Highlights
-
•
In this study, the immunolocalization for glucocorticoid receptor (NR3C1) in goat ovarian follicles and the effect of cortisol on in vitro development of preantral follicles was evaluate.
-
•
The NR3C1 was strongly expressed in oocytes of primary and antral follicles.
-
•
A progressive increase of immunostaining for NR3C1 in granulosa cells from primordial to antral follicles was observed.
-
•
In conclusion, it was observed the presence of NR3C1 in the oocyte and granulosa cells in all follicular categories.
-
•
The in vitro culture showed that high cortisol concentration (10 ng/ml) exerts a deleterious effect on follicular survival.
Keywords: Caprine, Cortisol, NR3C1, Preantral follicles, Stress
Abstract
The aim of this study was to evaluate the immunolocalization for glucocorticoid receptor (NR3C1) in goat ovarian follicles and the effect of cortisol on in vitro development of preantral follicles. Goat ovarian fragments were cultured for 7 days under different cortisol concentrations (0, 1, 5 and 10 ng/ml). Before and after culture, the protein expression of NR3C1 was analyzed in ovarian tissue by immunohistochemical analysis. Moreover, the endpoints follicular morphology, viability, activation as well as follicular and oocyte diameter were also analyzed. The NR3C1 was strongly expressed in oocytes of primordial and antral follicles. A progressive increase of immunostaining for NR3C1 in granulosa cells from primordial to antral follicles was observed regardless of the treatment. After in vitro culture, it was observed a significant reduction in the rate of normal preantral follicles rate in the 10 ng/ml cortisol treatment when compared to the other treatments. Moreover, follicular and oocyte diameter significantly decreased in all treatments (cortisol 0, 1, 5 and 10 ng/ml) compared to the fresh control. After culture, the activation rate significantly increased when the follicles were exposed to 1, 5 and 10 ng/ml cortisol compared to the fresh control. In conclusion, it was observed the presence of NR3C1 in the oocyte and granulosa cells in all follicular categories, except in granulosa cells of primordial follicles. The in vitro culture showed that high cortisol concentration (10 ng/ml) exerts a deleterious effect on follicular survival.
1. Introduction
Goat production is economically and socially important activity with an increasing interest in milk, meat and skin production around the world. Although goats are well adapted to many different environments, improper handling and feeding practices may produce stress reducing their productivity and profitability (Kannan, Kouakou, Terrill, & Gelaye, 2013). Conditions of stress lead to an increase of cortisol inducing suppression of reproductive system function (Griekspoor et al., 2002, Tilbrook et al., 2000) with subsequent economic impact on the livestock.
Cortisol, commonly known as hormone stress, is produced in the adrenal gland and is responsible for the regulation of several physiological processes, including metabolism, immunological response, and female or male reproductive function. High level of cortisol in response to stress, acts directly or indirectly on the reproductive functionality resulting in low reproductive rates (Chaves et al., 2013, Kannan et al., 2013, Tilbrook et al., 2000). The rise of cortisol acts on the hypothalamic -pituitary suppressing GnRH secretion, which in turn inhibits FSH release and reduce the LH surge frequency in sheep (Breen et al., 2005, Macfarlane et al., 2000). Furthermore, excessive cortisol levels can inhibit the estradiol secretion, inducing the disruption of the follicular development and ovulation (Breen et al., 2005). Recent research showed that longer-term administration of adrenocorticotropic hormone (ACTH) increased the cortisol and affected the development and ovulatory process of ovarian follicles (Natsumi, Hiroaki, Larasati, & Tomomi, 2018).
In physiological conditions, several studies have demonstrated the role of cortisol on the ovary during the oocyte maturation as well as granulosa cell differentiation and steroidogenesis (Breen et al., 2005, González et al., 2010). Moreover, this hormone is associated with oocyte maturation in human because human follicular fluid from matured oocytes had greater cortisol level than immature oocytes (Fateh, Ben-Rafael, Benadiva, Mastroianni, & Flickinger, 1989). In addition, cortisol increases lipid metabolism in pre-ovulatory follicle and promoting meiotic resumption in human (Simerman et al., 2015). The concentrations of circulating cortisol in female small ruminant was around 5–10 ng/ml in health condition (Kaneko, Harvey, & Bruss, 1997) and 10–19 ng/ml under stress (Nazifi, Saebi, Rowghani, & Kaveh, 2003).
Despite some studies reporting the role of cortisol in reproduction, it is unclear the effect of this hormone on the early folliculogenesis (primordial and primary follicles). In addition, there is no information on the localization of cortisol receptor in the goat ovarian follicles. Therefore, the aims of this study were to investigate the immunolocatization for cortisol receptor, NR3C1, and the effect of cortisol on the survival and activation of caprine preantral follicles cultured in vitro.
2. Material and methods
2.1. Chemicals
Unless mentioned otherwise, all chemicals used in the present study were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
2.2. Source of ovaries
Ovaries (n = 10) from 5 adults, mixed-breed goats between 1 and 3 years old were collected at a local slaughterhouse. Immediately after slaughter, the ovaries were washed in 70% ethanol, followed by two washes in washing medium consisting of Minimum Essential Medium (MEM) buffered with 25 mM HEPES (MEM-HEPES) supplemented with 100 µg/ml penicillin and 100 µg/ml streptomycin. Ovaries were transported to the laboratory within 1 h in 50 ml tubes containing wash medium at 4 °C (Chaves et al., 2008).
2.3. Immunohistochemical localization of glucocorticoid receptor (NR3C1)
In the laboratory, before culture (fresh control) and after 7 days of culture, all of the ovarian fragments cultured with different concentration of cortisol (0, 1, 5, 10 ng/ml) were fixed in 4% paraformaldehyde for 12 h, dehydrated, and embedded in paraffin. For detections of NR3C1, immunohistochemistry was performed according to the protocol described previously by Sales et al. for aquaporin 3 (Sales et al., 2014). Briefly, sections of 5 µm from each block were obtained and mounted in poly-L-lysine slides and dried overnight at 37 °C, deparaffinised in xylene and rehydrated in graded ethanol series, followed by antigen retrieval with citric acid buffered solution (pH 6.0) and Tween 20. After blocking solution endogenous peroxidase activity with 3% hydrogen peroxide and methanol for 10 min, the sections were incubated for 1 h with blocking solution (25 ml PBS containing 1.25% bovine serum albumin and 3% Triton X) at room temperature and overnight with rabbit polyclonal primary antibody (NR3C1) (1:800 Abcam Inc., USA) at 4°C. After repeated washing in PBS for 5 min, slides were incubated with secondary antibody rabbit anti-rabbit IgG (1:500 Abcam Inc., USA) for 30 min at room temperature followed by washing in PBS for 5 min. Then the sections were incubated with horseradish peroxidase-conjugated avidin (ABC kit, Vector Laboratories, Burlingame, CA, USA). The positive reactions were visualized with 3,3′ -diaminobenzidine tetrahydrochloride (DAB) solution. Finally, the sections were counterstained with hematoxylin, dehydrated, and mounted in Depex. Replacing the primary antibody for IgG of the same species in which the primary antibody was produced performed negative controls (Goat pAb to Rb IgG – Biotin). The preantral follicles were classified as primordial (oocyte surrounded by one layer of squamous granulosa cells), primary (oocyte surrounded by one layer of cuboidal granulosa cells), or secondary (oocyte surrounded by two or more layers of cuboidal granulosa cells). The presence of small antral follicles (presence of cavity filled with follicular fluid and well-developed layers of granulosa cells) was also noted. In the various follicular compartments (oocyte, granulosa or theca cells), the immunostaining was classified as absent (−), weak (+), moderate (++) or strong (+++).
2.4. In vitro culture of goat preantral follicle
In vitro culture of caprine follicles was performed according to the protocol described previously by Chaves et al. (2008). Briefly, in the laboratory, the surrounding fatty tissues and ligaments were stripped from the ovaries. Ovarian cortical slices (3 × 3 × 1 mm) were cut from the ovarian surface using a surgical blade under sterile conditions and subsequently placed in holding medium consisting of MEM-HEPES with antibiotics (100 mg/ml penicillin and 100 mg/ml streptomycin). Subsequently, half the ovarian fragments were randomly fixed for histological analysis (fresh control or noncultured fragments). The remaining fragments were cultured individually in vitro in 24-well culture dishes (Corning, Corning, NY, USA) containing 1 ml culture medium at 39 °C in presence of 5% CO2 in air for 7 days. The basic culture medium consisted of α-MEM (pH 7.2–7.4) supplemented with 10 ng/ml insulin, 5.5 ng/ml transferrin, 5 ng/ml selenium, 2 mM glutamine, 2 mM hypoxanthine and 1.25 mg/ml bovine serum albumin. This medium was called MEM+ and was supplemented with different cortisol (Hydrocortisone) concentrations (0, 1, 5 and 10 ng/ml). Before (fresh control) and after culture, the samples were fixed and destined for histological analysis. Fresh medium was prepared immediately before use. Every 2 days, the culture medium was replaced by fresh medium. Before (fresh control) and after in vitro culture for 7 days, ovarian fragments were processed as described below to evaluate the morphology of goat preantral follicles. The concentrations tested in this study represent the concentration of circulating cortisol in female small ruminant (Kaneko et al., 1997, Nazifi et al., 2003).
2.5. Morphological analysis of goat preantral follicles
Ovarian fragments from each treatment, including the fresh control or non-cultured fragments, were fixed individually in 4% paraformaldehide for 12 h. Subsequently, fragments were dehydrated in a graded series of ethanol, clarified with xylene and embedded in paraffin wax (Bruno et al., 2008). Tissues were sectioned serially at a thickness of 7 μm and sections were stained using standard protocols with periodic acid-Schiff and haematoxylin (PAS staining system; Sigma). Sections were examined by light microscopy (Zeiss, Jena, Germany) at 400 × magnification.
Preantral follicles were counted and evaluated in the section where the oocyte nucleus was visible. According to the developmental stage, preantral follicles were classified as primordial (oocyte surrounded by one layer of flattened pre-granulosa cells) or developing, with the latter category further subdivided into intermediate (oocyte surrounded by one layer of flattened pregranulosa and cuboidal granulosa cells), primary (oocyte surrounded by one layer of cuboidal granulosa cells) or secondary (oocyte surrounded by two or more layers of cuboidal granulosa cells). Each follicle was evaluated according to the following morphological parameters: (1) integrity of oocyte and granulosa cells; (2) the presence or absence of pyknotic bodies; (3) ooplasmic retraction; and (4) the organisation of granulosa cells. Based on this evaluation, preantral follicles were classified as normal, when a morphologically normal oocyte with a non-pyknotic nucleus was surrounded by granulosa cells organised in discrete layers. Degenerated follicles were defined as those with a retracted oocyte, with a pyknotic nucleus and/or surrounded by disorganised granulosa cells, which were detached from the basement membrane. The tissue analysis was performed by a single player using the same optical microscope (Nikon, Tokyo, Japan). Slide analysis was made blind reading (reviewing the slides without knowledge of treatment group). Overall, 150 follicles were evaluated for each treatment (30 follicles per treatment in 1 repetition x 5 repetitions = 150 follicles).
2.6. Primordial follicle activation and growth
To evaluate primordial follicle activation and growth, only normal follicles with a visible oocyte nucleus were recorded and the proportion of primordial and developing follicles was calculated for fresh control and for all treatments where the ovarian fragments were cultured for 7 days with different cortisol concentrations (0, 1, 5 and 10 ng/ml). In addition, for each follicle category, oocyte and follicle diameters were measured using an ocular micrometer and the average of the minor and major axes was reported as oocyte and follicle diameter, respectively.
2.7. Statistical analysis
Data were initially submitted to Shapiro-Wilk and Bartlett tests to verify normality of residues distribution and homoscedasticity, respectively. Confirmed both requirements underlying analysis of variance (ANOVA), it was performed according to a completely randomized design using General Linear Model Procedure (GLM) of SAS (2002) and Student-Newman-Keuls (SNK) test was applied to compare means when a significant effect of treatment (cortisol concentration) was present. Results were expressed as mean ± standard deviation and differences were considered significant when P < 0.05.
3. Results
3.1. Immunohistochemical localization of glucocorticoid receptor (NR3C1) in the caprine ovarian tissue
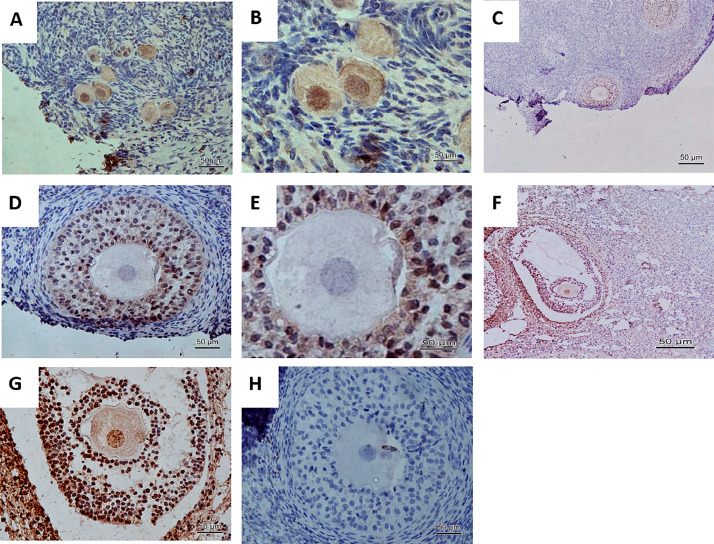
The results from immunohistochemistry demonstrated the presence of the NR3C1 in all ovarian follicular categories in caprine, except in granulosa cells of primordial follicles, varying only the intensity and location (Fig. 1). There was no difference in the intensity of immunoreaction to NR3C1 among the treatments. However, when it was evaluated the intensity of immunoreaction to NR3C1 among follicular structures in preantral follicles, some interesting findings can be related. The oocyte of primordial follicles showed a moderate immunostaining in the cytoplasm and strong immunoreaction in the nucleus; the oocyte of a primary follicles showed a weak immunostaining in cytoplasm and nucleus and oocyte of secondary follicles showed a moderate immunostaining in the cytoplasm and nucleus (Table 1). Regarding the granulosa cells, there was a progressive increase of immunostaining for NR3C1 in primordial, primary and secondary follicles, which were classified as absent, weak and moderate immunoreaction, respectively (Table 1). In antral follicles, there was a strong immunoreaction in the oocyte (cytoplasm and nucleus) as well as in the granulosa cells. In theca cells, it was not detected the presence of NR3C1 in the secondary follicles, however, the antral follicles had a weak immunoreaction.
Fig. 1.
Immunohistochemical detection of NR3C1 in caprine follicles enclosed in ovarian tissue. (A), primordial follicles. (B), higher magnification of area A. (C), secondary follicle. (D,E), higher magnification of area D. (F), antral follicle. (G), higher magnification of area G. (H), Negative control.
Table 1.
Relative intensity of immunohistochemical staining for NR3C1 in caprine ovarian follicles.
| Follicular structure | Follicular category |
|||
|---|---|---|---|---|
| Primordial | Primary | Secondary | Antral | |
| Oocyte | +++ | + | ++ | +++ |
| Granulosa | - | + | ++ | +++ |
| Theca | NA | NA | - | + |
NA, not applicable. (-) absent; (+) weak; (++) moderate; (+++) strong immunoreaction.
3.2. Effect of cortisol on follicular survival, activation and growth
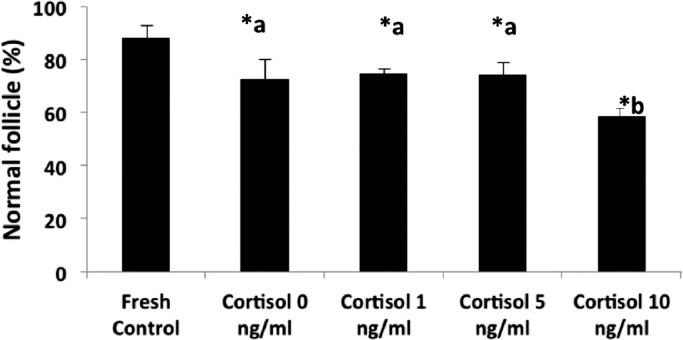
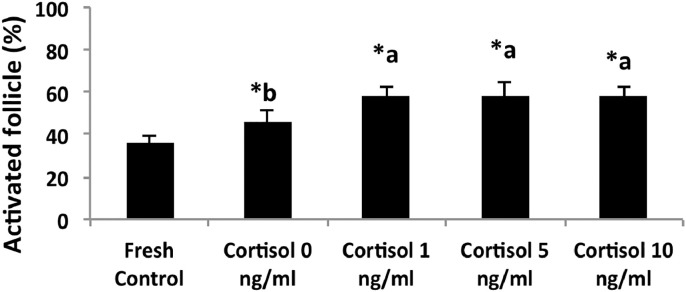
A total of 4470 preantral follicles were analyzed. After seven days of culture, regardless of the presence or absence of cortisol, a reduction (P < 0.05) in the percentage of normal follicles was observed when compared to the fresh control. Among the treatments, the 10 ng/ml cortisol treatment showed the lowest (P < 0.05) percentage of morphologically normal follicles at day 7 of culture (Fig. 2 and Table 2). With respect to the follicular activation, a significant increase in the percentage of activated follicles (intermediate, primary and secondary) after 7 days of culture was observed in all treatments when compared to the fresh control (Fig. 3). Furthermore, the percentage of activated follicles in all treatments containing cortisol was significantly higher than that observed in the 0 ng/ml cortisol treatment. However, we observed a few number of secondary follicles after 7 days of culture.
Fig. 2.
Effect of different cortisol concentrations on the percentage of normal caprine preantral follicles after 7 days of in vitro culture.
* Significant difference compared to the fresh control. (P < 0.05).
a,b Significant difference among treatments. (P < 0.05).
Table 2.
Effect of different cortisol concentrations on the percentage of normal caprine preantral follicles according to the follicular stage after 7 days of in vitro culture.
| Treatment | Percentage of normal follicles by follicular stage |
|||
|---|---|---|---|---|
| Primordial | Intermediate | Primary | Secondary | |
| Fresh control (D0) | 61,4 | 24,2 | 10,8 | 3,6 |
| Cortisol 0 ng/ml (D7) | 50,8 *a | 32,4 | 14,3 | 2,5 |
| Cortisol 1 ng/ml (D7) | 40,7 *b | 40,4 | 18,9 | 0,0 |
| Cortisol 5 ng/ml (D7) | 41,1 *b | 40,1 | 15,9 | 2,9 |
| Cortisol 10 ng/ml (D7) | 39,8 *b | 43,5 | 16,7 | 0,0 |
Significant difference compared to the fresh control. (P < 0.05).
Significant difference among treatments. (P < 0.05).
Fig. 3.
Effect of different cortisol concentrations on the activation of caprine preantral follicles after 7 days of in vitro culture. The percentages of activated follicles were calculated by dividing the number of normal developing follicles by total number follicles evaluated.
* Significant difference compared to the fresh control. (P < 0.05).
a,b Significant difference among treatments. (P < 0.05).
The oocyte and follicular diameters in the fresh control and in the cultured ovarian tissue are shown (Table 3). There was a decrease (P < 0.05) in the follicular and oocyte diameters in all treatments when compared to the fresh control. However, no significant difference in follicular and oocyte diameters was observed among the cultured treatments.
Table 3.
Effect of different cortisol concentrations on follicular and oocyte diameters (mean ± S.E.) of caprine preantral follicles during in vitro culture.
| Treatment | Follicle diameter (µm) | Oocyte diameter (µm) |
|---|---|---|
| Fresh control | 36,95 ± 6,93 | 24,09 ± 3,19 |
| Cortisol 0 ng/ml | 30,62 ± 5,20* | 19,44 ± 3,28* |
| Cortisol 1 ng/ml | 31,72 ± 6,37* | 19,37 ± 3,83* |
| Cortisol 5 ng/ml | 29,71 ± 6,49* | 17,94 ± 2,99* |
| Cortisol 10 ng/ml | 26,77 ± 6,06* | 17,72 ± 3,72* |
Significant difference compared to the fresh control. (P < 0.05).
4. Discussion
The current study showed, for the first time, the immunolocalization for NR3C1 in goat ovarian follicles. After the receptor is bound to cortisol, this protein is activated and translocated to the nucleus acting as a transcription factor (Griekspoor et al., 2002). The mRNA for glucocorticoid receptor has been detected in other species (Tetsuka, Nishimoto, Myamoto, Okuda, & Hamano, 2010), using the RT-PCR. However, in goats, there were no studies on glucocorticoid receptor in the ovary. In the current study, the NR3C1 was expressed in all follicular categories and compartments, except in granulosa cells of primordial follicles, especially in the oocyte (cytoplasm and nucleus) and in the granulosa cell of secondary and antral follicles. The NR3C1 expression increased in advanced stages of folliculogenesis. In bovine, mRNA was detected for the glucocorticoid receptor in all follicular categories, both in granulosa and theca cells (Tetsuka et al., 2010). Furthermore, in primordial follicles, our results showed that NR3C1 was expressed with a strong immunoreaction in the oocyte, suggesting an important role for the maintenance of follicular quiescence or activation. Although the cortisol receptor is strongly expressed in the oocyte of early follicles, the form it participates in follicular activation still remains unclear. It is known that one of the functions of this substance in ovarian function in preantral follicles is the differentiation of granulosa cells (Andersen, 2002). In the secondary follicles, granulosa cells express the protein receptor for glucocorticoids, however it was not possible to classify the intensity of the immunostaining in the theca cells, probably due to the reduction in cytoplasmic volume during the histology procedure (Almeida et al., 2012). According Adcock (2000) the glucocorticoid receptors are expressed in almost all tissues.
The present study was the first to demonstrate the effect of different concentrations of cortisol on in vitro culture of goat preantral follicles. After the culture, it was observed that the cortisol affected in a concentration-dependent manner the follicular survival and growth. Regarding the survival, the exposure to 10 ng/ml cortisol resulted in a lower percentage of normal preantral follicles when compared to the other treatments and the fresh control. One possible explanation for this fact is that the glucocorticoids may impair ovarian steroidogenesis, inhibiting the biosynthesis of steroids in granulosa cells, probably through decreasing the expression of StAR protein (Ben-Rafall et al., 1988, Huang and Li, 2001). In cattle, during induction of stress by ACTH administration, there was a decrease in the expression of P450 aromatase and CYP17 enzyme, reducing the production of estradiol and androstenedione (Biran, Braw-Tal, Gendelman, Lavon, & Roth, 2015), and therefore contributing to the reduction of follicle viability. The data from the current study were consistent with previous in vivo studies on the effect of cortisol caused by the stress. In sheep, the cortisol was correlated with the inhibition of follicular recruitment and selection phase in the first half of the follicular phase (Whirledge & Cidlowski, 2010). A recent study showed that the stress increased the cortisol and affected the development and ovulatory process of goat ovarian follicles (Natsumi et al., 2018).
In the current study, a significant increase in the percentage of activated follicles after in vitro culture was observed in all treatments in the presence of cortisol. This result suggests that cortisol does affect primordial follicles promoting their activation. Moreover, a strong immunoreaction in oocytes of primordial follicles was observed in the present study.
One of the alternatives to assess the growth of histologically normal follicles after in vitro culture is the measurement of follicle and oocyte diameters. In the present study, it was observed a significant reduction in the follicle and oocyte diameter of preantral follicles cultured either in the presence or absence of cortisol when compared to the fresh control. During the in vitro culture process, part of the ovarian follicles become atretic, especially more advanced follicle stage (primary and secondary) (Meresman, 2011). However, most of the ovarian follicles measured in the current experiment, after in vitro culture, were intermediate which are, in general, smaller than the aforementioned follicular categories (Aguiar et al., 2017). This may explain the decrease in follicle and oocyte diameter in this study.
5. Conclusion
In conclusion, it was observed the presence of NR3C1 in the oocyte and granulosa cells in all follicular categories, except in granulosa cells of primordial follicles. The in vitro culture showed that high cortisol concentration (10 ng/ml) exerts a deleterious effect on follicular survival, reducing the percentage of normal follicles.
Acknowledgments
Conflict of interest
None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Funding
This research was supported by grants from the National Council for Scientific and Technological Development (CNPq-79/2013 linha 3) – Rede Nordeste de Biotecnologia(Rede de pesquisa do ovário artificial) – Processo (N° 407594/2013-2). Juliân da Trindade Pontes is the recipient of a grant from CAPES/CE (Brazil).
Acknowledgments
The authors thank Dr. Maria Helena Tavares Matos by ceding BIOFOV (Nucleus of Biotechnology Applied to Ovarian Follicle Development, Federal University of San Francisco Valley, Petrolina, PE, Brazil) to carry out this experiment.
References
- Adcock I.M. Molecular mechanisms of glucocorticosteroid actions. Pulmonary Pharmacology & Therapeutics. 2000;13:115–126. doi: 10.1006/pupt.2000.0243. [DOI] [PubMed] [Google Scholar]
- Aguiar F.L.N., Lunardi F.O., Lima L.F., Bruno J.B., Alves B.G., Magalhães-Padilha D.M., et al. Role of EGF on in situ culture of equine preantral follicles and metabolomics. Research in Veterinary Science. 2017;115:155–164. doi: 10.1016/j.rvsc.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Almeida A.P., Saraiva M.V.A., Alves Filho J.G., Silva G.M., Gonçalves R.F.B., Brito I.R., et al. Gene expression and immunolocalization of fibroblast growth factor 2 in the ovary and its effects on the in vitro culture of caprine preantral ovarian follicle. Reproduction in Domestic Animals. 2012;47:20–25. doi: 10.1111/j.1439-0531.2011.01793.x. [DOI] [PubMed] [Google Scholar]
- Andersen C.Y. Possible new mechanism of cortisol action in female reproductive organs: Physiological implications of the free hormone hypothesis. Journal of Endocrinology. 2002;173:211–217. doi: 10.1677/joe.0.1730211. [DOI] [PubMed] [Google Scholar]
- Ben-Rafall Z., Benadiva C.A., Garcia C.J., Flickinger G.L. Cortisol stimulation of estradiol and progesterone secretion by human granulosa cells is independent of follicle-stimulating hormone effect. Fertility and Sterility. 1988;49:813–816. [PubMed] [Google Scholar]
- Biran D., Braw-Tal R., Gendelman M., Lavon Y., Roth Z. ACTH administration during formation of preovulatory follicles impairs steroidogenesis and angiogenesis in association with ovulation failure in lactating cows. Domestic Animal Endocrinology. 2015;53:52–59. doi: 10.1016/j.domaniend.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Breen K.M., Billings H.J., Wagenmaker E.R., Wessinger E.W., Karsch F.J. Endocrine basis for disruptive effect of cortisol on preovulatory events. Endocrinology. 2005;146:2107–2115. doi: 10.1210/en.2004-1457. [DOI] [PubMed] [Google Scholar]
- Bruno J.B., Lima-Verde I.B., Martins F.S., Matos M.H.T., Lopes C.A.P., Maia-JR J.E., et al. Característica histológica, ultra-estrutural e produção de nitrito de folículos pré-antrais caprinos cultivados in vitro na ausência ou presença de soro. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 2008;60:1329–1337. [Google Scholar]
- Chaves R.M., Santos E.R., Neves J.P., Moura M.T., Silva J.C.F., Lima P.F. Influência das estações seca e chuvosa na capacidade de desenvolvimento de oócitos e produção in vitro de embriões da espécie caprina. Ciência Animal Brasileira. 2013;14:135–142. [Google Scholar]
- Chaves R.N., Martins F.S., Saraiva M.V.A., Celestino J.J.H., Lopes C.A.P., Correia J.C., et al. Chilling ovarian fragments during transportation improves viability and growth of goat preantral follicles cultured in vitro. Reproduction, Fertility and Development. 2008;20:640–647. doi: 10.1071/rd07195. [DOI] [PubMed] [Google Scholar]
- Fateh M., Ben-Rafael Z., Benadiva C.A., Mastroianni L.J.R., Flickinger G.L. Cortisol levels in human follicular fluid. Fertility and Sterility. 1989;51:538–541. doi: 10.1016/s0015-0282(16)60572-1. [DOI] [PubMed] [Google Scholar]
- González R., Ruiz-León Y., Gomendio M., Roldan E.R.S. The effect of glucocorticoids on ERK-1/2 phosphorylation during maturation of lamb oocytes and their subsequent fertilization and cleavage ability in vitro. Reproductive Toxicology. 2010;29:198–205. doi: 10.1016/j.reprotox.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Griekspoor A., Zwart W., Neefjes J., Michalides R. Visualizing the action of steroid hormone receptors in living cells. Nuclear Receptor Signaling. 2002;5:e003. doi: 10.1621/nrs.05003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T.J., Li P.S. Dexamethasone inhibits luteinizing hormone-induced synthesis of steroidogenic acute regulatory protein in cultured rat preovulatory follicles. Biology of Reproduction. 2001;64:163–170. doi: 10.1095/biolreprod64.1.163. [DOI] [PubMed] [Google Scholar]
- Kaneko J.J., Harvey J.W., Bruss M.L. 5th ed. Academic Press; California: 1997. Clinical biochemistry of domestic animals; p. 932. [Google Scholar]
- Kannan G., Kouakou B., Terrill T.H., Gelaye S. Endocrine, blood metabolite, and meat quality changes in goats as influenced by short-term, preslaughter stress. Journal of Animal Science. 2013;81:1499–1507. doi: 10.2527/2003.8161499x. [DOI] [PubMed] [Google Scholar]
- Macfarlane M.S., Breen K.M., Sakurai H., Adams B.M., Adams T.E. Effect of duration of infusion of stress-like concentrations of cortisol on follicular development and the preovulatory surge of LH in sheep. Animal Reproduction Science. 2000;63:167–175. doi: 10.1016/s0378-4320(00)00179-2. [DOI] [PubMed] [Google Scholar]
- Meresman G. Relevance of apoptosis in the female reproductive system. Investigacion Clinica. 2011;52:274–290. [PubMed] [Google Scholar]
- Natsumi E., Hiroaki Y., Larasati P.R., Tomomi T. Effect of repeated adrenocorticotropic hormone administration on reproductive function and hair cortisol concentration during the estrous cycle in goats. General and Comparative Endocrinology. 2018;259:207–212. doi: 10.1016/j.ygcen.2017.11.027. [DOI] [PubMed] [Google Scholar]
- Nazifi A.S., Saebi M., Rowghani E., Kaveh K. The influences of thermal stress on serum biochemical parameters of Iranian fat-tailed sheep and their correlation with triiodothyronine (T3), thyroxine (T4) and cortisol concentrations. Comparative Clinical Pathology. 2003;12:135–139. [Google Scholar]
- Sales A.D., Brito I.R., De Lima L.F., Lobo C.H., Duarte A.B., Souza C.E., et al. Expression and localization of Aquaporin 3 (AQP3) in folliculogenesis of ewes. Acta Histochemica. 2014;116:831–837. doi: 10.1016/j.acthis.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Simerman A.A., Hill D.L., Grogan T.R., Elashoff D., Clarck N.J., Goldstein E.H., et al. Intrafolliclar cortisol levels inversely correlate with cumulus cell lipid content as a possible energy source during oocyte meiotic resumption in women undergoing ovarian stimulation for in vitro fertilization. Fertility and Sterility. 2015;103:249–257. doi: 10.1016/j.fertnstert.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsuka M., Nishimoto H., Myamoto A., Okuda K., Hamano S. Gene Expression of 11β-HSD and glucocorticoids receptor in the bovine (Bos Taurus) follicle during follicular maturation and atresia: The role of follicular stimulating hormone. Journal of Reproduction and Development. 2010;56:616–622. doi: 10.1262/jrd.10-019k. [DOI] [PubMed] [Google Scholar]
- Tilbrook A.J., Turner A.I., Clarke I.J. Effects of stress on reproduction in non-rodent mammals: The role of glucocorticoids and sex differences. Reviews of Reproduction. 2000;5:105–113. doi: 10.1530/ror.0.0050105. [DOI] [PubMed] [Google Scholar]
- Whirledge S., Cidlowski J.A. Glucocorticoids, stress, and fertility. Minerva endocrinologica. 2010;35:109–125. [PMC free article] [PubMed] [Google Scholar]