mechanism of virulence gene regulation may be commonplace.
AauS is a predicted extracytoplasmic sensor kinase with an N-terminal periplasmic domain and a C-terminal intracellular histidine kinase domain. Based on well-established models of two-component system activation [37], as well as previous functional characterization of the aat/aau locus in P. putida [20, 21], the presence of aspartate and glutamate in the periplasm likely causes AauS to auto-phosphorylate, followed by trans-phosphorylation and activation of AauR. Multiple mechanisms of AauS activation by aspartate and glutamate are possible. The N-terminus of AauS possesses a predicted CACHE (CA2+ channels, chemotaxis receptors) domain involved in small molecule binding (Pfam.xfam.org, [38]), and has structural similarity to C4-dicarboxylic transport B (DctB) (Phyre2, [39]), a sensor kinase in Rhizobia that recognizes plant-derived dicarboxylates [40, 41]. Based on this similarity to DctB, aspartate and glutamate may directly bind to the periplasmic domain of AauS to activate this pathway. Alternatively, AauS may perceive aspartate and glutamate indirectly through interactions with AatJ. In this regard, ligand-bound SBPs can function in signal transduction by directly interacting with and activating an associated chemotaxis receptor or sensor kinase [34, 42–44]. Therefore, AauS may recognize an AatJ-metabolite complex. This latter model is consistent with our observation that aatJ is required for maximal induction of hrpRS by aspartate and glutamate. Additional experiments to assess metabolite binding to AatJ and AauS, as well as in vivo studies of possible AatJ-AauS interactions, will be necessary to differentiate between these possible activation mechanisms.
Our observation that T3SS deployment by ΔaatJ, ΔaatS and ΔaauR strains is reduced in planta suggests that aspartate and/or glutamate are key virulence-inducing signals for DC3000 within the leaf apoplast. Furthermore, the conserved nature of Rbm upstream of hrpRS suggests that essentially all pathogenic strains of P. syringae use AauS-AauR to regulate their virulence. This possibility is supported by our evidence that hrpL expression was induced by aspartate in strain B728a, and that aauR was required for maximal growth of strain B728a in bean. Similar to targeting of PAMPs by PRRs, we reason that P. syringae may use aspartate and glutamate as virulence-inducing cues because these metabolites are abundant in plant tissues and are essential for plant viability. Across a broad phylogeny of plant species, aspartate and glutamate are the most abundant amino acids in plants [45]. At the cellular level, both metabolites play central roles in amino acid biogenesis and catabolism pathways, including the assimilation of nitrogen into organic compounds [46, 47]. At the whole plant level, aspartate and glutamate participate in nitrogen transport from roots to shoots, and between source and sink tissues [48]. Glutamate may also function as a trigger for long-distance wounding signals in plants [49]. Within the leaf apoplast, the steady state levels of extracellular metabolites likely reflect the combined rates of uptake and release from vascular tissue as well as from local mesophyll cells. Interestingly, aspartate and pyroglutamic acid, a derivative of glutamate, were both decreased in exudates from the defense-heightened Arabidopsis mkp1 mutant [9], suggesting exudation of acidic amino acids from plants may be defense-regulated. In addition to regulating T3SS-encoding genes within the apoplast, metabolites that leach onto the leaf surface may serve as virulence signals. Recently, the aspartate- and glutamate-specific chemotaxis receptor PscA in DC3000 was shown to be required for maximal virulence of bacteria inoculated onto the surface of tomato leaves [50], indicating aspartate and glutamate may also be important chemotactic signals during motile stages of infection on the leaf surface.
Identifying AauR as a direct regulator of hrpRS is an important step towards addressing how the HrpR-HrpS-HrpL signaling module is activated during infection. However, aspartate- and glutamate-dependent induction of this pathway was only partially abrogated in ΔaatJ, ΔaatS and ΔaauR mutants, indicating that additional Aat/Aau-independent signaling pathways are involved in perceiving these metabolites. Furthermore, loss of AauS-AauR had little to no impact on hrpL expression in DC3000 in response to fructose, citrate, or 4-hydroxybenzoic acid. Therefore, these metabolites must also induce T3SS-associated genes through AauS/AauR-independent mechanisms. Collectively, these data suggest that regulation of type III secretion in P. syringae is complex, with contributions from redundant pathways that respond to the same metabolite, as well as from pathways that function independently in response to distinct metabolic signals. Previously, the two-component system RhpS/RhpR (Regulator of hrp genes, Sensor and Regulator) was identified as a regulator of hrpRS expression in P. savastanoi and P. syringae [17]. RhpR binds to the hrpRS promoter in a region located approx. 300 nucleotides further upstream from the Rbm [51]. In rhpS- mutants, RhpR constitutively represses hrpRS expression, resulting in decreased T3SS deployment and reduced virulence [17]. However, mutants lacking both rhpS and rhpR have wild-type levels of hrpRS expression and are fully virulent, indicating that RhpS/RhpR does not directly regulate T3SS genes in response to host-specific signals [17]. We hypothesize that additional host metabolite-responsive two-component systems, apart from AauS-AauR, may provide signaling inputs. Alternatively, metabolite-mediated regulation of T3SS may also occur intracellularly. In this regard, we recently identified a predicted metabolite-sensing transcription regulator SetA that is genetically required for sugar-induced expression of hrpL [18]. Because both sugars and amino acids are present within the leaf apoplast, AauS/AauR- and SetA-dependent host perception mechanisms likely function simultaneously during infection. How T3SS induction by different classes of host metabolites may be coordinately regulated in P. syringae is an open question for future investigation.
Materials and methods
Bacterial strains and growth conditions
Bacterial strains and plasmids are listed in S1 Table. Pseudomonas syringae were cultured in a modified King’s B (KB) [52] medium (1% (w/v) peptone, 1% tryptone, 0.1% MgSO4-7H2O, 0.1% K2HPO4, and 1% (v/v) glycerol). For in vitro GFP reporter assays, P. syringae were cultured in a modified hrp-inducing minimal medium (MM, [9]) or M9 medium [53] amended with appropriate carbon sources. Aspartate, glutamate, citrate and 4-hydroxybenzoic acid were prepared as 20 mM stocks in water, 0.2 μm filtered and stored at -20°C. Fructose was prepared as a 1 M stock in water, 0.2 μm filtered and stored at room temperature. Escherichia coli were cultured in Luria-Bertani (LB) [54] broth at 28°C. Prior to aatJ promoter activity experiments, P. syringae were cultured at OD600 = 5.0 in standard M9 medium at 28°C with shaking at 200 rpm for four weeks, with bacteria collected by centrifugation and resuspended in fresh M9 medium every two days. For all other assays P. syringae were cultured for two days on KB agar (1.5%) at room temperature prior to use.
Tn5 transposon mutagenesis screen
DC3000 carrying an avrRpm1promoter:gfp::pBBR1-MCS2 construct [55,56] were mutagenized by transposon Tn5 through conjugative transfer of suicide vector pUT:mini-Tn5 Sm/Sp as described previously [18]. Briefly, to select for Tn5+ colonies, conjugation mixtures were plated onto the surface of a nitrocellulose filter placed on the surface of KB agar (1.5%) containing spectinomycin (150 μg/mL), rifampicin (50 μg/mL) and kanamycin (30 μg/mL). The plate was kept at room temperature for three days until colonies became visible. To remove residual KB medium, the nitrocellulose membrane was transferred to 1.5% agar solidified with water. After 24 hours, the membrane was transferred to 1.5% agar plates containing 50 mM fructose and 5 mM aspartate. After six hours, GFP fluorescence of colonies was visually scored using a Leica MZFLIII stereomicroscope. Approximately 20,000 Tn5+ colonies were screened in this manner, and 400 selected for further study. As a second round of screening, a Tecan Spark 10M plate reader was used to measure the GFP fluorescence of each mutant strain cultured at a starting OD600 = 0.1 for eight hours in liquid MM supplemented with 50 mM fructose and 1 mM aspartate. Approximately two hundred mutant strains with a >20% reduction in GFP fluorescence relative to a wild type DC3000 culture were selected, and Tn5 insertion sites identified by Illumina sequencing of genomic DNA as described previously [18].
Construction of P. syringae deletion mutants
Strains of P. syringae with deletions of aauS, aauR, aatJ, aatQM, aatP, aauSR or the aauR binding motif (Rbm) were generated by suicide vector-mediated allelic exchange. DNA fragments flanking each targeted gene were PCR amplified from DC3000 genomic DNA, fused into a single PCR product by a second round of overlapping PCR, and subcloned into suicide vector pEX18Km [57]. Deletion constructs were introduced into P. syringae by tri-parental mating. Briefly, 2 mL overnight cultures of E. coli containing either the pEX18Km deletion construct or helper plasmid pRK600 [58] were incubated at 28°C with a shaking at 200 rpm. The overnight cultures were washed with sterile water, mixed at a volumetric ratio of 1:1:10 (E. coli pRK600: E.coli pEX18Km: P. syringae), and spread onto a KB agar plate without antibiotics. After incubating overnight at 28°C, the conjugation mixtures were resuspended in sterile water and plated onto KB agar amended 50 μg/mL rifampicin and 30 μg/mL kanamycin to select for the first recombination event. The resulted merodiploid colonies were then plated on KB agar supplemented with 15% v/v sucrose for two days to counter-select for presence of sacB and identify deletion mutants. Gene deletions were confirmed by sensitivity of mutant strains to kanamycin and by PCR genotyping using oligonucleotides that anneal to regions that flank the targeted genes. A list of all deletion constructs generated is provided in S1 Table, and sequences of oligonucleotides used for vector construction are provided in S2 Table.
Construction of transcriptional reporter plasmids and gene expression plasmids
Reporter plasmids avrRpm1promoter:gfp::pBBR1-MCS2 and hrpLpromoter:gfp::pProbe-NT were described previously [59]. To make the hrpRSpromoter:gfp reporter, oligonucleotides hrpR-F1_pro and hrpR-1a were used to PCR amplify a 1222-bp DNA fragment containing the hrpR promoter region from DC30000 genomic DNA. The resulting PCR fragment was subcloned into BamHI and KpnI sites of pPROBE-NT [60]. The resulted plasmid was named phrpRSpromoter-gfp. To delete the AauR-binding motif (Rbm) in phrpRSpromoter-gfp, oligonucleotide pairs hrpR-F1/hrpR-R7-OVLP and hrpR-R1/hrpR-F6-OVLP were used to PCR amplify two DNA fragments flanking the Rbm site. The resulting DNA fragments were fused together by overlapping PCR using oligonucleotides hrpR-F1-pro and hrpR-R1-pro. NEBuilder HiFi mix (NEB) was used to ligate the resulting PCR product into BamHI/KpnI-digested pPROBE-NT. To make the aatJpromoter:gfp construct, oligonucleotides aatJ_f2 and aatJ_R2a were used to PCR amplify a 506-bp DNA fragment from DC3000 genomic DNA. The resulting PCR fragment was digested with HindIII and EcoRI, and ligated into pPROBE-NT. All constructs used for gene complementation were made using broad host range vector pME6010 [61]. To prepare a linearized vector for subcloning, oligonucleotides pairs pME6010-F1/pME6010-R1 and pME6010-F2/ pME6010-R2 were used to PCR amplify two DNA fragments 2.8 kb and 5.5 kb in length from pME6010. Gene-specific oligonucleotides were used to PCR amplify aauS, aauR and aatJ-containing DNA fragments from DC3000 genomic DNA. NEBuilder HiFi mix (NEB) was used to assemble the resulting PCR products into an intact plasmid. Correct assembly of plasmids was confirmed by PCR and Sanger sequencing. All constructs were confirmed by PCR genotyping and Sanger sequencing. Sequences of oligonucleotides used for constructing plasmids are provided in S2 Table.
In vitro and in planta GFP transcriptional reporter assays
To measure GFP fluorescence of P. syringae cultured in T3SS-inducing media, bacteria were scraped from the surface of KB agar, resuspended in 1 mL of H2O and washed twice with 1 mL of H2O using a centrifuge to pellet bacteria between washes. After washing, the suspensions of bacteria were adjusted to an optical density at λ = 600 nm (OD600) of 1.0 and inoculated into wells of black μClear 96-well plates (Greiner Bio-One) containing 90 μL of MM amended with 200 μM of aspartate, glutamate, citrate, 4-hydroxybenzoic acid and/or 10 mM fructose. The final density of bacteria in each well was OD600 = 0.1. A Spark 10M plate reader (Tecan) was used to measure GFP fluorescence and OD600 of each well. Prior to measurements, the plate was incubated in the plate reader at 25°C or ambient (if higher) and shaken at 216 rpm. A humidity cassette (Tecan) was used to prevent sample evaporation. Fluorescence from each well was measured using excitation and emission wavelengths of 485nm and 535nm, respectively, with manual gain set to 100 and settle time set to 1000 ms. OD600 was measured using default settings with a 1000 ms settle time. Each fluorescence measurement was divided by the corresponding OD600 of each well to determine a culture density-normalized GFP level. Further, culture density-normalized fluorescence values from wells containing empty pPROBE-NT control strains were used for background correction to calculate final normalized GFP fluorescence values. All normalized fluorescence values were divided by 10,000 to simplify graphing of data. Measurements of hrpLpromoter:gfp activity in leaf tissue and immunoblot detection of AvrPto were done as described previously [18,59].
Measurements of P. syringae growth in culture
For each culture, 10 μL of P. syringae suspended in sterile water was added to 90 μL of either KB broth, M9 medium containing 0.4% glucose and 0.1% ammonium chloride, or a modified M9 medium lacking glucose/NH4Cl and supplemented with 10 mM glutamate. Cultures were maintained in wells of a 96-well plate incubated at 28°C and shaken at 216 rpm. A Spark 10M plate reader was used to measure the OD600 of each well every 30–60 minutes using default settings.
Measurements of P. syringae growth in planta
Arabidopsis Col-0 seeds were surface sterilized, stratified and plated onto MS agar as described previously [9]. After two weeks, Arabidopsis seedlings were transferred to Sunshine mix soil. Seeds of Phaseolus vulgaris (bush bean) cultivar Blue Lake seeds (Ferry-Morse Seed) were germinated directly in Sunshine mix. All plants were maintained at 22°C in a 10-hour-day growth chamber. For Arabidopsis infection, inoculums of P. syringae in water were adjusted to OD600 = 0.002, then infiltrated into fully expanded leaves of five-week-old plants. For bean infections, inoculums were adjusted to OD600 = 0.001, then infiltrated into leaves of five-week-old plants. The infected plants were kept at 22°C in 10-hour-day growth chamber. Bacterial populations in infected leaves were measured as previously described [18]. Each sample is bacteria isolated from three leaf disks taken from three leaves of a single plant.
Expression and purification of recombinant AauR
Recombinant AauR with a C-terminal 6xHis epitope tag was produced in E. coli. To prepare an expression construct, oligonucleotides aauR_28a_F and aauR_28a_R were used to PCR-amplify aauR from DC3000 genomic DNA, and oligonucleotides pET28a_F and pET28a_R were used to amplify a linearized pET28a fragment. NEBuilder HiFi reaction mix (NEB) was used to assemble the resulting aauR and pET28a DNA fragments into an intact plasmid. Correct assembly of plasmids was confirmed by PCR and Sanger sequencing. Sequences of oligonucleotides used for plasmid construction are provided in S2 Table. For protein expression, E. coli strain BL21(DE3)(pLysS) harboring the pET28a::aauR plasmid were cultured overnight at 37°C, transferred to 200 mL of LB broth and cultured at 37°C until OD600 of the culture reached 0.6–0.8. AauR expression was induced by addition of IPTG to a final concentration of 0.2 mM. After incubating at 20°C for 16 hrs, cells were pelleted by centrifugation. The cell pellet was resuspended into 5 mL of lysis buffer (25 mM Tris-HCl pH 7.5, 500 mM NaCl, 20 mM imidazole) and sonicated. The total cell lysate was then centrifuged at 12,000 x g for 30 min at 4°C. The resulting supernatant was added to pre-equilibrated Profinity IMAC Ni-charged resin (Bio-Rad), and the mixture gently mixed by shaking at 4°C for 1 hr. After loading the mixture onto a column, the resin was washed with 10 column volumes of wash buffer (2.5 mM Tris-HCl pH 7.5, 500 mM NaCl, 50 mM Imidazole). The bound protein was eluted with elution buffer (25 mM Tris-HCl p H 7.5, 300 mM NaCl, 200 mM Imidazole). Elution fractions were analyzed by SDS-PAGE and AauR abundance quantified using Pierce BCA Protein Assay Kit.
In vitro detection of AauR-DNA interactions
The AlphaScreen histidine (nickel chelate) detection kit (Perkin Elmer) was used to detect interactions between recombinant AauR and DNA. Single-stranded biotinylated and non-biotinylated DNA oligonucleotides (Integrated DNA Technologies) were reconstituted into dsDNA probes using the method described in [62]. Sequences of oligonucleotides are provided in S2 Table. Interactions between AauR and DNA were assayed in wells of half-volume white 96-well plates (Greiner Bio-One). In each well, 13 μL containing 1x kit buffer, 0.01% Tween 20, 2% BSA was mixed with 2 μL of 6xHis tagged AauR (in 300 nM-1000 nM range) and 2 μL of 625 nM biotinylated or non-biotinylated dsDNA probe. After incubating at room temperature for 2 hr or 4°C overnight, 4 μL of 125 μg/ml acceptor beads was added to each well. The mixture was incubated at room temperature for 1 hr, then 4 μL of 125 μg/ml donor beads solution added to each well. After the addition of donor beads, the plate was kept in the dark at room temperature for 1 hr before measuring luminescence from each well using a BioTek Synergy 2 plate reader. All reactions were performed in triplicate and results were successfully repeated with independent batches of purified AauR protein.
Bioinformatics and phylogenetic analyses
Genome sequences and locus identifiers of P. syringae strains analyzed are listed in S3 Table. Strains were chosen to encompass all diversity of the canonical type III secretion system, with strains differentiated by phylogroup using the citrate synthase (cts) partial coding sequence [24,25]. All genome sequences in NCBI listed under the ‘Pseudomonas syringae group’ (taxid 136849) were downloaded (May 20, 2019). The cts gene sequence was extracted from BLASTN+ (v 2.7.1) search results using the P. syringae DC3000 cts gene sequence as the query, and the complete genome sequences of all other strains as the target BLAST database [63]. The DC3000 cts gene sequence and other reference phylogroup cts gene sequences were obtained from Supplementary File S1 from [24]. Complete genomes were chosen, when available, for each phylogroup. When possible, potential intra-phylogroup diversity was also captured in the strains selected for analysis based on the cts phylogeny; general quality of genome assembly was estimated by comparing total numbers of contigs and total assembly length. Individual representatives from each phylogroup were selected to present in Fig 6A; these strains are listed in S3 Table. The amino acid sequence from P. syringae DC3000 for HrpR (PSPTO_1379), AatJ (PSPTO_4171), and AauS (PSPTO_4175) were used as queries for BLASTP+ (v 2.7.1) searches against the predicted proteomes of all other genomes [63]. BLASTP hits were manually filtered using empirically derived percent identity cut-offs. Nucleotide sequences for these protein coding regions were used in the phylogenetic tree reconstruction; amino acid sequences for AauS were used as they provided sufficient resolution of intra-phylogroup relationships. The intergenic region upstream hrpR, including the predicted AauR binding motif, was extracted using BioPython (v 1.70) [64]; the aligned motif was taken as a subset from the full alignment of the intergenic region. We used MAFFT (v 7.402) specifying the G-INS-i algorithm to align the sequences, and IQ-TREE (v 1.6.9) to generate the maximum likelihood phylogenetic trees; bootstrap supports were computed using both ultrafast bootstraps (-bb 1000) and the SH-aLRT test (-alrt 1000) [65–68]. Trees were visualized using the itol web server v4 (https://itol.embl.de/, [69]). The aligned AauR binding motif was visualized using the texshade (v 1.25) package in TeX Live 2019 (https://www.ctan.org/pkg/texshade;https://www.tug.org/texlive/index.html). Trees and alignments were annotated using Inkscape v0.92 (https://inkscape.org/).
Statistics
All statistical analyses were done using Minitab, R and Excel software.
Supporting information
GFP fluorescence of DC3000, ΔaatJ, ΔaauS, ΔaauR and ΔaauSΔaauR strains carrying (A) avrRpm1promoter:gfp, (B) hrpLpromoter:gfp or (C) hrpRSpromoter:gfp reporter plasmids. Bacteria were cultured in a minimal medium (MM) with 10 mM fructose (open bars) and 10 mM fructose plus 200 μM glutamate (filled bars) for 6 hours. Graphed are means ± SE of GFP fluorescence normalized to OD600 and background fluorescence from empty vector strains; n = 3. Asterisks denote significant difference based on two-sample t-test comparison with DC3000 treated with fructose and aspartate, P < 0.05. Data are representative of three independent experiments.
(TIF)
GFP fluorescence of DC3000 and ΔaauSΔaauR carrying hrpLpromoter:gfp reporter plasmids. Bacteria were cultured for eight hours in minimal medium containing 10 mM fructose and with or without 200 μM glutamate, citrate, or 4-hydroxybenzoic acid. Graphed are means ± SD of the change in GFP fluorescence at T = 8 hours post-treatment. Fluorescence values were normalized to OD600 and background fluorescence from empty vector strains; n = 12. Data are pooled from 3 independent experiments, n = 4 per experiment. Small-case letters denote statistical significance based on ANOVA with Tukey’s post-hoc HSD test, P < 0.05.
(TIF)
(A) 1 x 108 cfu/mL of DC3000, ΔaauR, ΔaauS or ΔaatJ were syringe-infiltrated in Arabidopsis leaves. Leaf bacteria populations were measured by serial dilution plating of leaf extracts six hours post-infection. Graphed are means ± SE of bacteria colonies isolated from infected tissue, n = 3. Abbreviation ns is not significant based on ANOVA with Tukey’s HSD, α = .05. (B) 5 x 108 cfu/mL of DC3000 or ΔaauR were syringe-infiltrated into Arabidopsis leaves. Leaf bacteria populations were measured by serial dilution plating of leaf extracts 6 hours post-infection. Graphed are means ± SE of bacteria colonies isolated from infected tissue, n = 3. Abbreviation ns is not significant based on t-test, P = .444. (C-D) 1 x 106 cfu/mL of DC3000, ΔaauS, ΔaauR, or ΔaatJ and respective complemented strains were syringe-infiltrated into Arabidopsis leaves. Leaf bacteria populations were enumerated on day 0 and day 3 by serial dilution plating of leaf extracts. Graphed are log-transformed means ± SE of bacteria colony-forming units (cfus) isolated from infected tissue, n = 6 for day 0 and n = 8 for day 3. Small-case letters denote significance groupings based on ANOVA with Tukey’s HSD, P < 0.01. Data shown were pooled from two independent experiments.
(TIF)
DC3000, ΔaatJ, ΔaauS, and ΔaauR were individually inoculated into 100 μL of (A) Lysogeny broth (LB) or (B) King’s B (KB) broth and grown for 24 hours at 28°C. OD600 readings of each well were taken at 30 min intervals. Graphed are means ± SD of OD600 readings, n = 3. Results are representative of 3 independent experiments.
(TIF)
(A) DC3000 and ΔaauS carrying empty vector (EV) pME6010 or aauS::pME6010; (B) DC3000 and ΔaauR carrying empty vector (EV) pME6010 or aauR::pME6010; and (C) DC3000 and ΔaatJ carrying empty vector (EV) pME6010 or aatJ::pME6010 were inoculated into 100 μL of M9 medium supplemented with 10 mM glutamate as the sole carbon source. Optical density of each culture was measured every 30 minutes from 6 to 18 hours post-inoculation. Graphed are means ± SD of OD600 readings, n = 4. Results are representative of 3 independent experiments.
(TIF)
Timecourse analysis of DC3000 and ΔaatP growth in King’s B (KB) broth. Cultures were inoculated at an optical density at λ = 600 nm (OD600) of 0.05 and grown for 24 hours at 28°C. Graphed are means ± SD of OD600 readings, n = 3. Results are representative of 3 independent experiments.
(TIF)
Growth of DC3000 and ΔaatQaatM strains in KB medium (A) or M9 minimal medium with glutamate as a sole carbon and nitrogen source (B). Cultures were inoculated at an optical density (λ = 600 nm) of 0.05 and grown for 24 hours at 28°C in wells of a 96-well plate. Optical density at λ = 600 nm (OD600) readings of each well were taken at 30 min intervals. Graphed are means ± SD of OD600 readings, n = 3. (C) Analysis of GFP fluorescence from DC3000 and ΔaatQaatM avrRpm1promoter:gfp reporter strains incubated with 10 mM fructose (open bars) or 10 mM fructose and 200 μM glutamate (filled bars) for 6 hours. Graphed are means ± SD of GFP fluorescence normalized to OD600 and background fluorescence from empty vector strains; n = 4. Abbreviation ns is not significant based on t-test with α = 0.05. (D) Growth of DC3000 and ΔaatQaatM strains in Arabidopsis. A 1 x 106 cfu/mL inoculum of each strain was syringe-infiltrated into Arabidopsis leaves. Leaf bacteria populations were enumerated on day 0 and day 3 by serial dilution plating of leaf tissue extracts. Graphed are means ± SE of bacteria from 4 infected plants, n = 3 for day 0 and n = 4 for day 3. Abbreviation ns is not significant based on t-test with α = 0.05.
(TIF)
AlphaScreen assay of E. coli-expressed recombinant DC3000 AauR protein binding to 50 bp of aatJ promoter DNA including the Rbm sequence. Assays containing non-biotinylated DNA were included as negative controls to demonstrate specificity of detected interactions. Graphed are means ± SD of luminescence from assay wells, n = 3. Asterisks is P < 0.001 based on t-test. Data are representative of 3 independent experiments.
(TIF)
Alignment of genomic DNA containing Rbm upstream of hrpRS from 38 P. syringae isolates. Sequences are labeled with phylogroup and strain name. Full names can be found in S3 Table. Upper and lower case letters below alignment are consensus sequence of nucleotides based on 100% and ≥85% identity, respectively; dots on the consensus line indicate no consensus based on either criteria. Highlighted in blue is AauR-binding motif (Rbm).
(TIF)
Nucleotide sequences of hrpR from all canonical tripartite type III secretion system encoding P. syringae strains examined in this study are included in the phylogeny. Ultrafast bootstraps (>95% support) calculated by IQ-TREE are reported. Within phylogroup supports are all above the 95% bootstrap support threshold. Branch lengths indicate the number of mutations per site. The tree is artificially rooted on representatives from PG9 for reference.
(TIF)
Nucleotide sequences corresponding to the intergenic regions between hrpH and hrpR from all canonical tripartite type III secretion system encoding P. syringae strains examined in this study are included in the phylogeny. Ultrafast bootstraps (>95% support) calculated by IQ-TREE are reported. Within phylogroup supports are all above the 95% bootstrap support threshold. Branch lengths indicate the number of mutations per site. The tree is artificially rooted on representatives from PG9 for reference.
(TIF)
Nucleotide sequences of aatJ from diverse P. syringae isolates and other pseudomonads are included in the phylogeny. Ultrafast bootstraps (>95% support) calculated by IQ-TREE are reported. Within phylogroup supports are all above the 95% bootstrap support threshold. Branch lengths indicate the number of mutations per site. The tree is artificially rooted on branches with representative Pseudomonas spp. outside of P. syringae for reference.
(TIF)
Amino acid sequences of AauS from diverse P. syringae isolates and other pseudomonads are included in the phylogeny. Ultrafast bootstraps (>95% support) calculated by IQ-TREE are reported. Within phylogroup supports are all above the 95% bootstrap support threshold. Branch lengths indicate the number of mutations per site. The tree is artificially rooted on branches with representative Pseudomonas spp. outside of P. syringae for reference.
(TIF)
Timecourse analysis of B728a and ΔaauR growth in (A) standard M9 medium supplemented with glucose and ammonium chloride or (B) lysogeny broth (LB). Cultures were inoculated at an optical density at λ = 600 nm (OD600) of 0.05 and grown for 24 hours at 28°C in a Tecan Spark 10M plate reader. Graphed are means ± SD of OD600 readings, n = 3. Results are representative of 3 independent experiments.
(TIF)
Plant-exuded aspartate and glutamate enter the periplasm, mostly likely through an outer membrane porin, and bind to AatJ, an ABC transporter-associated substrate-binding protein. AatJ, in complex with aspartate or glutamate, binds to AatQM to deliver these metabolites for transport across the inner membrane, and also binds to AauS to activate signaling of the two-component system AauSR. Alternatively, aspartate/glutamate may directly bind to and activate AauS, with AatJ functioning indirectly in promoting AauSR activation (not shown in model). Activated AauS phosphorylates and activates AauR, which in turn binds to a conserved motif (AauR binding motif, Rbm) present within the promoter region of aatJ to increase expression of aat/aau genes. Activated AauR also binds to an Rbm upstream of the operon encoding enhancer-binding proteins HrpR and HrpS. Increased abundance of HrpR and HrpS results in increased expression of the gene encoding the alternative sigma factor HrpL, which in turn activates expression of genes encoding type III secretion system (T3SS) structural components and effectors. See discussion for additional details of model.
(TIF)
(PDF)
(PDF)
(PDF)
(XLSX)
Acknowledgments
We thank Tom Wolpert and Jeff Chang for helpful discussions and critical reading of this manuscript, and the Department of Botany and Plant Pathology at Oregon State University for its generous support of the computing cluster.
Data Availability
All relevant data are within the manuscript and Supporting Information data file.
Funding Statement
This study was funded by National Science Foundation award IOS-1557694 to JCA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sturm A, Heinemann M, Arnoldini M, Benecke A, Ackermann M, Benz M, et al. The cost of virulence: retarded growth of Salmonella typhimurium cells expressing type III secretion system 1. PloS Path. 2011; 7: e1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segonzac C, Zipfel C. Activation of plant pattern-recognition receptors by bacteria. Curr Opin Microbiol. 2011; 14: 54–61. 10.1016/j.mib.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 3.Boutrot F, Zipfel C. Function, discovery, and exploitation of plant pattern recognition receptors for broad-spectrum disease resistance. Ann Rev Phytopathol. 2017; 55: 257–286. [DOI] [PubMed] [Google Scholar]
- 4.Brencic A, Winans SC. Detection of and response to signals involved in host-microbe interactions by plant-associated bacteria. Microbiol Mol Biol Rev. 2005; 69: 155–194. 10.1128/MMBR.69.1.155-194.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xin X-F, Kvitko B, He SY. Pseudomonas syringae: what it takes to be a pathogen. Nat Rev Microbiol. 2018; 16: 316 10.1038/nrmicro.2018.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang JH, Desveaux D, Creason AL. The ABCs and 123s of bacterial secretion systems in plant pathogenesis. Annu Rev Phytopathol. 2014; 52: 317–45. 10.1146/annurev-phyto-011014-015624 [DOI] [PubMed] [Google Scholar]
- 7.Büttner D. Protein export according to schedule: architecture, assembly, and regulation of type III secretion systems from plant-and animal-pathogenic bacteria. Microbiol Mol Biol Rev. 2012; 76: 262–310. 10.1128/MMBR.05017-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan M, Seto D, Subramaniam R, Desveaux D. Oh, the places they’ll go! A survey of phytopathogen effectors and their host targets. The Plant Journal 2018; 93: 651–663. 10.1111/tpj.13780 [DOI] [PubMed] [Google Scholar]
- 9.Anderson JC, Wan Y, Kim YM, Pasa-Tolic L, Metz TO, Peck SC. Decreased abundance of type III secretion system-inducing signals in Arabidopsis mkp1 enhances resistance against Pseudomonas syringae. Proc Natl Acad Sci USA. 2014; 111: 6846–6851. 10.1073/pnas.1403248111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang X, Xiao Y, Zhou JM. Regulation of the type III secretion system in phytopathogenic bacteria. Mol Plant-Microbe Interact. 2006; 19: 1159–66. 10.1094/MPMI-19-1159 [DOI] [PubMed] [Google Scholar]
- 11.Grimm C, Panopoulos NJ. The predicted protein product of a pathogenicity locus from Pseudomonas syringae pv. phaseolicola is homologous to a highly conserved domain of several procaryotic regulatory proteins. J Bacteriol. 1989; 171: 5031–8. 10.1128/jb.171.9.5031-5038.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahme L, Mindrinos M, Panopoulos N. Genetic and transcriptional organization of the hrp cluster of Pseudomonas syringae pv. phaseolicola. J Bacteriol. 1991; 173: 575–586. 10.1128/jb.173.2.575-586.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutcheson SW, Bretz J, Sussan T, Jin S, Pak K. Enhancer-binding proteins HrpR and HrpS interact to regulate hrp-encoded type III protein secretion in Pseudomonas syringae strains. J Bacteriol. 2001; 183: 5589–98. 10.1128/JB.183.19.5589-5598.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jovanovic M, James EH, Burrows PC, Rego FG, Buck M, Schumacher J. Regulation of the co-evolved HrpR and HrpS AAA+ proteins required for Pseudomonas syringae pathogenicity. Nat Commun. 2011; 2: 177 10.1038/ncomms1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bretz J, Losada L, Lisboa K, Hutcheson SW. Lon protease functions as a negative regulator of type III protein secretion in Pseudomonas syringae. Mol Microbiol. 2002; 45: 397–409. 10.1046/j.1365-2958.2002.03008.x [DOI] [PubMed] [Google Scholar]
- 16.Preston G, Deng WL, Huang HC, Collmer A. Negative regulation of hrp genes in Pseudomonas syringae by HrpV. J Bacteriol. 1998; 180: 4532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao Y, Lan L, Yin C, Deng X, Baker D, Zhou JM, et al. Two-component sensor RhpS promotes induction of Pseudomonas syringae type III secretion system by repressing negative regulator RhpR. Mol Plant-Microbe Interact. 2007; 20: 223–34. 10.1094/MPMI-20-3-0223 [DOI] [PubMed] [Google Scholar]
- 18.Turner SE, Pang YY, O'Malley MR, Weisberg AJ, Fraser VN, Yan Q, et al. A DeoR-type transcription regulator is required for sugar-induced expression of type III secretion-encoding genes in Pseudomonas syringae pv. tomato DC3000. Mol Plant-Microbe Interact. 2020; 33:509–518. [DOI] [PubMed] [Google Scholar]
- 19.Huynh TV, Dahlbeck D, Staskawicz BJ. Bacterial blight of soybean: regulation of a pathogen gene determining host cultivar specificity. Science. 1989; 245: 1374–1377. 10.1126/science.2781284 [DOI] [PubMed] [Google Scholar]
- 20.Singh B, Rӧhm K-H. Characterization of a Pseudomonas putida ABC transporter (AatJMQP) required for acidic amino acid uptake: biochemical properties and regulation by the Aau two-component system. Microbiology. 2008; 154: 797–809. 10.1099/mic.0.2007/013185-0 [DOI] [PubMed] [Google Scholar]
- 21.Sonawane AM, Singh B, Rӧhm KH. The AauR-AauS two-component system regulates uptake and metabolism of acidic amino acids in Pseudomonas putida. Appl Environ Microbiol. 2006; 72: 6569–6577. 10.1128/AEM.00830-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson AL, Chen J. ATP-binding cassette transporters in bacteria. Annu Rev Biochem. 2004; 73: 241–268. 10.1146/annurev.biochem.73.011303.073626 [DOI] [PubMed] [Google Scholar]
- 23.Winsor GL, Griffiths EJ, Lo R, Dhillon BK, Shay JA, Brinkman FS. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res. 2015; 44: D646–D653. 10.1093/nar/gkv1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dillon MM, Thakur S, Almeida RND, Wang PW, Weir BS, Guttman DS. Recombination of ecologically and evolutionarily significant loci maintains genetic cohesion in the Pseudomonas syringae species complex. Genome Biol. 2019; 20: 3 10.1186/s13059-018-1606-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berge O, Monteil CL, Bartoli C, Chandeysson C, Guilbaud C, Sands DC, et al. A user’s guide to a database of the diversity of Pseudomonas syringae and its application to classifying strains in this phylogenetic complex. PloS One. 2014; 9: e105547 10.1371/journal.pone.0105547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feil H, Feil WS, Chain P, Larimer F, DiBartolo G, Copeland A, et al. Comparison of the complete genome sequences of Pseudomonas syringae pv. syringae B728a and pv. tomato DC3000. Proc Natl Acad Sci USA. 2005; 102: 11064–11069. 10.1073/pnas.0504930102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochman H, Lawrence JG, Groisman EA. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000; 405: 299 10.1038/35012500 [DOI] [PubMed] [Google Scholar]
- 28.Groisman EA, Ochman H. Pathogenicity islands: bacterial evolution in quantum leaps. Cell. 1996; 87: 791–794. 10.1016/s0092-8674(00)81985-6 [DOI] [PubMed] [Google Scholar]
- 29.Sawada H, Suzuki F, Matsuda I, Saitou N. Phylogenetic analysis of Pseudomonas syringae pathovars suggests the horizontal gene transfer of argK and the evolutionary stability of hrp gene cluster. J Mol Evol. 1999; 49: 627–644. 10.1007/pl00006584 [DOI] [PubMed] [Google Scholar]
- 30.Mohr TJ, Liu H, Yan S, Morris CE, Castillo JA, Jelenska J, et al. Naturally occurring nonpathogenic isolates of the plant pathogen Pseudomonas syringae lack a type III secretion system and effector gene orthologues. J Bacteriol. 2008; 190: 2858–2870. 10.1128/JB.01757-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stone JR, Wray GA. Rapid evolution of cis-regulatory sequences via local point mutations. Mol Biol Evol. 2001; 18: 1764–1770. 10.1093/oxfordjournals.molbev.a003964 [DOI] [PubMed] [Google Scholar]
- 32.Groisman EA, Mouslim C. Sensing by bacterial regulatory systems in host and non-host environments. Nat Rev Microbiol. 2006; 4: 705 10.1038/nrmicro1478 [DOI] [PubMed] [Google Scholar]
- 33.Groisman EA. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol. 2001; 183: 1835–1842. 10.1128/JB.183.6.1835-1842.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cangelosi GA, Ankenbauer RG, Nester EW. Sugars induce the Agrobacterium virulence genes through a periplasmic binding protein and a transmembrane signal protein. Proc Natl Acad Sci USA. 1990; 87: 6708–6712. 10.1073/pnas.87.17.6708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He F, Nair GR, Soto CS, Chang Y, Hsu L, Ronzone E, et al. Molecular basis of ChvE function in sugar binding, sugar utilization, and virulence in Agrobacterium tumefaciens. J Bacteriol. 2009; 191: 5802–5813. 10.1128/JB.00451-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu X, Zhao J, DeGrado WF, Binns AN. Agrobacterium tumefaciens recognizes its host environment using ChvE to bind diverse plant sugars as virulence signals. Proc Natl Acad Sci USA. 2013; 110: 678–683. 10.1073/pnas.1215033110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West AH, Stock AM. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem Sci. 2001; 26: 369–376. 10.1016/s0968-0004(01)01852-7 [DOI] [PubMed] [Google Scholar]
- 38.El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, Potter SC, et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2018; 47: D427–D432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015; 10: 845 10.1038/nprot.2015.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yurgel SN, Kahn ML. Dicarboxylate transport by rhizobia. FEMS Microbiol Rev. 2004; 28: 489–501. 10.1016/j.femsre.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 41.Zhou YF, Nan B, Nan J, Ma Q, Panjikar S, Liang YH, et al. C4-dicarboxylates sensing mechanism revealed by the crystal structures of DctB sensor domain. J Mol Biol. 2008; 383: 49–61. 10.1016/j.jmb.2008.08.010 [DOI] [PubMed] [Google Scholar]
- 42.Shimoda N, Toyoda-Yamamoto A, Aoki S, Machida Y. Genetic evidence for an interaction between the VirA sensor protein and the ChvE sugar-binding protein of Agrobacterium. J Biol Chem. 1993; 268: 26552–26558. [PubMed] [Google Scholar]
- 43.Kossmann M, Wolff C, Manson M. Maltose chemoreceptor of Escherichia coli: interaction of maltose-binding protein and the tar signal transducer. J Bacteriol. 1988; 170: 4516–4521. 10.1128/jb.170.10.4516-4521.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antoine R, Huvent I, Chemlal K, Deray I, Raze D, Locht C, et al. The periplasmic binding protein of a tripartite tricarboxylate transporter is involved in signal transduction. J Mol Biol. 2005; 351: 799–809. 10.1016/j.jmb.2005.05.071 [DOI] [PubMed] [Google Scholar]
- 45.Kumar V, Sharma A, Kaur R, Thukral AK, Bhardwaj R, Ahmad P, et al. Differential distribution of amino acids in plants. Amino Acids. 2017; 49: 821–869. 10.1007/s00726-017-2401-x [DOI] [PubMed] [Google Scholar]
- 46.Forde BG, Lea PJ. Glutamate in plants: metabolism, regulation, and signaling. J Exp Bot. 2007; 58: 2339–2358. 10.1093/jxb/erm121 [DOI] [PubMed] [Google Scholar]
- 47.Azevedo RA, Lancien M, Lea PJ. The aspartic acid metabolic pathway, an exciting and essential pathway in plants. Amino Acids. 2006; 30: 143–62. 10.1007/s00726-005-0245-2 [DOI] [PubMed] [Google Scholar]
- 48.Tegeder M, Masclaux-Daubresse C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018; 217: 35–53. 10.1111/nph.14876 [DOI] [PubMed] [Google Scholar]
- 49.Toyota M, Spencer D, Sawai-Toyota S, Jiaqi W, Zhang T, Koo AJ, et al. Glutamate triggers long-distance, calcium-based plant defense signaling. Science. 2018; 361: 1112–1115. 10.1126/science.aat7744 [DOI] [PubMed] [Google Scholar]
- 50.Cerna-Vargas JP, Santamaría-Hernando S, Matilla MA, Rodríguez-Herva JJ, Daddaoua A, Rodríguez-Palenzuela P, et al. Chemoperception of specific amino acids controls phytopathogenicity in Pseudomonas syringae pv. tomato. mBio. 2019; 10: e01868–19. 10.1128/mBio.01868-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng X, Liang H, Chen K, He C, Lan L, Tang X. Molecular mechanisms of two-component system RhpRS regulating type III secretion system in Pseudomonas syringae. Nucleic Acids Res. 2014; 42: 11472–11486. 10.1093/nar/gku865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.King EO, Ward MK, Raney DE. Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med. 1954; 44: 301–307. [PubMed] [Google Scholar]
- 53.Cold Spring Harbor Laboratory, M9 minimal medium (standard). Cold Spring Harb Protoc. 2010; 10.1101/pdb.rec12295 [DOI] [Google Scholar]
- 54.Luria SE, Burrous JW. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957; 74: 461–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang JH, Urbach JM, Law TF, Arnold LW, Hu A, Gombar S, Grant SR, Ausubel FM, and Dangl JL. A high-throughput, near-saturating screen for type III effector genes from Pseudomonas syringae. Proc Natl Acad Sci USA. 2005; 102: 2549–54. 10.1073/pnas.0409660102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rogan C J, Anderson JC. Isolation and characterization of plant metabolite signals that induce type III secretion by the plant pathogen Pseudomonas syringae. Methods Mol Biol. 2019; 1991:115–126. 10.1007/978-1-4939-9458-8_13 [DOI] [PubMed] [Google Scholar]
- 57.Li R, Oliver RA, Townsend CA. Identification and characterization of the sulfazecin monobactam biosynthetic gene cluster. Cell Chem Biol. 2017; 24: 24–34. 10.1016/j.chembiol.2016.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kessler B, De Lorenzo V, Timmis KN. A general system to integrate lacZ fusions into the chromosomes of Gram-negative eubacteria: regulation of the Pm promoter of the TOL plasmid studied with all controlling elements in monocopy. Mol Gen Genet. 1992; 233:293–301. 10.1007/BF00587591 [DOI] [PubMed] [Google Scholar]
- 59.Yan Q, Rogan CJ, Anderson JC. Development of a Pseudomonas syringae-Arabidopsis suspension cell infection system for investigating host metabolite-dependent regulation of type III secretion and pattern-triggered immunity. Mol Plant-Microbe Interact. 2019; 32: 527–539. 10.1094/MPMI-10-18-0295-FI [DOI] [PubMed] [Google Scholar]
- 60.Miller WG, Leveau JH, Lindow SE. Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol Plant-Microbe Interact. 2000; 13: 1243–1250. 10.1094/MPMI.2000.13.11.1243 [DOI] [PubMed] [Google Scholar]
- 61.Heeb S, Itoh Y, Nishijyo T, Schnider U, Keel C, Wade J, et al. Small, stable shuttle vectors based on the minimal pVS1 replicon for use in gram-negative, plant-associated bacteria. Mol Plant-Microbe Interact. 2000; 13: 232–237. 10.1094/MPMI.2000.13.2.232 [DOI] [PubMed] [Google Scholar]
- 62.Nomoto M, Tada Y, Tsukagoshi H. In vitro Protein-DNA Binding Assay (AlphaScreen Technology). Bio-Protocol. 2019; 9: e3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009; 10: 421 10.1186/1471-2105-10-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cock PJ, Antao T, Chang JT, Chapman BA, Cox CJ, Dalke A, et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics. 2009; 25: 1422–1423. 10.1093/bioinformatics/btp163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013; 30: 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalyaanamoorthy S, Minh BQ, Wong TK, von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017; 14: 587 10.1038/nmeth.4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2014; 32: 268–274. 10.1093/molbev/msu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. UFBoot2: Improving the ultrafast bootstrap approximation. Mol Biol Evol. 2018; 35:518–522. 10.1093/molbev/msx281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Letunic I, Bork P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 2019; 47: W256–W259. 10.1093/nar/gkz239 [DOI] [PMC free article] [PubMed] [Google Scholar]