Abstract
Indiscriminate antimicrobial use (AMU) is a factor contributing to antimicrobial resistance (AMR). The objectives of this study were to (1) identify factors influencing AMU practices of veterinary clinicians at The University of Tennessee Veterinary Medical Center (UTVMC), (2) analyze the clinicians’ preferential choices of antimicrobials, and (3) evaluate their perceptions, opinions, and concerns regarding AMU and AMR. A total of 121 clinicians were surveyed. Among the 62 respondents, culture and susceptibility test results and pressure from clients were the most and least important factors in their antimicrobial prescription decision-making, respectively. Compared to clinicians who obtained their veterinary degree from 1970 to 1999, those who graduated from 2000 to 2009 and 2010–2016 were 3.96 (P = 0.034) and 5.39 (P = 0.01) times less concerned about AMR, respectively. There is a critical need to increase awareness about judicious AMU practices among clinicians, increase emphasis about AMR in the present veterinary curriculum, and implement antimicrobial stewardship program (AMS) in this institution. Educational activities in combination with awareness campaigns and the stewardship programs could be used to improve AMU practices at this hospital. More client education on AMR is needed.
Keywords: Antimicrobial resistance, Antimicrobial stewardship, Ordinal logistic regression, Questionnaire, Survey
1. Introduction
Antimicrobial drugs in veterinary practice are primarily prescribed for the purposes of maintaining or improving animal health and increasing productivity (Marshall & Levy, 2011). However, the emergence and spread of antimicrobial resistant microorganisms is eroding the value of antimicrobial drugs (Dyar et al., 2016, Guardabassi and Prescott, 2015). Although antimicrobial resistance (AMR) is an ancient phenomenon (D'costa et al., 2011, Perry et al., 2016), indiscriminate antimicrobial use (AMU) is an important risk factor for the development of AMR (McKay, Mah, Law, McGrail, & Patrick, 2016). The increase in the prevalence of microorganisms resistant to antimicrobials, both in veterinary and human medicine, is now widely attributed to AMU (De Briyne et al., 2013, Holmes et al., 2016).
Shedding of drug resistant microorganisms by animals can directly (through contact) or indirectly lead to human infections/colonization by commensal bacteria (Guardabassi et al., 2004, Loeffler et al., 2005, Marshall and Levy, 2011). These bacteria carry transferable resistance genes across species through multiple pathways like food, water, fomites, sludge and manure applications to food crop soils (Chung et al., 2017, Marshall and Levy, 2011, McEachran et al., 2015, Van Boeckel et al., 2015), as well as house hold environments with pets carrying resistant bacteria and other environments contaminated with pet feces (Pomba et al., 2017). Multi-drug resistant infections exert a huge burden on veterinary medical care (Kuzi et al., 2016) and pose public health risks (Walther et al., 2017, Weese et al., 2015).
To reduce indiscriminate use and to improve AMU practices, veterinary practices are encouraged to develop and implement antimicrobial stewardship (AMS) programs. Such stewardship programs include effective infection control, bacteriologic culture and antimicrobial susceptibility testing, and the use of individual practice guidelines for AMS (Prescott and Boerlin, 2016, Weese, 2006). According to the American Veterinary Medical Association (AVMA, 2018) and the U.S. Food and Drug Administration (FDA, 2017), veterinarians in the U.S. are required to direct AMU only within the context of a valid veterinarian-client-patient-relationship (VCPR) to ensure judicious use. In the context of VCPR, the veterinarian can write a prescription or dispense prescription drugs only when all of the following five requirements are observed (1) the veterinarian assumes the responsibility of providing health care for the patient and the client agrees to follow the veterinarian's instructions, (2) the veterinarian is sufficiently knowledgeable of the patient to initiate care and is well acquainted with the keeping and care provided to the patient either through patient evaluation or through timely visits to the operation where the patient is managed, (3) the veterinarian is available for follow-up evaluations or has planned for emergency health coverage, continuing veterinary care and treatment, (4) the veterinarian provides oversight of treatment, compliance and outcome, and (5) patient records are well kept. The VCPR can be applied to individual animals as well as a group or groups of animals within an operation (production system).
Research conducted from May 2008 to May 2009 at a veterinary teaching hospital in the north eastern U.S. suggests clinicians are frequently prescribing antimicrobials without proper documentation in medical records or without indicating their use (Wayne, McCarthy, & Lindenmayer, 2011). In a 2014 survey, veterinarians in North Carolina State University veterinary teaching hospital believed the veterinary practice over-prescribed antimicrobials, were concerned about AMR, and supported the idea of restricting the use of certain antimicrobial classes in companion animals (Jacob et al., 2015). Prior to the present study, the factors that influenced AMU practices of veterinary clinicians at University of Tennessee Veterinary Medical Center (UTVMC) were unknown. Similarly, their perceptions, opinions, and concerns about AMU, AMS, and AMR were undocumented. Additionally, the association between the effort allocation to veterinary clinical practice and the frequency of antimicrobial prescriptions for therapeutic treatment of infectious diseases had not been explored. This study contributes to the wider knowledge of AMU by providing insights into the AMU practices of clinicians at a veterinary teaching hospital.
The objectives of this study were to (1) identify factors influencing AMU practices of veterinary clinicians at the UTVMC, (2) analyze the clinicians’ preferential choices of antimicrobials, and (3) evaluate their perceptions, opinions, and concerns regarding AMU, AMS, and AMR. These findings will be beneficial in improving AMS programs and educational training on judicious AMU. Ultimately, these efforts could prolong the efficacy of current antimicrobials and reduce the burden of AMR within veterinary medicine and public health.
2. Materials and methods
2.1. Study design and administration of survey
A questionnaire was developed and validated by four professionals with expertise in survey design and the University of Tennessee Knoxville Institutional Review Board for the Protection of Human Subjects in Research approved the study (Protocol number: UTK IRB-16- 103 02956-XP). A survey software (Qualtrics software, Provo, UT) housed the 36-questions questionnaire, which were adapted for computer, tablets, and cell phone responses. These questions targeted the respondent's demographics and their antimicrobial prescription practices, perceptions, opinions, and concerns about AMU, AMS, and AMR. The anonymize function in the software was optimized, so responses were not attached to any personal identifiers. Next, the questionnaire was pre-tested among four veterinary clinicians at UTVMC and their comments were used to improve questionnaire clarity.
Targeted demographic information included gender, the nature of the clinical position (faculty versus house officers), the primary type of patients seen (small animal, food animal, equine, etc.), where the veterinary degree was obtained (U.S. versus non-U.S.), and year of graduation from veterinary school/ total number of years in clinical practice from time of graduation. Biological age of respondents was not included because year of graduation and number of years in clinical practice were considered to be more clinically relevant to the research question. This demographic information were our explanatory variables of interest. Our two outcomes of interest were (1) the frequency of antimicrobial prescription and (2) the degree of concern about antimicrobial resistant infections. Most of the survey questions were closed-ended while a few were free-text (open questions). Three-point scales and ordinal Likert scales were used to capture participant responses to most of the closed-ended survey questions relating to perceptions about AMU practices and AMR. Regarding antimicrobial class preference based on clinician's frequency of prescription, participants were asked to rank medically important classes of antimicrobials on a five-point Likert scale ranging from a strong dislike (never prescribed) to a strong preference (always prescribed).
During departmental meetings at about a week before the study's start date, eligible participants (all faculty members with clinical appointments, residents, and interns at UTVMC) were notified about our upcoming survey, in an effort to increase response rate. Subsequently, a notification email was sent to all potential respondents an hour before the survey went live. Afterwards, all 121 eligible participants received an email invitation about the survey, which was optimized to accept only one response from each respondent. To minimize potential selection bias, the survey was sent to all clinicians at the hospital irrespective of whether their primary clinical duties directly or indirectly involved AMU. The survey remained open for 6 weeks (January 27, 2017 through March 10, 2017). Weekly follow-up email reminders were sent to non-respondents. No incentive was provided to clinicians for participation or completion but a thank you message was sent to all respondents at the end of the study.
2.2. Statistical analysis
Descriptive and inferential analyses was completed using commercial statistical software (SAS, version 9.4, SAS Institute Inc, Cary, NC). Descriptive statistics (frequencies and proportions) were used to summarize the data. Side-by-side bar charts and stacked bar charts for responses on the three-point scales and on the Likert scales were created using another commercial software (Tableau software, version 8.2, Seattle, WA). No corrections were made on missing data.
To test for associations between the captured demographic information and the two outcomes of interest, both univariable and multivariable analysis were performed using ordinal logistic regression. Specifically, ordinal logistic regression was used to investigate the effects of antimicrobial class on clinicians’ frequency of prescription and to identify differences in preference between classes of antimicrobials. To validate the data on ranking of classes of antimicrobials based on frequency of prescription, the commonly prescribed antimicrobial drugs captured as free text (generic or trades names) from clinician responses were further grouped into classes as described previously (Green et al., 2010, Jacob et al., 2015). From these classes, we isolated the medically important antimicrobial classes as grouped by the United States Food and Drug Administration (FDA, 2015). These medically important classes included: aminoglycosides e.g. gentamicin; cephalosporins e.g. ceftriaxone, cefazolin; fluoroquinolones e.g. ciprofloxacin; lincosamides e.g. clindamycin, lincomycin; macrolides e.g. erythromycin; penicillins e.g. amoxicillin, ampicillin; sulfonamides e.g. sulfadiazine, sulfathiazole; and tetracyclines e.g. doxycycline, oxytetracycline. The United States Food and Drug Administration groups antimicrobials as medically important in line with the World Health Organization's classification of antimicrobials. Preferential ordering of the medically important antimicrobial classes was analyzed based on the main categories of patients seen by clinicians. The preference ordering was assessed based on the relative magnitudes of the parameter estimates from the model. Preferential ordering refers to the order in which antimicrobial classes were preferred from the least preferred to the most preferred. During the modeling, tetracyclines was selected as the reference class and the probability of disliking another class of antimicrobial in comparison to tetracyclines was estimated. Spearman's rank correlation was used to evaluate for correlations and quantify the strength of association between two ranked variables: for example, the proportion of total professional activity dedicated to clinical practice (effort allocation to clinical practice) and the frequency of prescription of antimicrobials for therapeutic purposes; number of years in clinical practice from the time of graduation from veterinary school and year of graduation from veterinary school.
In assessing the clinicians’ degree of concern about AMR, a multivariable ordinal logistic regression model was manually fitted using backwards elimination method. Briefly, potential predictors at a P ≤ 0.20 from the univariable analyses were included in the multivariable model building and variables were dropped if they were either non-significant (P > 0.05) or non-confounders. Possible effects of confounding were evaluated by comparing a change in parameter estimates with and without the suspected variables (Okwechime et al., 2015, Qekwana et al., 2017). A predictor variable that caused a ≥ 20% change in another parameter estimate upon removal from the model was considered a confounder and was retained in the final model regardless of its statistical significance (Dohoo, Martin, & Stryhn, 2003). For two predictor variables that were highly correlated (e.g. number of years in clinical practice from the time of graduation from veterinary school and year of graduation from veterinary school), only one variable was used in the multivariable model building based on completeness of data or ease in clinical interpretation. Year of graduation was captured as a free text and was later classified into 3 quantiles (1970 – 1999, 2000 – 2009, and 2010 – 2016) as done in a previous study (Jacob et al., 2015). In the final model, two-way interactions (e.g., year of graduation and clinician's primary patient load) were assessed based on plausibility and standard multiple pairwise comparisons were obtained. Finally, the model fit was assessed using the score test for the proportional odds assumption, deviance, and Pearson goodness-of-fit statistics, and a plot of the empirical cumulative logit function. A plot yielding an approximate parallel empirical cumulative logits was indicative of an appropriate proportional odds model.
3. Results
3.1. Study site
The UTVMC is the veterinary teaching hospital of UTCVM and the only academic veterinary medical center in the US state of Tennessee. This veterinary college is under the Institute of Agriculture at the University of Tennessee and employs a total of 99 faculty members and 174 staff. There are currently three academic departments at UTCVM namely: biomedical and diagnostic sciences (29 faculty members and 54 Staff), large animal clinical sciences (21 faculty members and 29 Staff) and small animal clinical sciences (49 faculty members and 91 Staff). As of fiscal year, 2017, the average annual large animal caseload (both clinic and ambulatory) was 15,031 patients. The annual small animal case load was estimated to be more than 15,000 patients and the avian caseload was estimated to be 1500 per year.
3.2. Descriptive statistics
Of the 121 invited participants, 62 (51.2%) responded to the survey. Complete responses were provided in most questions except for a few responses that were unanswered. The demographic information of the 62 respondents is presented in Table 1.
Table 1.
Demographics of clinicians (n = 62) on an online survey to identify determinants of antimicrobial use practices at the University of Tennessee Veterinary Medical Center, 2017.
| Variable | Number (%) of respondents |
|---|---|
| Gender | |
| Female | 37 (59.7) |
| Male | 21 (33.9) |
| Preferred not to report gender | 4 (6.5) |
| Nature Clinical Position | |
| Faculty members | 44 (71) |
| House officers | 17 (27.4) |
| Not reported | 1 (1.6) |
| Year of graduation from veterinary school | |
| 1970–1999 | 21 (33.9) |
| 2000–2009 | 22 (35.5) |
| 2010–2016 | 19 (30.7) |
| College where veterinary degree was obtained | |
| U.S. veterinary school | 51 (82.3) |
| Non-U.S. veterinary school | 11 (17.7) |
| Primary patient load | |
| Small animal | 37 (59.7) |
| Equine | 8 (12.9) |
| Food animal | 7 (11.3) |
| Others (mixed animal, exotics) | 10 (16.1) |
| Specialty board certification | |
| Obtained specialty board certification | 43 (69.4) |
| No specialty board certification | 19 (30.6) |
Among the factors that influence the choice of antimicrobial drug(s) for clinical use at UTVMC (Fig. 1), results from bacteriological culture and antimicrobial susceptibility tests were the most important. Pressure from clients/producers to the clinician to prescribe antimicrobials and the fear of litigation by the client/producer in the event of an undesirable clinical outcome were the two least important factors. Peer-reviewed scientific literature and textbooks/drug handbooks were the most important sources of information on antimicrobial drugs for these clinicians while pharmaceutical company representatives and online resources (e.g., blogs or media searches) were the least important sources of information (Fig. 2). Frequency of prescriptions differed among these clinicians. Twenty clinicians (32.3%) prescribed antimicrobials for therapeutic purposes more than five times a week, while 35 of 62 (56.5%) clinicians prescribed antimicrobials for prophylactic purposes (Fig. 3). Of these 35 clinicians, 23 (65.7%) prescribed antimicrobials for pre-operative surgical prophylaxis, 29 (85.3%) for post-operative surgical prophylaxis, and 29 (82.9%) for peri‑operative surgical prophylaxis (Fig. 4).
Fig. 1.
Distribution of factors that influence the initiation and the choice of antimicrobials used by clinicians at the University of Tennessee Veterinary Medical Center, 2017 (n = 62).
Fig. 2.
Distribution of sources of information influencing the choice of antimicrobials used by clinicians at the University of Tennessee Veterinary Medical Center, 2017 (n = 62).
Fig. 3.
Self-reported antimicrobial prescription practices of clinicians at the University of Tennessee Veterinary Medical Center, 2017 (n = 62).
Fig. 4.
Self-reported antimicrobial prescription practices for surgical prophylaxis by clinicians at the University of Tennessee Veterinary Medical Center, 2017 (n = 62).
Clinicians’ opinions on AMU practices at UTVMC differed. One clinician (1.6%) believed antimicrobials were prescribed based only on confirmed infections, 21 (33.9%) believed antimicrobials were sometimes prescribed based on no documented evidence of infection, 38 (61.3%) believed that antimicrobials were sometimes prescribed for suspected (but not confirmed) infections, and two (3.2%) were not sure. As per prescription rate at UTVMC, one clinician (1.6%) believed antimicrobials were under-prescribed while 29 (46.8%) and 32 (51.6%) believed antimicrobials were optimally prescribed and over-prescribed, respectively. Overall, two (3.2%) clinicians believed UTVMC had an AMS program, 51 (82.3%) were not sure, while nine (14.5%) mentioned that none existed. Within the faculty cohort (n = 44), eight (13.1%) believed there was no AMS program, 34 (55.7%) were not sure, and two (3.3%) mentioned that one existed. However, of the 17 house officers, 16 (26.2%) were not sure if AMS program existed and one individual (1.6%) believed none existed. The respondent who did not disclose the nature of their clinical position was also not sure of the existence of AMS program at UTVMC. Of the nine clinicians who believed no AMS program currently exists, seven (77.8%) mentioned that development and implementation of AMS program in the hospital was necessary while the other two (22.2%) clinicians mentioned the opposite.
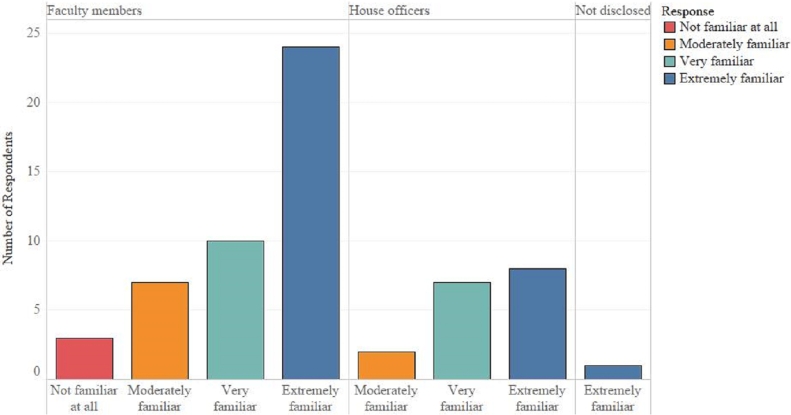
Regarding the clinicians’ familiarity with Veterinarian Client Patient Relationship (VCPR), three (4.8%) were not familiar at all, nine (14.5%) were moderately familiar, 17 (27.4%) were very familiar, 33 (53.2%) were extremely familiar. A comparison of the level of familiarity with the VCPR between faculty members and house officers is shown in Fig. 5. Overall, 10 (16.1%) mentioned that they never utilized VCPR in their antimicrobial prescription practice, three (4.8%) rarely used VCPR, four (6.5%) sometimes utilized VCPR, 10 (16.3%) often utilized VCPR, and 35 (56.5%) always utilized VCPR in their antimicrobial prescription practice. A comparison of the use of VCPR in antimicrobial prescription practice of clinicians based on the nature of clinical position is shown in Fig. 6.
Fig. 5.
Self-reported level of familiarity with Veterinarian Client Patient Relationship by clinicians at the University of Tennessee Veterinary Medical Center, 2017 (n = 62).
Fig. 6.
Self-reported use of Veterinarian Client Patient Relationship in antimicrobial prescription practice by clinicians at the University of Tennessee Veterinary Medical Center, 2017 (n = 62).
The extent to which the Doctor of Veterinary Medicine (or equivalent veterinary degree) training adequately equipped clinicians with knowledge on rational use of antimicrobials varied. For one clinician (1.6%), it was “not at all,” three (4.8%) mentioned “a little,” 22 (35.5%) responded “somewhat,” 28 (45.2%) believed “quite a bit,” and eight (12.9%) said “very much.” Similarly, the extent to which the present-day veterinary curriculum adequately trains students on rational use of antimicrobials varied. One clinician felt that present-day veterinary medical students do not receive any adequate training on rational use of antimicrobials, nine (14.8%) stated the students received “a little,” 28 (45.9%) responded “somewhat,” 21 (34.4%) responded “quite a bit,” and two (3.3%) responded “very much.” Seventeen (27.4%) clinicians had never read the FDA / American Veterinary Medical Association (AVMA) guidelines for judicious use of antimicrobials, 19 (30.7%) rarely did, 20 (32.3%) sometimes did, and six (9.7%) very often read the guidelines.
In rating other veterinarians’ concerns about AMR, 18 clinicians (29.1%) believed other veterinarians were slightly concerned about AMR, 36 (58.1%) believed that others were moderately concerned, five (8.1%) believed that others were quite concerned, and three (4.8%) believed others were very concerned. With respect to their clients’ concern about AMR, 27 clinicians (43.6%) believed their clients were not concerned, 25 (40.3%) believed they were slightly concerned, eight (12.9%) believed the clients were moderately concerned, and two (3.2%) believed they were quite concerned. Twelve clinicians (19.4%) strongly disagreed with the statement “antimicrobial classes commonly used in human medicine should not be used in veterinary medicine because their use in veterinary medicine selects for AMR in microbes affecting humans.” Thirty-two (51.6%) disagreed with this statement, 11 (17.7%) neither disagreed nor agreed, and seven (11.3%) agreed with this statement. For the statement “antimicrobial drug use in veterinary practice may lead to AMR in pathogens affecting humans,” one (1.6%) strongly disagreed, 8 (12.9%) disagreed, 17 (27.4%) neither disagreed nor agreed, 24 (38.7%) agreed, and 12 (19.4%) strongly agreed. One respondent (1.6%) was not concerned about antimicrobial resistant infections. Two (3.2%) were slightly concerned; 27 (43.6%) were moderately concerned. Nineteen clinicians (30.7%) were quite concerned, and 13 (21%) were very concerned about antimicrobial-resistant infections.
3.3. Preferential ordering of the medically important antimicrobial classes by small animal clinicians
Based on the frequency of prescriptions, the small animal clinicians preferred the following medically important antimicrobial classes in an increasing order: lincosamides, aminoglycosides, macrolides, sulfonamides, fluoroquinolones, tetracyclines, and penicillins/cephalosporins (Table 2). Compared to the tetracycline, the lincosamides, aminoglycosides, macrolides, and sulfonamide classes were significantly less preferred classes but there were no significant differences in the preference for fluoroquinolones, penicillins, and cephalosporins by small animal clinicians (Table 2).
Table 2.
Increasing order of preference of medically important antimicrobial classes based on self-reported frequency of prescription by small animal clinicians at the University of Tennessee Veterinary Medical Center, 2017 (n = 37).
| Antimicrobial class† | Parameter estimate | Standard error | Odds ratio (95% CI) | P value |
|---|---|---|---|---|
| Lincosamides | 2.6468 | 0.4637 | 14.11 (5.69 – 35.01) | <0.001 |
| Aminoglycosides | 2.6050 | 0.4522 | 13.53 (5.58 – 32.83) | <0.001 |
| Macrolides | 1.8271 | 0.4518 | 6.22 (2.56 – 15.07) | <0.001 |
| Sulfonamides | 1.7709 | 0.4411 | 5.88 (2.48–13.95) | <0.001 |
| Fluoroquinolones | 0.1857 | 0.4374 | 1.20 (0.51 – 2.84) | 0.671 |
| Tetracyclines* | – | – | – | – |
| Penicillins | −0.3091 | 0.4768 | 0.73 (0.29 – 1.87) | 0.517 |
| Cephalosporins | −0.3086 | 0.4425 | 0.73 (0.31 – 1.75) | 0.486 |
Reference class.
The least preferred class had the highest odds ratio because the probability of disliking a class was modeled.
3.4. Univariable analyses
Number of years in clinical practice (clinical experience), year of graduation from veterinary school, and nature of clinical position were the only explanatory demographic variables that were significantly associated with the outcome variable (Table 3). Compared to clinicians with more years in clinical practice, those with less were significantly less concerned about AMR (OR = 0.95). In other words, that the estimated odds of being less concerned about AMR decreased by 5% for every year in clinical practice. Similarly, compared to clinicians who graduated from 1970 to 1999, those who graduated from 2000 to 2009 and 2010–2016 were 2.83 and 4.55 times less concerned about AMR, respectively. However, there was no significant difference observed between graduates of 2000–2009 and those of 2010–2016. House officers were 3 times less concerned about AMR in comparison to faculty members. There was no significant correlation between proportion of total professional activity dedicated to clinical practice (effort allocation to clinical practice) and frequency of prescription of antimicrobials for therapeutic treatment of infectious diseases (r = 0.20211, P = 0.1152). Likewise, there was no significant correlation between period of graduation from veterinary school and frequency of prescription of antimicrobials for therapeutic treatment of infectious diseases (r = 0.1654, P = 0.1989). However, number of years in clinical practice and year of graduation from veterinary school were highly correlated (r = 0.915, P < 0.001).
Table 3.
Univariable analyses for associations between various demographic predictors and clinicians’ degree of concern about antimicrobial resistant infections at University of Tennessee Veterinary Medical Center, 2017.
| Variable | Category | OR (95% CI) | P Value |
|---|---|---|---|
| Gender | Male vs *Female | 1.01 (0.37 – 2.74) | 0.307 |
| Number of years in clinical practice | 1-year increase | 0.95 (0.91 – 0.99) | 0.018 |
| Nature of clinical position | House officers vs *Faculty members | 3.19 (1.04 – 9.79) | 0.043 |
| Year of graduation from veterinary school | ††Overall | – | 0.040 |
| 2000–2009 vs *1970–1999 | 2.83 (0.91 – 8.77) | 0.071 | |
| 2010–2016 vs *1970–1999 | 4.55 (1.35 – 15. 38) | 0.015 | |
| 2010–2016 vs *2000–2009 | 1.61 (0.49 – 5.25) | 0.431 | |
| Where veterinary degree was obtained | U.S. vs *Non-U.S. | 1.79 (0.54 – 5.94) | 0.343 |
| Specialty board certification | No vs *Yes | 2.84 (0.98 – 8.19) | 0.054 |
| Primary patient load | ††Overall | – | 0.164 |
| Food animal vs *Small animal | 4.14 (0.82 – 21) | 0.086 | |
| Equine vs *Small animal | 2.34 (0.55 – 9.97) | 0.251 | |
| Food animal vs *Others† | 1.36 (0.2 – 9.16) | 0.755 | |
| Food animal vs *Equine | 1.77 (0.24 – 12.91) | 0.573 | |
| Others† vs *Equine | 1.31 (0.22 – 7.77) | 0.768 | |
| Others† vs *Small animal | 3.06 (0.77 – 11.88) | 0.107 |
Reference category.
A combination of mixed animal and exotics.
Overall = overall effect of predictor on outcome variable.
3.5. Multivariable analyses
In the multivariable cumulative logit model, year of graduation from veterinary school was significantly associated (P = 0.025) with clinicians’ degree of concern about AMR, after controlling for clinicians’ primary patient load which was a confounder in the model (Table 4). Compared to clinicians who obtained their veterinary degree from 1970 to 1999, those who graduated from 2000 to 2009 and 2010 to 2016 were 3.96 (P = 0.034) and 5.39 (P = 0.01) times less concerned about AMR, respectively.
Table 4.
Cumulative logit model of multivariable analyses of factors associated with clinicians’ degree of concern about antimicrobial resistant infections at the University of Tennessee Veterinary Medical Center, 2017.
| Variable | Category | OR (95% CI) | P Value |
|---|---|---|---|
| Year of graduation from veterinary school | 2000–2009 vs *1970–1999 | 3.69 (1.104 – 12.33) | 0.034 |
| 2010–2016 vs *1970 – 1999 | 5.39 (1.49 – 19. 51) | 0.010 | |
| 2010–2016 vs *2000 – 2009 | 1.46 (0.44 – 4.87) | 0.537 | |
| Primary patient load | Food animal vs *Small animal | 3.32 (0.64 – 17.25) | 0.153 |
| Equine vs *Small animal | 3.9 (0.83 – 18.36) | 0.085 | |
| Others† vs *Food animal | 1.14 (0.16 – 8.22) | 0.894 | |
| Equine vs *Food animal | 1.18 (0.15 – 9.44) | 0.879 | |
| Equine vs *Others† | 1.03 (0.16 – 6.49) | 0.977 | |
| Others† vs *Small animal | 3.8 (0.91 – 15.80) | 0.067 |
A combination of mixed animal and exotics;
Reference category.
4. Discussion
In the present study, we have shown that controlling for UTVMC clinicians’ primary patient load, clinicians’ concern about AMR decreased among those who graduated after 1999 compared to those that had been in clinical practice for longer. There are two possible explanations for this finding. Firstly, clinicians who graduated from 1970-1999 could have been more experienced and had received greater exposure and awareness about the risks associated with AMR than those who graduated after 1999. Alternatively, the result perhaps reflects an inadequate emphasis on the judicious use of antimicrobial drugs in the veterinary curriculum over the recent years. The latter may be true because teaching of AMR and antimicrobial pharmacology in most veterinary schools has been described as inadequate (Guardabassi & Prescott, 2015). In fact, most clinicians in the present study expressed less enthusiasm about the adequacy of training on rational AMU practices received by present day veterinary students. Before a generalized conclusion can be made from the observed results, further evaluation of the tested associations is needed from other veterinary teaching hospitals as well as from primary care veterinary hospitals. In the interim, educational interventions, such as an increased educational emphasis about AMS approaches for veterinary students and continuing professional development for practicing veterinarians aimed at promoting prudent AMU by veterinary clinicians at all levels of clinical experience, would be helpful in modifying prescription behaviors and practices of clinicians. Also, in this study, we found that many clinicians believed their clients were either not concerned about AMR, or were slightly concerned, suggesting a need for more client education on AMR.
The use of bacteriological culture and antimicrobial susceptibility test results, along with other Good Stewardship Practices (GSP), is very important in the practice of evidence-based antimicrobial therapy (Guardabassi and Prescott, 2015, Prescott and Boerlin, 2016, Rubin, 2013). Based on predisposition for choice of and source of information for antimicrobial drugs, clinicians in the present study utilized evidence-based approach in their prescription practices. Firstly, 47 clinicians (75.8%) reported results from bacteriological culture and susceptibility tests to be an extremely important factor in deciding their choice of antimicrobial. This is consistent with the findings of other studies, (De Briyne et al., 2013, Jacob et al., 2015) where veterinarians rated bacteriologic culture and antimicrobial susceptibility among the most important factors in clinical decision-making. Next, cultural measures of uncertainty avoidance and wide power distance between the clinician and client/producer may influence antimicrobial prescribing practices (Cheng and Worth, 2015, Hulscher et al., 2010). Clinicians with high uncertainty avoidance would probably prescribe antimicrobials in the event of undesirable clinical outcomes. Likewise, fear of litigation by the client/producer could influence the clinician to yield to client's requests on AMU. However, these factors were not identified as major drivers in AMU practice in the present study. Pressure from clients or producers to the clinician to prescribe antimicrobials was not at all important to over 45% of the clinicians in the present study. Similarly, fear of litigation by the client or producer was not an important factor. Evidently, power distance (the extent to which power is distributed between the clinician and the client or producer based on their hierarchical distance in the society) is narrow in the UTVMC. Thus, uncertainty avoidance may not be a very influential factor in prescription decision-making in this hospital. Furthermore, aggressive marketing by pharmaceutical companies is believed to influence clinicians’ information about antimicrobials. In a survey of small animal veterinarians in the UK, 331 clinicians (70%) ranked pharmaceutical companies as an important source of information on antimicrobial drugs (Hughes et al., 2012). However, among the 62 clinicians in the present study, 55% rated pharmaceutical company representatives as “not at all important” but over 56% rated peer-reviewed literature as “extremely important” sources of information for antimicrobial products. A survey at another U.S. veterinary teaching hospital identified peer-reviewed literature as an important source of antimicrobial information utilized by most clinicians in determining their choice of antimicrobial (Jacob et al., 2015). But, in a survey of all companion animal veterinarians in Australia 260 clinicians (36%) reported using peer-reviewed literature as a source of information on antimicrobials (Hardefeldt et al., 2017). Possibly, compared to veterinarians in general care hospitals, those in referral hospitals rely more on peer-reviewed literature for their sources of antimicrobial information. In summary, it is reassuring that clinicians in the present study utilize evidence-based approach in their prescription practices, an attitude that would improve success of an AMS program.
To promote judicious AMU practices, FDA and AVMA developed guidelines for judicious antimicrobials by veterinary clinicians. However, the uptake of this AMU guidelines among the clinicians at UTVMC appear low. Although a few clinicians were either not at all familiar with or never used VCPR, coincidentally these clinicians had clinical duties that did not directly involve antimicrobial prescription. Nevertheless, this observation does not justify a non-judicious AMU practice. Only six clinicians (9.7%) read very often the FDA/AVMA guidelines for judicious use of antimicrobials while the rest either never read or infrequently read the guidelines. Apparently, little awareness exists among these clinicians about the existing guidelines for judicious use of antimicrobials. A recent survey of U.S. veterinarians (Grayzel et al., 2015) found that 218 of 247 (88%) clinicians were unaware of the available guidelines for judicious AMU practices. However, implementation of AMU guidelines led to significant decrease in antimicrobial prescription rates in some human pediatric emergency departments (Ouldali et al., 2017) and compliance with AMU guidelines may have led to a reduction in overall AMU at a veterinary teaching hospital (Weese, 2006). Therefore, more awareness and compliance is needed about the available AMU guidelines for veterinary clinicians. Furthermore, only nine clinicians (14.5%) knew that UTVMC does not have an AMS program currently. Others were either uncertain or believed that an AMS program existed. These disparities might be due to variations in knowledge and awareness among clinicians about what constitutes an AMS program, suggesting a need for more training and awareness on AMS and GSP.
Antimicrobial stewardship programs involve multifaceted approaches that aim to sustain the efficacy of antimicrobial drugs, while minimizing the emergence of AMR (Prescott & Boerlin, 2016). Clinician preference for certain antimicrobials is justified in certain situations e.g. based on knowledge of drug toxicity such as aminoglycoside toxicity; when the characteristics of the infecting bacteria at a given infection site are known; when knowledge of the usual susceptibility profile of the suspected pathogens is available; when the cost of treatment is an issue; when observation of AMU regulations is required (Giguère, 2013). Also, a clinician may prefer a certain antimicrobial when based on his or her judgment, culture and susceptibility testing shows that it is the only treatment option (Aidara-Kane et al., 2018). Frequent use of preferred antimicrobial classes will lead to prolonged exposure of bacteria to these drugs and subsequently select for resistance. In the present study, β-lactams, were the most preferred antimicrobial classes by small animal clinicians. Recent studies of veterinary antimicrobial prescribing practices in the U.S. also showed that β-lactams are the most commonly prescribed antimicrobials by veterinarians (Baker et al., 2012, Fowler et al., 2016). The antimicrobial preference ordering for food animal, equine and other clinicians was not reported because of the few respondents in these categories which did not allow for meaningful analysis. Similarly, our study did not evaluate the preference for specific drugs within an antimicrobial class. Future studies could benefit from evaluating clinicians’ preference for specific drugs within antimicrobial classes. Such scrutiny could provide additional details about prudent AMU. Implementation of AMS strategies (Weese et al., 2015), such as de-escalation (reduction in the spectrum of antimicrobials used through the discontinuation of antimicrobials or switching to a narrow-spectrum antimicrobial) and antimicrobial cycling (rotational use of two or more antimicrobial classes on a specified time scale) could minimize likely buildup of AMR to the most preferred classes at this hospital. Additionally, non-judicious AMU for surgical prophylaxis may exert selection pressure leading to AMR. In routine surgical practice, antimicrobials may be given prophylactically: pre-operatively, peri‑operatively or post-operatively, often based on the judgment of the surgeon. These AMU for surgical prophylaxis is especially important when surgeries are performed either in suboptimal conditions, such as in farm animal practice, (Dumas et al., 2016) or when the surgical procedure is classified as contaminated (Boothe & Boothe, 2015). Surgical prophylaxis is not recommended for neutering and routine uncomplicated dental procedures (Hardefeldt et al., 2017). In the present study, most clinicians used antimicrobials for surgical prophylaxis in more than half of their surgical cases. Although we did not ascertain the types of surgical cases for which antimicrobials were used, we contend that an AMS program at this hospital would provide guidance on AMU for surgical prophylaxis. There is need to develop and implement an AMS program at UTVMC based on the findings of the knowledge gaps or current AMU practice at this hospital. Through training, antimicrobial prescribers are more likely to accept and implement AMS after benefits are evident; this approach reduces their non-judicious AMU practices (Guardabassi & Prescott, 2015). In the absence of an AMS program and training programs, AMR challenge could be evident in this hospital. Also, it would be useful to explore the AMU practices among other primary and tertiary veterinary hospitals in the U.S. Such nationwide survey would provide details on the feasibilities of reducing AMR burden at a national scale.
A 2004/2005 observational study of Norwegian general medical practitioners found that antimicrobial prescribing rates of physicians significantly increased with increased number of consultations (Gjelstad et al., 2011). Findings from this Norwegian study suggested that busy physicians may rely on antimicrobials in presence of diagnostic uncertainty, as the consultation duration may be short to conduct a proper clinical investigation. At the design of this present study, we had hypothesized that busy veterinary clinicians with less effort allocation to clinical practice and more effort allocation to other non-clinical duties would perhaps play safe by prescribing broad-spectrum antimicrobials as a timesaving strategy in the face of diagnostic uncertainties. However, effort allocation to clinical practice was not significantly correlated with frequency of prescription of antimicrobials at UTVMC. Possibly, the difference in these observations could be from the nature of patients seen or the expertise level of the clinicians. We contend that the findings of this study cannot be extrapolated to first opinion (primary care) veterinary practices because clinicians in primary care may have different AMU practices than those in tertiary hospitals who are mostly comprised of specialists in their fields. An evaluation of the association between effort allocation and frequency of antimicrobial prescription at other veterinary schools and in primary care veterinary hospitals would be useful in providing a better justification for this disparity.
There is a growing perception among veterinarians that non-judicious AMU practices occur in veterinary practice. In this study, 21 clinicians (33.9%) mentioned that antimicrobials were sometimes prescribed based on no documented evidence of infection, while 38 (61.3%) mentioned that antimicrobials were sometimes prescribed for suspected (but not confirmed) infections. A recent retrospective study (Wayne et al., 2011) from a veterinary school showed similar findings: 38% of antimicrobial prescription did not have documented evidence of infection, while 45% of antimicrobial prescriptions at that hospital were for suspected infections. In the present study, 32 clinicians (51.6%) believed that antimicrobials were over-prescribed. Clinicians in another U.S. teaching hospital (Jacob et al., 2015) also held a similar view that antimicrobials were overprescribed. Therefore, it is necessary to conduct a targeted study evaluating actual prescription records in these hospitals to validate or dispute the perceived non-judicious AMU practices.
Communicating the importance of the survey along with sending reminders to respondents through diverse media has been suggested to improve response rates (Postma et al., 2016). Response rate in the present study was higher than other surveys among veterinarians in the U.S. and elsewhere (Chipangura et al., 2017, Fowler et al., 2016, Grayzel et al., 2015, Jacob et al., 2015, Postma et al., 2016). Attending departmental and weekly clinical rounds meetings before the survey as well as sending out weekly email reminders to participants during the survey duration could have contributed to the observed high response rate of 51.2%.
Although bias was not assessed, results of this study could have been influenced by response and or non-response bias. Social desirability bias (which is a form of response bias) and non-response bias can be issues in any survey (Sax, Gilmartin, & Bryant, 2003). Possibly, the clinicians provided answers that they deemed socially acceptable (social desirability bias) rather than their true opinions, perceptions and practices. Alternatively, the survey answers of the respondents could have differed from those of non-respondents. Non-responder analysis was not performed because it would breach the confidentiality and anonymity of the study. Furthermore, results of this study may be more reflective of opinion and perceptions of small animal clinicians than other clinicians because of the over representation of small animal clinicians in the study. However, this observation is a true representation of the clinician demographics in this hospital and could not have been improved by any other method.
5. Conclusions
After controlling for UTVMC clinicians’ primary patient load, clinicians’ concern about AMR decreased among those who graduated after 1999 compared to those that have been in clinical practice for longer. Most clinicians utilize evidence-based approach in their choice of antimicrobials but are unaware or underutilize the FDA/AVMA guidelines for judicious use of antimicrobials. Some practices and perceptions are suggestive of non-judicious AMU practices. Therefore, there is a critical need to increase awareness about judicious AMU practices among clinicians, increase emphasis about AMR in the present veterinary curriculum, and implement AMS program in this institution. Educational activities in combination with awareness campaigns and the stewardship programs could be used to improve AMU practices of veterinary clinicians at this hospital. Also, more client education on AMR is needed. Prospectively, evaluation of AMU practices across other veterinary hospitals in the U.S is necessary to provide details on the feasibilities of reducing AMR burden at a national scale.
Competing interests
The authors declare that they have no financial or personal relationships which may have inappropriately influenced them in writing this article.
Acknowledgments
Acknowledgments
The authors thank Ms. Cary Springer, Drs. Nancy Howell, J. Mark Fly, and Agricola Odoi for technical assistance.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
John Eddie Ekakoro, Email: jekakoro@vols.utk.edu.
Chika C. Okafor, Email: okaforch@utk.edu.
References
- Aidara-Kane A., Angulo F.J., Conly J.M., Minato Y., Silbergeld E.K., McEwen S.A. World Health Organization (WHO) guidelines on use of medically important antimicrobials in food-producing animals. Antimicrobial Resistance & Infection Control. 2018;7:7. doi: 10.1186/s13756-017-0294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AVMA VCPR: The veterinarian-client-patient relationship. 2018. https://www.avma.org/KB/Resources/Reference/Pages/VCPR.aspx Accessed 18 July 2018.
- Baker S.A., Van-Balen J., Lu B., Hillier A., Hoet A.E. Antimicrobial drug use in dogs prior to admission to a veterinary teaching hospital. Journal of the American Veterinary Medical Association. 2012;241(2):210–217. doi: 10.2460/javma.241.2.210. [DOI] [PubMed] [Google Scholar]
- Boothe D.M., Boothe H.W. Antimicrobial considerations in the perioperative patient. Veterinary Clinics of North America: Small Animal Practice. 2015;45(3):585–608. doi: 10.1016/j.cvsm.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Cheng A.C., Worth L.J. Cultural dimensions relevant to antimicrobial stewardship: The contribution of individualism and power distance to perioperative prescribing practices in European hospitals. Healthcare Infection. 2015;20(3–4):124–127. [Google Scholar]
- Chipangura J.K., Eagar H., Kgoete M., Abernethy D., Naidoo V. An investigation of antimicrobial usage patterns by small animal veterinarians in South Africa. Preventative Veterinary Medicine. 2017;136:29–38. doi: 10.1016/j.prevetmed.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Chung Y.S., Hu Y.S., Shin S., Lim S.K., Yang S.J., Park Y.H. Mechanisms of quinolone resistance in Escherichia coli isolated from companion animals, pet-owners, and non-pet-owners. Journal of Veterinary Science. 2017;18(4):449–456. doi: 10.4142/jvs.2017.18.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'costa V.M., King C.E., Kalan L., Morar M., Sung W.W. Antibiotic resistance is ancient. Nature. 2011;477(7365):457. doi: 10.1038/nature10388. [DOI] [PubMed] [Google Scholar]
- De Briyne N., Atkinson J., Pokludova L., Borriello S.P., Price S. Factors influencing antibiotic prescribing habits and use of sensitivity testing amongst veterinarians in Europe. Veterinary Record. 2013;173(19):475. doi: 10.1136/vr.101454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohoo I.R., Martin W., Stryhn H. Second ed. Charlottetown, PE; Canada: VER: 2003. Veterinary epidemiologic research. Inc, (Chapter 13) [Google Scholar]
- Dumas S.E., French H.M., Lavergne S.N., Ramirez C.R., Brown L.J., Bromfield C.R. Judicious use of prophylactic antimicrobials to reduce abdominal surgical site infections in periparturient cows: Part 1 – a risk factor review. Veterinary Record. 2016;178(26):654. doi: 10.1136/vr.i103677. [DOI] [PubMed] [Google Scholar]
- Dyar O.J., Obua C., Chandy S., Xiao Y., Stalsby Lundborg C., Pulcini C. Using antibiotics responsibly: Are we there yet. Future Microbiology, 2016;11:1057–1071. doi: 10.2217/fmb-2016-0041. [DOI] [PubMed] [Google Scholar]
- FDA 2013 summary report on antimicrobials sold or distributed for use in food-producing animals. 2015. https://www.fda.gov/downloads/ForIndustry/UserFees/AnimalDrugUserFeeActADUFA/UCM440584.pdf/ (April 2015) Accessed July 22 2018.
- FDA Judicious use of antimicrobials for beef cattle veterinarians. 2017. https://www.fda.gov/downloads/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/JudiciousUseofAntimicrobials/UCM095568.pdf Acessed 22 July 2018.
- Fowler H., Davis M., Perkins A., Trufan S., Joy C., Buswell M. A survey of veterinary antimicrobial prescribing practices, Washington State 2015. Veterinary Record. 2016;179(25):651. doi: 10.1136/vr.103916. [DOI] [PubMed] [Google Scholar]
- Giguère S. Principles of antimicrobial drug selection and use. In: Giguère S., Prescott J.F., Dowling P.M., editors. In antimicrobial therapy in veterinary medicine. Fifth Edition. John Wiley & Sons, Inc; 2013. pp. 105–115. [DOI] [Google Scholar]
- Gjelstad S., Straand J., Dalen I., Fetveit A., Strøm H., Lindbæk M. Do general practitioners’ consultation rates influence their prescribing patterns of antibiotics for acute respiratory tract infections? Journal of Antimicrobial Chemotherapy. 2011;66(10):2425–2433. doi: 10.1093/jac/dkr295. [DOI] [PubMed] [Google Scholar]
- Grayzel S.E., Bender J.B., Glore R.P., Gumley N., Sykes J.E., Whichard J.M. Understanding companion animal practitioners’ attitudes toward antimicrobial stewardship. Journal of the American Veterinary Medical Association. 2015;247(8):883–884. doi: 10.2460/javma.247.8.883. [DOI] [PubMed] [Google Scholar]
- Green A.L., Carpenter L.R., Edmisson D.E., Lane C.D., Welborn M.G., Hopkins F.M. Producer attitudes and practices related to antimicrobial use in beef cattle in Tennessee. Journal of the American Veterinary Medical Association. 2010;237(11):1292–1298. doi: 10.2460/javma.237.11.1292. [DOI] [PubMed] [Google Scholar]
- Guardabassi L., Loeber M.E., Jacobson A. Transmission of multiple antimicrobial-resistant Staphylococcus intermedius between dogs affected by deep pyoderma and their owners. Veterinary Microbiology. 2004;98(1):23–27. doi: 10.1016/j.vetmic.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Guardabassi L., Prescott J.F. Antimicrobial stewardship in small animal veterinary practice. Veterinary Clinics of North America: Small Animal Practice. 2015;45(2):361–376. doi: 10.1016/j.cvsm.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Hardefeldt L.Y., Browning G.F., Thursky K., Gilkerson J.R., Billman-Jacobe H., Stevenson M.A. Antimicrobials used for surgical prophylaxis by companion animal veterinarians in Australia. Veterinary Microbiology. 2017;203:301–307. doi: 10.1016/j.vetmic.2017.03.027. [DOI] [PubMed] [Google Scholar]
- Holmes A.H., Moore L.S., Sundsfjord A., Steinbakk M., Regmi S., Karkey A. Understanding the mechanisms and drivers of antimicrobial resistance. The Lancet. 2016;387(10014):176–187. doi: 10.1016/s0140-6736(15)00473-0. [DOI] [PubMed] [Google Scholar]
- Hughes L.A., Williams N., Clegg P., Callaby R., Nuttall T., Coyne K. Cross-sectional survey of antimicrobial prescribing patterns in UK small animal veterinary practice. Preventive Veterinary Medicine. 2012;104(3):309–316. doi: 10.1016/j.prevetmed.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Hulscher M.E., van der Meer J.W., Grol R.P. Antibiotic use: How to improve it. International Journal of Medical Microbiology. 2010;300(6):351–356. doi: 10.1016/j.ijmm.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Jacob M.E., Hoppin J.A., Steers N., Davis J.L., Davidson G., Hansen B. Opinions of clinical veterinarians at a US veterinary teaching hospital regarding antimicrobial use and antimicrobial-resistant infections. Journal of the American Veterinary Medical Association. 2015;247(8):938–944. doi: 10.2460/javma.247.8.938. [DOI] [PubMed] [Google Scholar]
- Kuzi S., Blum S., Kahane N., Adler A., Hussein O., Segev G. Multi‐drug‐resistant Acinetobacter calcoaceticus‐Acinetobacter baumannii complex infection outbreak in dogs and cats in a veterinary hospital. Journal of Small Animal Practice. 2016;57(11):617–625. doi: 10.1111/jsap.12555. [DOI] [PubMed] [Google Scholar]
- Loeffler A., Boag A.K., Sung J., Lindsay J.A., Guardabassi L., Dalsgaard A. Prevalence of methicillin-resistant Staphylococcus aureus among staff and pets in a small animal referral hospital in the UK. Journal of Antimicrobial Chemotherapy. 2005;56(4):692–697. doi: 10.1093/jac/dki312. [DOI] [PubMed] [Google Scholar]
- Marshall B.M., Levy S.B. Food animals and antimicrobials: Impacts on human health. Clinical Microbiology Reviews. 2011;24(4):718–733. doi: 10.1128/cmr.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachran A.D., Blackwell B.R., Hanson J.D., Wooten K.J., Mayer G.D., Cox S.B. Antibiotics, bacteria, and antibiotic resistance genes: Aerial transport from cattle feed yards via particulate matter. Environmental Health Perspectives. 2015;123(4):337. doi: 10.1289/ehp.1408555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay R., Mah A., Law M.R., McGrail K., Patrick D.M. Systematic review of factors associated with antibiotic prescribing for respiratory tract infections. Antimicrobial Agents and Chemotherapy. 2016;60(7):4106–4118. doi: 10.1128/aac.00209-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okwechime I.O., Roberson S., Odoi A. Prevalence and predictors of pre-diabetes and diabetes among adults 18 years or older in Florida: A multinomial logistic modeling approach. PloS one. 2015;10(12) doi: 10.1371/journal.pone.0145781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouldali N., Bellettre X., Milcent K., Guedj R., de Pontual L., Cojocaru B. Impact of implementing national guidelines on antibiotic prescriptions for acute respiratory tract infections in pediatric emergency departments: an interrupted time series analysis. Clinical Infectious Diseases. 2017;65(9):1469–1476. doi: 10.1093/cid/cix590. [DOI] [PubMed] [Google Scholar]
- Perry J., Waglechner N., Wright G. The prehistory of antibiotic resistance. Cold Spring Harbor Perspectives in Medicine. 2016;6(6) doi: 10.1101/cshperspect.a025197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomba C., Rantala M., Greko C., Baptiste K.E., Catry B., van Duijkeren E. Public health risk of antimicrobial resistance transfer from companion animals. Journal of Antimicrobial Chemotherapy. 2017;72(4):957–968. doi: 10.1093/jac/dkw481. [DOI] [PubMed] [Google Scholar]
- Postma M., Speksnijder D.C., Jaarsma A.D., Verheij T.J., Wagenaar J.A., Dewulf J. Opinions of veterinarians on antimicrobial use in farm animals in Flanders and the Netherlands. Veterinary Record. 2016;179(3):68. doi: 10.1136/vr.103618. [DOI] [PubMed] [Google Scholar]
- Prescott J.F., Boerlin P. Antimicrobial use in companion animals and Good Stewardship Practice. Veterinary Record. 2016;179(19):486–488. doi: 10.1136/vr.i5908. [DOI] [PubMed] [Google Scholar]
- Qekwana D.N., Oguttu J.W., Sithole F., Odoi A. Burden and predictors of Staphylococcus aureus and S. pseudintermedius infections among dogs presented at an academic veterinary hospital in South Africa (2007–2012) PeerJ. 2017;5:e3198. doi: 10.7717/peerj.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J.E. Antimicrobial susceptibility testing methods and interpretation of results. In: Giguère S., Prescott J.F., editors. Antimicrobial Therapy in Veterinary Medicine. 5th ed. John Wiley & Sons, Inc; 2013. pp. 11–20. [DOI] [Google Scholar]
- Sax L.J., Gilmartin S.K., Bryant A.N. Assessing response rates and nonresponse bias in web and paper surveys. Research in Higher Education. 2003;44(4):409–432. doi: 10.1023/a:1024232915870. [DOI] [Google Scholar]
- Van Boeckel T.P., Brower C., Gilbert M., Grenfell B.T., Levin S.A., Robinson T.P. Global trends in antimicrobial use in food animals. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(18):5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther B., Tedin K., Lübke-Becker A. Multidrug-resistant opportunistic pathogens challenging veterinary infection control. Veterinary Microbiology. 2017;200:71–78. doi: 10.1016/j.vetmic.2016.05.017. [DOI] [PubMed] [Google Scholar]
- Wayne A., McCarthy R., Lindenmayer J. Therapeutic antibiotic use patterns in dogs: Observations from a veterinary teaching hospital. Journal of Small Animal Practice. 2011;52(6):310–318. doi: 10.1111/j.1748-5827.2011.01072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese J.S. Investigation of antimicrobial use and the impact of antimicrobial use guidelines in a small animal veterinary teaching hospital: 1995–2004. Journal of the American Veterinary Medical Association. 2006;228(4):553–558. doi: 10.2460/javma.228.4.553. [DOI] [PubMed] [Google Scholar]
- Weese J.S., Giguère S., Guardabassi L., Morley P.S., Papich M., Ricciuto D.R. ACVIM consensus statement on therapeutic antimicrobial use in animals and antimicrobial resistance. Journal of Veterinary Internal Medicine. 2015;29(2):487–498. doi: 10.1111/jvim.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]