Highlights
-
•
Mixed tumors are common lesions in female dogs and present variable biological behavior.
-
•
Carcinomas in mixed tumors are neoplasms that present good biological behavior.
-
•
Even at an advanced stage, female dogs with carcinomas in mixed tumors exhibit a longer survival compared with bitches with carcinosarcomas.
-
•
Female dogs with carcinosarcomas undergoing surgery only presented a lower overall survival rate.
Keywords: Carcinosarcoma, Carcinoma in mixed tumor, Chemotherapy, Neoplasms
Abstract
Mixed tumors are the most frequent mammary gland neoplasms in bitches; however, studies that thoroughly describe their clinicopathological data, treatment approaches, and the survival of bitches with mixed tumors are scarce. This study evaluated the epidemiological and clinicopathological data, prognostic factors, and therapeutic approaches for bitches with mixed tumors. In all, 162 benign mixed tumors, 682 carcinomas in mixed tumors, and 60 carcinosarcomas were included. Regarding tumor size, T3 lesions were predominantly associated with carcinosarcomas, while T1 and T2 lesions occurred more frequently in benign mixed tumors and in carcinomas in mixed tumors. Based on clinical staging, most bitches with benign mixed tumors presented with stage I tumors; 92% of bitches with carcinomas in mixed tumors presented with stage I–III tumors, while 8% presented with stage IV–V tumors; and 70% of bitches with carcinosarcomas presented with stage I–III tumors, while 30% presented with stage IV–V tumors. Surgery was curative for bitches with benign mixed tumors and for those with stage I–III carcinomas in mixed tumors. Combination therapy in bitches with carcinomas in mixed tumors (IV–V) and carcinosarcomas resulted in a higher overall survival compared with bitches who underwent surgery only. Carcinosarcomas presented higher relapse rates and distant metastases than carcinomas in mixed tumors did.
Introduction
Mixed tumors are the most frequent mammary gland neoplasms in bitches (Cassali et al., 2012). These tumors are characterized by epithelial, myoepithelial, and mesenchymal elements, which may undergo malignant transformation, as well as by their origins in carcinomas in mixed tumors and carcinosarcomas (Cassali et al., 2012, Misdorp, 1999, 2014).
Microscopically, benign mixed tumors are characterized by benign epithelial (ductal and/or acinar cells), myoepithelial, and mesenchymal elements, along with the presence of cartilage and/or bone (Misdorp et al., 1999). Carcinomas in mixed tumors are composed of malignant epithelial and benign mesenchymal components, which may be represented by cartilage, bone, adipose tissue or all three. However, carcinosarcomas are mammary neoplasms with both malignant epithelial and mesenchymal components (Misdorp et al., 1999).
Mammary tumor prognosis is influenced by several factors including age, histological type and grade, clinical staging, tumor size, tumor biological behavior, mitotic index, regional or distant metastases, microvessel density, and molecular markers (Sorenmo, 2003, Cassali et al., 2014). Surgical treatment is the first choice for all bitches with mammary tumors (Sorenmo, 2003, Sorenmo et al., 2013). Adjuvant chemotherapy is indicated for patients with regional or distant metastases and for those with mammary neoplasms with poor prognoses (Cassali et al., 2014). Combining antiangiogenic strategies and antineoplastic chemotherapeutic protocols may benefit bitches with malignant neoplasms (Pierini, Bocci, Giorgi, Owen, & Marchetti, 2012).
Studying the epidemiological and clinicopathological characteristics of mixed tumors provides important information that helps in understanding their biological behavior. This study evaluated the epidemiological and clinicopathological data, prognostic factors, and treatment approach in bitches with mixed tumors and is the first study to present these evaluations using a large number of cases.
Methods
All procedures were performed under the appropriate guidelines and were approved by the Ethics Committee for Animal Experimentation of the Federal University of Minas Gerais (CETEA/UFMG), protocol number 338/2012.
In total, 904 bitches were included and diagnosed with mixed tumors that were histologically classified as follows: 162 benign mixed tumors, 682 carcinomas in mixed tumors, and 60 carcinosarcomas. The histopathological classifications followed the criteria proposed by Goldschmidt, Peña, and Zappulli (2017) and were standardized per Cassali et al. (2017). All bitches were seen in the oncology department of the Veterinary Hospital of the Federal University of Minas Gerais (Universidade Federal de Minas Gerais UFMG), Brazil and received mastectomies from 2000 to 2015. The surgical technique was chosen based on the number of lesions and the lesion site, respecting lymphatic drainage and established prognostic factors, such as lesion size and presence of cutaneous or muscular adhesions, including simple mastectomy, regional mastectomy, and unilateral radical mastectomy. All the histopathological tests were performed at the Laboratory of Comparative Pathology of the UFMG Biological Sciences Institute.
Epidemiological, clinical, and pathological data were obtained from admission records. The time of tumor development was defined as the period in days from the time of perception by the owners until the time of diagnosis. All patients underwent complementary chest radiographic examinations with projections (lateral-lateral right, lateral-lateral left, and ventral-dorsal) and serum tests (complete blood count, clotting tests, and liver and kidney panels).
Staging was performed per a modified version of the original tumor, node, metastases (TNM) system established by the World Health Organization (WHO). This system enables categorizing the animals into 5 stages, as shown in Table 1 (Owen, 1980, Sorenmo et al., 2013). Next, the animals' tumors were classified during clinical staging as early (I–III) or advanced (IV–V). (Table 2).
Table 1.
Clinical staging of bitches with mammary tumors according to the modified version of the original TNM system established by the WHO (5).
| Clinical stage | Tumor size (T) | Regional lymph nodes (N) | Distant metastases (M) |
|---|---|---|---|
| I | T1 | N0 | M0 |
| II | T2 | N0 | M0 |
| III | T3 | N0 | M0 |
| IV | T1,2,3 | N1 | M0 |
| V | T1,2,3 | N0,1 | M1 |
Table 2.
Epidemiological, clinical, and pathological characteristics according to the histological classification of mixed tumors in bitches.
| Variables | Benign mixed tumor | Carcinomasin mixed tumors | Carcinosarcoma | P value |
|---|---|---|---|---|
| Number of cases | 162 (18%) | 682 (75%) | 60 (7%) | |
| Time of development (days) | 297.25 ± 420.13 | 367.8 ± 430.98 | 570.5 ± 599.47 | 0.034 |
| Age | 8.89 ± 2.69 | 9.68 ± 2.74 | 10.50 ± 2.76 | <0.001 |
| Breed | ||||
| Pure | 131 (84%) | 502 (78.2%) | 41 (70.7%) | 0.860 |
| Mixed | 25 (16%) | 140 (21.8%) | 17 (29.3%) | |
| Ovariohysterectomy | ||||
| Yes | 16 (26.2%) | 87 (20.5%) | 11 (26.2%) | 0.453 |
| No | 45 (73.8%) | 337 (79.5%) | 31 (73.8%) | |
| Pseudocyesis | ||||
| Present | 27 (55.1%) | 124 (39.6%) | 9 (32.1%) | 0.075 |
| Absent | 22 (44.9%) | 189 (60.4%) | 19 (67.9%) | |
| Estrous cycle | ||||
| Regular | 33 (71.7%) | 243 (73.4%) | 22 (68.8%) | 0.838 |
| Irregular | 13 (28.3%) | 88 (26.6%) | 10 (31.3%) | |
| Hormone administration | ||||
| Yes | 3 (6.8%) | 23 (7.4%) | 3 (9.4%) | 0.907 |
| No | 41 (93.2%) | 287 (92.6%) | 29(90.6%) | |
| Location | ||||
| Cranial thoracic | 7 (5.1%) | 35 (6.0%) | 4 (7.8%) | 0.251 |
| Caudal thoracic | 20 (14.5%) | 84 (14.5%) | 1 (2.0%) | |
| Cranial abdominal | 15 (10.9%) | 110 (19%) | 11 (21.6%) | |
| Caudal abdominal | 44 (31.9%) | 161 (27.8%) | 15 (29.4%) | |
| Inguinal | 52 (37.7%) | 189 (32.6%) | 20 (39.2%) | |
| Adherence | ||||
| Absent | 160 (98.8) | 616 (91.4%) | 47 (78.3%) | <0.001 |
| Present | 2 (1.2%) | 58 (8.6%) | 13 (21.7%) | |
| Ulceration | ||||
| Absent | 161 (99.4%) | 646 (95.8%) | 52 (86.7%) | <0.001 |
| Present | 1 (0.6%) | 28 (4.2%) | 8 (13.3%) | |
| Relapse and/or distant metastases | ||||
| Absent | 162 (100%) | 646(95.8%) | 44 (71.1%) | <0.001 |
| Present | 0 | 28 (4.2%) | 16(27.8%) | |
| Size | ||||
| T1 < 3.0 cm | 97 (76.4%) | 328 (56.3%) | 5 (10.4%) | <0.001 |
| T2 3.0–5.0 cm | 18 (14.2%) | 142 (24.4%) | 11 (22.9%) | |
| T3 > 5.0 cm | 12 (9.4%) | 113 (19.4%) | 32 (66.7%) | |
| Clinical stage | ||||
| I | 97 (76.4%) | 318 (54.5%) | 3 (6%) | <0.001 |
| II | 18 (14.2%) | 132 (22.6%) | 8 (16%) | |
| III | 12 (9.4%) | 90 (15.4%) | 24 (48%) | |
| IV | - | 42 (7.2%) | 13 (26%) | |
| V | - | 2 (0.3%) | 2 (4%) | |
| Histological grade | ||||
| I | 59 (67%) | 3 (17.7%) | <0.001 | |
| II | 26 (29.6%) | 6 (35.3%) | ||
| III | 3 (3.4%) | 8 (47.1%) | ||
After surgical excision, tumors were fixed in 10% buffered formalin for 48 hours, processed using routine paraffin embedding, and cut into 4-μm-thick histological sections, which were stained with hematoxylin and eosin (HE).
For histological classification, depending on tumor size, three or five fragments, representing intratumor and peripheral tumor areas were randomly selected and removed from each tumor, excluding necrotic areas (Estrela-lima et al., 2010). The tumors were graded using the Nottingham System (Elston & Ellis, 1998). Surgical excision was the initial therapeutic approach for all bitches with mammary tumors. Combination adjuvant therapy was indicated for bitches diagnosed with carcinomas in mixed tumors at advanced stages (IV-V) and bitches with carcinosarcoma regardless of stage. The proposed protocols consisted of 4 cycles of carboplatin (300 mg/m²) or carboplatin (300 mg/m²) intercalated with doxorubicin (30 mg/m²) at 21-day intervals. Following adjuvant chemotherapy, 6 months of antiangiogenic therapy was proposed with thalidomide or firocoxib (Campos et al., 2017, Lavalle et al., 2012). For patients diagnosed with distant metastases, antiangiogenic treatment was maintained without interruption.
All patients underwent clinical evaluations, chest radiography examinations, and abdominal ultrasound scans before surgery and every 3 months throughout the entire follow-up period. After surgery, the patients were followed up every 3 months during the first year and every 6 months for a minimum of 24 months. For cases in which the patient's clinical follow-up was discontinued, the owners were contacted by phone to obtain information on the animal.
Overall survival was defined as the time in days between the tumor's surgical excision and the animal's death from disease progression. Animals that died of unknown causes or causes unrelated to the tumor and those that were lost to follow-up were not included in the analysis.
Overall survival was estimated by the Kaplan–Meier curve using GraphPad Prism ® v.5.0. Comparisons between groups were made using the Mantel-Cox logrank test. Comparisons between categorical variables were performed using the chi-square test, and comparisons between continuous variables were performed by an analysis of variance (ANOVA) using the statistical software, SPSS. Values were considered significant when P < 0.05.
Results
Carcinomas in mixed tumors were the most frequent, representing 75% (682/904) of cases, followed by benign mixed tumors at 18% (162/904) and carcinosarcomas at 7% (60/904).
Bitches diagnosed with benign mixed tumors were younger (mean 8.89 ± 2.69 years of age) than those with carcinomas in mixed tumors and carcinosarcomas, for which the means were 9.78 ± 2.71 and 10.50 ± 2.76 years, respectively. Bitches with benign mixed tumors had a lower mean tumor development time at diagnosis (297 days) than those with carcinomas in mixed tumors and carcinosarcomas, for which the means were 367 and 570 days, respectively.
Most bitches with mixed mammary neoplasms were purebred, intact, presented regular estrous cycles, and had never received hormones. Pseudocyesis was observed in more than half the benign mixed tumor cases but was absent in most bitches with carcinomas in mixed tumors and carcinosarcomas.
The caudal and inguinal abdominal mammary glands were the most frequently affected, and approximately 70% of the benign mixed tumors, 60% of the carcinomas in mixed tumors, and 68% of the carcinosarcomas were in those glands. Regarding tumor size, most benign mixed tumors and carcinomas in mixed tumor lesions were T1 (<3.0 cm), including 76% (97/127) of benign mixed tumors and 56% (328/583) of carcinomas in mixed tumors. The next most frequent was T2 (3.0–5.0 cm), which included 14% (18/127) of benign mixed tumors and 24% (142/583) of carcinomas in mixed tumors. The least frequent was T3 (>5.0 cm), which included 9% (12/127) of benign mixed tumors and 19% (113/583) of carcinomas in mixed tumors. However, approximately 67% (32/48) of the carcinosarcomas were T3 (>5.0 cm), 23% (11/48) were T2 (3.0–5.0 cm), and 10% (5/48) were T1 (<3.0 cm).
In bitches with benign mixed tumors, stage I was the predominant clinical stage (76%; 97/127), followed by stage II (14%; 18/127), and stage III (9%; 12/127). Most of the bitches diagnosed with carcinomas in mixed tumors (92%; 540/584), presented with early stage (I–III) tumors, and evidence of metastases (IV–V) was observed in 8% (44/584) of the cases. However, in bitches with carcinosarcoma, 70% (35/50) of the cases were stage I–III, and 30% (15/50) were stage IV–V.
In patients with carcinoma in mixed tumors, stages IV–V presented lesions larger than 3.0 cm. Adherence was observed in only 2 cases of benign mixed tumors, which were both larger than 5.0 cm in diameter, and one presented ulcerated areas. Adherence and ulceration were present in 9% (58/674) and 4% (28/674) of cases of carcinomas in mixed tumors and in 22% (13/60) and 13% (8/60) of the carcinosarcomas.
In 35 cases of carcinomas in mixed tumors, different proliferation patterns, including aggressive histological types, were observed in association with the carcinomatous area as follows: 31% (11/35) with in situ micropapillary areas, 28% (10/35) with invasive micropapillary areas, 20% (7/35) with solid areas, 6% (2/35) with areas of apocrine metaplasia, 6% (2/35) with malignant myoepithelial areas, 6% (2/35) with tubular areas, and 3% (1/35) with sebaceous differentiation areas. In the overall survival evaluation, bitches with carcinoma in mixed tumors with micropapillary areas and solid carcinomatous areas presented shorter survivals, with medians at 613 days and 186 days, respectively (P = 0.0001).
Most carcinomas in mixed tumors were histological grade I, (67%; 59/88) or grade II (29%; 26/88), while only 3.4% (3/88) were classified as grade III. However, greater frequencies of histological grades II and III (35% [6/17] and 47% [8/17], respectively) were observed in carcinosarcomas.
Bitches with benign mixed tumors and carcinomas in mixed tumors presented longer survival times but did not reach the median. However, bitches with carcinosarcomas presented a shorter overall survival with a median of 232 days (P < 0.0001) (Fig. 1).
Fig. 1.
Kaplan–Meier survival curve for bitches with mixed mammary tumors, treated only with surgery, according to histological type. BMT: benign mixed tumor (n = 31) did not reach the median; CMT: carcinomas in mixed tumor (n = 220) did not reach median; and CS: carcinosarcoma (n = 19), median of 232 days (P < 0.0001).
Bitches with carcinomas in mixed tumors at stages I–III presented a longer overall survival but did not reach the median. For stages IV–V, the median was 613 days. Bitches with carcinosarcomas had a shorter overall survival, and the median survival times at stages I–III and IV–V were 338 and 78 days, respectively (P < 0.0001) (Fig. 2).
Fig. 2.
Kaplan–Meier survival curve for bitches with malignant mixed neoplasms of the mammary glands, treated only with surgery, according to clinical stage. CMT I–III: carcinomas in mixed tumor at an early stage did not reach the median (n = 196 cases); CMT IV–V: carcinomas in mixed tumor at an advanced stage reached the median of 613 days (n = 17 cases); CS I–III: carcinosarcoma at an early stage reached the median of 368 days (n = 11 cases); CS IV–V: carcinosarcoma at an advanced stage reached the median of 78 days (n = 4 cases) (P < 0.0001).
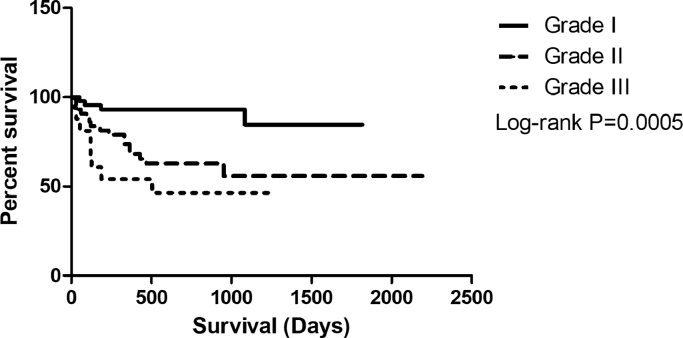
Bitches with histological grades I and II mixed malignant tumors presented longer overall survival but did not reach the median. However, evidence of histological grade III is associated with shorter survival at a median of 504 days (P = 0.0005) (Fig. 3).
Fig. 3.
Kaplan–Meier overall survival curve for bitches with malignant mixed tumors, Kaplan–Meier survival curve for bitches with malignant mixed neoplasms of the mammary glands, treated only with surgery, according to clinical stage, according to histological grade. Grade I: did not reach the median, n = 44 cases; Grade II: did not reach the median, n = 43 cases; Grade III: median of 504 days, n = 18 cases (P = 0.0005).
All bitches with benign mixed tumors and carcinomas in mixed tumors at stages I-III were treated with surgery only. In all, 37% of carcinosarcoma cases (22/60) and 32% of cases of stage IV-V carcinomas in mixed tumors (14/44) underwent adjuvant treatment.
Overall survival did not significantly differ by surgical technique (nodulectomy, simple mastectomy, regional mastectomy, or unilateral radical mastectomy (P = 0.1290)). Bitches with carcinoma in mixed tumors and carcinosarcomas with free surgical margins presented greater overall survival than did bitches with compromised surgical margins (P = 0.0010).
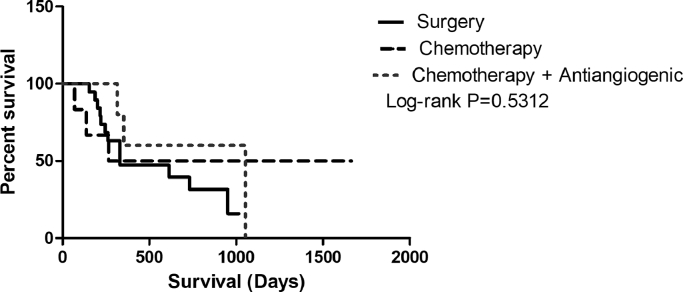
In bitches with stages IV and V carcinomas in mixed tumors, a median survival of 330 days was observed when surgery was the only therapeutic approach. Patients undergoing combination therapy with antineoplastic agents or antiangiogenic therapy had higher overall survival rates, of which, the median survivals were 964 and 1052 days, respectively (P = 0.0499) (Fig. 4).
Fig. 4.
Kaplan–Meier overall survival curve for bitches with stage IV-V carcinomas in mixed tumors according to treatment: surgery only, median of 331 days (n = 19 cases); surgery and adjuvant chemotherapy, median of 964 days (n = 6 cases); and surgery, adjuvant chemotherapy and antiangiogenic therapy, median of 1052 days (n = 5 cases) (P = 0.5312).
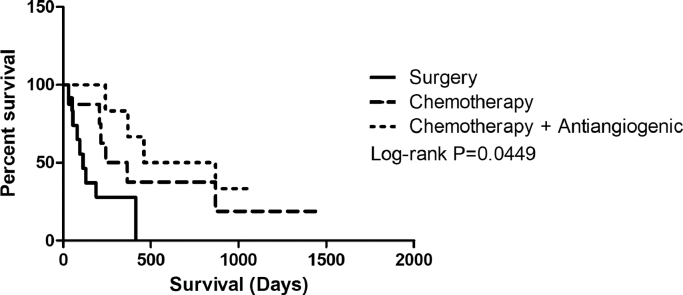
Bitches with carcinosarcomas undergoing surgery presented lower overall survival rates (median, 113 days). However, when treated with adjuvant chemotherapy or adjuvant chemotherapy and antiangiogenic therapy after surgery, higher overall survival rates were observed, with medians of 414 and 664 days, respectively (P = 0.0095) (Fig. 4). (Fig. 5).
Fig. 5.
Kaplan–Meier survival for bitches with carcinosarcomas according to therapeutic approach: surgery only, median of 113 days (n = 14 cases); surgery and adjuvant chemotherapy, median of 303 days (n = 8 cases); and surgery, adjuvant chemotherapy and antiangiogenic therapy, median of 664 days (n = 6 cases) (P = 0.0499).
In this study, the maximum follow-up period was 5 years. The relapse rate of carcinomas in mixed tumors was 1.5% (10/682); of these, 4 cases presented clinically as inflammatory carcinoma, and 2.6% (18/682) progressed to distant metastases, with the lungs (99%; 17/18) and the brain (1%; 1/18) as the main sites. Carcinosarcoma relapses were observed in 3.3% of the cases (2/60), and 26.6% (16/268) presented distant metastases in the lungs (99%; 15/16) and liver (1%; 1/16). The mean disease-free survivals of bitches with carcinomas in mixed tumors and carcinosarcomas were 425 and 253 days, respectively.
Discussion
The mixed tumors presented high heterogeneity regarding morphological characteristics, clinical progression, and response to combination therapy. Thus, we highlight the importance of standardizing criteria for morphologically characterizing these tumors to enable differentiating the histological types of mixed tumors.
Microscopic classification of these tumors allowed predicting their prognosis and clinical progression, with benign mixed tumors and carcinomas in mixed tumors having better prognoses and longer overall survival than neoplasms of malignant epithelial and mesenchymal origin. This demonstrates the higher aggressiveness of the carcinosarcoma subtype. Methods for classifying canine mammary tumors vary considerably, making it difficult to compare studies based on the biological characteristics of malignant neoplasms (Benjamin et al., 1999, Sarli et al., 2002).
Tumor size is an independent and well-established prognostic factor in canine mammary neoplasms (Bostock, 1986, Misdorp and Hart, 1976, Gilbertson et al., 1983, Yamagami et al., 1996). In our study, smaller lesions (T1 and T2) occurred mainly in benign mixed tumors and carcinomas in mixed tumors, and more T3 lesions were observed among carcinosarcomas, corroborating the results of Damasceno et al. (2016). Longer progression times, increased malignancy, tumor progression, and the owners delaying seeking care may explain this finding. Ferreira, Bertagnolli, Cavalcanti, Schmitt, and Cassali (2009) evaluated canine mammary tumors and observed that most lesions larger than 5.0 cm (T3) were malignant, presented higher proliferation indexes, and had lower hormone receptor positivity than did smaller tumors (T1, T2).
Clinical stage was an important prognostic factor, and bitches with advanced-stage tumors exhibited worse prognoses in the overall survival analysis. Prior studies reported lower survival rates in bitches with regional lymph node metastases than did those without lymph node involvement, and worse prognoses were associated with distant metastases (Karayannopoulou et al., 2005, Yamagami et al., 1996). Bitches with advanced-stage carcinomas in mixed tumors showed higher overall survival rates and better prognoses than did bitches with carcinosarcomas, which demonstrates their decreased aggressiveness. The increased malignancy of the mixed neoplasms evaluated in this study was also associated with histological grade, and the carcinosarcomas were predominantly grades II and III. Karayannopoulou et al. (2005) and Dutra, Azevedo, Schmitt, and Cassali (2008) observed that bitches with undifferentiated carcinomas (grade III) exhibited worse prognoses than did those with carcinomas that were grades I or II.
Adherence and ulceration indicate poor prognosis (Cassali et al., 2014). In malignant mixed neoplasms, the increased frequencies of adherence and ulceration were proportional to the increased malignancy. However, these characteristics were observed in some cases of benign mixed neoplasms, which may be explained by the larger tumor size and longer tumor development time. This demonstrates that adherence and ulceration, although indicative of malignancy, must be evaluated with caution. Ulceration may be caused by invasive tumor growth or trauma, ischemia, or skin infection, which are characteristics not necessarily associated with aggressive biological behavior (Santos, Correia-Gomes, Santos, De Matos, & Lopes, 2015).
Misdorp, Cotchin, Hampe, Jabara, and Sandersleben (1973) observed that more than one type of carcinoma may be recognized within the same tumor and that different types of differentiation also occur to tissues in sarcomas. Carcinomatous areas with aggressive histological types associated with carcinomas in mixed tumors may affect their biological behavior. Therefore, thoroughly characterizing the malignant components associated with carcinomas in mixed tumors is important for prognosis. According to Cassali et al. (2014), micropapillary carcinomas, solid carcinomas, and high-grade tubular carcinomas are considered aggressive histological types, and bitches with those diagnoses must undergo combination therapy with adjuvant chemotherapy. Thus, a differentiated therapeutic approach may benefit patients with carcinomas in mixed tumors that contain areas of aggressive histological types.
Surgery is the treatment of choice for all bitches with mammary tumors except those with inflammatory carcinomas (Sorenmo, 2003, Sorenmo et al., 2013). The results showed that surgery was curative for bitches with benign mixed tumors and early-stage (I–III) carcinomas in mixed tumors. Early diagnosis, in addition to an improved prognosis, allow planning conservative surgeries. Surgical margins are recommended in cases of compromised margins. Regarding combination therapy, some adjuvant protocols are suggested in the literature, and these consist of administering doxorubicin combined with cyclophosphamide, doxorubicin combined with carboplatin, carboplatin combined with gemcitabine, and paclitaxel as a single agent (Cassali et al., 2014). Per Lavalle et al. (2012), based on the advanced clinical stage and immunophenotypic characteristics, bitches with mammary neoplasms must undergo complementation with adjuvant chemotherapy, resulting in increased survival compared with bitches undergoing surgical excision. This agrees with the present study's results, which showed that complementation with adjuvant chemotherapy benefitted bitches with stages IV–V carcinomas in mixed tumors and for those with carcinosarcomas. Karayannopoulou et al. (2001) reported increased disease-free survival and overall survival in bitches treated with surgery and chemotherapy consisting of 5-fluoracil and cyclophosphamide compared with bitches treated with surgery only.
Angiogenesis has been associated with metastasis development and tumor progression in solid tumors (Raje & Anderson, 2002). This study showed that antiangiogenic therapy resulted in improved overall survival and interfered with disease progression. Angiogenesis is a crucial factor for tumor growth and for metastatic development. Lavalle, Bertagnolli, Tavares, and Cassali (2009) study of 46 mammary carcinomas in bitches found a correlation between COX-2 expression and angiogenesis as well as shorter survival. The authors suggested that using COX-2 inhibitors in these cases could be an alternative for treating and controlling advanced carcinomas in bitches.
The literature indicates that carcinosarcomas feature poor prognosis and usually result in metastases in the first year after surgery (Benjamin et al., 1999), which confirms this study's findings. Bitches with carcinomas in mixed tumors and carcinosarcomas must be evaluated periodically, for a minimum of 2 years after surgery, since the relapse rates and/or metastases in carcinosarcomas were higher than those for carcinomas in mixed tumors. Studies indicate that 50%–75% of bitches with carcinosarcomas present with lung metastases (Misdorp et al., 1973, Moulton et al., 1986). A general and specific clinical assessment must be performed every 3 months during the first year of follow-up and subsequently every 6 months. Complementary exams are important for following disease progression and investigating metastases in distant organs. Distant metastases are more commonly found in the lungs (Sorenmo, 2003, Von Euler, 2011), followed by the liver, kidneys, spleen, bones, central nervous system, and pleura (Von Euler, 2011), as observed in this study.
Here, carcinomas in mixed tumors were the most frequent neoplasms, followed by benign mixed tumors and carcinosarcomas. Benign mixed tumors are frequent mammary gland neoplasms in bitches, and per the current veterinary classification system, they represent 40%–50% of benign tumors in canines (Cassali et al., 2014). Carcinomas in mixed tumors arise from epithelial malignant transformation in benign mixed tumors (Misdorp, 1999, Moulton et al., 1970) and are the most frequent lesions, representing approximately 42%–56.7% of all malignant neoplasms of the canine mammary glands (Cassali et al., 2009, Toríbio et al., 2012).
Studies indicate that benign mixed tumors affect younger bitches, aged 3 to 9 years (Bertagnolli et al., 2011, Cassali et al., 2012), which is similar to this study's findings. However, malignant mammary neoplasms occur in older bitches, aged 9 to 11 years (Sorenmo et al., 2013). Misdorp et al. (1973) evaluated canine mammary carcinosarcomas and reported that the mean age for bitches diagnosed with this histological type was 11 years (range: 4–15 years), which agrees with the data in this study, as we observed advanced ages in bitches with carcinomas in mixed tumors and carcinosarcomas.
Tumor development time is a subjective tool since some owners are unaware of the precise time when spontaneous neoplasms develop in their dogs. However, a longer mean development time was observed for carcinosarcomas than for carcinomas in mixed tumors, and of these, compared with benign mixed tumors, carcinosarcomas showed a positive correlation between progression time and malignant transformation. Studies indicate that mixed tumors may undergo malignant transformation and that they primarily originate as carcinomas in mixed tumors and more rarely as carcinosarcomas and sarcomas in mixed tumors (Cassali et al., 2012, Misdorp, 1999). The prolonged time suggests that mammary neoplasms progress from benign lesions to invasive malignant lesions (Moulton et al., 1970, Sorenmo, 2009; Goldschmidt, Peña, Rasotto, & Zappulli, 2011). Bertagnolli et al. (2009) observed protein changes that may contribute to the malignant transformation of benign mixed tumors such as the loss of p63, ΔNp63, E-cadherin, and β-catenin expressions.
Conclusion
Carcinomas in mixed tumors are neoplasms that present good biological behavior, but depending on their epithelial histological subtypes, their prognoses may vary. However, even at advanced stages, bitches with carcinomas in mixed tumors survive longer than bitches with carcinosarcomas. Given these mixed tumors’ different biological behaviors, evaluating each patient and considering all prognostic factors are important for adopting adequate and individualized therapeutic protocols.
Acknowledgments
The authors wish to thank the Minas Gerais State Research Foundation (FAPEMIG - OET-00354-15), the Coordination for the Improvement of Higher Education Personnel (CAPES - 1783546) and the National Council for Scientific and Technological Development (CNPq - 302449/2013-2) of Brazil for financial support.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.vas.2018.09.003.
Appendix. Supplementary materials
References
- Benjamin S.A., Lee A.C., Saunders W.J. Classification and behavior of canine mammary epithelial neoplasms based on life-span observations in beagles. Veterinary Pathology. 1999;36:423–436. doi: 10.1354/vp.36-5-423. [DOI] [PubMed] [Google Scholar]
- Bertagnolli A.C., Cassali G.D., Genelhu M.C.U.S., Costa F.U.M.A., Oliveira J.F.C., Gonçalves P.B.D. Immunohistochemical expression of p63 and _np63 in mixed tumors of canine mammary glands and its relation with p53 expression. Vet. Pathol. 2009;46:407–415. doi: 10.1354/vp.08-VP-0128-C-FL. [DOI] [PubMed] [Google Scholar]
- Bertagnolli A.C., Ferreira E., Dias E.J., Cassali G.D. Canine mammary mixed tumours: Immunohistochemical expressions of EGFR and HER-2. Australian Veterinary Journal. 2011;89:312–317. doi: 10.1111/j.1751-0813.2011.00803.x. [DOI] [PubMed] [Google Scholar]
- Bostock D.E. Canine and feline mammary neoplasms. British Veterinary Journal. 1986;142:506–515. doi: 10.1016/0007-1935(86)90107-7. [DOI] [PubMed] [Google Scholar]
- Campos C.B., Lavalle G.E., Ligório S.F., Monteiro L.N., Amorim R.L., Cassali G.D. Thalidomide treatment in a canine mammary gland carcinosarcoma presenting pulmonary metastasis. Advances in Animal and Veterinary Sciences. 2017;5:120–126. [Google Scholar]
- Cassali G.D., Bertagnolli A.C., Lavalle G.E., Tavares W.L.F., Ferreira E., Silva A.E., Campos C.B. Perspectives for diagnosis, prognosis and treatment of mammary neoplasias in dogs. In 34th World Small Animal Veterinary Congress - WSAVA. 2009;14:173. [Google Scholar]
- Cassali G.D., Bertagnolli C.A., Ferreira E., Gamba C.O., Araujo K., Campos B. Canine mammary mixed tumours : A review. Veterinary Medicine International. 2012:1–7. doi: 10.1155/2012/274608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassali G.D., Lavalle G.E., Nardi A.B., Ferreira E., Bertagnolli A.C., Estrela-Lima A., De Nardi A.B. Consensus for the diagnosis, prognosis and treatment of canine mammary tumors. Brazilian J. Vet. Pathol. 2014;4:153–180. [Google Scholar]
- Cassali G., Damasceno K., Bertagnolli A., Estrela-Lima A., Lavalle G., Di Santis G. Consensus regarding the diagnosis, prognosis and treatment of canine mammary tumors: Benign mixed tumors, carcinomas in mixed tumors and carcinosarcomas. Brazilian Journal of Veterinary Pathology. 2017;10:87–99. [Google Scholar]
- Damasceno K.A., Ferreira E., Estrela-Lima A., Gamba C.O., Miranda F.F., Alves M.R. HER-2 and EGFR mRNA expression and its relationship with versican in malignant matrix-producing tumors of the canine mammary gland. Plos One. 2016;11(8) doi: 10.1371/journal.pone.0160419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra A.P., Azevedo J.G.M., Schmitt F.C., Cassali G.D. Assessment of cell proliferation and prognostic factors in canine mammary gland tumors. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 2008;60:1403–1412. [Google Scholar]
- Elston C.W., Ellis I.O. Systemic pathology. The breast. 3rd ed. Churchill Livingstone; London: 1998. Assessment of histological grade; pp. 365–384. [Google Scholar]
- Estrela-lima A., Araújo M.S.S., Costaneto J.M., Teixeira-Carvalho A., Barrouin-Melo S.M., Cardoso S.V. Immunophenotypic features of tumor infiltrating lymphocytes from mammary carcinomas in female dogs associated with prognostic factors and survival rates. BMC Cancer [Electronic Resource] 2010;10:1–14. doi: 10.1186/1471-2407-10-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira E., Bertagnolli A.C., Cavalcanti M.F., Schmitt F.C., Cassali G.D. The relationship between tumour size and expression of prognostic markers in benign and malignant canine mammary tumours. Veterinary and Comparative Oncology. 2009;7:230–235. doi: 10.1111/j.1476-5829.2009.00193.x. [DOI] [PubMed] [Google Scholar]
- Gilbertson S.R., Kurzman I.D., Zachrau R.E., Hurvitz A.I., Black M.M. Canine mammary epithelial neoplasms: biologic implications of morphologic characteristics assessed in 232 dogs. Veterinary Pathology. 1983;20:127–142. doi: 10.1177/030098588302000201. [DOI] [PubMed] [Google Scholar]
- Goldschmidt M., Peña L., Rasotto R., Zappulli V. Classification and grading of canine mammary tumors. Veterinary Pathology. 2011;48:117–131. doi: 10.1177/0300985810393258. [DOI] [PubMed] [Google Scholar]
- Goldschmidt M.H., Peña L., Zappulli V. Tumors of the mammary gland. In: Meuten D.J., editor. Tumors in domestic animals. 5th ed. 2017. pp. 731–773. [Google Scholar]
- Karayannopoulou M., Kaldrymidou E., Constantinidis T.C., Dessiris A. Adjuvant post‐operative chemotherapy in bitches with mammary cancer. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 2001;48:85–96. doi: 10.1046/j.1439-0442.2001.00336.x. [DOI] [PubMed] [Google Scholar]
- Karayannopoulou M., Kaldrymidou E., Constantinidis T.C., Dessiris A. Histological grading and prognosis in dogs with mammary carcinomas: Application of a human grading method. Journal of Comparative Pathology. 2005;133:246–252. doi: 10.1016/j.jcpa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Lavalle G.E., Bertagnolli A.C., Tavares W.L.F., Cassali G.D. Cox-2 expression in canine mammary carcinomas: Correlation with angiogenesis and overall survival. Veterinary Pathology. 2009;46:1275–1280. doi: 10.1354/vp.08-VP-0226-C-FL. [DOI] [PubMed] [Google Scholar]
- Lavalle G.E., Campos C.B., Bertagnolli A.C., Cassali G.D. Canine malignant mammary gland neoplasms with advanced clinical staging treated with carboplatin and cyclooxygenase inhibitors. In Vivo (Athens, Greece) 2012;26:375–379. [PubMed] [Google Scholar]
- Misdorp W., Cotchin E., Hampe J.F., Jabara A.G., Sandersleben J.V. Canine malignant mammary tumors special types of carcinomas, malignant mixed tumors. Veterinary Pathology. 1973;10:241–256. doi: 10.1177/030098587301000307. [DOI] [PubMed] [Google Scholar]
- Misdorp W., Hart A.A. Prognostic factors in canine mammary cancer. Journal of the National Cancer Institute. 1976;56:779–786. doi: 10.1093/jnci/56.4.779. [DOI] [PubMed] [Google Scholar]
- Misdorp W. Histological classification of the mammary tumors of the dog and the cat. In: Series S., editor. WHO international histological classification tumors of domestic animals. AFIP; Washington, DC: 1999. [Google Scholar]
- Moulton J., Rosenblatt L., Goldman M. Mammary tumors in a colony of beagle dogs. Veterinary Pathology. 1986;23:741–749. doi: 10.1177/030098588602300613. [DOI] [PubMed] [Google Scholar]
- Moulton J.E., Taylor D.O., Dorn C.R. Andersen AC. Canine mammary tumors. Pathologia Veterinaria. 1970;7:289–320. doi: 10.1177/030098587000700401. [DOI] [PubMed] [Google Scholar]
- Owen L. World Health Organisation; 1980. TNM classification of tumours in domestic animals; pp. 1–52. [Google Scholar]
- Pierini A., Bocci G., Giorgi M., Owen H., Marchetti V. From humans to dogs and back: The translational lesson of metronomic chemotherapy. American Journal of Animal and Veterinary Sciences. 2012;7:198–1212. [Google Scholar]
- Raje N., Anderson K.C. Thalidomide and immunomodulatory drugs as cancer therapy. Current Opinion in Oncology. 2002;14:635–640. doi: 10.1097/00001622-200211000-00008. [DOI] [PubMed] [Google Scholar]
- Santos M., Correia-Gomes C., Marcos R., Santos A., De Matos A., Lopes C. Value of the nottingham histological grading parameters and nottingham prognostic index in canine mammary carcinoma. Anticancer Research. 2015;35:4219–4228. [PubMed] [Google Scholar]
- Sarli G., Preziosi R., Benazzi C.G.C., Marcato P.S. Prognostic value of histological stage and proliferative activity in canine malignant mammary tumors. Journal of Veterinary Diagnostic Investigation. 2002;14:24–32. doi: 10.1177/104063870201400106. [DOI] [PubMed] [Google Scholar]
- Sorenmo K. Canine mammary gland tumors. Veterinary Clinics of North America. Small Animal Practice. 2003;33:573–596. doi: 10.1016/s0195-5616(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Sorenmo K.U., Kristiansen V.M., Cofone M.A. Canine mammary gland tumours: a histological continuum from benign to malignant; clinical and histopathological evidence. Vet. Comp. Oncol. 2009;7:162–172. doi: 10.1111/j.1476-5829.2009.00184.x. [DOI] [PubMed] [Google Scholar]
- Sorenmo K.U., Worley D., Goldschmidt M.H. Tumors of the mammary gland. In: Withrow S.J., Vail D.M., Page R.L., editors. Withrow and MacEwen's small animal clinical oncology. 5th ed. Saunders Elsevier; St. Louis: 2013. pp. 538–556. [Google Scholar]
- Toríbio J.M.D.M.L., Lima A.E., Martins F.E.F., Ribeiro L.G.R., D'Assis M.J.M.H., Teixeira R.G. Clinical characterization, histopathologic diagnosis and geoprocessing of mammary tumours in bitches from the city of Salvador, Bahia State. Revista Ceres. 2012;59:427–433. [Google Scholar]
- Von Euler H. Tumors of the mammary gland. In: Dobson J.M., Lascelles B., editors. BSAVA Manual of canine and feline oncology. 3rd ed. British Small Animal Veterinary Association; Gloucester: 2011. pp. 237–247. [Google Scholar]
- Yamagami T., Kobayashi T., Takahashi K., Sugiyama M. Prognosis for canine malignant mammary tumors based on TNM and histologic classification. Journal of Veterinary Medical Science. 1996;58:1079–1083. doi: 10.1292/jvms.58.11_1079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.