Highlights
-
•
It is concluded that the feline oral flora is highly diverse than canine oral flora.
-
•
Porphyromonas gingivalis, Prevotella nigrescens and Porphyromonas gulae were the dominant species in cats and dogs.
-
•
T. forsythia, C. ochracea, and C. sputigena in cats and T. forsythia, C. ochracea, C. sputigena T. denticola and, E. corrodens, in dogs showed that the prevalence was lower than 10%. E. corrodens in cats and, P. intermedia, A. actinomycetemcomitans, and C. rectus in dogs were also isolated from the swab samples with less than 30% percentage.
-
•
A balanced diet for cats and dogs should be provided to reduce the formation of residues in the oral flora. Daily rinsing with antiseptic solutions may also be helpful against the development of periodontal pathogens.
Keywords: Periodontal disease, Cat, Dog, PCR
Abstract
Periodontal disease is the most common infectious disease of cats and dogs which are strongly associated with periodontal pathogens. The primary etiologic factor in the formation of periodontal disease is microbial dental plaque accumulation on teeth. In our research, we aimed to investigate the presence of periodontal disease-related bacterial species in dental plaques of cats and dogs. Specimens collected from 50 cats and 51 dogs with periodontal disease examined in terms of periodontal pathogens by polymerase chain reaction (PCR) using primers directed to 16S rRNA and tdpA genes. Our findings indicate the presence of periodontal disease-related pathogens, especially Porphyromonas gingivalis (cats 96%, dogs 88%), Prevotella nigrescens (cats 90%, dogs 57%) and, Porphyromonas gulae (cats 70%, dogs 39%). In addition, the prevalence of Tannerella forthysia (cats 2%, dogs 4%) well-known pathogen in cats and dogs were isolated with an extremely low percentage.
Furthermore, our results suggest that the feline oral cavity microbiota has considerably more diversity than dogs. Consequently, daily oral hygiene practices may become essential for controlling the pathogenic bacteria which have clinical importance and in preventing the propagation of microorganisms in the oral cavity of cats and dogs.
1. Introduction
The microbial population colonizing on the teeth begins dental infections such as periodontal diseases, gingivitis, and pulpitis in humans, cats and dogs (Hale, 2009, Munemasa et al., 2000). Periodontal disease is a set of inflammatory conditions affecting the tissues surrounding the teeth. Feline and canine specifications as age, species, breed, genetics, diet, health status, habitat, the frequency of dental care and bacterial flora condition of the oral cavity may have a role in the development of diseases (Kim and Amar, 2006, Niemiec, 2012). The disease is common in cats and dogs with a prevalence of 70% and 80%, respectively (Booij-Vrieling et al., 2010). While Gram-positive bacteria species are predominant in healthy dogs, Gram-negative anaerobes prevail in supragingival and subgingival plaques in dogs in the course of periodontal diseases (Ebrahimi et al., 2010, Forsblom et al., 2002, Harvey et al., 1995 ). Both Gram-positive and Gram-negative bacteria may lead to inflammation and the gingival destruction of periodontal tissue as well as the loss of alveolar bone in humans and animals with periodontal disease. Also, anaerobe bacteria may cause releasing of enzymes and endotoxins during the formation of periapical lesions. Porphyromonas sp. and Prevotella sp. can be found in dental plaque and periodontal pockets. In particular, P. gingivalis contributes to chronic periodontal disease and inhibits the migration of PMNs that pass through the epithelial barrier (Dahlen, 2002, Forsblom et al., 2002). The Porphyromonas sp. species have also appropriate virulence factors that can cause periodontal disease and stimulate an appropriate humoral immune response (Adler, Malik & Gina, 2016).
Many studies indicated that diet consumption has an important effect on the formation of the oral microbiome and periodontal disease. Soften wet diets have been associated with the prevalence and severity of periodontal disease in cats and dogs. Therefore, it is recommended that feeding with dry food diet has a positive effect on oral health and reduces the formation of dental residues and periodontal disease (Adler et al., 2016, Gawor et al., 2006).
The participation of potential zoonotic and periodontopathic bacteria in the oral flora of cats and dogs may cause public health problems due to bite wound infections (Booij-Vrieling et al., 2010, Khazandi et al., 2014, Yamasaki et al., 2012). The infection rates are between 4–25% and 20–50% in the case of cats and dogs bite wounds, and the symptoms appear within 24 h. Furthermore, bites can also cause a systemic infection which results in 6.7% death annually (Griego et al., 1995, Talan et al., 1999). On average, up to 15–20% of dog bites and approximately 30–50% of cat bites have been infected (Brook, 2003, Centers for Disease Control and Prevention (CDC) 2015, Rothe et al., 2015).
The periodontal pathogens such as C. sputigena, P. gingivalis, P. nigrescens, E. corrodens, C. rectus, C. ochracea, A. actinomycetemcomitans, T. forsythia, and T. denticola have been reported to be isolated from saliva samples of humans (Piau et al., 2013, Tamura et al., 2006). Additively, these bacteria species from oral microbiome of dogs and cats can cause many diseases in humans such as Aggregatibacter actinomycetemcomitans (brain abscesses, endocarditis, rheumatoid arthritis-a potential trigger of the autoimmune disease) (Henderson et al., 2002, Konig et al., 2016), Campylobacter rectus (periodontal disease-because of increased salivary estradiol concentrations during pregnancy) (Mahlen & Clarridge, 2009), Capnocytophaga ochracea (intrauterine infections, endocarditis), Capnocytophaga sputigena (iliopsoas abscess) (Desai, Harrison & Murphy, 2007), Eikenella corrodens (sinusitis, arthritis, endocarditis, pancreatic abscesses, vertebral osteomyelitis) (Paul & Patel, 2001), Porphyromonas gingivalis (rheumatoid arthritis, bacterial vaginosis, osteomyelitis) (Gaetti-Jardim et al., 2010, Venkataraman and Almas, 2015, Wegner et al., 2010), Porphyromonas gulae (periodontal disease; bind to human oral epithelial cells) (Hamada et al., 2008, Yamasaki et al., 2012), Prevotella intermedia (gingivitis-during pregnancy, cystic fibrosis) (Borgo et al., 2014, Gilpin et al., 2017), Prevotella nigrescens (carotid atherosclerosis) (Yakob et al., 2011), Tannerella forsythia (atherosclerosis, osteomyelitis) (Ardila et al., 2015, Gaetti-Jardim et al., 2010), Treponema denticola (bacterial vaginosis, bone infections) (Africa, Nel & Stemmet, 2014). Therefore, a meticulous oral hygiene application on pet animals is of paramount importance.
The aim of this study was to determine the distribution of periodontal pathogens (A. actinomycetemcomitans, C. rectus, C. ochracea, C. sputigena, E. corrodens, P. gingivalis, P. gulae, and P. intermedia) in cats and dogs dental plaque samples by using primers directed to 16S rRNA and tdpA genes with PCR.
2. Materials and methods
2.1. Collection of samples
In this study, samples were collected from veterinary clinics in İzmir province and districts between November 2017 and March 2018. Dental plaque swab samples were obtained and transported in Stuart agar from the maxillary molar region of 51 dogs and 50 cats with periodontal disease. Meanwhile, specimens were transferred into plastic falcon tubes and crushed in 5 ml of sterile distilled water. Then, they were brought to Aydın Adnan Menderes University Veterinary Faculty Microbiology Department under the cold chain.
2.1.1. DNA extraction of bacteria
Centrifugation was applied to the samples at 10,000 xg for 5 min and, remaining sediment gathered at the bottom of the tube was dissolved in 100 µl sterile saline. DNA was extracted from bacteria colonies of Porphyromonas gingivalis (ATCC 33,277), Treponema denticola (ATCC 35,405), Tannerella forsythia (ATCC 43,037), Capnocytophaga ochracea (ATCC 27,872), Capnocytophaga sputigena (ATCC33612) Prevotella intermedia (ATCC 25,611), Prevotella nigrescens (ATCC 33,563), Campylobacter rectus (ATCC 33,238), Aggregatibacter actinomycetemcomitans (ATCC 33,384), Eikenella corrodens (ATCC 23,834) and, Porphyromonas gulae (ATCC 51,700) and used for positive control. Subsequently, the bacterial DNA extraction process was carried out for each sample by using a DNA extraction kit (Thermo Fisher®) as indicated by the manufacturer.
2.1.2. Design of primer
PCR assay conducted by using species-specific primers after the extraction (Table 1).
Table 1.
PCR primer sets used for the detection of the bacterial species.
| Target species | Sequences (5′-3′) | Target gene | Size (bp) | References |
|---|---|---|---|---|
| Universal primer (positive control) | AGA GTT TGA TCM TGG CTC AG CTG CTG CSY CCC GTA G |
16S rRNA |
315 | Doungudomdacha et al., 2000 |
| Porphyromonas gingivalis | CCG CAT ACA CTT GTA TTA TTG CAT GAT ATT AAG AAG TTT ACA ATC CTT AGG ACT GTC T |
16S rRNA |
267 | Kato et al., 2011. |
| Treponema denticola | AAG GCG GTA GAG CCG CTC A AGC CGC TGT CGA AAA GCC CA |
tdpA | 311 | Watanabe and Frommel, 1996 |
| Tannerella forsythia | GCG TAT GTA ACC TGC CCG CA TGC TTC AGT GTC AGT TAT ACC T |
16S rRNA |
641 | Ashimoto et al., 1996 |
| Capnocytophaga ochracea | AGA GTT TGA TCC TGG CTC AG GAT GCC GTC CCT ATA TAC TAT GGG G |
16S rRNA |
185 | Conrads et al., 1996 |
| Capnocytophaga sputigena | AGA GTT TGA TCC TGG CTC AG GAT GCC GCT CCT ATA TAC CAT TAG G |
16S rRNA |
185 | Conrads et al., 1996 |
| Prevotella intermedia | TTT GTT GGG GAG TAA AGC GGG TCA ACA TCT CTG TAT CCT GCG T |
16S rRNA |
575 | Ashimoto et al., 1996 |
| Prevotella nigrescens | ATG AAA CAA AGG TTT TCC GGT AAG CCC ACG TCT CTG TGG GCT GCG A |
16S rRNA |
804 | Ashimoto et al., 1996 |
| Campylobacter rectus | TTT CGG AGC GTA AAC TCC TTT TC TTT CTG CAA GCA GAC ACT CTT |
16S rRNA |
598 | Ashimoto et al., 1996 |
| Aggregatibacter actinomycetemcomitans | CTA GGT ATT GCG AAA CAA TTT G CCT GAA ATT AAG CTG GTA ATC |
16S rRNA |
262 | Kuboniwa et al., 2004 |
| Eikenella corrodens | CTA ATA CCG CAT ACG TCC TAA G CTA CTA AGC AAT CAA GTT GCC C |
16S rRNA |
688 | Ashimoto et al., 1996 |
| Porphyromonas gulae | TTG CTT GGT TGC ATG ATC GG GCT TAT TCT TAC GGT ACA TTC ACA |
16S rRNA |
314 | Doungudomdacha et al., 2000 |
The universal primer sets designed for use as a positive control for the detection of bacteria (Doungudomdacha, Rawlinson & Douglas, 2000), P. gingivalis, T. denticola, T. forsythia, C. ochracea, C. sputigena, P. intermedia, P. nigrescens, A. actinomycetemcomitans, C. rectus, and E. corrodens from cats and dogs oral cavity (Ashimoto et al., 1996, Conrads et al., 1996, Kuboniwa et al., 2004, Watanabe and Frommel, 1996). Moreover, it was planned to identify of P. gulae which can be isolated from gingival cavities in cats and dogs, excluded from human originated P. gingivalis strains in the study (Hamada et al., 2008, Kato et al., 2011).
2.1.3. Polymerase chain reaction (PCR) stage
5 µl DNA sample and 45 µl PCR master mixes were used in the amplification of the universal primer sets to the detection of total bacteria. Thereafter, the amplification was applied under the conditions was pre-denaturation at 95 °C for 5 min, 1 min denaturation at 95 °C, 1 min annealing at 55 °C, 1 min elongation at 72 °C with 30 cycles and a final elongation at 72 °C for 10 min with 1 cycle (Doungudomdacha et al., 2000). DNAs of the samples identified as positive for evaluation of the presence of total bacteria as a result of amplification by universal primers. Then, all positive samples practiced in multiplex PCR, including 5 µl of DNA sample and 45 µl of PCR master mix. Afterwards, amplification was carried out under the following conditions as 95 °C for 5 min for initial denaturation, 94 °C for 30 s, 62 °C for 30 s, 72 °C with 30 cycles for 30 s, 72 °C for 5 min at a final elongation 1 cycle. The PCR products were soon after electrophoresed at 80 V/cm power for 40 min with a 2% agarose gel which containing ethidium bromide. At the end of the electrophoresis, the gel screened via Vilber Lourmat UV transilluminator system and band size was searched at the base ranges of target size (Table 1) (Hamada et al., 2008, Kato et al., 2011).
3. Results
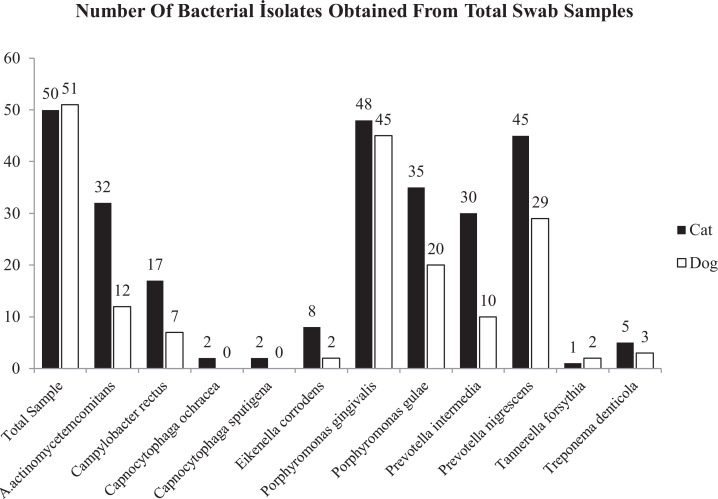
Analysis of all dental plaque swab samples collected from cats and dogs by using primers directed to 16S rRNA and tdpA genes with PCR yielded a great number of positive results in this study. The isolates obtained from cats (48/50, 45/50) and dogs (45/51, 29/51) swap samples were identified as Porphyromonas gingivalis and Prevotella nigrescens, respectively. In the present study, Capnocytophaga ochracea and Capnocytophaga sputigena were also detected from only 4 cats. Besides, Porphyromonas gingivalis was detected in almost all cats and dogs. Forty-eight of 50 cats (96%) and forty-five of 51 dogs (88, 23%) were shown possess to that species (Fig 1).
Fig. 1.
Distribution of isolated periodontal bacteria in cats and dogs.
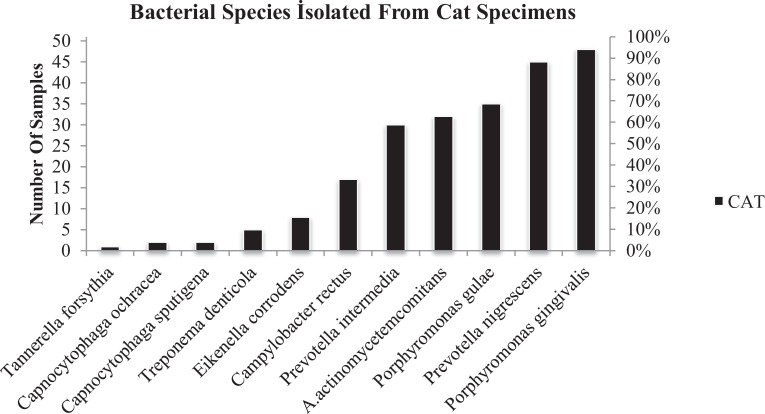
In contrast, the detection rates of 3 species (T.forsythia, C. ochracea, and C. sputigena) in cats and of 5 species (T. forsythia, C. ochracea, C. sputigena T. denticola and, E. corrodens) in dogs showed that the prevalence was lower than 10%. E. corrodens in cats and, P. intermedia, A. actinomycetemcomitans, and C. rectus in dogs were also isolated from the swab samples with less than 30% percentage. Remarkably, C. sputigena and C. ochracea species that were not detected in dogs swab specimens (0%), although it was detected in 2% of cats even if with low percentages (Fig. 2).
Fig. 2.
Bacterial isolates identified from cats by PCR.
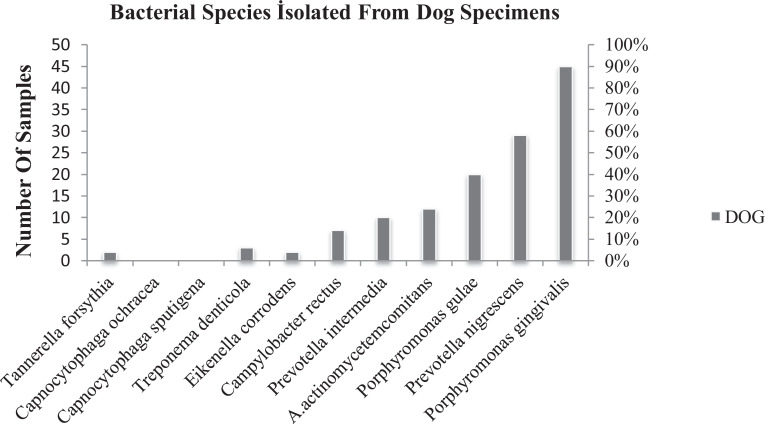
P. gulae, P. gingivalis, and P. nigrescens were also the most frequently detected species in dogs. The detection percentages of these bacteria were 39, 2%, 88, 2% and 56, 8%, respectively (Fig 3).
Fig. 3.
Bacterial isolates identified from dogs by PCR.
4. Discussion
Periodontal disease is one of the most common infectious disorders in cats and dogs (Niemiec, 2012). Gram-negative bacteria such as A. actinomycetemcomitans, T. forsythia, Campylobacter spp., Capnocytophoga spp., E. corrodens, P. gingivalis, P. intermedia, and T. denticola can contribute to forming of subgingival plaque and particularly have importance in bite wounds (He and Shi, 2009). Some studies have shown that the cat's oral cavity is shifted towards anaerobic gram-negative rods with higher gingival index scores (Adler et al., 2016). The most prevalent species were detected as A. actinomycetemcomitans (64%), P. gulae (70%), P. gingivalis (96%), P. intermedia (60%), P. nigrescens (90%) in cats and P. gulae (39%), P. gingivalis (88%) and Prevotella nigrescens (57%) in dogs in our study, respectively. It has been reported that the combinations of A. actinomycetemcomitans and P. gingivalis contributed to the formation of deepened pockets in periodontal disease (Samaranayake, 2012). We observed the prevalence of Prevotella intermedia (60%; 20%), A. actinomycetemcomitans (64%; 24%), Porphyromonas gulae (70%; 39%), Prevotella nigrescens (90%; 57%) in cats much higher than dogs, respectively. In addition to these results, P. nigrescens (57%) interestingly found highly prevalent.
The acquired data from this study confirmed that C. ochracea and C. sputigena species were not encountered in dogs but found in cats with a low rate (4%) which this bacterium can be associated with periodontal disease in cats. Although T. denticola and E. corrodens were identified with 6% and 4% from all dogs plaque samples, it could be regarded as determining. The prevalence of P. gulae, P. nigrescens, and P. gingivalis were detected highly in cats plaque samples (70%, 90%, 96%) and dogs plaque samples (39%, 57%, 88%) by using PCR. Findings of P. gingivalis from dental plaque samples were noticeably high in cats and dogs. Therefore, P. gingivalis can be evaluated an opportunistic pathogen which initiates the infection (Fujise et al., 2002, van Winkelhoff et al., 2002).
T. forsythia has also been implicated as significant periodontopathogens and conceivably can be found 90% with varying stages in periodontal disease in Booij-Vrieling et al. (2010), Perez-Salcedo, Herrera and Esteban-Saltiveri (2013), Zarco, Vess and Ginsburg (2012). In a recent study, we were isolated 2% in cats dental plaque samples. These discrepancies can common likely be attributed to the fact that cats are contingently at different stages of periodontal disease.
P. gulae reported as the most common type in dogs (Forsblom et al., 2002, Hale, 2009). However, in our study, we were rarely (39%) identified this bacterium from dogs dental samples. C. rectus (67%) has been described as the most common genera isolated from dental plaque specimens collected from dogs (Yamasaki et al., 2012). However, we were also rarely detected C. rectus (14%) in our samples. P. gingivalis, P. intermedia, and P. nigrescens are known as Black-pigmented anaerobes (BPA) have been associated as common pathogens with the periodontal disease in both genera. Especially, P. gingivalis and P. nigrescens showed a correlation in 90% of the cases.
Some studies showed that Tannerella sp. and Porphyromonas sp. were the most common oral flora bacteria isolated from cats. However, Porphyromonas sp. found to be the dominant species in cats besides the low percentage of Tannerella sp. (2%) (Kasempimolporn, Benjavongkulchai, Saengseesom & Sitprija, 2003). In addition, P. gulae has been evaluated as one of the most dominant pathogen in the oral cavity (Allaker et al., 1994, Kato et al., 2011). The 38–76% of anaerobic bacteria, for instance, Prevotella sp., Porphyromonas sp., has been reported to be present bite wounds of cats and dogs. The isolation of P. gulae, P. nigrescens, and P. gingivalis from dental plaque samples supports our findings (Arakawa et al., 2000, Aydin, 2004, Foschi et al., 2005, Munemasa et al., 2000). Moreover, Senhorinho et al. (2011) reported that 92% of P. gulae has been isolated in their study. However, we were interestingly detected in 39% in our study. We also demonstrate that the results obtained from PCR support the presence of P. gulae, P. nigrescens and P. gingivalis in cats which are significantly associated with periodontal disease.
Although the genetic literature for periodontal disease is more important than caries, micronutrient deficiencies such as vitamin C, vitamin D or vitamin B12 may be associated with the onset and progressive in periodontal disease in cats and dogs. Furthermore, genes involved in enamel formation in humans (AMELX, AMBN, ENAM, TUFT, MMP20, and KLK4), saliva characteristics (AQP5), have the greatest effect on caries (Chapple, Bouchard, Cagetti, Campus & Carra, 2017). Therefore, it would be beneficial to investigate these formation features in cats and dogs presenting with periodontal disease.
5. Conclusions
Our results suggest that the feline oral cavity considerably has more diversity of microbiota than dogs. Bacteria such as P. gulae, P. nigrescens, and P. gingivalis were the major species in dental plaque samples collected from cats and dogs. Similarly, P. gingivalis and P. nigrescens known to be important pathogens for periodontitis in humans and they were highly identified in this study. Thus, the periodontal pathogens detected in cats and dogs should be eliminated by improving oral hygiene.
In addition to oral health control, high protein-based nutrient consumption promotes bacterial composition in oral flora and the oral cavity. Besides, feeding with a well-formulated dry food diet may be a positive effect on oral health and reduces the formation of dental residues and periodontal disease. Bacterial synergism in conjunction with virulence factors of periodontal diseases and the effects of nutrition on the development of the oral microbiome in pet animals should be investigated.
Finally, the results of the study can also provide measures by which veterinary doctors specialized in dentistry can monitor the risk of developing periodontal infections from cats and dogs oral pathogens through both diet control and assessment of plaque and calculus.
Declaration of Competing Interest
There is not any commercial firm played role in the study design nor in the collection, analysis and interpretation of data, nor in the decision to submit the manuscript for publication. None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Acknowledgments
This research was funded by the Scientific Research Council of Yozgat Bozok University (Project No: 6602c ZF/16-40).
References
- Adler C.J., Malik Richard, Gina V. Diet may influence the oral microbiome composition in cat. Microbiome. 2016;4(1):23. doi: 10.1186/s40168-016-0169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Africa C.W., Nel J., Stemmet M. Anaerobes and bacterial vaginosis in pregnancy: Virulence factors contributing to vaginal colonisation. International Journal of Environmental Research and Public Health. 2014;11(7):6979–7000. doi: 10.3390/ijerph110706979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaker R.P., Langlois T., Hardie J.M. Prevalence of eikenella corrodens and actinobacillus actinomycetemcomitans in the dental plaque of dogs. The Veterinary Record. 1994;134(20):519–520. doi: 10.1136/vr.134.20.519. [DOI] [PubMed] [Google Scholar]
- Arakawa S., Nakajima T., Ishikura H., Ichinose S., Ishikawa I., Tsuchida N. Novel apoptosis-inducing activity in bacteroides forsythus: A comparative study with three serotypes of actinobacillus actinomycetemcomitans. Infection and Immunity. 2000;68(8):4611–4615. doi: 10.1128/iai.68.8.4611-4615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila C.M., Perez-Valencia A.Y., Rendon-Osorio W.L. Tannerella forsythia is associated with increased levels of atherogenic low density lipoprotein and total cholesterol in chronic periodontal disease. Journal of Clinical and Experimental Dentistry. 2015;7:e254–e260. doi: 10.4317/jced.52128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashimoto A., Chen C., Bakker I., Slots J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontal disease lesions. Oral Microbiology and Immunology. 1996;11(4):266–273. doi: 10.1111/j.1399-302x.1996.tb00180.x. [DOI] [PubMed] [Google Scholar]
- Aydin M. Vol. 72. Günes Yayin evi; Ankara, Turkey: 2004. Porphyromonas gingivalis; pp. 633–644. (Tip ve Dis Hekimliginde Genel ve Özel Mikrobiyoloji). [Google Scholar]
- Booij-Vrieling H.E., van der Reijden W.A., Houwers D.J., de Wit W.E., Bosch-Tijhof C.J., Penning L.C. Comparison of periodontal pathogens between cats and their owners. Veterinary Microbiology. 2010;144(1–2):147–152. doi: 10.1016/j.vetmic.2009.12.046. [DOI] [PubMed] [Google Scholar]
- Borgo P.V., Rodrigues V.A., Feitosa A.C., Xavier K.C., Avila-Campos M.J. Association between periodontal condition and subgingival microbiota in women during pregnancy: A longitudinal study. Journal of Applied Oral Science: Revista FOB. 2014;22(6):528–533. doi: 10.1590/1678-775720140164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I. Microbiology and management of human and animal bite wound infections. Primary Care. 2003;30(1):25–39. doi: 10.1016/s0095-4543(02)00056-8. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Centers for Disease Control and Prevention (CDC); 2015. Preventing dog bites. [Google Scholar]
- Chapple I.L., Bouchard P., Cagetti M.G., Campus G., Carra M.C., Cocco F. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. Journal of Clinical Periodontology. 2017;44(Suppl 18):S39–S51. doi: 10.1111/jcpe.12685. Mar. [DOI] [PubMed] [Google Scholar]
- Conrads G., Mutters R., Fischer J., Brauner A., Lutticken R., Lampert F. PCR reaction and dot-blot hybridization to monitor the distribution of oral pathogens within plaque samples of periodontally healthy individuals. Journal of Periodontology. 1996;67(10):994–1003. doi: 10.1902/jop.1996.67.10.994. [DOI] [PubMed] [Google Scholar]
- Dahlen G. Microbiology and treatment of dental abscesses and periodontal-endodontic lesions. Periodontology 2000. 2002;28:206–239. doi: 10.1034/j.1600-0757.2002.280109.x. [DOI] [PubMed] [Google Scholar]
- Desai S.S., Harrison R.A., Murphy M.D. Capnocytophaga ochracea causing severe sepsis and purpura fulminans in an immunocompetent patient. The Journal of Infection. 2007;54(2):e107–e109. doi: 10.1016/j.jinf.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Doungudomdacha S., Rawlinson A., Douglas C.W. Enumeration of porphyromonas gingivalis, prevotella intermedia and actinobacillus actinomycetemcomitans in subgingival plaque samples by a quantitative-competitive PCR method. Journal of Medical Microbiology. 2000;49(10):861–874. doi: 10.1099/0022-1317-49-10-861. [DOI] [PubMed] [Google Scholar]
- Ebrahim A., Oskuiezadeh K., Khoshnevisan R. A study on the prevalent bacterial population in the oral cavity of owned healthy dogs and cats. Intas Polivet. 2010;11:271–273. [Google Scholar]
- Forsblom B., Sarkiala-Kessel E., Kanervo A., Vaisanen M.L., Helander M., Jousimies-Somer H. Characterisation of aerobic gram-negative bacteria from subgingival sites of dogs potential bite wound pathogens. Journal of Medical Microbiology. 2002;51(3):207–220. doi: 10.1099/0022-1317-51-3-207. [DOI] [PubMed] [Google Scholar]
- Foschi F., Cavrini F., Montebugnoli L., Stashenko P., Sambri V., Prati C. Detection of bacteria in endodontic samples by polymerase chain reaction assays and association with defined clinical signs in Italian patients. Oral Microbiology and Immunology. 2005;20(5):289–295. doi: 10.1111/j.1399-302X.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- Fujise O., Hamachi T., Inoue K., Miura M., Maeda K. Microbiological markers for prediction and assessment of treatment outcome following non-surgical periodontal therapy. Journal of Periodontology. 2002;73(11):1253–1259. doi: 10.1902/jop.2002.73.11.1253. [DOI] [PubMed] [Google Scholar]
- Gaetti-Jardim E.C., Marqueti A.C., Faverani L.P., Gaetti-Jardim E., Júnior Antimicrobial resistance of aerobes and facultative anaerobes isolated from the oral cavity. Journal of Applied Oral Science. 2010;18(6):551–559. doi: 10.1590/S1678-77572010000600004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawor J.P., Reiter A.M., Jodkowska K., Kurski G., Wojtacki M.P., Kurek A. Influence of diet on oral health in cats and dogs. The Journal of Nutrition. 2006;136(7):2021S–2023S. doi: 10.1093/jn/136.7.2021S. [DOI] [PubMed] [Google Scholar]
- Gilpin D.F., Nixon K.A., Bull M., McGrath S.J., Sherrard L., Rolain J.M. Evidence of persistence of prevotella spp. in the cystic fibrosis lung. Journal of Medical Microbiology. 2017;66(6):825–832. doi: 10.1099/jmm.0.000500. [DOI] [PubMed] [Google Scholar]
- Griego R.D., Rosen T., Orengo I.F., Wolf J.E. Dog, cat, and human bites: A review. Journal of American Academy of Dermatology. 1995;33(6):1019–1029. doi: 10.1016/0190-9622(95)90296-1. [DOI] [PubMed] [Google Scholar]
- Hale F.A. Dental caries in the dog. Canadian Veterinary Journal. 2009;50(2):1301–1304. [PMC free article] [PubMed] [Google Scholar]
- He X.S., Shi W.Y. Oral microbiology: past, present and future. International Journal of Oral Science. 2009;1(2):47–58. doi: 10.4248/ijos.09029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada N., Takahashi Y., Watanabe K., Kumada H., Oishi Y., Umemoto T. Molecular and antigenic similarities of the fimbrial major components between porphyromonas gulae and P. gingivalis. Veterinary Microbiology. 2008;128(1–2):108–117. doi: 10.1016/j.vetmic.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Harvey C.E., Thornsberry C., Miller B.R. Subgingival bacteria comparison of culture results in dogs and cats with gingivitis. Journal of Veterinary Dentistry. 1995;12:147–150. [PubMed] [Google Scholar]
- Henderson B., Wilson M., Sharp L., Ward J.M. Actinobacillus actinomycetemcomitans. Journal of Medical Microbiology. 2002;51:1013–1020. doi: 10.1099/0022-1317-51-12-1013. [DOI] [PubMed] [Google Scholar]
- Kasempimolporn S., Benjavongkulchai M., Saengseesom W., Sitprija V. Oral bacterial flora of dogs with and without rabies: A preliminary study in thailand. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2003;86:1162–1166. [PubMed] [Google Scholar]
- Kato Y., Shirai M., Murakami M., Mizusawa T., Hagimoto A., Wada K. Molecular detection of human periodontal pathogens in oral swab specimens from dogs in japan. Journal of Veterinary Dentistry. 2011;28:84–89. doi: 10.1177/089875641102800204. [DOI] [PubMed] [Google Scholar]
- Khazandi M., Bird P.S., Owens J., Wilson G., Meyer J.N., Trott D.J. In vitro efficacy of cefovecin against anaerobic bacteria isolated from subgingival plaque of dogs and cats with periodontal disease. Anaerobe. 2014;28:104–108. doi: 10.1016/j.anaerobe.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Kim J., Amar S. Periodontal disease and systemic conditions: A bidirectional relationship. Odontology / The Society of the Nippon Dental University. 2006;94:10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig M.F., Abusleme L., Reinholdt J., Palmer R.J., Teles R.P., Sampson K. Aggregatibacter actinomycetemcomitans induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Science Translational Medicine. 2016;8 doi: 10.1126/scitranslmed.aaj1921. 369RA176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboniwa M., Amano A., Kimura K.R., Sekine S., Kato S., Yamamoto Y. Quantitative detection of periodontal pathogens using real-time polymerase chain reaction with Taqman probes. Oral Microbiology and Immunology. 2004;19:168–176. doi: 10.1111/j.0902-0055.2004.00135.x. [DOI] [PubMed] [Google Scholar]
- Mahlen S.D., Clarridge J.E., 3rd Oral abscess caused by campylobacter rectus: Case report and literature review. Journal of Clinical Microbiology. 2009;47:848–851. doi: 10.1128/JCM.01590-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa T., Takemoto T., Dahlen G., Hino T., Shiba H., Ogawa T. Adherence of bacteroides forsythus to host cells. Microbios. 2000;101:115–126. [PubMed] [Google Scholar]
- Niemiec, B. (2012). Etiology and pathogenesis of periodontal disease, 18–37 pp.
- Paul K., Patel S.S. Eikenella corrodens infections in children and adolescents: Case reports and review of the literature. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2001;33:54–61. doi: 10.1086/320883. [DOI] [PubMed] [Google Scholar]
- Perez-Salcedo L., Herrera D., Esteban-Saltiveri D., León R., Jeusette I., Torre C. Isolation and identification of porphyromonas spp. and other putative pathogens from cats with periodontal disease. Journal of Veterinary Dentistry. 2013;30:208–213. doi: 10.1177/089875641303000402. [DOI] [PubMed] [Google Scholar]
- Piau C., Arvieux C., Bonnaure-Mallet M., Jolivet-Gougeon A. Capnocytophaga spp. involvement in bone infections: A review. International Journal of Antimicrobial Agents. 2013;41:509–515. doi: 10.1016/j.ijantimicag.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Rothe K., Tsokos M., Handrick W. Animal and human bite wounds. Deutsches Arzteblatt International. 2015;112:433–443. doi: 10.3238/arztebl.2015.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayake L. Normal oral flora, the oral ecosystem and plaque biofilms. Essential Microbiology for Dentistry. 2012;392:291–294. [Google Scholar]
- Talan D.A., Citron D.M., Abrahamian F.M., Moran G.J., Goldstein E.J. Bacteriologic analysis of infected dog and cat bites. Emergency medicine animal bite infection study group. The New England Journal of Medicine. 1999;340:85–92. doi: 10.1056/NEJM199901143400202. [DOI] [PubMed] [Google Scholar]
- Tamura K., Nakano K., Hayashibara T., Nomura R., Fujita K., Shintani S. Distribution of 10 periodontal bacteria in saliva samples from Japanese children and their mothers. Archives of Oral Biology. 2006;51:371–377. doi: 10.1016/j.archoralbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- van Winkelhoff A.J., Loos B.G., van der Reijden W.A., van der Velden U. Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destruction. Journal of Clinical Periodontology. 2002;29(11):1023–1028. doi: 10.1034/j.1600-051x.2002.291107.x. [DOI] [PubMed] [Google Scholar]
- Venkataraman A., Almas K. Rheumatoid arthritis and periodontal disease. an update. The New York State Dental Journal. 2015;81:30–36. [PubMed] [Google Scholar]
- Watanabe K., Frommel T.O. Porphyromonas gingivalis, actinobacillus actinomycetemcomitans and treponema denticola detection in oral plaque samples using the polymerase chain reaction. Journal of Clinical Periodontology. 1996;23:212–219. doi: 10.1111/j.1600-051x.1996.tb02078.x. [DOI] [PubMed] [Google Scholar]
- Wegner N., Wait R., Sroka A., Eick S., Nguyen K.A., Lundberg K. Peptidylarginine deiminase from porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: Implications for autoimmunity in rheumatoid arthritis. Arthritis and Rheumatism. 2010;62:2662–2672. doi: 10.1002/art.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakob M., Soder B., Meurman J.H., Jogestrand T., Nowak J., Soder P.O. Prevotella nigrescens and porphyromonas gingivalis are associated with signs of carotid atherosclerosis in subjects with and without periodontal disease. Journal of Periodontal Research. 2011;46:749–755. doi: 10.1111/j.1600-0765.2011.01398.x. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y., Nomura R., Nakano K., Naka S., Matsumoto-Nakano M., Asai F. Distribution of periodontopathic bacterial species in dogs and their owners. Archives of Oral Biology. 2012;57:1183–1188. doi: 10.1016/j.archoralbio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Zarco M.F., Vess T.J., Ginsburg G.S. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral Diseases. 2012;18:109–120. doi: 10.1111/j.1601-0825.2011.01851.x. [DOI] [PubMed] [Google Scholar]