Abstract
Background
Previous clinical trials and institutional studies have demonstrated that surgery for the treatment of obesity (termed bariatric or metabolic surgery) reduces all-cause mortality and the development of obesity-related diseases such as type 2 diabetes mellitus (T2DM), hypertension, and dyslipidaemia. The current study analysed large-scale population studies to assess the association of bariatric surgery with long-term mortality and incidence of new-onset obesity-related disease at a national level.
Methods and findings
A systematic literature search of Medline (via PubMed), Embase, and Web of Science was performed. Articles were included if they were national or regional administrative database cohort studies reporting comparative risk of long-term mortality or incident obesity-related diseases for patients who have undergone any form of bariatric surgery compared with an appropriate control group with a minimum follow-up period of 18 months. Meta-analysis of hazard ratios (HRs) was performed for mortality risk, and pooled odds ratios (PORs) were calculated for discrete variables relating to incident disease. Eighteen studies were identified as suitable for inclusion. There were 1,539,904 patients included in the analysis, with 269,818 receiving bariatric surgery and 1,270,086 control patients. Bariatric surgery was associated with a reduced rate of all-cause mortality (POR 0.62, 95% CI 0.55 to 0.69, p < 0.001) and cardiovascular mortality (POR 0.50, 95% CI 0.35 to 0.71, p < 0.001). Bariatric surgery was strongly associated with reduced incidence of T2DM (POR 0.39, 95% CI 0.18 to 0.83, p = 0.010), hypertension (POR 0.36, 95% CI 0.32 to 0.40, p < 0.001), dyslipidaemia (POR 0.33, 95% CI 0.14 to 0.80, p = 0.010), and ischemic heart disease (POR 0.46, 95% CI 0.29 to 0.73, p = 0.001). Limitations of the study include that it was not possible to account for unmeasured variables, which may not have been equally distributed between patient groups given the non-randomised design of the studies included. There was also heterogeneity between studies in the nature of the control group utilised, and potential adverse outcomes related to bariatric surgery were not specifically examined due to a lack of available data.
Conclusions
This pooled analysis suggests that bariatric surgery is associated with reduced long-term all-cause mortality and incidence of obesity-related disease in patients with obesity for the whole operated population. The results suggest that broader access to bariatric surgery for people with obesity may reduce the long-term sequelae of this disease and provide population-level benefits.
Author summary
Why was this study done?
Surgical treatment for obesity (known as bariatric or metabolic surgery) has been suggested to reduce risk of death in people with obesity and decrease development of other medical conditions including diabetes, heart disease, and certain forms of cancer.
This study aimed to analyse pooled data from previously published studies utilising data from national or regional administrative databases in order to examine the effect of bariatric surgery at a population level.
What did the researchers do and find?
This study pooled data from published research articles utilising large administrative databases (with information from over 1.5 million patients)
The results suggested that bariatric surgery was associated with reduced risk of death, and decreased incidence of new-onset diabetes, high blood pressure, high cholesterol, and heart disease.
What do these findings mean?
These findings suggest that bariatric surgery is associated with reduced risk of premature death or developing other medical conditions in people with obesity at a population level.
Increased availability of bariatric surgery may help to improve health outcomes for these individuals.
Introduction
Obesity is a worldwide pandemic, with 39% of the worldwide adult population being considered overweight [1]. Mean worldwide body mass index (BMI) has been steadily increasing since 1975, and current trends predict that 20% of the global population will be classified as having obesity (BMI ≥ 30 kg/m2) by 2030 [1,2]. In the UK, 27% of the population is already classified as having obesity [3]. Billions of dollars are spent annually worldwide to curb this health crisis, alongside the development of government public health initiatives [2,4].
Secondary to smoking, obesity has been identified as the leading cause of preventable premature death in the US [5]. Obesity predisposes individuals to a plethora of adverse conditions and is an established risk factor for overall all-cause mortality, cardiovascular disease, and type 2 diabetes mellitus (T2DM) [6–9]. Although multiple therapeutic options for the treatment of patients with obesity are available, bariatric surgery has been established as the most effective method of achieving sustained weight loss in these patients [10,11]. Bariatric surgery has also been associated with reduced overall mortality rates in patients with obesity [12] and leads to remission of obesity-related disease [10,11,13]. Bariatric surgery has also been associated with an overall reduction in the risk of developing multiple cancer types [14–16].
Current national guidance in the UK recommends that bariatric surgery be considered for patients who have a BMI of 40 kg/m2 or more, or a BMI between 35 kg/m2 and 40 kg/m2 if other significant obesity-related comorbidities are present [17]. Patients with new-onset T2DM may also be considered for surgery at lower BMI (≥30 kg/m2). Despite the presence of these criteria and evidence that patients are healthier and more functional following bariatric surgery, far less than 1% of eligible patients receive bariatric surgery [18,19]. Data from the UK National Bariatric Surgery Registry establish that at the time of primary surgery 53.9% of men and 41.4% of women already have a high level of co-existing disease (defined as 4 or more obesity-related diseases) [20]. Previous studies have also demonstrated that improving pathways to bariatric surgery can reduce the healthcare burden upon individuals and lead to overall healthcare cost savings [21].
Results from previous pooled analyses have demonstrated that bariatric surgery reduces long-term all-cause mortality [22,23]. However, these studies also included data from clinical trials and single-institution studies, which may lack external validity compared to data collected independently in population studies undertaken using administrative datasets. The present study aimed to investigate, in people with severe and complex obesity, the influence of bariatric surgery on overall long-term mortality and prevention of incident obesity-related disease at a population level.
Methods
A systematic literature search of Medline (via PubMed), Embase, and Web of Science was performed using the search criteria as follows: (“Bariatric Surgery”[MeSH] OR bariatric surgery) AND (mortality OR survival OR diabetes OR metabolic OR hypertension OR sleep apnoea OR cardiac OR angina OR heart disease OR myocardial infarction OR dyslipidaemia OR dyslipidemia OR thromboembolism) AND (national OR registry OR population) (S1 Text). Two authors (TW and NG) performed the electronic literature search independently, which was last updated in January 2020. The literature search dates included any studies published between 1 January 2000 and 31 January 2020. Studies published prior to 2000 were excluded to ensure all data were contemporaneous and applicable to present-day bariatric surgical practice. The electronic search was supplemented by a hand-search of published abstracts from relevant specialist conference meetings. Reference lists of all relevant studies were also reviewed to identify potentially relevant studies. The protocol for this study was not prospectively registered in any repository for systematic reviews.
Identified abstracts were independently scrutinised by 2 reviewers (TW and NG) to determine eligibility for inclusion. Any discrepancies regarding study inclusion from the literature search were settled by discussion with a third author (SRM). Studies were included if they were either national or regional administrative database cohort studies reporting comparative risk of long-term mortality or incident obesity-related diseases for patients who have undergone any form of bariatric surgery compared to an appropriate control group (i.e., with a clinical diagnosis of obesity) with a minimum follow-up period of 18 months. For the purposes of this study, obesity-related diseases were defined as T2DM, hypertension, obstructive sleep apnoea, cardiac disease (ischemic heart disease or cardiac failure), dyslipidaemia, and venous thromboembolism. Studies that only provided details on remission of obesity-related diseases existing prior to bariatric surgery (as opposed to new onset) were not included. Any study that was not a population-based study utilising data from an administrative database (including randomised controlled trials and single-institution studies) was excluded. This selection criterion was imposed to ensure that results from all included studies could be directly applied at a population level. This led to randomised controlled studies, and cohort studies that did not utilise a population-registry-based data design, being excluded as results from these studies may be representative of only the highly specialised units participating in such studies and would lack external validity when applied to the general population of patients receiving bariatric surgery. In the situation where 2 studies utilised the same registry data and reported on the same outcome measure, and therefore potentially contained duplicate data, the most recent study was selected for inclusion. Only English-language studies were included.
Data from eligible studies were extracted into a computerised spreadsheet for analysis. Data were collected for overall all-cause long-term mortality and new-onset obesity-related comorbidities. Authors of individual studies were not specifically contacted to obtain more detailed results than those available in the main publication and published appendices. Mortality was evaluated according to reported HRs to minimise the effect of patient dropouts, whereas obesity-related diseases were primarily analysed directly according to event rates in each group. Event rate analysis was used for the primary analysis of obesity-related disease, as a greater proportion of included studies reported these data, but outcomes were also analysed according to adjusted odds ratios (ORs) where these data were available, for confirmatory purposes.
Statistical analysis was performed using StatsDirect 3.2.9. Pooled outcome measures were determined using random effects models as described by DerSimonian and Laird [24]. Heterogeneity amongst the trials was assessed by Cochran Q statistic, a null hypothesis test in which p < 0.05 is taken to indicate the presence of heterogeneity, and the I2 statistic, which describes the percentage of variation across studies due to heterogeneity. The Egger test was used to assess the funnel plot for asymmetry, indicating possible publication or other biases.
Results
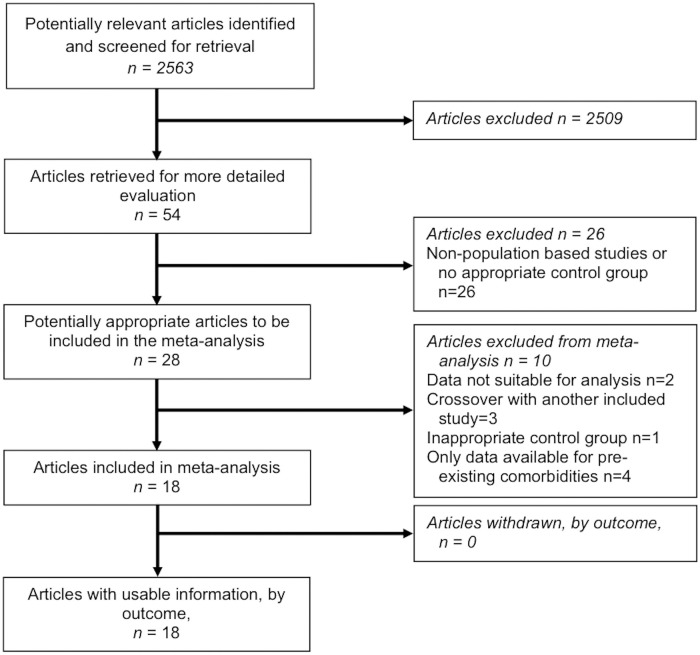
The literature search identified 18 studies suitable for inclusion [25–42]. Fig 1 provides details of the PRISMA flowchart for the literature search. In total there were 1,539,904 patients included in the analysis, with 269,818 patients receiving bariatric surgery and 1,270,086 control patients. The types of surgery were gastric bypass (n = 137,578, 51%), sleeve gastrectomy (n = 58,916, 22%), adjustable gastric band (n = 52,973, 20%), vertical banded gastroplasty (n = 6,397, 2%), biliopancreatic diversion (with or without duodenal switch) (n = 1,002, 0.4%), and an alternative procedure or unspecified operation (n = 12,952, 5%) (S1 Table). Median follow-up across all studies was 59 months (range 18 to 144 months).
Fig 1. PRISMA flow chart with details of literature search.
Patient demographic details for all studies are provided in Table 1. Quality assessment of studies was undertaken with the Newcastle–Ottawa Scale (S2 Table) [43]. The majority of studies used patients with a diagnosis of obesity as the control group, although a group of patients without a diagnosis of obesity was utilised as the control group in 1 study [26]. All studies except this one scored 4 stars for patient selection (rated out of 4) [25,27–42]. Studies that did not report BMI for control and surgery groups scored only 1 star for comparability [26,28,29,39–42]. All studies scored the maximum 3 stars for exposure.
Table 1. Patient demographics for the studies included in the pooled analysis.
| Study | Nation or region of origin | Included study dates | Median follow-up (months) | Number of patients | Mean ± SD or median (range) age (years) | Female sex, n (%) | Mean BMI ± SD (kg/m2) or n (%) with BMI ≥ 40 kg/m2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery | Control | Surgery | Control | Surgery | Control | Surgery | Control | ||||
| Arterburn [25] | US | 2000–2011 | 60 | 2,500 | 7,462 | 52 ± 8.8 | 53 ± 8.7 | 651 (26%) | 1,920 (26%) | 47 ± 7.9 | 46 ± 7.3 |
| Backman [26] | Sweden | 2007–2012 | 55 | 18,418 | 175,138 | 39 ± 10.5 | 39 ± 10.5 | 14,518 (79%) | 138,082 (79%) | 42.2 ± 5.8 | — |
| Bailly [35] | France | 2008–2016 | 44 | 102,627 | 225,882 | 37.3 ± 10.5 | 44.0 ± 11.7 | 88,464 (86%) | 151,566 (67%) | 47,516 (46.3%) | 37,948 (16.8%) |
| Ceriani [36] | Lombardy region, Italy | 1999–2008 | 144 | 472 | 1,405 | 43.1 ± 10.6 | 43.5 ± 12.5 | 354 (75%) | 995 (71%) | 47.3 ± 7.5 | 46.8 ± 3.8 |
| Douglas [37] | UK | Up to 2014 | 36 | 3,882 | 3,882 | 45 ± 11 | 45 ± 11 | 3,126 (81%) | 3,166 (82%) | 44.7 ± 8.8 | 42.1 ± 6.5 |
| Eliasson [38] | Sweden | 2007–2014 | 60 | 6,132 | 6,132 | 48.5 ± 9.8 | 50.5 ± 12.7 | 3,678 (61%) | 3,768 (61%) | 42.0 ± 5.7 | 41.4 ± 5.7 |
| Flum [39] | Washington State, US | 1987–2001 | 120 | 3,328 | 62,781 | 43.1 ± 10.1 | 47.0 ± 6.2 | 2,679 (81%) | 40,368 (64%) | — | — |
| Johnson [40] | South Carolina, US | 1996–2009 | 60 | 2,580 | 13,371 | 47.5 ± 10.6 | 52.1 ± 12.8 | 1,987 (77%) | 9,012 (67%) | — | — |
| Kauppila [41] | Nordic countries | 1980–2012 | 48 | 49,977 | 494,842 | — | — | 37,247 (75%) | 334,407 (68%) | — | — |
| Moussa [42] | UK | Up to 2017 | 129 | 3,842 | 177,973 | — | — | — | — | — | — |
| Moussa [27] | UK | Up to 2017 | 60 | 4,073 | 4,073 | 50 (42–58) | 50 (43–58) | — | — | 40.2 (37.0–45.2) | 40.4 (36.7–45.6) |
| Perry [28] | US | 2001–2014 | 18 | 11,903 | 11,901 | — | — | 9,237 (78%) | 9,236 (78%) | — | — |
| Persson [29] | Sweden | 1987 onwards | 44 | 22,295 | 25,564 | 40.7 ± 10.7 | 44.3 ± 13.2 | 16,921 (76%) | 17,077 (67%) | — | — |
| Pontiroli [30] | Lombardy region, Italy | 1995–2001 | 59 | 385 | 681 | 39.2 ± 10.4* | 40.2 ± 12.0* | 292 (76%) | 509 (75%) | 41.1 ± 5.4* | 40.9 ± 7.3* |
| Singh [31] | UK | 1990–2018 | 43 | 5,170 | 9,995 | — | — | 4,158 (80%) | 8,105 (81%) | 3,634 (70.3%) | 6,780 (67.8%) |
| Reges [32] | Israel | 2005–2014 | 48 | 8,385 | 25,155 | 46 (37–54) | 46 (37–54) | 5,490 (66%) | 16,470 (66%) | 4,980 (59%) | 14,940 (59%) |
| Thereaux [33] | France | 2008–2015 | 72 | 15,650 | 15,650 | 38.9 ± 11.2 | 39.4 ± 11.2 | 13,241 (85%) | 13,241 (85%) | 9,449 (60%) | 9,449 (60%) |
| Thereaux [34] | France | 2009 | 72 | 8,199 | 8,199 | 39.9 ± 11.5 | 40.5 ± 11.6 | 6,728 (82%) | 6,728 (82%) | 6,092 (74%) | 6,092 (74%) |
*Study subgroup with no type 2 diabetes at baseline.
All-cause mortality
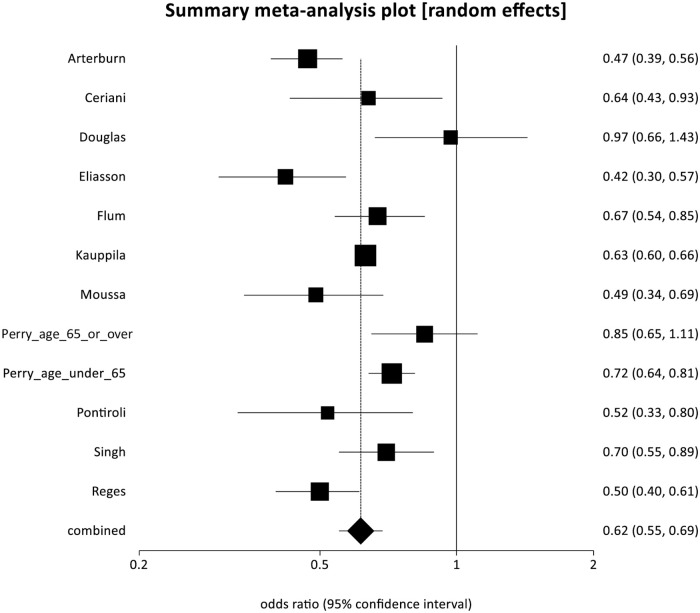
Eleven studies reported a significant reduction in relative risk of long-term all-cause mortality for patients following bariatric surgery compared to controls [25,28,30–32,36,37,38,39,41,42], with a pooled OR (POR) of 0.62 (95% CI 0.55 to 0.69, p < 0.001). There was some evidence of statistical heterogeneity (Cochran Q = 39.03, p < 0.001; I2 = 71.8%) but no evidence of bias (Egger intercept = −0.31, p = 0.714) (Fig 2).
Fig 2. Forest plot of all-cause mortality (pooled odds ratio 0.62, 95% CI 0.55 to 0.69, p < 0.001).
Cardiovascular mortality
Three studies reported significantly reduced relative risk of cardiovascular mortality for patients following bariatric surgery compared to controls [36,38,41] (POR 0.50, 95% CI 0.35 to 0.71, p < 0.001). There was no evidence of statistical heterogeneity (Cochran Q = 2.82, p = 0.24; I2 = 29.2%), and too few strata to assess for bias.
Data for overall and cardiovascular mortality are presented in Table 2.
Table 2. Outcomes for overall mortality rates relative to controls.
| Study | Overall mortality risk | Cardiovascular mortality risk | ||
|---|---|---|---|---|
| Hazard ratio or POR | 95% CI | Hazard ratio or POR | 95% CI | |
| Arterburn [25] | 0.47 | 0.39–0.56 | — | — |
| Ceriani [36] | 0.64 | 0.29–0.97 | 0.26 | 0.09–0.72 |
| Douglas [37] | 0.97 | 0.66–1.43 | — | — |
| Eliasson [38] | 0.42 | 0.30–0.57 | 0.41 | 0.19–0.90 |
| Flum [39] | 0.67 | 0.54–0.85 | — | — |
| Kauppila [41] | 0.63 | 0.60–0.66 | 0.57 | 0.52–0.63 |
| Moussa [42] | 0.49 | 0.34–0.69 | — | — |
| Perry (age 65 years or over) [28] | 0.85 | 0.65–1.11 | — | — |
| Perry (age under 65 years) [28] | 0.72 | 0.64–0.81 | — | — |
| Pontiroli [30] | 0.52 | 0.33–0.80 | — | — |
| Singh [31] | 0.70 | 0.55–0.89 | — | — |
| Reges [32] | 0.50 | 0.40–0.61 | — | — |
| POR | 0.62 | 0.55–0.69 | 0.50 | 0.35–0.71 |
POR, pooled odds ratio.
Details of incident comorbidities
Details of incident comorbidities in each study are provided in Table 3.
Table 3. Outcomes for incident obesity-related diseases.
| Study | T2DM | Hypertension | Obstructive sleep apnoea | Dyslipidaemia | Ischemic heart disease | Cardiac failure | VTE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery | Control | Surgery | Control | Surgery | Control | Surgery | Control | Surgery | Control | Surgery | Control | Surgery | Control | |
| Arterburn [25] | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Backman [26] | 189 (1.0%) | 2,319 (1.3%) | — | — | — | — | — | — | — | — | — | — | — | — |
| Bailly [35] | 2,091 (2.0%) | 29,855 (13.2%) | — | — | — | — | — | — | — | — | — | — | — | — |
| Ceriani [36] | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Douglas [37] | 158 (6.6%) | 237 (9.3%) | 79 (3.2%) | 219 (8.8%) | 36 (1.1%) | 71 (2.0%) | — | — | 40 (1.2%) | 68 (1.9%) | — | — | — | — |
| Eliasson [38] | — | — | — | — | — | — | — | — | 24 (0.4%) | 67 (1.1%) | — | — | — | — |
| Flum [39] | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Johnson [40] | — | — | — | — | — | — | — | — | — | — | ||||
| Kauppila [41] | — | — | — | — | — | — | — | — | 8 (0.3%) | 241 (1.8%) | — | — | — | — |
| Moussa [42] | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Moussa [27] | — | — | — | — | — | — | — | — | — | — | — | — | 71 (1.7%) | 179 (4.4%) |
| Perry [28] | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Persson [29] | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Pontiroli [30] | 15 (9.7%) | 75 (20.8%) | 47 (30.5%) | 174 (48.3%) | — | — | — | — | — | — | 89 (0.4%) | 944 (3.7%) | — | — |
| Singh [31] | — | — | 118 (3.3%) | 567 (8.0%) | — | — | — | — | 14 (9.1%) | 52 (14.4%) | — | — | — | — |
| Reges [32] | 265 (3.2%) | 2,038 (8.1%) | 265 (3.1%) | 2038 (8.1%) | — | — | 570 (6.8%) | 3,100 (12.3%) | 49 (1.0%) | 123 (1.3%) | 19 (0.4%) | 71 (0.7%) | — | — |
| Thereaux [33] | 68 (0.4%) | 213 (1.4%) | — | — | — | — | — | — | — | — | — | — | — | — |
| Thereaux [34] | — | — | 305 (5.6%) | 760 (15.8%) | — | — | 144 (2.1%) | 565 (9.1%) | — | — | — | — | — | — |
Data are given as n (%).
T2DM, type 2 diabetes mellitus; VTE, venous thromboembolism.
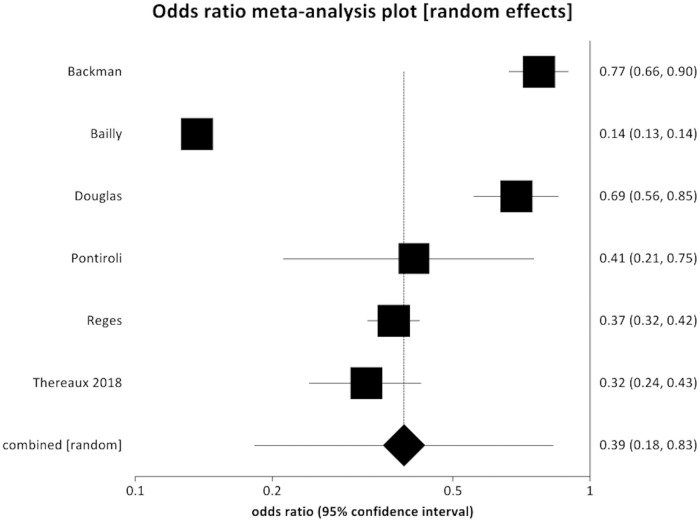
New-onset type 2 diabetes
Six studies reported a reduction in incident T2DM after bariatric surgery compared to controls [26,30,32,33,35,37] (POR 0.39, 95% CI 0.18 to 0.83, p = 0.010) (Fig 3). There was significant evidence of statistical heterogeneity (Cochran Q = 824.8, p < 0.001; I2 = 99.4%) but no evidence of bias (Egger intercept = 8.18, p = 0.069).
Fig 3. Forest plot of incident diabetes (pooled odds ratio 0.39, 95% CI 0.183 to 0.831, p = 0.010).
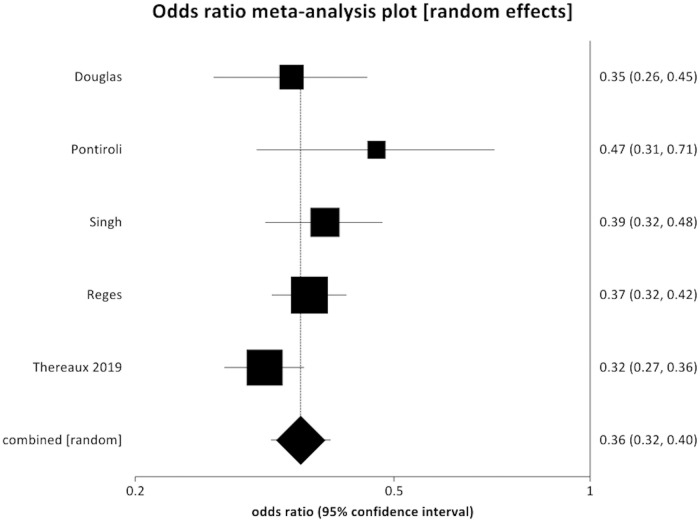
New-onset hypertension
Five studies reported that incident hypertension was reduced after bariatric surgery compared to controls [30–32,34,37] (POR 0.36, 95% CI 0.32 to 0.40, p < 0.001) (Fig 4). There was no evidence of statistical heterogeneity (Cochran Q = 5.90, p = 0.21; I2 = 32.2%) and no evidence of bias (Egger intercept = −0.32, p = 0.92).
Fig 4. Forest plot of new-onset hypertension (pooled odds ratio 0.36, 95% CI 0.32 to 0.40, p < 0.001).
New-onset obstructive sleep apnoea
Only 1 study reported incident obstructive sleep apnoea relative to controls, and therefore it was not possible to do a pooled analysis [37]. This individual study reported a reduced rate of new-onset obstructive sleep apnoea in patients undergoing bariatric surgery (new-onset obstructive sleep apnoea rate of 1.1%) compared to controls (2.0%) (HR 0.55, 95% CI 0.37–0.82, p = 0.004) [37].
New-onset dyslipidaemia
Two studies reported significantly reduced incident dyslipidaemia following bariatric surgery compared to controls [32,34] (POR 0.33, 95% CI 0.14 to 0.80, p = 0.010) (S1 Fig). There was evidence of statistical heterogeneity (Cochran Q = 69.5, p < 0.001; I2 = 98.6%), and too few strata to assess for bias.
New-onset ischemic heart disease
Five studies reported significantly reduced incident ischemic heart disease after bariatric surgery compared to controls [30,31,37,38,40] (POR 0.46, 95% CI 0.29 to 0.73, p = 0.001). There was evidence of statistical heterogeneity (Cochran Q = 18.9, p < 0.001; I2 = 78.8%) but no evidence of bias (Egger intercept = −5.62, p = 0.119) (S2 Fig).
New-onset cardiac failure
Two studies reported the rate of incident cardiac failure [29,31] and found no statistically significant protective association with bariatric surgery (POR 0.23, 95% CI 0.05 to 1.10, p = 0.066). There was significant evidence of statistical heterogeneity (Cochran Q = 32.5, p < 0.001; I2 = 96.9%), and too few strata to assess for bias.
Development of venous thromboembolism
Only 1 study reported incident venous thromboembolism in bariatric surgery patients relative to controls, and therefore it was not possible to undertake a pooled analysis [27]. This individual study demonstrated a reduced incidence of new-onset venous thromboembolism in bariatric surgery patients (1.7%) compared to controls (4.4%) (HR 0.60, 95% CI 0.43 to 0.84, p = 0.003) [27].
Comorbidity analysis by adjusted ORs
Adjusted ORs for incident comorbidities were analysed separately in order to confirm results identified during event rate analysis. These results demonstrated the same patterns identified in the event rate data, with reduced incidence of T2DM (POR 0.28, 95% CI 0.11 to 0.73, p = 0.009), hypertension (POR 0.32, 95% CI 0.21 to 0.47, p < 0.001), ischemic heart disease (POR 0.67, 95% CI 0.49 to 0.90, p = 0.009), and cardiac failure (POR 0.43, 95% CI 0.29 to 0.64, p < 0.001) in bariatric surgical patients compared to controls. These data are presented in S3 Table.
Discussion
The results of the present study suggest that bariatric surgery is associated with reduced all-cause long-term mortality compared to appropriate control patients at a population level. This is consistent with the findings of the Swedish Obese Subjects observational study, which demonstrated decreased overall mortality in patients with obesity who received bariatric surgery [12]. This association was also identified in previous meta-analyses that had included data from non-population-based clinical trials [22,23,44]. The current results establish that these findings are generalisable to the population at large and are not just limited to patients receiving bariatric surgery within specialised centres participating in clinical trials or publishing individual series.
The present study also suggests that bariatric surgery is associated with reduced incidence of obesity-related disease including T2DM, hypertension, and dyslipidaemia. The relative risk reductions associated with bariatric surgery were 61%, 64%, and 77% for the development of T2DM, hypertension, and dyslipidaemia, respectively. These data demonstrate the potential protective association of bariatric surgery with prevention of these obesity-related diseases. Bariatric surgery was also specifically associated with a reduction in cardiovascular mortality and development of ischemic heart disease. Bariatric surgery has previously been associated with a reduction in both macrovascular (including myocardial infarction and cerebrovascular events) and microvascular (including retinal complications, diabetic kidney disease, and peripheral neuropathy) complications relating to T2DM [10,40,45]. The reduction in cardiovascular mortality is likely accounted for by the modification of comorbidities that are known cardiovascular risk factors (T2DM, hypertension, and dyslipidaemia), as suggested in the present study.
In addition to prevention of new-onset disease, bariatric surgery effectively improves pre-existing diseases including T2DM [10,11,46,47], dyslipidaemia [46,47], and hypertension [47]. Some of the included studies also identified improvements in remission of these diseases at a population level. Thereaux et al. [33] established that discontinuation of antidiabetic medications for patients in France with pre-existing T2DM was significantly greater in patients receiving bariatric surgery compared to controls (−49.9% versus −9.0%, p < 0.001) [33]. Population studies from Israel and the UK Clinical Practice Research Datalink have identified similar results [32,37]. In a separate study also included in the present analysis, Thereaux et al. [34] identified that bariatric surgery was significantly associated with discontinuation of therapy for pre-existing hypertension and dyslipidaemia relative to having obesity but not having bariatric surgery [34].
Following surgery, most patients remain in the overweight or class 1 obesity BMI category (BMI 25–35 kg/m2). From data currently available, it is unclear if the overall mortality rate for patients following surgery decreases to that of the general population, decreases to that of the new equivalent BMI category, or is persistently elevated due to the period of time spent at an even higher BMI. In data from 5 Nordic countries, the standardised mortality rate (SMR) of patients receiving bariatric surgery was improved compared to non-operated patients with obesity (SMR 0.63, 95% CI 0.60 to 0.66), although mortality for these patients was still significantly increased relative to the general non-obese population (SMR 1.94, 95% CI 1.83 to 2.05) [41]. Increased BMI class has been strongly associated development of disease and increased mortality risk [48–50]; therefore, elevated mortality relative to the general population may relate to ongoing effects of existing disease before surgery. Determining the ongoing mortality rate of patients following bariatric surgery relative to non-operated individuals within their new BMI classification remains an area for future population-based research.
Data from the UK National Bariatric Surgery Registry indicate that over 80% of patients receiving surgery have several obesity-related diseases [20]. Although most therefore have the chance to improve existing disease with bariatric surgery, our data enable us to quantify the relative risk reduction for developing disease in those disease-free at presentation. Knowledge of the relative risk reductions of 61%, 64%, and 77% for the diagnosis of incident T2DM, hypertension, and dyslipidaemia, respectively, for bariatric surgery patients relative to controls may aid the discussions between healthcare professionals and people with obesity as to whether bariatric surgery should be undertaken.
Strengths and limitations
Our study has limitations that may affect interpretation of the results. All the studies included were large-scale, non-randomised comparative studies based upon registry data. It is therefore not possible to account for potentially important confounding variables, which may not have been equally distributed between patient groups given the non-randomised design of the studies included. One potential example is socioeconomic status, which is known to be an important predictor of all-cause mortality [51]. Previous evidence indicates that patients receiving bariatric surgery may have higher education levels and income than controls, and socioeconomic status may have influenced results [52,53]. Another example would be the higher rate of alcohol abuse disorders and schizophrenia within the control group of the study by Arterburn et al. [25] included in the present analysis. However, these large-scale studies, a strength of the paper, were selected to explore the association of bariatric surgery with long-term mortality and incidence of new obesity-related disease at a population level, with this natural variation in patient factors. The majority of studies compared results from patients receiving bariatric surgery to those of an appropriate control group, with only 1 study unable to select control group patients who also had a diagnosis of obesity [26].
An additional potential source of bias is that patients receiving bariatric surgery would have been managed within a weight loss management pathway including non-surgical treatments for obesity and close monitoring of obesity-related diseases. As the control cohorts for the studies included were taken from the general population, these individuals would likely not have been receiving any non-surgical treatment of obesity, and obesity-related diseases would likely have been less closely monitored, potentially influencing the risk of developing these diseases. It is not possible from the current dataset to evaluate separately the influence of bariatric surgery and non-surgical interventions for treatment of obesity within weight loss management programmes on rates of incident disease, and this may have led to an overestimate of the beneficial effects of bariatric surgery.
This potential source of bias can be reduced by comparing outcomes of patients receiving bariatric surgery to patients seeking treatment for obesity within a specialised medical treatment service including individual- or group-based lifestyle intervention programmes. This approach has been undertaken in a previous study by Jakobsen et al. [54] investigating long-term medical complications in a single-institution cohort of patients receiving bariatric surgery or specialised medical treatment for obesity. However, due to the population-based nature of the studies included in our analysis, it was not possible to identify which patients within the non-surgical control group may have received non-surgical treatment of obesity.
Due to heterogeneity in the definition of pre-existing disease, along with differences in the definition of remission, we did not examine disease remission rates. It was also not possible to perform a specific analysis of outcomes by procedure type in the current analysis. Our study also did not examine specific procedure-related complications or measures of adverse outcomes associated with bariatric surgery. Bariatric surgery is known to be extremely safe, with low peri-operative mortality and complication rates [55–57]. Although other long-term potential complications of bariatric surgery exist (including gastroesophageal reflux [58], internal herniation [59], and trace element malnutrition [60]), these issues are relatively uncommon in modern bariatric surgical practice, and management strategies are available to treat these conditions should they occur. Finally, from the perspective of statistical analysis, the Egger test was utilised to measure for the presence of potential bias. This test is considered to be most reliable when greater than 10 studies are included within the analysis. Due to the limited number of studies included in some of the analyses presented here, some of these bias assessments must be interpreted carefully, although we have chosen to report these results as they still provide an indication of the presence of any potential bias.
To our knowledge, this is the first published study of pooled data from population-based studies of incident disease following bariatric surgery. Our results represent real-world data that may be generalisable to routine clinical practice. As further data accumulate, it may become clearer whether bariatric surgery reduces the incidence of new-onset obstructive sleep apnoea or venous thromboembolism. Another significant strength of the current study is that due to the nature of data collection from administrative datasets, follow-up data (such as mortality and rate of incident obesity-related disease) are externally validated outside each of the individual studies as part of the administrative database collection. This allows for completeness of data collection regarding these aspects and removes the inherent difficulties with patient follow-up data within single-institution-based studies. One method utilised to circumvent this issue within institutional studies has been to link study participants directly to data contained within administrative datasets, and this methodology was utilised successfully by Adams et al. for patients in Utah [47]. This study was not included in the present meta-analysis as all surgical patients had been collected from a single centre, although results were consistent with those identified in the current analysis (OR of incident obesity-related diseases in the surgical cohort 0.08 [95% CI 0.03 to 0.24] for T2DM, 0.23 [95% CI 0.11 to 0.49] for hypertension, and 0.12 [95% CI 0.03 to 0.46] for dyslipidaemia [47]). Results from the Swedish Obese Subjects study were also excluded from the present analysis as this study did not use a population-based design methodology, rather individuals within the control group responded to a nationwide advert [61,62]. However, results from this study also support the findings identified here, with bariatric surgery being associated with reduced long-term mortality (HR 0.71, 95% CI 0.54 to 0.92) [12], decreased development of T2DM (OR 0.17, 95% CI 0.13 to 0.21) [63], and reduced incidence of cardiovascular events (OR 0.67, 95%CI 0.54 to 0.83) [64].
Conclusion
This meta-analysis of large-scale registry studies indicates that patients receiving bariatric surgery have improved long-term mortality rates compared to controls at a population level. They also have significantly reduced incidence of obesity-related disease including T2DM, hypertension, dyslipidaemia, and ischemic heart disease. Healthcare providers may use the data on relative risk reduction as part of the discussion with patients considering bariatric surgery.
Supporting information
(TIF)
(TIF)
BPD, biliopancreatic diversion; DS, duodenal switch; VBG, vertical banded gastroplasty.
(DOCX)
(DOCX)
Data in parentheses represent 95% confidence interval. T2DM, type 2 diabetes; VTE, venous thromboembolism.
(DOCX)
(DOCX)
(DOC)
Abbreviations
- BMI
body mass index
- HR
hazard ratio
- OR
odds ratio
- POR
pooled odds ratio
- T2DM
type 2 diabetes mellitus
Data Availability
All relevant data are available within the manuscript and its Supporting information files.
Funding Statement
SRM - National Institute of Health Research (NIHR) Imperial Biomedical Research Centre grant (Grant number NIHR-IAT-EAN/021/00146/C). Sponsors played no role in study design, data collection or analysis, decision to publish or manuscript preparation.
References
- 1.World Health Organization. Global Health Observatory (GHO) data: overweight and obesity. Geneva: World Health Organization; 2020. [cited 2020 Apr 30]. http://www.who.int/gho/ncd/risk_factors/overweight/en/. [Google Scholar]
- 2.Smith KB, Smith MS. Obesity statistics. Prim Care. 2016;43(1):121–35. 10.1016/j.pop.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 3.NHS Digital. Statistics on obesity, physical activity and diet. Leeds: NHS Digital; 2017. [cited 2020 Apr 30]. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/613532/obes-phys-acti-diet-eng-2017-rep.pdf. [Google Scholar]
- 4.Scheelbeek PFD, Cornelsen L, Marteau TM, Jebb SA, Smith RD. Potential impact on prevalence of obesity in the UK of a 20% price increase in high sugar snacks: modelling study. BMJ. 2019;366:I4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–45. 10.1001/jama.291.10.1238 [DOI] [PubMed] [Google Scholar]
- 6.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359(20):2105–20. 10.1056/NEJMoa0801891 [DOI] [PubMed] [Google Scholar]
- 7.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. 10.1016/S0140-6736(09)60318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C, Rexrode KM, Van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117(13):1658–67. 10.1161/CIRCULATIONAHA.107.739714 [DOI] [PubMed] [Google Scholar]
- 9.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–14. 10.1016/S0140-6736(11)60813-1 [DOI] [PubMed] [Google Scholar]
- 10.Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219–34. 10.1111/joim.12012 [DOI] [PubMed] [Google Scholar]
- 11.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376(7):641–51. 10.1056/NEJMoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52. 10.1056/NEJMoa066254 [DOI] [PubMed] [Google Scholar]
- 13.Kristensson FM, Andersson-Assarsson JC, Svensson P-A, Carlsson B, Peltonen M, Carlsson LMS. Effects of bariatric surgery in early- and adult-onset obesity in the prospective controlled Swedish Obese Subjects study. Diabetes Care. 2020;43(4):860–6. 10.2337/dc19-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiggins T, Antonowicz SS, Markar SR. Cancer risk following bariatric surgery—systematic review and meta-analysis of national population-based cohort studies. Obes Surg. 2019;29(3):1031–9. 10.1007/s11695-018-3501-8 [DOI] [PubMed] [Google Scholar]
- 15.Mackenzie H, Markar S, Askari A, Faiz O, Hull M, Purkayastha S, et al. Obesity surgery and cancer risk: an English population-based cohort study. Br J Surg. 2018;105(12):1650–7. 10.1002/bjs.10914 [DOI] [PubMed] [Google Scholar]
- 16.Sjöström L, Gummesson A, Sjöström CD, Narbro K, Peltonen M, Wedel H, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10(7):653–62. 10.1016/S1470-2045(09)70159-7 [DOI] [PubMed] [Google Scholar]
- 17.National Institute for Health and Care Excellence. NICE pathways: surgery for obese adults. London: National Institute for Health and Care Excellence; 2020 [cited 2020 Jun 22]. https://pathways.nice.org.uk/pathways/obesity#path=view%3A/pathways/obesity/surgery-for-obese-adults.xml&content=view-index.
- 18.Welbourn R, le Roux CW, Owen-Smith A, Wordsworth S, Blazeby JM. Why the NHS should do more bariatric surgery; how much should we do? BMJ. 2016;353:i1472 10.1136/bmj.i1472 [DOI] [PubMed] [Google Scholar]
- 19.Miras AD, Kamocka A, Patel D, Dexter S, Finlay I, Hopkins JC, et al. Obesity surgery makes patients healthier and more functional: real world results from the United Kingdom National Bariatric Surgery Registry. Surg Obes Relat Dis. 2018;14(7):1033–40. 10.1016/j.soard.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welbourn R, Sareela A, Small P, Somers S, Finlay I, Mahawar K. The United Kingdom National Bariatric Surgery Registry: second registry report. London: British Obesity & Metabolic Surgery Society; 2014. [cited 2020 Apr 30]. http://www.bomss.org.uk/wp-content/uploads/2018/11/Extract_from_the_NBSR_2014_Report-2.pdf. [Google Scholar]
- 21.Davis JA, Saunders R. Comparison of comorbidity treatment and costs associated with bariatric surgery among adults with obesity in Canada. JAMA Netw Open. 2020;3(1):e1919545 10.1001/jamanetworkopen.2019.19545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, Yu J, Li L, Gloy VL, Nordmann A, Tiboni M, et al. Effects of bariatric surgery on mortality, cardiovascular events, and cancer outcomes in obese patients: systematic review and meta-analysis. Obes Surg. 2016;26(11):2590–601. 10.1007/s11695-016-2144-x [DOI] [PubMed] [Google Scholar]
- 23.Cardoso L, Rodrigues D, Gomes L, Carrilho F. Short- and long-term mortality after bariatric surgery: a systematic review and meta-analysis. Diabetes Obes Metab. 2017;19(9):1223–32. 10.1111/dom.12922 [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 25.Arterburn DE, Olsen MK, Smith VA, Livingston EH, Van Scoyoc L, Yancy WS, et al. Association between bariatric surgery and long-term survival. JAMA. 2015;313(1):62–70. 10.1001/jama.2014.16968 [DOI] [PubMed] [Google Scholar]
- 26.Backman O, Bruze G, Näslund I, Ottosson J, Marsk R, Neovius M, et al. Gastric bypass surgery reduces de novo cases of type 2 diabetes to population levels: a nationwide cohort study from Sweden. Ann Surg. 2019;269(5):895–902. 10.1097/SLA.0000000000002983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moussa O, Ardissino M, Tang A, Lazzari L, Millar O, Ziprin P, et al. Long-term impact of bariatric surgery on venous thromboembolic risk. Ann Surg. 2019. December 17 10.1097/SLA.0000000000003750 [DOI] [PubMed] [Google Scholar]
- 28.Perry CD, Hutter MM, Smith DB, Newhouse JP, McNeil BJ. Survival and changes in comorbidities after bariatric surgery. Ann Surg. 2008;247(1):21–7. 10.1097/SLA.0b013e318142cb4b [DOI] [PubMed] [Google Scholar]
- 29.Persson CE, Björck L, Lagergren J, Lappas G, Giang KW, Rosengren A. Risk of heart failure in obese patients with and without bariatric surgery in Sweden—a registry-based study. J Card Fail; 2017;23(7):530–7. 10.1016/j.cardfail.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 30.Pontiroli AE, Zakaria AS, Fanchini M, Osio C, Tagliabue E, Micheletto G, et al. A 23-year study of mortality and development of co-morbidities in patients with obesity undergoing bariatric surgery (laparoscopic gastric banding) in comparison with medical treatment of obesity. Cardiovasc Diabetol. 2018;17(1):161 10.1186/s12933-018-0801-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh P, Subramanian A, Adderley N, Gokhale K, Singhal R, Bellary S, et al. Impact of bariatric surgery on cardiovascular outcomes and mortality: a population-based cohort study. Br J Surg. 2020;14:15–7. [DOI] [PubMed] [Google Scholar]
- 32.Reges O, Greenland P, Dicker D, Leibowitz M, Hoshen M, Gofer I, et al. Association of bariatric surgery using laparoscopic banding, Roux-en-Y gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. JAMA. 2018;319(3):279–90. 10.1001/jama.2017.20513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thereaux J, Lesuffleur T, Czernichow S, Basdevant A, Msika S, Nocca D, et al. Association between bariatric surgery and rates of continuation, discontinuation, or initiation of antidiabetes treatment 6 years later. JAMA Surg. 2018;153(6):526–33. 10.1001/jamasurg.2017.6163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thereaux J, Lesuffleur T, Czernichow S, Basdevant A, Msika S, Nocca D, et al. Multicentre cohort study of antihypertensive and lipid-lowering therapy cessation after bariatric surgery. Br J Surg. 2019;106(3):286–95. 10.1002/bjs.10999 [DOI] [PubMed] [Google Scholar]
- 35.Bailly L, Schiavo L, Sebastianelli L, Fabre R, Morisot A, Pradier C, et al. Preventive effect of bariatric surgery on type 2 diabetes onset in morbidly obese inpatients: a national French survey between 2008 and 2016 on 328,509 morbidly obese patients. Surg Obes Relat Dis. 2019;15(3):478–87. 10.1016/j.soard.2018.12.028 [DOI] [PubMed] [Google Scholar]
- 36.Ceriani V, Sarro G, Micheletto G, Giovanelli A, Zakaria AS, Fanchini M, et al. Long-term mortality in obese subjects undergoing malabsorptive surgery (biliopancreatic diversion and biliointestinal bypass) versus medical treatment. Int J Obes. 2019;43(6):1147–53. [DOI] [PubMed] [Google Scholar]
- 37.Douglas IJ, Bhaskaran K, Batterham RL, Smeeth L. Bariatric surgery in the United Kingdom: a cohort study of weight loss and clinical outcomes in routine clinical care. PLoS Med. 2015;12(12):e1001925 10.1371/journal.pmed.1001925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eliasson B, Liakopoulos V, Franzén S, Näslund I, Svensson AM, Ottosson J, et al. Cardiovascular disease and mortality in patients with type 2 diabetes after bariatric surgery in Sweden: a nationwide, matched, observational cohort study. Lancet Diabetes Endocrinol. 2015;3(11):847–54. 10.1016/S2213-8587(15)00334-4 [DOI] [PubMed] [Google Scholar]
- 39.Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population-based analysis. J Am Coll Surg. 2004;199(4):543–51. 10.1016/j.jamcollsurg.2004.06.014 [DOI] [PubMed] [Google Scholar]
- 40.Johnson BL, Blackhurst DW, Latham BB, Cull DL, Bour ES, Oliver TL, et al. Bariatric surgery is associated with a reduction in major macrovascular and microvascular complications in moderately to severely obese patients with type 2 diabetes mellitus. J Am Coll Surg. 2013;216(4):545–56. 10.1016/j.jamcollsurg.2012.12.019 [DOI] [PubMed] [Google Scholar]
- 41.Kauppila JH, Tao W, Santoni G, von Euler-Chelpin M, Lynge E, Tryggvadóttir L, et al. Effects of obesity surgery on overall and disease-specific mortality in a 5-country population-based study. Gastroenterology. 2019;157(1):119–27.e1. 10.1053/j.gastro.2019.03.048 [DOI] [PubMed] [Google Scholar]
- 42.Moussa OM, Erridge S, Chidambaram S, Ziprin P, Darzi A, Purkayastha S. Mortality of the severely obese: a population study. Ann Surg. 2019;269(6):1087–91. 10.1097/SLA.0000000000002730 [DOI] [PubMed] [Google Scholar]
- 43.Wells GA, Shea B, O’Connell D, Paterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital; 2019[cited 2020 Apr 30]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 44.Pontiroli AE, Morabito A. Long-term prevention of mortality in morbid obesity through bariatric surgery. A systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann Surg. 2011;253(3):484–7. 10.1097/SLA.0b013e31820d98cb [DOI] [PubMed] [Google Scholar]
- 45.Carlsson LMS, Sjöholm K, Karlsson C, Jacobson P, Andersson-Assarsson JC, Svensson PA, et al. Long-term incidence of microvascular disease after bariatric surgery or usual care in patients with obesity, stratified by baseline glycaemic status: a post-hoc analysis of participants from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol. 2017;5(4):271–9. 10.1016/S2213-8587(17)30061-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Courcoulas AP, King WC, Belle SH, Berk P, Flum DR, Garcia L, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) study. JAMA Surg. 2018;153(5):427–34. 10.1001/jamasurg.2017.5025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adams TD, Davidson LE, Litwin SE, Kim J, Kolotkin RL, Nanjee MN, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017;377(12):1143–55. 10.1056/NEJMoa1700459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nyberg ST, Batty GD, Pentti J, Virtanen M, Alfredsson L, Fransson EI, et al. Obesity and loss of disease-free years owing to major non-communicable diseases: a multicohort study. Lancet Public Health. 2018;3(10):e490–7. 10.1016/S2468-2667(18)30139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y, Dugué PA, Lynch BM, Hodge AM, Karahalios A, MacInnis RJ, et al. Trajectories of body mass index in adulthood and all-cause and cause-specific mortality in the Melbourne Collaborative Cohort Study. BMJ Open. 2019;9(8):e030078 10.1136/bmjopen-2019-030078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitahara CM, Flint AJ, Berrington de Gonzalez A, Bernstein L, Brotzman M, MacInnis RJ, et al. Association between class iii obesity (BMI of 40–59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med. 2014;11(7):e1001673 10.1371/journal.pmed.1001673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mackenbach JP, Stirbu I, Roskam AJR, Schaap MM, Menvielle G, Leinsalu M, et al. Socioeconomic inequalities in health in 22 European countries. N Engl J Med. 2008;358(23):2468–81. 10.1056/NEJMsa0707519 [DOI] [PubMed] [Google Scholar]
- 52.Martin M, Beekley A, Kjorstad R, Sebesta J. Socioeconomic disparities in eligibility and access to bariatric surgery: a national population-based analysis. Surg Obes Relat Dis. 2010;6(1):8–15. 10.1016/j.soard.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 53.Keating C, Backholer K, Moodie M, Stevenson C, Peeters A. Differences in the rates of treatment of severe obesity using bariatric surgery across socioeconomic groups. JAMA Surg. 2015;150(4):367–8. 10.1001/jamasurg.2014.3180 [DOI] [PubMed] [Google Scholar]
- 54.Jakobsen GS, Småstuen MC, Sandbu R, Nordstrand N, Hofsø D, Lindberg M, et al. Association of bariatric surgery vs medical obesity treatment with long-term medical complications and obesity-related comorbidities. JAMA. 2018;319(3):291–301. 10.1001/jama.2017.21055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ladak F, Dang JT, Switzer NJ, Mocanu V, Birch DW, Karmali S. Rates of reoperation and nonoperative intervention within 30 days of bariatric surgery. Surg Obes Relat Dis. 2019;15(3):431–40. 10.1016/j.soard.2018.12.035 [DOI] [PubMed] [Google Scholar]
- 56.Inaba CS, Koh CY, Sujatha-Bhaskar S, Silva JP, Chen Y, Nguyen DV, et al. One-year mortality after contemporary laparoscopic bariatric surgery: an analysis of the Bariatric Outcomes Longitudinal Database. J Am Coll Surg. 2018;226(6):1166–74. 10.1016/j.jamcollsurg.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alam M, Bhanderi S, Matthews JH, McNulty D, Pagano D, Small P, et al. Mortality related to primary bariatric surgery in England. BJS Open. 2017;1(4):122–7. 10.1002/bjs5.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melissas J, Braghetto I, Molina JC, Silecchia G, Iossa A, Iannelli A, et al. Gastroesophageal reflux disease and sleeve gastrectomy. Obes Surg. 2015;25(12):2430–5. 10.1007/s11695-015-1906-1 [DOI] [PubMed] [Google Scholar]
- 59.Stenberg E, Szabo E, Ågren G, Ottosson J, Marsk R, Lönroth H, et al. Closure of mesenteric defects in laparoscopic gastric bypass: a multicentre, randomised, parallel, open-label trial. Lancet. 2016;387:1397–404. 10.1016/S0140-6736(15)01126-5 [DOI] [PubMed] [Google Scholar]
- 60.Billeter AT, Probst P, Fischer L, Senft J, Kenngott HG, Schulte T, et al. Risk of malnutrition, trace metal, and vitamin deficiency post Roux-en-Y gastric bypass—a prospective study of 20 patients with BMI < 35 kg/m2. Obes Surg. 2015;25(11):2125–34. 10.1007/s11695-015-1676-9 [DOI] [PubMed] [Google Scholar]
- 61.Torgerson JS, Sjöström L. The Swedish Obese Subjects (SOS) study—rationale and results. Int J Obes Relat Metab Disord. 2001;25(Suppl 1):S2–4. [DOI] [PubMed] [Google Scholar]
- 62.Sjöström L, Lindroos A-K, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93. 10.1056/NEJMoa035622 [DOI] [PubMed] [Google Scholar]
- 63.Carlsson LMS, Peltonen M, Ahlin S, Anveden Å, Bouchard C, Carlsson B, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367(8):695–704. 10.1056/NEJMoa1112082 [DOI] [PubMed] [Google Scholar]
- 64.Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65. 10.1001/jama.2011.1914 [DOI] [PubMed] [Google Scholar]