Highlights
-
•
Dogs poorly tolerate rectal temperature measurements with a contact thermometer.
-
•
Existing alternative approaches used uncalibrated infrared thermometers.
-
•
Gum and inguinal temperature are correlated moderately to rectal temperature.
-
•
Hyperthermia was detected with sensitivity and specificity up to 90.0% and 78.6%.
-
•
Future studies should include a calibrated thermometer and control external factors.
Keywords: Body surface temperature, Dog, Infrared thermometer, Health status, Rectal temperature
Abstract
Because dogs tolerate conventional rectal temperature measurements poorly, a calibrated infrared thermometer was tested for assessing canine body surface temperature. Body surface temperature of 204 dogs was estimated on various sites (digit, snout, axilla, eye, gum, inguinal region, and anal verge). Having rectal temperature as the gold standard, temperature difference, Spearman's correlation coefficient, hyperthermia and hypothermia detection sensitivity and specificity, and stress response score was calculated for each measurement site. Although the canine body surface temperature was considerably lower than the rectal temperature, there was a moderate correlation between both temperatures. Spearman's coefficients were 0.60 (p < 0.001) for the inguinal region with a single operator and 0.50 (p < 0.001) for the gum with multiple operators. Measurement site on the gum additionally guaranteed hyperthermia detection sensitivity and specificity up to 90.0% (95% CI: [66.7 100]) and 78.6% (95% CI: [71.6 85.2]), respectively. Measurements with the infrared thermometer provoked a statistically significant lower stress response (mean stress scores between 1.89 and 2.48/5) compared to the contact rectal measurements (stress score of 3.06/5). To conclude, the correct body surface temperature measurement should include a calibrated thermometer, reliable sampling, and the control of external factors such as ambient temperature influence. The transformation of body surface temperature to the recognized rectal temperature interval allows more straightforward data interpretation. The gum temperature exhibited the best clinical potential since the differences to rectal temperatures were below 1°C, and hyperthermia was detected with the sensitivity of up to 90%.
Introduction
Body temperature measurement is an essential part of the clinical examination in dogs since deviations from the normal temperature (due to infection or shock for example) significantly influence clinical decisions (Gomart et al., 2014; Smith et al., 2015). Because invasive core body temperature measurements cannot be performed in clinical settings (Greer et al., 2007; Hayes et al., 1996), the clinicians opt for measuring rectal temperature (RT) with a digital contact thermometer (DCT). Although RT is in close agreement with the core body temperature, RT is slightly lower and often lags after body temperature changes due to intestinal air, faeces, and masses (Greer et al., 2007; Kreissl and Neiger, 2015; Sousa, 2016). Importantly, RT measurements are often poorly tolerated, especially by fractious patients and patients with recto-anal and pelvic conditions (Gomart et al., 2014; Hall and Carter, 2017, Hall et al., 2019; Kreissl and Neiger, 2015). Gomart et al. (Gomart et al., 2014) showed that the RT compared to axillar or auricular temperature measurement provoked more extensive animal defensive behaviour, which can affect various physiological variables like blood pressure and pulse rate (Bragg et al., 2015). Furthermore, the rhythmicity of body temperature is an important physiological process, which depends on body size (Piccione et al., 2009) and location (Giannetto et al., 2015). Ideally, an alternative temperature measurement approach should be accurate but less stressful (Sousa, 2016).
Because of the RT measurement disadvantages mentioned above, the correlation and agreement between rectal and better-tolerated body surface temperature measurements are actively investigated (Kunkle et al., 2004). Taking auricular temperature by a non-contact infrared thermometer (IRT) is common due to the tympanic membrane potential to reflect core body temperature (Lamb and McBrearty, 2013; Smith et al., 2015). However, the correlation between auricular and RT ranged from high (González et al., 2002; Hall and Carter, 2017; Southward et al., 2006; Zanghi, 2016) to poor (Cichocki et al., 2017; Greer et al., 2007; Konietschke et al., 2014; Sousa et al., 2011). It seems that the correct thermometer placement is crucial to obtain real body temperature. Inappropriate IRT positioning probably samples cooler ear canal wall, resulting in anomalous tympanic membrane temperature reading. Similarly, conflicting results were reported when ocular and axillar temperature was studied (Gomart et al., 2014; E. Hall et al., 2019; Kreissl and Neiger, 2015; Rizzo et al., 2017; Sousa, 2016; Zanghi, 2016).
Certainly, conflicting results cannot be attributed only to the thermometer operators. Pušnik and Drnovšek (Pušnik and Drnovšek, 2005) showed that uncalibrated IRTs could cause faulty temperature readings with the error up to 3°C. The existing studies on comparing canine body surface and RT employed various, even human, contact (DCT) and infrared thermometers (IRT), which did not offer the possibility to be clinically calibrated. Therefore, the retrieved thermometric data was evaluated in the incomparable temperature ranges, leading to conflicting conclusions.
In this study, due to the poor canine tolerance towards conventional rectal temperature measurements, we wanted to test a calibrated infrared thermometer for assessing body surface temperature on several body sites, which could be easily used in the clinic. First, we had built a custom-made IRT, which was calibrated in an expected canine temperature range. Measurements were performed on several easily accessible and well-tolerated body sites, which have potential as an alternative temperature measurement for clinicians and animal owners (Hall and Carter, 2017). To access the clinical value of our results, RT additionally served for the calculation of hyperthermia and hypothermia detection sensitivity and specificity. Finally, the animal stress response to the IRT and rectal measurement was estimated.
Materials and methods
Animals and measurements
We conducted a prospective multicenter and multi-operator study, which was approved by the Administration of the Republic of Slovenia for Food Safety, Veterinary Sector and Plant Protection. Additionally, the owner's written permission was collected. The study evolved in two stages:
(1) Hospital stage (realized at the University's Small animal clinic with a single operator) served to identify normal temperature ranges and correlations to RT of several measurement sites (Fig. 1, Table 1). The leading surgeon took temperature readings of 51 dogs (21 males, 30 females) before, during, or after the anesthesia for the scheduled surgeries. There were 46 purebred dogs. Out of 29 breeds, golden retrievers (n = 5), German shepherds (4), and Border collies (3) were the most common. Dogs’ age ranged from 3 months to 15 years, with a median of 7.25 years. The number of young dogs (below 1 y) was 8. At least one hour-long acclimatization to the room temperature was strictly followed (Rizzo et al., 2017). First, infrared measurements in random order were conducted on eight body sites. Finally, the rectal temperature was estimated by DCT (DT-10, rigid rectal thermometer, Advanced Monitors Corporation, San Diego, USA). For each measurement, the ambient temperature was recorded.
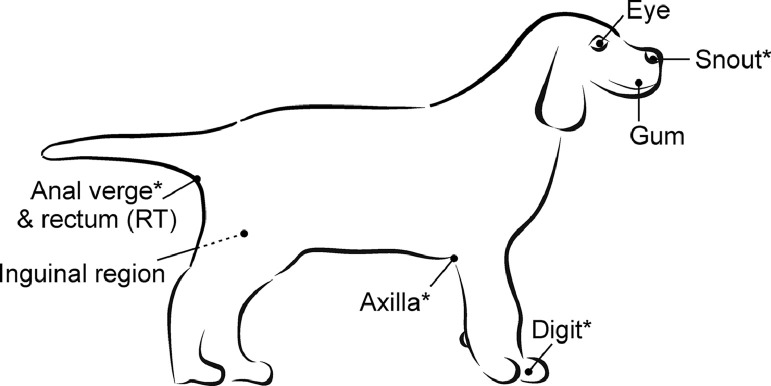
Figure 1.
Measurement sites (*investigated only in the hospital stage).
Table 1.
Description of measurement sites with the corresponding thermometer.
| Short name | Precise location | Thermometer |
|---|---|---|
| Digit* | the second digit, lateral side, left forelimb | infrared (IRT) |
| Snout* | rostral plane of the snout | |
| Axilla* | left side, fold between forelimb and body | |
| Eye | left eye, the pupil was aimed | |
| Gum | left side, gum above the canine tooth | |
| Inguinal region | left side, fold between hind limb and body | |
| Anus* | anal verge | |
| Rectum (RT) | rectal | contact (DCT) |
- investigated only in the hospital stage
(2) Clinical stage (realized with multiple operators). Temperature measurements were taken from 153 dogs (84 males, 69 females) – 71 at private clinics, 82 at animal shelters. Ninety-three dogs were mongrels; the rest were purebred. The average age was 3.2 years (full range from 1.5 months to 13 years). The number of young dogs (below 1 y) was 41. For each measurement site separately, three dogs were excluded from further analysis because either the infrared or rectal measurement was missing. Multiple veterinarians performed measurements during a regular clinical examination right after the animals were admitted to the facility. Therefore, acclimatization to the room temperature was not guaranteed. Infrared measurements on the eye, the gum, and the inguinal region were taken randomly. Finally, RT was acquired. For each measurement, animal hairiness, body condition, body mass, and ambient temperature were recorded.
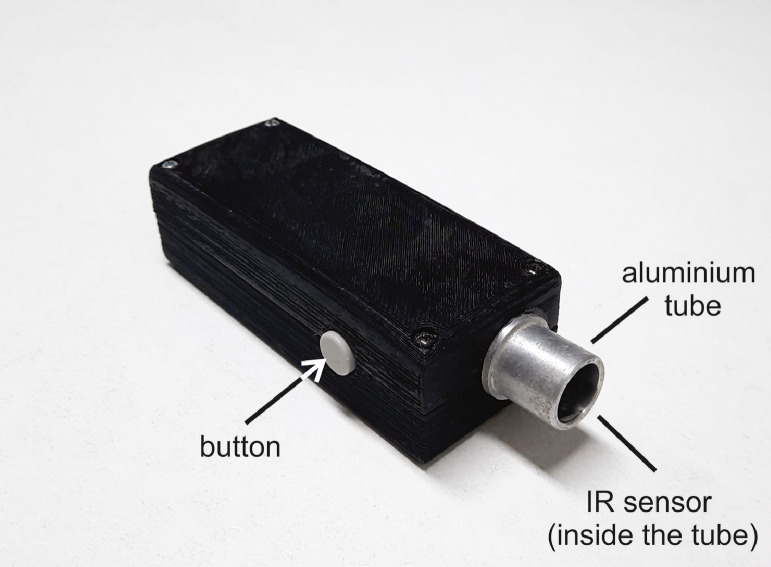
For non-contact measurements, we built a custom-made IRT (Fig. 2). IRT was based on a miniature infrared sensor (MLX90615, Melexis, Ypern, Belgium), which ensured the medical accuracy of 0.2°C in a limited temperature range. To guarantee actual temperature values, we calibrated IRT in the range between 28 and 41°C (9 evenly distributed measurements) with the black body calibrator (Miklavec et al., 2013). The thermometer additionally included a tiny aluminium tube (diameter = 1.5 cm, length = 2.1 cm), which was placed above the sensor in order to reduce the sensor's field of view (FOV) and to remove possible ambient IR sources. To minimize material emissivity, the tube was additionally polished. During the measurements, the IRT thermometer was held approximately 0.5 cm away from the body surface, which resulted in 2.7 cm2 of the circular sampling area. IRT ran on the open-source Arduino platform. Rectal temperature was acquired by a conventional electronic DCT. First, the thermometer was lubricated and then inserted approximately 1.5 cm into the rectum. During the measurement, the thermometer was gently pressed against the rectal wall. In the end, animal body condition and measurement site hairiness were scored with grades from 1 to 5. Age, body mass, and ambient temperature were obtained additionally.
Figure 2.
We used a custom-made infrared thermometer (IRT) calibrated in the temperature range between 28 and 41°C.
Stress assessment
According to animal defensive behaviour, the leading clinician estimated each measurement acceptance with so-called stress score (Gomart et al., 2014; Kreissl and Neiger, 2015). Scores were given in steps of 0.5 in the range between 1 and 5 (Table 2). Stress scores were assessed in the clinical stage for all dogs from shelters (82) and 44 dogs from the private clinic. For the rest of the animals, the clinicians did not provide data.
Table 2.
Stress score criteria.
| Scores | Criteria |
|---|---|
| 1 | Dog is unconcerned by the measurement |
| 2 | Dog is attentive to the measurement but does not show any anxiety and remains still |
| 3 | Dog exhibits nervousness but does not try to slip away |
| 4 | Dog tries to slip away, and in order to perform a measurement, assistance is needed |
| 5 | Dog growls or is aggressive with attempts of biting or is desperate to escape, restraint is needed to measure temperature |
Statistical analysis
The results were summarized with means, standard deviations (STD), medians, and percentiles. First, the monotonic relationship between RT and the infrared temperature was assessed with Spearman's correlation coefficient. We additionally tested a possible impact of gender on the correlation between body and rectal temperature, and between body temperature and external factors (hairiness, body condition, ambient temperature, age, and body mass). To assess the agreement between rectal and IR temperature, Bland-Altman (BA) plots were used (Martin Bland and Altman, 1986). In order to operate with common temperature values and offer comparable results, the infrared temperatures were additionally transformed (based on linear regression) to the RT temperature range. For all BA plots, we plotted 95% limits of agreement, i.e., mean ± 1.95•STD.
Three different rectal temperature thresholds were used for hyperthermia (39.0, 39.3, and 39.6°C) and two for hypothermia (37.7 and 38.0°C) (Couto, 2009; Lorenz, 1995; Refinetti and Piccione, 2003). For each threshold, we calculated cut-off temperature in the specific infrared range (optimizing the geometric mean between sensitivity and specificity), detection sensitivity and specificity, and area under receiver operating characteristic curve (AUC, ROC). 95% confidence intervals (CI) were estimated based on a bootstrap approach with 100,000 repetitions. We analyzed stress scores separately for dogs from the shelters and the private clinic. When comparing all measurement sites, we used repeated-measures ANOVA since the distributions of differences between separate measurement sites were symmetric. For posthoc comparisons between two measurement sites, paired t-tests and adjusted p values with Holm's method were applied. The difference was considered statistically significant if p < 0.05. The post-hoc power analyses were performed for t-tests at α=0.05. In addition, we reported the expected sample size at 80% power for each variable, assuming that the means and standard deviations from our sample reflect the true population. We processed the results in the Matlab environment (R2016a, MathWorks, Natick, USA).
Results
Hospital stage (single operator)
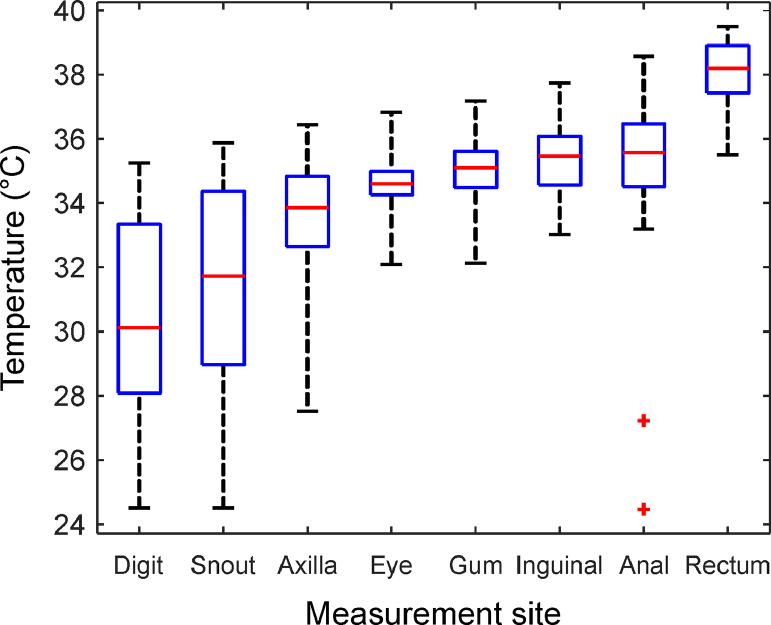
We measured the body surface and rectal temperature (RT) of 51 dogs (Table 3 and Fig. 3). Rectal temperature (RT) had the highest mean value (38.0°C). Oppositely, the mean temperature of the digit and the snout was just 30.50°C and 31.41°C, respectively. Standard deviation below 1.3°C appeared on the eye, on the gum, in the inguinal region, on the anal verge, and in the rectum. Greater variability was seen when temperature was measured on the digit and snout. Inguinal temperature exhibited the best correlation with rectal measurements; the correlation coefficient was 0.60. Finally, we did not discover any significant correlation between the body temperature and age or ambient temperature (all correlation coefficients were below 0.29, all p > 0.09). Additionally, there was no gender-related impact on correlation coefficients between body and rectal temperature.
Table 3.
Hospital stage. Temperature mean value, standard deviation (STD), median, 5th and 95th percentile (in square brackets), and Spearman's correlation coefficient (with 95% confidence intervals in square brackets) against rectal measurements (Corr. with RT).
| Measurement site | Mean (STD) | Median | Corr. with RT |
|---|---|---|---|
| Digit | 30.43 (2.79) | 29.85 [26.19, 34.80] | -0.28 [-0.51, -0.01] |
| Snout | 31.20 (3.02) | 31.46 [26.54, 35.71] | -0.34 [-0.55, -0.09] |
| Axilla | 33.66 (1.96) | 33.98 [30.37, 36.17] | 0.10 [-0.15, 0.35] |
| Eye | 34.54 (0.81) | 34.61 [33.07, 35.88] | 0.46 [0.22, 0.67] |
| Gum | 35.06 (0.97) | 35.09 [33.64, 36.32] | 0.38 [0.15, 0.56] |
| Inguinal region | 35.40 (1.14) | 35.52 [33.29, 36.98] | 0.60 [0.41, 0.74] |
| Anal verge | 35.63 (1.18) | 35.57 [34.09, 37.61] | 0.43 [0.16, 0.67] |
| Rectum (RT) | 38.1 (1.0) | 38.30 [36.06, 39.30] | / |
Figure 3.
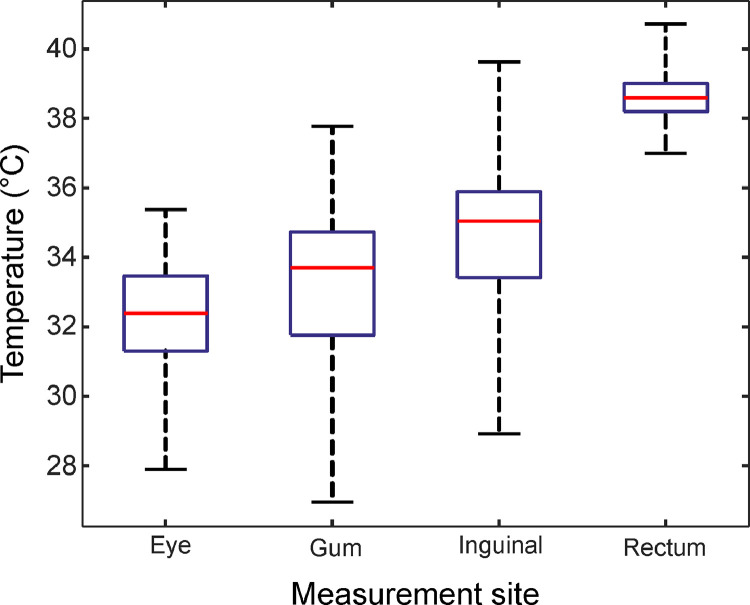
Temperature ranges as boxplots for all measurement sites from the hospital stage. The central mark (red) represents median, the bottom and top box edges indicate the 25th and 75th percentiles (blue), respectively. Whiskers extend to the most extreme data point, which is no more than 1.5 times the interquartile range from the box. Outliers are presented as red crosses.
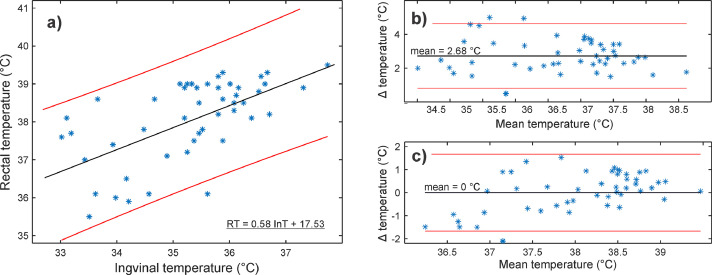
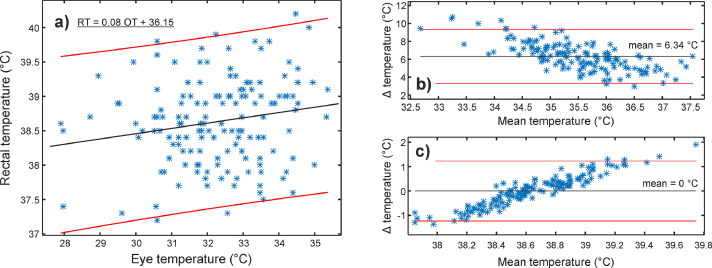
Fig. 4 shows the relationship between inguinal (InT) and rectal temperature (RT). Fig. 4(a) additionally includes a fitted linear function between InT and RT. The original data is presented as a Bland-Altman (BA) plot in Fig. 4(b). The mean difference between InT and RT was 2.68°C. In the end, we transformed the inguinal temperature into the RT range (Fig. 4(c)) based on the linear function from Fig. 4(a). The transformation lowered the average difference to zero (95% limits of agreement [-1.67, 1.67]), and most of the values were well under 1°C. Additionally, 40% of differences were below 0.5°C.
Figure 4.
Hospital stage with a single operator. (a) Rectal (RT) and corresponding inguinal temperature (InT) with a fitted linear regression line (black line) and 95% prediction intervals (red lines). Bland-Altman plots (Δ = RT – InT) (b) before and (c) after the transformation of InT to RT range based on the fitted linear function (Fig. 4 (a)). Mean difference (mean) and 95% limits of agreement are marked as black and red lines, respectively.
In the clinical stage, only the best-performing hospital measurement sites were kept: eye, gum, and inguinal region. The choice was based on the temperature variability (i.e., STD) and the correlation with RT (Table 3).
Clinical stage (multiple operators)
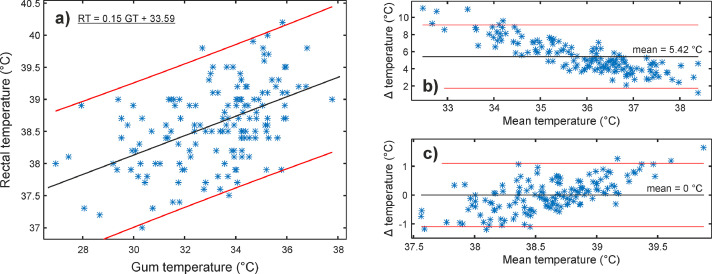
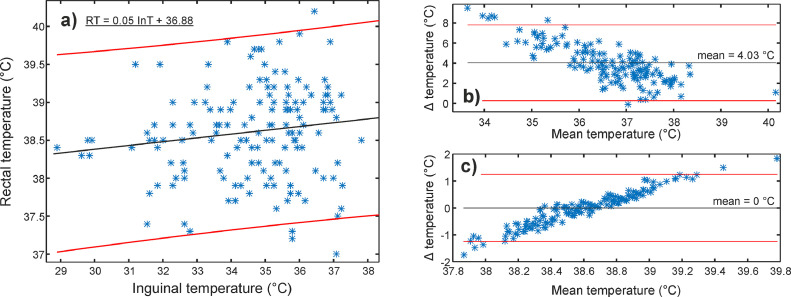
Table 4 and Fig. 5 list the temperatures from the 153 dogs involved. Compared to the hospital stage, mean temperatures of the eye, gum, and inguinal region were lower (Table 4). Conversely, the mean RT was slightly higher. Furthermore, correlation coefficients were lower for the eye and inguinal region (0.46/0.60 vs. 0.17/0.13), whereas the coefficient increased for the gum from 0.38 to 0.50, showing a moderate correlation. The negative correlation was additionally found between inguinal temperature (InT) and hairiness, body condition, and ambient temperature. On the other hand, RT correlated weakly with body mass (Table 4). Animal age did not exhibit any correlation with the body or rectal temperature.
Table 4.
Clinical stage. Temperature mean value, standard deviation (STD), median, 5th and 95th percentile (in squared brackets) and Spearman's correlation coefficient and p value (with 95% confidence intervals in square brackets) against rectal measurements (Corr. with RT) and external factors (Corr. with EF).
| Measurement site | Mean ± STD | Median | Corr. with RT | Corr. with EF* |
|---|---|---|---|---|
| Eye | 32.30 (1.52) | 32.39 [29.62, 34.53] | 0.17, p = 0.04 [0.00, 0.32] | / |
| Gum | 33.20 (2.12) | 33.70 [29.39, 35.97] | 0.50, p < 0.001 [0.37, 0.61] | / |
| Inguinal region | 34.58 (1.91) | 35.02 [31.19, 37.08] | 0.13, p = 0.11 [-0.04, 0.29] | -0.45a -0.51b -0.38c |
| Rectum (RT) | 38.62 (0.65) | 38.60 [37.60, 39.70] | / | 0.35d |
External factors are denoted with the following letters.
– hairiness (p < 0.001)
– body condition (p < 0.001)
– ambient temperature (p < 0.001)
– body mass (p = 0.96).
Correlation coefficients against external factors with values between -0.30 and 0.30 are not listed.
Figure 5.
Temperature ranges as boxplots from the clinical stage. The central mark (red) represents median, the bottom and top box edges indicate the 25th and 75th percentiles (blue), respectively. Whiskers extend to the most extreme data point.
Figure 6, Figure 7, Figure 8 show the relationship between infrared (eye, gum, and inguinal region) and rectal temperature (RT). Fig. 6-8(a) include scatter plots and fitted linear functions (black line), which served for the transformation of infrared temperatures to the RT range. The agreement between infrared and RT is shown with Bland-Altman plots before (Figure 6, Figure 7, Figure 8(b)) and after (Figure 6, Figure 7, Figure 8(c)) temperature range transformation. The purpose of the transformation is in 1) presenting data in the range veterinarians are used to; 2) setting mean difference close to zero; and 3) removing trends of dependence between the infrared in RT (e.g., Fig. 7(b)). In Fig. 7(b), the mean difference was more than 5.4°C, with the 95 % limits of agreement between 1.7 and 9.1°C. After the transformation (Fig. 7(c)), the mean was zero, and most of the differences (91.3%, 137/150) between gum (GT) and RT were up to 1°C.
Figure 6.
Clinical stage with multiple operators. (a) Rectal (RT) and corresponding eye/ocular temperature (OT) with a fitted linear regression line (black line) and 95% prediction intervals (red lines). Bland-Altman plots (Δ = RT – OT) (b) before and (c) after the transformation of OT to RT range based on the fitted linear function (Fig. 6 (a)). Mean difference (mean) and 95% limits of agreement are marked as black and red lines, respectively.
Figure 7.
Clinical stage with multiple operators. (a) Rectal (RT) and corresponding gum temperature (GT) with a fitted linear regression line (black line) and 95% prediction intervals (red lines). Bland-Altman plots (Δ = RT – GT) (b) before and (c) after the transformation of GT to RT range based on the fitted linear function (Fig. 7 (a)). Mean difference (mean) and 95% limits of agreement are marked as black and red lines, respectively.
Figure 8.
Clinical stage with multiple operators. (a) Rectal (RT) and corresponding inguinal temperature (InT) with a fitted linear regression line (black line) and 95% prediction intervals (red lines). Bland-Altman plots (Δ = RT – InT) (b) before and (c) after the transformation of InT to RT range based on the fitted linear function (Fig. 8 (a)). Mean difference (mean) and 95% limits of agreement are marked as black and red lines, respectively.
Table 5 lists the thresholds for clinical hyperthermia and hypothermia detection together with the corresponding sensitivities and specificities, all with 95% confidence intervals. The gum exhibited the best potential to correctly detect hyperthermia since the sensitivity was up to 90.0 %. On the other hand, we could not detect hypothermia very well.
Table 5.
Hyperthermia and hypothermia detection characteristics at three measurement sites from the clinical stage. The predetermined thresholds of rectal temperature: 39.6, 39.3, 39.0, 38.0, and 37.7°C, corresponding infrared cut-off temperature, sensitivity and specificity, and AUC with 95% confidence intervals (in squared brackets) are listed.
| Measurement site | Hyperth.(↑) Hypoth. (↓) |
Rectal (°C) | Infrared (°C) | Sensitivity (%) | Specificity (%) | AUC |
|---|---|---|---|---|---|---|
| Eye | ↑ | 39.6 | 33.3 [31.8, 34.4] | 54.6 [22.2, 85.7] | 74.8 [67.4, 81.9] | 0.63 [0.43, 0.81] |
| 39.3 | 32.6 [31.8, 34.2] | 59.1 [37.5, 80.0] | 57.0 [48.4, 65.6] | 0.57 [0.43, 0.71] | ||
| 39.0 | 32.6 [31.8, 33.0] | 66.7 [53.0, 80.0] | 64.7 [55.2, 73.9] | 0.66 [0.56, 0.75] | ||
| ↓ | 38.0 | 32.0 [31.4, 33.5] | 61.3 [43.5, 78.3] | 41.2 [32.4, 50.0] | 0.48 [0.37, 0.60] | |
| 37.7 | 32.6 [32.0, 33.6] | 50.0 [20.0, 80.0] | 52.2 [43.8, 60.6] | 0.44 [0.24, 0.63] | ||
| Gum | ↑ | 39.6 | 34.7 [34.7, 35.1] | 90.0 [66.7, 100] | 78.6 [71.6, 85.2] | 0.84 [0.70, 0.93] |
| 39.3 | 34.7 [32.7, 35.1] | 61.9 [40.8, 82.6] | 79.8 [72.7, 86.5] | 0.76 [0.65, 0.86] | ||
| 39.0 | 34.0 [33.0, 34.7] | 72.3 [59.0, 84.8] | 68.0 [58.8, 76.9] | 0.75 [0.66, 0.83] | ||
| ↓ | 38.0 | 33.3 [30.5, 35.2] | 31.3 [15.6, 48.2] | 33.9 [25.4, 42.5] | 0.24 [0.15, 0.33] | |
| 37.7 | 32.8 [30.3, 33.7] | 38.5 [11.8, 66.7] | 32.9 [24.8, 40.6] | 0.24 [0.15, 0.35] | ||
| Inguinal | ↑ | 39.6 | 34.6 [33.9, 36.4] | 90.0 [66.7, 100] | 42.9 [34.7, 51.1] | 0.64 [0.47, 0.79] |
| 39.3 | 34.6 [34.6, 36.0] | 76.2 [56.3, 93.8] | 43.4 [34.9, 51.9] | 0.53 [0.40, 0.66] | ||
| 39.0 | 34.7 [34.5, 35.9] | 71.7 [58.1, 84.4] | 50.0 [39.6, 59.1] | 0.60 [0.50, 0.70] | ||
| ↓ | 38.0 | 35.7 [33.8, 35.8] | 40.6 [23.5, 58.1] | 67.8 [59.1, 76.0] | 0.52 [0.41, 0.64] | |
| 37.7 | 35.3 [34.1, 37.1] | 61.5 [33.3, 88.9] | 62.0 [53.0, 69.6] | 0.59 [0.40, 0.77] |
The stress scores were statistically significantly different for measurement sites (repeated measures ANOVA, p < 0.001 for the clinic and shelters). The mean score and p values of posthoc paired t-tests (i.e., comparing two measurement sites), adjusted for multiple comparisons, are listed in Table 6. The inguinal measurements were tolerated best, mean stress score was 2.45 for the shelter dogs and 1.32 for the clinic dogs, respectively. On the other hand, rectal measurements provoked the most stress – the mean score was around 3. The difference analysis is presented in Table 7.
Table 6.
Stress scores from the clinical stage, separately for the shelters and clinic: mean (with standard deviation - STD) and p values from posthoc tests for comparisons of stress scores evaluated on two different measurement sites.
| M. site | Shelters (n = 82) | Clinic (n = 44) | ||
|---|---|---|---|---|
| Mean (STD) | adjusted p values | Mean (STD) | adjusted p values | |
| 1: Eye | 3.20 (0.72) | p1-4 = 0.07 p1-2, p1-3 < 0.01 p2-3, p2-4 < 0.01 p3-4 < 0.01 |
1.75 (0.92) | p1-2 = 0.11 p1-3, p1-4 < 0.01 p2-3, p2-4 < 0.01 p3-4 < 0.01 |
| 2: Gum | 2.89 (0.67) | 1.95 (1.08) | ||
| 3: Inguinal | 2.45 (0.59) | 1.32 (0.60) | ||
| 4: Rectum | 3.37 (0.90) | 2.75 (1.06) | ||
Table 7.
Mean and standard deviation (STD) and 95 % confidence intervals (CI) of stress score differences between rectal and infrared measurements. The post-hoc power (α=0.05) and the expected sample size with a power of 80% are included.
| Measurement site | Shelters (n = 82) | Clinic (n=44) | ||||
|---|---|---|---|---|---|---|
| Mean (STD) | 95% CI | Power / Size | Mean (STD) | 95% CI | Power / Size | |
| Rectum – Eye | 0.16 (0.80) | -0.01 – 0.34 | 43% / 199 | 1.00 (1.01) | 0.69 – 1.31 | 100% / 11 |
| Rectum – Gum | 0.48 (0.85) | 0.29 – 0.66 | 100% / 27 | 0.80 (1.00) | 0.49 – 1.10 | 100% / 15 |
| Rectum – Inguinal | 0.92 (0.91) | 0.72 – 1.12 | 100% / 10 | 1.43 (1.11) | 1.09 – 1.77 | 100% / 7 |
Discussion
The mean rectal temperature (RT) of hospital animals was lower for 0.62°C compared to the clinical stage. The reason for the difference could lie in the fact that temperature measurement in approximately one-third of hospital dogs took place under general anaesthesia, which can lower the body temperature (Rigotti et al., 2015). The rest of the measurement sites in the hospital stage exhibited higher mean body surface temperature, probably due to the strict acclimatization procedure followed. Conversely, winter months during which the clinical stage with mostly walk-in patients was executed, hindered a proper temperature acclimatization process, typically taking up to one hour (Rizzo et al., 2017). Our assumption was supported by the increased correlation between inguinal and ambient temperature in the clinical stage (Table 4).
The existing studies reported many discrepancies in differences and correlations between the body surface and RT. Since some measurement sites are well vascularized and isolated (e.g., tympanic membrane, axillar region), many authors expectedly reported small temperature differences to RT of up to 1.3°C (Cichocki et al., 2017; Goic et al., 2014; Gomart et al., 2014; Greer et al., 2007; Hall and Carter, 2017; Konietschke et al., 2014; Lamb and McBrearty, 2013; Mathis and Campbell, 2015; Piccione et al., 2011; Sousa et al., 2011; Southward et al., 2006; Wiedemann et al., 2006; Yanmaz et al., 2015). On the other hand, our study recorded a higher difference between axillar and RT of 4.32°C, similar to the study of Rizzo et al (2017). Significant temperature differences to RT were also reported on the eye (our study: 3.41-6.30°C, Rizzo et al.: 6°C (Rizzo et al., 2017)), and in the digital (our study: 7.50°C) and inguinal region (our study: 2.68°C). The differences above are in the same range as the digit and torso temperature of the human, where the values were 7.5°C and 4.9°C, respectively (Taylor et al., 2014). Many discrepancies can also be found in the reported correlation. The correlation between auricular and RT ranged from weak (0.3) to strong (0.89) (Gomart et al., 2014; Konietschke et al., 2014; Wiedemann et al., 2006; Yanmaz et al., 2015). A strong correlation (0.7) was found between axillar and RT (Gomart et al., 2014). However, our study and the study of Mathis and Campbell (2015) reported almost no correlation (ρ = 0.16 and 0.24). Regarding eye temperature, the reported correlations were moderate (0.38-0.59) (Kreissl and Neiger, 2015; Rizzo et al., 2017; Yanmaz et al., 2015), similar to the hospital stage of our study (0.50). However, the correlation in the clinical stage, which included multiple operators, was weaker.
According to the available data, we believe that conflicting results can be attributed to the following factors:
-
1)
Thermometers. The existing studies applied various contact (DCT) or infrared thermometers (IRT). DCT operates typically in the predictive technique, which does not measure but only estimates the final temperature. On the other hand, uncalibrated IRT can exhibit errors up to 3°C (Pušnik and Drnovšek, 2005). Therefore, many commercial thermometers are specified only for a specific species and measurement site (e.g., the axillar temperature in humans). As existing and our data show, thermometers should be calibrated for a specific temperature range and measurement site.
-
2)
Sampling. Since IRT samples a relatively small area, the operator can easily acquire the temperature of (cooler) surrounding tissues. Fig. 3(a) displays two measurements on the anal verge with significantly lower temperatures. Both measurements probably include the surrounding skin and hair. Furthermore, ocular measurements in conscious dogs can be challenging since animal movements can increase the probability of false sampling. Stable and standardized sampling is even more crucial if multiple operators are included in the study.
-
3)
External and body factors. Factors as ambient temperature, body mass, hairiness, gender, and age can crucially affect the difference or correlation between rectal (RT) and body surface temperature (Gomart et al., 2014). In dogs, the statistically significant correlation was found only between body surface temperature and mass, hairiness, and body condition (Lamb and McBrearty, 2013). The same factors proved influential in our study with the addition of ambient temperature (Table 4). In both studies, increased body condition score had a negative correlation coefficient. Surprisingly, the hairiness correlation was not consistent (positive vs. negative), which could be a consequence of different coat types (Kwon and Brundage, 2019) or ambient temperatures affecting the insulation function of the coat.
Comparing temperature differences directly does not contribute to the analysis of agreement between IRT and DCT. If original data is shown in Bland-Altman plots (Giavarina, 2015), substantial mean differences and apparent trends between temperatures can be noted (examples can be found in the previous studies (Greer et al., 2007; Kreissl and Neiger, 2015; Lamb and McBrearty, 2013) or Fig. 4(b), 6-8(b)). As suggested in our study, body surface temperature can be transformed into RT value range by, e.g., linear regression analysis. The approach presents data in the RT range that veterinary clinicians and researchers are accustomed to, and the data is suitable to be drawn in the Bland-Altman plot. However, the “opposite” trend can be seen also after the transformation (Figure 6, Figure 7, Figure 8(c)). It should be noted that this appears due to the fitting process. If a body surface temperature replaces the mean temperature on the x-axis, these trends disappear.
For clinical medicine, the detection of hyper- or hypothermia is the most relevant. In humans, the hyperthermia detection sensitivity under the armpit (axillar region) can reach up to 87.5 %. In dogs, the axillar sensitivity was lower, i.e., 57 % (specificity = 100 %). On the other hand, hypothermia was better detected (sensitivity = 86 %, specificity = 87 %) (Goic et al., 2014). Our study showed that only the mouth (i.e., gum) could be used for hyperthermia detection. When threshold temperature value was set to 39.6°C, up to 90 % (95% CI: [66.7, 100]) cases were discovered (specificity = 78.6 %, 95% CI: [71.6, 85.2]). With the lower threshold value (39.3°C), we could not detect hyperthermia as well; sensitivity and specificity were 61.9% and 79.8%, respectively. This fact can limit the usability of IRT to detect fever, especially with the marginal cases, which should be followed by the standard method (RT). When it comes to hypothermia, IRT did not prove very accurate. However, we should add that the recent work in cats, horses, and rabbits (Gallego, 2017; Hall et al., 2019; Levy et al., 2015) has suggested the lower limit for temperature ranges in healthy animals. Therefore, 37.3°C would probably be a more reasonable hypothermic limit, as applied in the study by Konietschke et al. (2014). However, in this case, the number of hypothermic samples would be only four, preventing of doing any reliable detection analysis.
Our work additionally confirmed that RT measurements by DCT are significantly more stressful than IRT. Lamb McBrearty showed that 45.5% of dogs needed additional restraint during rectal measurements (Lamb and McBrearty, 2013). With measurements on the eye, this percentage was only 20 %. In our study, RT measurements achieved the average stress score of 3, statistically significantly more than IRT on the inguinal (average score 1.32 for the clinic and 2.45 for the shelters), gum (average score 1.95 for the clinic and 2.89 for the shelters), and 1.75 for ocular measurements (only in the clinical setting, where pets were better accustomed to human handling).
Conclusions
In this study, we investigated canine surface body temperature with an infrared thermometer (IRT), and we compared the results to the rectal temperature (RT). The transformation with the estimated regression linear function kept most of the differences between the gum and rectal temperature (RT) below 1°C. What is more, hyperthermia (threshold value of 39.6°C) was detected quite well, with the sensitivity up to 90 % (95% CI: [66.7, 100]), which is comparable to the axillar region in humans. We also showed that RT measurements caused more stress than the approach with IRT.
We additionally discovered that due to different temperature ranges and uncalibrated thermometers applied in the previous studies, a direct comparison of results proved challenging (Sousa, 2016). We think that future research on new thermometers or measurement sites should guarantee: (1) a calibrated thermometer, (2) stable conditions (e.g., fixed ambient temperature, the same measuring approach for multiple operators), (3) strict animal acclimatization process, which can take up to one hour. However, ensuring acclimatization could be challenging if/when novel methods are applied in clinical settings. Additionally, we could introduce better-designed IRT thermometers adjusted for the specific veterinary use.
Ethical statement
The authors ensure that the work described has been carried out according to the NC3Rs ARRIVE Guidelines. Our study was approved by the Administration of the Republic of Slovenia for Food Safety, Veterinary Sector and Plant Protection. We additionally obtained the owner's agreement.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 745396 (DogSPEC), and from the Latvian State Education Development Agency under the grant agreement 1.1.1.2/VIAA/3/19/455 (GoBVM). We would also like to express our gratitude to veterinarians and all other personnel from Small Animal Clinic (University of Ljubljana), Veterinary clinic Primavet (Rače) and animal shelters Meli, Turk, Mala Hiša, Maribor, Obalno Zavetišče and Brežice. We thank prof. Dr. Igor Pušnik from the Laboratory of Metrology and Quality, University of Ljubljana, for the use of their equipment. There are no other conflicts of interest to declare.
References
- Bland M.J., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–310. [PubMed] [Google Scholar]
- Bragg R.F., Bennett J.S., Cummings A., Quimby J.M. Evaluation of the effects of hospital visit stress on physiologic variables in dogs. J. Am. Vet. Med. Assoc. 2015;246:212–215. doi: 10.2460/javma.246.2.212. [DOI] [PubMed] [Google Scholar]
- Cichocki B., Dugat D., Payton M. Agreement of Axillary and Auricular Temperature with Rectal Temperature in Systemically Healthy Dogs Undergoing Surgery. J. Am. Anim. Hosp. Assoc. 2017;53:291–296. doi: 10.5326/JAAHA-MS-6500. [DOI] [PubMed] [Google Scholar]
- Couto C. Fever of undetermined origin. In: Nelson R., Couto C., editors. Small Animal Internal Medicine. Mosby/Elsevier, St.Louis; 2009. pp. 1274–1277. [Google Scholar]
- Gallego M. Laboratory reference intervals for systolic blood pressure, rectal temperature, haematology, biochemistry and venous blood gas and electrolytes in healthy pet rabbits. Open Vet. J. 2017;7:203–207. doi: 10.4314/ovj.v7i3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannetto C., Fazio F., Panzera M., Alberghina D., Piccione G. Comparison of rectal and vaginal temperature daily rhythm in dogs (Canis familiaris) under different photoperiod. Biol. Rhythm Res. 2015;46:113–119. [Google Scholar]
- Giavarina D. Understanding Bland Altman analysis. Biochem. Medica. 2015;25:141–151. doi: 10.11613/BM.2015.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goic J.B., Reineke E.L., Drobatz K.J. Comparison of rectal and axillary temperatures in dogs and cats. J. Am. Vet. Med. Assoc. 2014;244:1170–1175. doi: 10.2460/javma.244.10.1170. [DOI] [PubMed] [Google Scholar]
- Gomart S.B., Allerton F.J.W., Gommeren K. Accuracy of different temperature reading techniques and associated stress response in hospitalized dogs. J. Vet. Emerg. Crit. Care. 2014;24:279–285. doi: 10.1111/vec.12155. [DOI] [PubMed] [Google Scholar]
- González A.M., Mann F.A., Preziosi D.E., Meadows R.L., Wagner-Mann C.C. Measurement of body temperature by use of auricular thermometers versus rectal thermometers in dogs with otitis externa. J. Am. Vet. Med. Assoc. 2002;221:378–380. doi: 10.2460/javma.2002.221.378. [DOI] [PubMed] [Google Scholar]
- Greer R.J., Cohn L.A., Dodam J.R., Wagner-Mann C.C., Mann F.A. Comparison of three methods of temperature measurement in hypothermic, euthermic, and hyperthermic dogs. J. Am. Vet. Med. Assoc. 2007;230:1841–1848. doi: 10.2460/javma.230.12.1841. [DOI] [PubMed] [Google Scholar]
- Hall E., Fleming A., Carter (Pullen) A. Investigating the use of non-contact infrared thermometers in cats and dogs. Vet. Nurse. 2019;10:109–115. [Google Scholar]
- Hall E.J., Carter A.J. Comparison of rectal and tympanic membrane temperature in healthy exercising dogs. Comp. Exerc. Physiol. 2017;13:37–44. [Google Scholar]
- Hall E.J., Carter A.J., Stevenson A.G., Hall C. Establishing a Yard-Specific Normal Rectal Temperature Reference Range for Horses. J. Equine Vet. Sci. 2019;74:51–55. [Google Scholar]
- Hayes J.K., Collette D.J., Peters J.L., Smith K.W. Monitoring body-core temperature from the trachea: comparison between pulmonary artery, tympanic, esophageal, and rectal temperatures. J. Clin. Monit. 1996;12:261–269. doi: 10.1007/BF00857648. [DOI] [PubMed] [Google Scholar]
- Konietschke U., Kruse B.D., Müller R., Stockhaus C., Hartmann K., Wehner A. Comparison of auricular and rectal temperature measurement in normothermic, hypothermic, and hyperthermic dogs. Tierärztl. Prax. Kleintiere. 2014;42:13–19. [PubMed] [Google Scholar]
- Kreissl H., Neiger R. Measurement of body temperature in 300 dogs with a novel noncontact infrared thermometer on the cornea in comparison to a standard rectal digital thermometer. J. Vet. Emerg. Crit. Care. 2015;25:372–378. doi: 10.1111/vec.12302. [DOI] [PubMed] [Google Scholar]
- Kunkle G.A., Nicklin C.F., Sullivan-Tamboe D.L. Comparison of Body Temperature in Cats Using a Veterinary Infrared Thermometer and a Digital Rectal Thermometer. J. Am. Anim. Hosp. Assoc. 2004;40:42–46. doi: 10.5326/0400042. [DOI] [PubMed] [Google Scholar]
- Kwon C.J., Brundage C.M. Quantifying body surface temperature differences in canine coat types using infrared thermography. J. Therm. Biol. 2019;82:18–22. doi: 10.1016/j.jtherbio.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Lamb V., McBrearty A.R. Comparison of rectal, tympanic membrane and axillary temperature measurement methods in dogs. Vet. Rec. 2013;173 doi: 10.1136/vr.101806. 524–524. [DOI] [PubMed] [Google Scholar]
- Levy J.K., Nutt K.R., Tucker S.J. Reference interval for rectal temperature in healthy confined adult cats. J. Feline Med. Surg. 2015;17:950–952. doi: 10.1177/1098612X15582081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz M. Pyrexia (Fever) In: Lorenz M., Cornelius L., editors. Small Animal Medical Diagnosis. Wiley; 1995. pp. 15–22. [Google Scholar]
- Mathis J.C., Campbell V.L. Comparison of axillary and rectal temperatures for healthy Beagles in a temperature- and humidity-controlled environment. Am. J. Vet. Res. 2015;76:632–636. doi: 10.2460/ajvr.76.7.632. [DOI] [PubMed] [Google Scholar]
- Miklavec A., Pušnik I., Batagelj V., Drnovšek J. A large aperture blackbody bath for calibration of thermal imagers. Meas. Sci. Technol. 2013;24 [Google Scholar]
- Piccione G., Fazio F., Giudice E., Refinetti R. Body size and the daily rhythm of body temperature in dogs. J. Therm. Biol. 2009;34:171–175. [Google Scholar]
- Piccione G., Giannetto C., Fazio F., Giudice E. Accuracy of auricular temperature determination as body temperature index and its daily rhythmicity in healthy dog. Biol. Rhythm Res. 2011;42:437–443. [Google Scholar]
- Pušnik I., Drnovšek J. Infrared ear thermometers—parameters influencing their reading and accuracy. Physiol. Meas. 2005;26:1075–1084. doi: 10.1088/0967-3334/26/6/016. [DOI] [PubMed] [Google Scholar]
- Refinetti R., Piccione G. Daily Rhythmicity of Body Temperature in the Dog. J. Vet. Med. Sci. 2003;65:935–937. doi: 10.1292/jvms.65.935. [DOI] [PubMed] [Google Scholar]
- Rigotti C.F., Jolliffe C.T., Leece E.A. Effect of prewarming on the body temperature of small dogs undergoing inhalation anesthesia. J. Am. Vet. Med. Assoc. 2015;247:765–770. doi: 10.2460/javma.247.7.765. [DOI] [PubMed] [Google Scholar]
- Rizzo M., Arfuso F., Alberghina D., Giudice E., Gianesella M., Piccione G. Monitoring changes in body surface temperature associated with treadmill exercise in dogs by use of infrared methodology. J. Therm. Biol. 2017;69:64–68. doi: 10.1016/j.jtherbio.2017.06.007. [DOI] [PubMed] [Google Scholar]
- Smith V.A., Lamb V., McBrearty A.R. Comparison of axillary, tympanic membrane and rectal temperature measurement in cats. J. Feline Med. Surg. 2015;17:1028–1034. doi: 10.1177/1098612X14567550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa M.G. Measuring body temperature: how do different sites compare? Vet. Rec. 2016;178:190–191. doi: 10.1136/vr.i893. [DOI] [PubMed] [Google Scholar]
- Sousa M.G., Carareto R., Pereira-Junior V.A., Aquino M.C. Comparison between auricular and standard rectal thermometers for the measurement of body temperature in dogs. Can. Vet. J. 2011;52:403–406. [PMC free article] [PubMed] [Google Scholar]
- Southward E.S., Mann F.A., Dodam J., Wagner-Mann C.C. A comparison of auricular, rectal and pulmonary artery thermometry in dogs with anesthesia-induced hypothermia. J. Vet. Emerg. Crit. Care. 2006;16:172–175. [Google Scholar]
- Taylor N.A.S., Tipton M.J., Kenny G.P. Considerations for the measurement of core, skin and mean body temperatures. J. Therm. Biol. 2014;46:72–101. doi: 10.1016/j.jtherbio.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Wiedemann G.G.S., Scalon M.C., Paludo G., Silva I.O., Boere V. Comparison between tympanic and anal temperature with a clinical infrared ray thermometer in dogs. Arq. Bras. Med. Veterinária E Zootec. 2006;58:503–505. [Google Scholar]
- Yanmaz L.E., Dogan E., Okumus Z., Şenocak M.G., Yildrim F. Comparison of Rectal, Eye and Ear Temperatures in Kangal Breed Dogs. Kangal Irkı Köpeklerde Kulak Göz Ve Rektal Isıların Karşıştırılması. 2015;21:615–617. [Google Scholar]
- Zanghi B.M. Eye and Ear Temperature Using Infrared Thermography Are Related to Rectal Temperature in Dogs at Rest or With Exercise. Front. Vet. Sci. 2016;3:111. doi: 10.3389/fvets.2016.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]