Abstract
The aim of this study was to evaluate the efficacy of treatment with estradiol benzoate (EB) at luteal phase prior to the ovum pick-up (OPU) during in vitro production of transferable embryos in Japanese Black cattle. A total of 15 cows were used as oocyte donors for OPU. Of those, four donors were randomly allocated (three times) into each of two treatment groups as a crossover study, and OPU session was carried out six times per one donor. Another eleven donors were used in a paired difference test by one crossover trial. Donors in the control group received no hormonal treatment; whereas, donors in the EB group received 1 mg of EB as a single injection. First, we observed dynamics of ovarian follicles and emergence of follicular wave after EB injection using transrectal ultrasonography. The number and proportion of medium-sized follicles with 4 to 6 mm in diameter increased gradually and achieved a peak at 72 and 96 hours after EB injection. The OPU was performed 88 hours after EB injection. The EB-treated donors had a higher proportion of follicles with 4 to 6 mm in diameters at the time of OPU. The stimulation with EB significantly increased the numbers of follicles aspirated, and the good quality cumulus-oocyte complexes per OPU. Furthermore, in the EB group, the percentage of transferable blastocysts was significantly greater than that in the control group (P<0.05). In conclusion, a single EB injection before OPU increases the number of medium-sized follicles and can produce more transferable embryos.
Keywords: Ovum pick up, In vitro embryo production, Estradiol benzoate, Bovine
Highlights
-
•
Efficacy of one-shot treatment with 1 mg of estradiol benzoate (EB) at luteal phase prior to the ovum pick-up (OPU) for in vitro production of transferable embryos in Japanese Black cattle was investigated.
-
•
The donors had a higher proportion of follicles with 4 to 6 mm in diameter at 72 and 96 hours after EB i.m. injection.
-
•
The stimulation with EB before OPU increased significantly the number of good quality cumulus-oocyte complexes, and the percentage of blastocysts or transferable embryos after in vitro fertilization.
1. Introduction
Embryo transfer (ET) of in vitro production (IVP) embryos enables cattle industries to enhance and accelerate the diffusion of production traits (Kruip et al., 1994, Numabe et al., 2000, Numabe et al., 2001; Stringfellow, Givens, 2010). The transfer of IVP embryos from Japanese Black donors to Holstein recipients increases the number of beef calves (Numabe et al., 2000, Numabe et al., 2001). Even if the cost of ET is twice more expensive than artificial insemination (AI), it is still profitable due to the increased revenue from beef calf sales in addition to the dairy production. Therefore, we are committed to the spread of ET. To achieve our aims, it was necessary to produce valuable embryos at a lower cost.
In the earliest years, bovine embryo production for ET was mainly in vivo-fertilized embryos from superovulated heifers or cows (Bousquet et al., 1990, Pontes et al., 2009). Currently, the retrieval of oocytes using ultrasound guided follicle puncture, or ovum pick-up (OPU), is linked to the procedures for IVP embryos, as it can exploit more Japanese Black cattle embryos (Numabe et al., 2000, Numabe et al., 2001). The OPU is a valuable technology that greatly enhances the potential of in vitro fertilization (IVF) systems in a variety of breeding conditions and species: Holstein (Ogata et al., 2016, Vieira et al., 2014), Angus cross (Chaubal et al., 2007), Nelore (Pontes et al., 2009), Buffalo (Presicce et al., 2002, Presicce, 2007) and Japanese Black (Numabe et al. 2001) cattle.
For effective production of more IVP embryos by OPU-IVF, FSH stimulation treatment can promote the development of multiple follicles in the ovaries and improve embryos yield in non-lactating or lactating Holstein donors (De Roover et al., 2005, De Roover et al., 2008, Vieira et al., 2014). On the other hand, non-lactating Holstein cows can produce a higher blastocyst rate and a higher number of transferable embryos than lactating cows regardless of FSH stimulation treatment (Sendag et al., 2008, Vieira et al., 2014). In OPU without hormonal stimulation, ovum collection can be repeated every 1 to 3 weeks (Kruip et al., 1994). When comparing the number of embryos produced by one donor in 1 year, more than 2-fold embryos are produced in vitro than in vivo (Goodhand et al., 1999, Kruip et al., 1994, Numabe et al., 2000, Pontes et al., 2009).
Breeding techniques which utilize gonadotropin-releasing hormone (GnRH) to control the follicular wave and ovulation synchronization methods, such as Ovsynch, have been developed (Pursley, Mee & Wiltbank, 1995). Previously, we reported that GnRH-stimulation before OPU improved the efficiency of embryo production in Holstein cows during early lactation (Ogata, Hidaka, Matzushige & Maeda,2015).
Ovarian follicular growth and development in bovines is characterized by two or three consecutive follicular waves per estrous cycle (Ginther et al., 1989, Sirois and Fortune, 1988). Each wave involves the recruitment of a cohort of follicles and the selection of a dominant follicle. The growth of follicular waves is initiated by a rise in circulating FSH. All follicles growing as a cohort contain specific receptors for FSH and depend on this gonadotropin when growing. Whereas, estradiol secretion is inversely related to FSH secretion and closely regulate the emergence and growth of follicular waves (Evans, Komar, Wandji & Fortune, 1997). Ovarian estradiol secretion is important for the final stages of dominant follicle growth (Evans et al., 1997). The dominant follicle is the major source of the fluctuations in circulating steroid concentrations and therefore is primarily responsible for the negative feedback effects of ovarian steroids during waves of follicular development (Araujo et al., 2009).
Administration of exogenous estradiol-17ß (E2) in progesterone-implanted cattle suppressed the dominant follicle and resulted in the consistent emergence of a new follicular wave, on average 4.3 days later, regardless of the stage of development of the dominant follicle (Araujo et al., 2009, Bo et al., 1995). Furthermore, the dynamics of ovarian follicular wave development during the estrous cycle can be manipulated by treating with estradiol benzoate (EB) to synchronize proestrous development of ovulatory follicle (Burke et al., 2000, Martinez et al., 2005). These data suggest that treatment with progesterone and E2, in combination, can be used to effectively control and synchronize follicular wave development. However, in OPU, there are few reports of treatment with EB alone, without the combination with progesterone source.
In this study, we hypothesized that the administration of EB prior to OPU would improve embryo production by OPU-IVF. We report here on the effectiveness of treatment with a single administration of EB prior to OPU for increasing the number of good quality embryos.
2. Materials and methods
2.1. Animals and Experimental design
Animal experiments in this study were approved by the Institutional Animal Experimental Committee of Hiroshima Prefectural Livestock Technology Research Center, where the experiments were performed. A total of four Japanese Black cows were used as donors for experiments. They were kept in stalls and fed grass silage and water.
Experiment 1 was designed to evaluate dynamics of ovarian follicles over time and emergence of follicular wave after one-shot EB (1 mg; estradiol benzoate, ASKA Animal Health Co., Ltd, Tokyo, Japan) intramuscular injection. In this experiment, four donors at luteal phase were stimulated with EB injection. At 0, 24, 48, 72, 96 and 120 hours after EB injection, ovarian follicles were visualized using a real-time ultrasound scanner (SSD-1000 type, Aloka Co. Ltd., Tokyo, Japan) equipped with a 7.5 MHz convex array transducer (UHT-9106 type, Aloka) and the number of follicles in ovaries were counted on ultrasound video images. All visible follicles were quantified and classified according to their diameters (small follicles: 2 to 3 mm, medium follicles: 4 and 6 mm and large follicles: more than 7 mm).
Experiment 2 was designed to evaluate effects of EB injection on the number and quality of oocytes aspirated by OPU. In this experiment, four donors were randomly allocated three times into each of two treatment groups (OPU without hormones as control group or with EB injection as experiment group). The experimental design was both group crossover study, and the OPU sessions were carried out total six times per one donor (Fig. 1A and B) The OPU was performed about 88 hours after EB injection according to the results of Experiment 1. Each OPU session was performed at more than four weeks intervals to avoid effects from repeated OPU and EB injection. In the preliminary study, we found that the number of COCs recovered decreased with the shorter intervals at less than 3-weeks.
Fig. 1.
Hormone treatment of four donors and ovum-pick up (OPU) schedule as the crossover design. Fig. 1A: control: The animals were not treated with hormone. OPU was performed on random days of estrous cycle. EB: Estradiol benzoate (EB) treatment at dosage of 1.0 mg IM was simultaneously administered. OPU was performed 88 hours after EB injection. Fig. 1B: Four donors were randomly allocated three times into each of two groups with/out the hormone (EB). OPU sessions was carried out total six times per one donor.
Immediately before the OPU session, both ovaries were examined by transrectal ultrasonography. All visible follicles were quantified and classified according to the criteria shown as above in experiment 1 for control and EB groups. Furthermore, we examined the number of cumulus-oocyte complexes (COCs) recovered, classified the quality of COCs, and then cultured them.
Then, using in vitro-matured oocytes obtained from living cattle by OPU in control and EB groups, we examined the embryo production following IVF. Embryo development and transferable blastocysts were evaluated under an inverted microscope according to the International Embryo Transfer Society (IETS) manual (4th Edition IETS, IL, USA) (Stringfellow, 2010). Evaluation of the quality of the embryo was based on its morphological integrity. Embryos classified as transferable were all of code 1(excellent/good).
Experiment 3 was designed to evaluate effects of EB injection on the number and quality of oocytes aspirated by OPU as a paired difference test by one crossover trial using eleven donors. The OPU was performed about 88 hours after EB injection according to the results of Experiment 1. We examined the number of COCs recovered, and the embryo development after IVF of COCs matured in vitro.
2.2. Follicle aspiration
Prior to follicular aspiration, all cows received a single dose of 80 mg Procaine hydrochloride (4 mL Adosan, Riken K.K., Tokyo, Japan) as epidural anesthetics to prevent abdominal straining and to relax the rectum, which was necessary for palpation of the ovaries for a long time. During the follicular aspiration, follicles were visualized using a real-time ultrasound scanner (SSD-1000 type, Aloka Co. Ltd., Tokyo, Japan) equipped with a 7.5 MHz convex array transducer (UHT-9106 type, Aloka). A 17-gauge disposable single lumen needle (COVA Needle, Misawa Medical, Tokyo, Japan) connected to a 50-mL conical tube via Teflon tubing was used for follicular puncture. Follicles greater than 2 mm in diameter were punctured using an aspirator (K-MAR-5115 type, Cook Medical Technology, Australia) equipped with a needle. The aspiration rate and vacuum pressure were 20 mL/min and 115 mmHg, respectively. Follicular fluids were collected in 50-mL conical centrifuge tubes (Sumitomo Bakelite, Tokyo, Japan). The collection medium was kept in a warm water bath at 35 °C. Lactate Ringer's Solution (Haruzen V, Zenoaq, Fukushima, Japan) supplemented with 0.3% fetal calf serum (FCS, Funakoshi, Tokyo, Japan), 10 units/mL heparin (Neotube, Nipro, Osaka, Japan) and 0.1 mg/mL kanamycin was used as the collection medium. The COCs were collected from the follicular aspirates.
2.3. Oocyte evaluation
The quality of COCs was graded according to morphologic criteria as described (De loos, Van, Vliet, Van, Maurik & Kurip, 1989). Briefly, grade A COCs had compacted and more than four layers of cumulus cells with a homogeneous ooplasm, grade B had a compacted and three or four layers of cumulus cells with a homogeneous ooplasm, grade C had a less compact cumulus cell layer with irregular ooplasm containing dark granules, grade D had denuded oocytes with no cumulus cells, and grade E had oocytes with expanded cumulus and a jelly-like matrix.
2.4. In vitro maturation (IVM), in vitro fertilization (IVF) and embryo culture
All COCs graded as A, B and C, were matured in IVM medium, as described by Kani et al. (Kani, Kuwahata, Ochi & Horiuchi, 2011) with minor modification. The IVM medium was tissue culture medium (TCM) 199 supplemented with pyruvate (0.25 mM), gentamycin sulfate (50 μg/mL), 10%FCS, 50 ng/mL epidermal growth factor (EGF, E-1264, Sigma-Aldrich, St. Louis, MO, U.S.A.), 10 µM dibutyryl-cyclic AMP (dbcAMP, D0260, Sigma-Aldrich) and 1 IU/ml FSH (0.12 Amour Unit/mL, Antrin 10, Kyoritu Seiyaku, Tokyo. Japan). Ten to 15 COCs were cultured in each 100-µl droplets of IVM medium under a layer of paraffin oil (Nacalai tesque, Kyoto, Japan) for 22 to 24 hours in a humidified atmosphere of 5% CO2 at 38.5 °C.
Frozen semen of the same lot of one bull was used in six OPU-IVF sessions of four donors in Experiment 2 to avoid effects from variations between different bulls. On the other hand, frozen semen of the same bull by one of six bulls was used in two OPU-IVF sessions of eleven donors in Experiment 3. The COCs were washed five times in fertilization medium (IVF-G, IFP, Yamagata, Japan) before being transferred to 10- to 50-μL droplets of IVF-G under mineral oil. They were co-incubated with spermatozoa at a concentration of 1.2×106 sperm/ml for 6 hours, and then the cumulus cells were removed (Ogata et al., 2015).
The presumptive zygotes were cultured in 50-μL droplets of modified synthetic oviduct fluid (m-SOF) medium supplemented with 6 mg/mL bovine serum albumin (BSA), 0.25 mg/mL linoleic acid albumin (L-8384, Sigma-Aldrich), 0.12 mg/mL glycine (G7126, Sigma-Aldrich), 0.25 mg/mL taurine (T8691, Sigma-Aldrich) and 10 µl/mL ITS (5 µg/mL Insulin, 5 µg/mL Transferrin and 5 ng/mL Selenium; I1884, Sigma) under a layer of paraffin oil (Nacalai tesque) in a humidified atmosphere of 5% O2, 5% CO2 and 90% N2 at 38.5 °C.
2.5. Statistical analyses
All statistical analyses were performed using GraphPad Prism software, Version 5.0 (GraphPad Software, Inc., San Diego, California, USA). Data were presented as means ± SEM in Table 1. Differences between two groups in the numbers of follicles, COCs, oocytes, and embryos were compared using student's t-test. Evaluation of COCs grade, distribution of follicle size and embryos development in Table 2, Table 3, Table 4, and Fig. 2, Fig. 3 were analyzed by Chi-square test. Significant differences were defined when P value was less than 0.05.
Table 1.
Effect of estradiol benzoate injection before ovum-pick up on the numbers of follicles aspirated and cumulus-oocyte complexes recovered.
| Treatment | No. of OPU trials* | No. of follicles aspirated (mean ± SE) | No. of COCs recovered (mean ± SE) | No. of COCs cultured† (mean ± SE) |
|---|---|---|---|---|
| None (control) | 12 | 334 (27.8±1.8)a | 262 (21.8±1.4)a | 180 (15.0±1.1)a |
| EB | 12 | 439 (36.6±3.0)b | 347 (28.9±2.9)b | 281 (23.4±2.4)b |
‡Abbreviations: COCs, cumulus-oocyte complexes; EB: estradiol benzoate; OPU, ovum pick-up.
a,b Values with different superscript letters in the same column are significantly different (P<0.05).
OPU were performed at more than three weeks interval using four donors at three times in each group randomly.
COCs at grade A to C were cultured for in vitro maturation of oocytes.
Table 2.
Morphological grade of cumulus-oocyte complexes retrieved by ovum-pick up from the donors pretreated with estradiol benzoate.
| Treatment | No. of COCs evaluated (No. of OPU session) | Grade A (%) | Grade B (%) | Grade C (%) | Grade D+E (%) |
|---|---|---|---|---|---|
| None (control) | 262 (12) | 22(8.4)a | 59 (22.5)a | 99 (37.8)a | 82 (31.3)a |
| EB | 347 (12) | 24(6.9)a | 110 (31.7)b | 147 (42.4)a | 66 (19.0)b |
* Abbreviations: COCs, cumulus-oocyte complexes; EB: estradiol benzoate; OPU, ovum pick-up.
† Grade A: more than four layers of cumulus cells with a homogeneous ooplasm, Grade B: a compacted and three or four layers of cumulus cells with a homogeneous ooplasm, Grade C: a less compact cumulus cell layer with irregular ooplasm containing dark granules, Grade D: denuded oocytes with no cumulus cells and Grade E: oocytes with expanded cumulus and a jelly-like matrix.
a-bValues with different superscript letters in the same column are significantly different by chi-square test (P<0.05).
Table 3.
Blastocyst development and transferable embryo production in donors pre-treated with estradiol benzoate.
| Treatment | No. of OPU trials | No. of oocytes inseminated | No. (%) of oocytes cleaved | No. (%) of blastocysts | No. (%) of transferable embryos |
|---|---|---|---|---|---|
| None (control) | 12 | 180 | 135 (75.0)a | 62 (34.4)a | 28 (15.6)a |
| EB | 12 | 281 | 217 (77.2)a | 142 (50.5)b | 92 (32.7)b |
* Abbreviations: EB: estradiol benzoate; OPU, ovum pick-up.
a,bValues with different superscript letters in the same column are significantly different (P<0.05).
Table 4.
Effect of pre-treatment with estradiol benzoate on blastocyst development and transferable embryo production: A paired difference test using 11 donors.
| Treatment | No. of OPU trials | No. of oocytes inseminated | No. (%) of oocytes cleaved | No. (%) of blastocysts | No. (%) of transferable embryos |
|---|---|---|---|---|---|
| None (control) | 11 | 242 | 143 (59.1)a | 82 (33.9)a | 38 (15.7)a |
| EB | 11 | 300 | 230 (76.7)b | 129 (43.0)b | 91 (30.3)b |
* Abbreviations: EB: estradiol benzoate; OPU, ovum pick-up.
a,bValues with different superscript letters in the same column are significantly different (P<0.05).
Fig. 2.
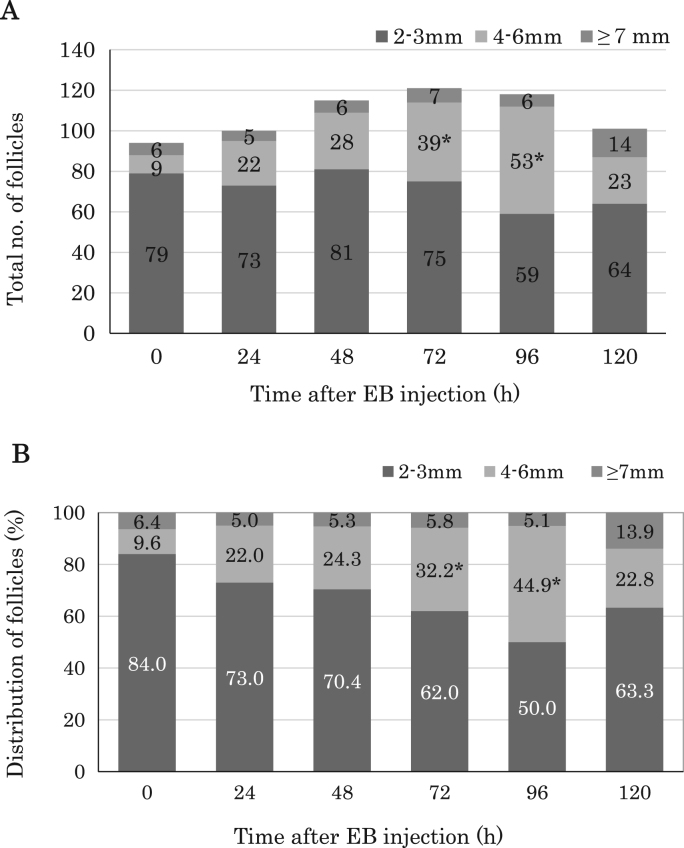
The number (A) and the percentage (B) of small (2–3 mm), medium (4–6 mm) and large follicles (≥7 mm) at 0, 24, 48, 72, 96 and 120 hours after EB injection in Japanese Black cows (n=4). *P <0.05, vs. 0 hour in medium follicles.
Fig. 3.
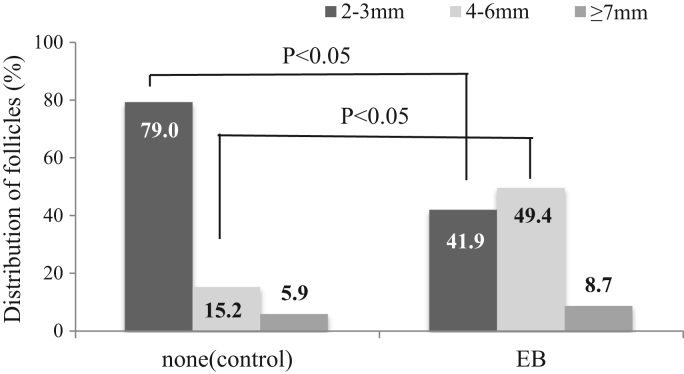
Distribution of a various size of follicles in the ovaries of donors pre-treated with estradiol benzoate (EB) at ovum-pick up (OPU). The percentages of follicles that were 4–6 mm in diameter in the EB groups were significantly higher than that in the control group without hormone injection (P <0.05, Chi-square test). Follicles were observed using a real-time ultrasound scanner at the time of OPU (n=12 OPU session in each group).
3. Results
3.1. Dynamics of ovarian follicles and emergence of follicular wave after EB injection
The mean number of follicles in ovaries increased gradually over time after one-shot EB injection (Fig. 2A). At 72 and 96 hours after EB injection, the mean number of follicles achieved a peak. As shown Fig. 2B, the proportion of medium follicles with 4 to 6 mm in diameter increased dramatically at 72 and 96 hours after EB injection (P<0.05, compared with 0 hour group).
3.2. The number and the quality of cumulus oocytes aspirated by OPU
The mean number of follicles aspirated by OPU was significantly greater in EB group than in control group without hormone injection (36.6±3.0 vs. 27.8±1.8, P<0.05, Table 1). Then, the mean numbers of COCs recovered and cultured, that is grade A to C were significantly higher in the EB group than in the control group (28.9±2.9 vs. 21.8±1.4, and 23.4±2.4 vs. 15.0±1.1, P<0.05). As shown Fig. 3, the proportion of medium follicles with 4–6 mm in diameter in the EB group increased remarkably compared with the control group (49 vs. 15%, P<0.05). Therefore, the percentage of grade B COCs in the EB group was significantly higher than that in the control group (P<0.05, Table 2).
Furthermore, at a paired difference test by one crossover trial using eleven donors, the mean numbers of follicles aspirated and COCs recovered were significantly higher in the EB group than in the control group (42.5±4.6 vs. 34.7±3.5 and 36.9±5.5 vs. 28.1±3.7, P<0.05, Fig. 4).
Fig. 4.
A paired difference test using 11 donors: Effect of pre-treatment with estradiol benzoate on the number of follicles aspirated and COCs recovered at each paired-donor.
3.3. Embryo development and transferable embryos produced by OPU-IVF
As shown in Table 3, the numbers of oocytes cleaved after IVF was not significantly different between the EB and the control groups. However, the number of blastocysts in the EB group was significantly higher than that in the control group (50.5 vs. 34.3%, P<0.05). In addition, the number of transferable embryos was significantly greater in the EB group compared with the control group (32.7 vs.15.6%, P<0.05).
Furthermore, at a paired difference test by one crossover trial, the numbers of blastocysts and transferable embryos were significantly higher in the EB group compared with the control group (43.0 vs. 33.9% and 30.3 vs. 15.7%, P<0.05, Table 4).
4. Discussion
The results of the present study showed that stimulating bovine donors with EB had a positive effect on the efficiency of embryo production in the OPU-IVF technology. The number of follicles in the ovaries increased gradually after EB injection. Furthermore, the number and proportion of medium follicles with 4 to 6 mm in diameter were the highest at 96 hours after EB injection. Donors treated with EB prior to OPU had a greater numbers of COCs suitable for culture with grade A to C. In addition, the numbers of blastocysts and transferable embryos were higher with EB stimulation than without the hormone treatment.
Many techniques have been evaluated for their ability to increase the mature follicle populations of bovine ovaries in OPU (Chaubal, 2007, De Roover et al., 2008, Presicce et al., 2011, Vieira, 2014). A number of studies have attempted to establish the most cost-effective procedure for retrieving the higher number of good quality oocytes, via OPU, that produce more blastocysts. The numbers of follicles observed by ultrasound and the COCs obtained by OPU were greater after GnRH treatment in the previous report (Ogata et al., 2015). These results suggested that OPU at 48 hours after GnRH administration, the presumed time for a new wave of follicular development, led to the increased number of COCs recovered.
As another approach, treatment with a combination of progestogen and E2 can effectively regulate and synchronize follicle wave development (Bo et al., 1993, Bo et al., 1995). In these studies, a wave of follicular development has been defined as a synchronous development of a large number of follicles with 4 to 6 mm in diameter, followed by selection and growth of the dominant follicle and suppression of the subordinates. The net result was that the interval from treatment to wave emergence was not different among E2 groups and occurred, on average, 4.3 days after E2 treatment. In our study, investigation of the effect of EB injection prior to OPU on embryo development was performed with reference to modified technique to control the follicle wave control by combination of progesterone and E2. In the case of donors with a normal estrous cycle, since most of the reproductive cycle is the luteal phase, in this study, the effect of the treatment based on an administration of estrogen alone was examined. In the donors treated with EB, the total number of follicles in ovaries of donors increased gradually over time after EB injection, and the number of follicles in ovaries was the highest at 96 hours after EB injection, and the proportion of the medium follicles with the diameter of 4–6 mm was the highest at this time. In addition, donors treated with EB only prior to OPU had increased numbers of follicles and a greater proportion of medium-sized follicles (Fig. 2). It is presumed that the regression of dominant follicles and resumption of a new follicle wave were occurred after the administration of EB. When the COC quality was examined, the mean number of COCs graded from A to C (considered suitable to culture for IVM) in the EB group was significantly greater than the control group. The number of COCs classified as grade B was significantly increased by EB treatment.
In a study of bovine follicle culture, E2 supplementation in culture medium improved the growth of oocytes derived from the early antral follicles (Endo, Kimura, Kuwayama, Monji & Iwata, 2014) and affected the gene expression profile of granulosa cells to support the in vitro development of oocyte-granulosa cell complexes (Endo et al., 2013). Although the situation in vitro is not the same as in vivo, EB treatment in our study might promote the gene expression of early antral follicles and stimulate follicle development. As a result, the quality of COCs might become higher via antrum formation during a new follicle wave after the administration of EB.
When considering parameters related to embryo development in this study, the number of oocytes cleaved in the EB group is the same as the control group. However, the numbers of blastocysts developed and transferable embryos in the EB group were significantly higher than that in the control group. The positive effect of EB stimulation might be related to a remarkable increase in the number of medium follicles with 4 to 6 mm in diameter (Fig. 3).
The outcome of IVP programs has also been associated with the stage of follicular growth at which OPU is performed (Blondin and Sirard, 1995, Fair et al., 1995, Hagemann et al., 1999, Hendriksen et al., 2004). The developmental potential of oocytes has been associated with follicular growth development and continues to be enhanced as the follicular diameter increases toward the luteinizing hormone (LH) surge (Chaubal, 2007, Humblot et al., 2005). EB treatment can manipulate the dynamics of dominant follicle during the estrous cycle and synchronize proestrous development of the ovulatory follicle (Burke et al., 2000). It was shown that the mean number of all counted follicles and all usable oocytes recovered per donor were similar, but the mean number of embryos per donor and the development rate of oocytes into blastocysts were higher in the growth phase than in the dominant phase (Maachatkova, Krausova, Jokesova & Tomanek, 2005). Moreover, synchronization of the follicular wave using progesterone implant and estradiol benzoate prior to OPU showed positive effects on in vitro embryo production as well as on pregnancy rates (Cavalieri et al., 2017). In this study, at the time of OPU, the number of medium-sized follicles with a diameter of 4–6 mm increased markedly, which is considered to be in the follicular growth phase. Therefore, EB injection prior to OPU induced a follicle growth phase, which is a new wave of follicular development and as a result, the number and quality of COCs recovered were improved, and the number of oocytes capable of developing to blastocysts was considered to have increased.
In conclusion, the results of our present experiments indicate that EB pretreatment prior to OPU to synchronize follicular wave emergence promotes blastocyst yield and the number of transferable embryos. Further studies should be conducted to optimize the timing and dose of EB injection.
Acknowledgements
The study was financed by Hiroshima Prefectural Technology Research Institute Livestock Technology Research Center, Hiroshima Prefecture. The authors have no conflicts of interest to declare.
References
- Araujo R.R., Ginther O.J., Ferreira J.C., Palhao M.M., Beg M.A., Wiltbank M.C. Role of follicular estradiol-17beta in timing of luteolysis in heifers. Biol. Reprod. 2009;81:426–437. doi: 10.1095/biolreprod.108.073825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondin P., Sirard M.A. Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes. Mol Reprod Dev. 1995;41:54–62. doi: 10.1002/mrd.1080410109. [DOI] [PubMed] [Google Scholar]
- Bo G.A., Adams G.P., Nasser L.F., Pierson R.A., Mapletoft R.J. Effect of estradiol valerate on ovarian follicles, emergence of follicular waves and circulating gonadotropins in heifers. Theriogenology. 1993;40:225–239. doi: 10.1016/0093-691x(93)90261-3. [DOI] [PubMed] [Google Scholar]
- Bo G.A., Adams G.P., Caccia M., Martinez M., Pierson R.A., Mapletoft R.J. Ovarian follicular wave emergence after treatment with progestogen and estradiol in cattle. Anim Reprod Sci. 1995;39:193–204. [Google Scholar]
- Bousquet D., Twagiramungu H., Morin N., Brisson C., Carboneau G., Durocher J. In vitro embryo production in the cow: an effective alternative to the conventional embryo production approach. Theriogenology. 1990;51:59–70. doi: 10.1016/s0093-691x(98)00231-3. [DOI] [PubMed] [Google Scholar]
- Burke C.R., Day M.L., Bunt C.R., Macmillan K.L. Use of a small dose of estradiol benzoate during diestrus to synchronize development of the ovulatory follicle in cattle. J Anim Sci. 2000;78:145–151. doi: 10.2527/2000.781145x. [DOI] [PubMed] [Google Scholar]
- Cavalieri F.L.B., Morotti F., Seneda M.M., Colombo A.H.B., Andreazzi M.A., Emanuelli I.P., Rigolon L.P. Improvement of bovine in vitro embryo production by ovarian follicular wave synchronization prior to ovum pick-up. Theriogenology. 2017 doi: 10.1016/j.theriogenology.2017.11.026. pii: S0093-691X(17)30567-8. [DOI] [PubMed] [Google Scholar]
- Chaubal S.A., Ferre L.B., Molina J.A., Faber D.C., Rezamand P., Tian X. Hormonal treatments for increasing the oocyte and embryo production in an OPU-IVP system. Theriogenology. 2007;67:719–728. doi: 10.1016/j.theriogenology.2006.07.022. [DOI] [PubMed] [Google Scholar]
- De loos F., Van, Vliet C., Van, Maurik P., Kurip T.A. Morphology of immature bovine oocytes. Gamete Res. 1989;24:197–204. doi: 10.1002/mrd.1120240207. [DOI] [PubMed] [Google Scholar]
- De Roover R., Genicot G., Leonard S., Bols P., Dessy F. Ovum pick up and in vitro embryo production in cows superstimulated with an individually adapted superstimulation protocol. Anim Reprod Sci. 2005;86:13–25. doi: 10.1016/j.anireprosci.2004.05.022. [DOI] [PubMed] [Google Scholar]
- De Roover R., Feugang J.M.N., Bols P.E.J., Genicot G., Hanzen C.H. Effects of ovum pick-up frequency and FSH stimulation: a retrospective study on seven years of beef cattle in vitro embryo production. Reprod Domest Anim. 2008;43:239–245. doi: 10.1111/j.1439-0531.2007.00873.x. [DOI] [PubMed] [Google Scholar]
- Endo M., Kawahara-Miki R., Cao F., Kuwayama T., Monji Y., Iwata H. Estradiol supports in vitro development of bovine early antral follicles. Reproduction. 2013;145:85–96. doi: 10.1530/REP-12-0319. [DOI] [PubMed] [Google Scholar]
- Endo M., Kimura K.R., Kuwayama T., Monji Y., Iwata H. Effect of estradiol during culture of bovine oocyte-granulosa cell complexes on the mitochondrial DNA copies of oocytes and telomere length of granulosa cells. Zygote. 2014;22:431–439. doi: 10.1017/S0967199412000603. [DOI] [PubMed] [Google Scholar]
- Evans A.C.O., Komar C.M., Wandji S.A., Fortune J.E. Changes in androgen secretion and luteinizing hormone pulse amplitude are associated with the recruitment and growth of ovarian follicles during the luteal phase of the bovine estrous cycle. Bio Reprod. 1997;57:394–401. doi: 10.1095/biolreprod57.2.394. [DOI] [PubMed] [Google Scholar]
- Fair T., Hyttel P., Greve T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Mol Reprod Dev. 1995;42:437–442. doi: 10.1002/mrd.1080420410. [DOI] [PubMed] [Google Scholar]
- Ginther O.J., Knopf L., Kastelic J.P. Temporal associations among ovarian events in cattle during oestrous cycles with two and three follicular waves. J Reprod Fertil. 1989;87:223–230. doi: 10.1530/jrf.0.0870223. [DOI] [PubMed] [Google Scholar]
- Goodhand K.L., Watt R.G., Staines M.E., Hutchinson J.S.M., Broadbent P.J. In vivo oocyte recovery and in vitro embryo production from bovine donors aspirated at different frequencies or following FSH treatment. Theriogenology. 1999;51:951–961. doi: 10.1016/s0093-691x(99)00041-2. [DOI] [PubMed] [Google Scholar]
- Hagemann L.J., Beaumont S.E., Berg M., Donnison M.J., Ledgard A., Peterson A.J. Development during single IVP of bovine oocytes from dissected follicles: interactive effects of estrous cycle stage, follicle size and atresia. Mol Reprod Dev. 1999;53:451–458. doi: 10.1002/(SICI)1098-2795(199908)53:4<451::AID-MRD11>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Hendriksen P.J., Steenweg W.N., Harkema J.C., Merton J.S., Bevers M.M., Vos P.L. Effect of different stages of the follicular wave on in vitro developmental competence of bovine oocytes. Theriogenology. 2004;61:909–920. doi: 10.1016/s0093-691x(03)00278-4. [DOI] [PubMed] [Google Scholar]
- Humblot P., Holm P., Lonergan P., Wrenzycki C., Lequarre A.S., Joly C.G. Effect of stage of follicular growth during superovulation on developmental competence of bovine oocytes. Theriogenology. 2005;63:1149–1166. doi: 10.1016/j.theriogenology.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Kani C., Kuwahata A., Ochi M., Horiuchi T. Effect of dibutyryl cAMP together with FSH and EGF during in vitro maturation on sperm aster formation and blastocyst development after intracytoplasmic sperm injection. J Mamm Ova Res. 2011;28:131–138. [Google Scholar]
- Kruip T.A., Boni R., Wurth Y.A., Roelofsen M.W.M., Pieterse M.C. Potential use of ovum pick-up for embryo production and breeding in cattle. Theriogenology. 1994;42:675–684. doi: 10.1016/0093-691x(94)90384-u. [DOI] [PubMed] [Google Scholar]
- Maachatkova M., Krausova K., Jokesova E., Tomanek M. Deveopmental competence of bovine oocytes: effects of follicle size and the phase of follicular wave on in vitro embryo production. Theriogenology. 2005;61:329–335. doi: 10.1016/s0093-691x(03)00216-4. [DOI] [PubMed] [Google Scholar]
- Martinez M.F., Kastelic J.P., Bo G.A., Caccia M., Mapletoft R.J. Effects of oestradiol and some of its esters on gonadotrophin release and ovarian follicular dynamics in CIDR-treated beef cattle. Anim Reprod Sci. 2005;86:37–52. doi: 10.1016/j.anireprosci.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Numabe T., Oikawa T., Kikuchi T., Horiuchi T. Production efficiency of Japanese black calves by transfer of bovine embryos produced in vitro. Theriogenology. 2000;54:1409–1420. doi: 10.1016/s0093-691x(00)00463-5. [DOI] [PubMed] [Google Scholar]
- Numabe T., Oikawa T., Kikuchi T., Horiuchi T. Birth weight and gestation length of japanese black calves following transfer of embryos produced in vitro with or without co-culture. J Vet Me. Sci. 2001;63:515–519. doi: 10.1292/jvms.63.515. [DOI] [PubMed] [Google Scholar]
- Ogata Y., Hidaka T., Matzushige T., Maeda T. Comparison of two biopsy methods in bovine embryos. J Adv Biol Biotech. 2015;2:16–23. [Google Scholar]
- Ogata Y., Yu G.M., Hidaka T., Matzushige T., Maeda T. Effective embryo production from Holstein cows treated with gonadotropin-releasing hormone during early lactation. Theriogenology. 2016;86:1421–1426. doi: 10.1016/j.theriogenology.2016.04.087. [DOI] [PubMed] [Google Scholar]
- Pontes J.H.F., Nonato-Junior I., Sanches B.V., Ereno-Junior J.C., Uvo S., Barreiros T.R. Comparison of embryo yield and pregnancy rate between in vivo and in vitro methods in the same Nelore (Bos indicus) donor cows. Theriogenology. 2009;71:690–697. doi: 10.1016/j.theriogenology.2008.09.031. [DOI] [PubMed] [Google Scholar]
- Presicce G.A., Senatore E.M., De Santis G., Stecco R., Terzano G.M., Borghese A. Hormonal stimulation and oocyte maturational competence in prepuberal Mediterranean Italian buffaloes (Bubalus bubalis) Theriogenology. 2002;57:1877–1884. doi: 10.1016/s0093-691x(02)00677-5. [DOI] [PubMed] [Google Scholar]
- Presicce G.A. Reproduction in the water buffalo. Reprod Domest Anim. 2007;42:24–32. doi: 10.1111/j.1439-0531.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- Presicce G.A., Xu J., Gong G., Moreno J.F., Chaubal S., Xue F. Oocyte source and hormonal stimulation for in vitro fertilization using sexed spermatozoa in cattle. Vet Med Int. 2011:145626. doi: 10.4061/2011/145626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursley J.R., Mee M.O., Wiltbank M.C. Synchronization of ovulation in dairy cows using PGF2α and GnRH. Theriogenology. 1995;44:915–923. doi: 10.1016/0093-691x(95)00279-h. [DOI] [PubMed] [Google Scholar]
- Sendag S., Cetin Y., Alan M., Hadeler K.G., Niemann H. Effects of eCG and FSH on ovarian response, recovery rate and number and quality of oocytes obtained by ovum pick-up in Holstein cows. Anim Reprod Sci. 2008;106:208–214. doi: 10.1016/j.anireprosci.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Sirois J., Fortune J.E. Ovarian follicular dynamics during the estrus cycle in heifers monitored by real-time ultrasonography. Bio Reprod. 1988;39:308–317. doi: 10.1095/biolreprod39.2.308. [DOI] [PubMed] [Google Scholar]
- Stringfellow D.A. Givens MD. Manual of the International Embryo Transfer Society (IETS). 4th ed. IETS; Champaign, IL: 2010. [Google Scholar]
- Vieira L.M., Rodrigues C.A., Castro, Netto. A., Guerreiro B.M., Silveira C.R.A., Moreira R.J.C. Superstimulation prior to the ovum pick-up in lactating and non-lactating Holstein cows. Theriogenology. 2014;82:318–324. doi: 10.1016/j.theriogenology.2014.04.013. [DOI] [PubMed] [Google Scholar]