Purpose of review
Despite the plethora of publications discussing the severe respiratory coronavirus 2 (SARS-CoV-2), evidence of viral secretion in urine is sparse.
Recent findings
We could identify 34 publications including a total of 2172 patients. Among those, 549 patients were tested for SARS-CoV-2 secretion in urine, which was detected in only 38 patients (6.9%). Within the seven studies displaying positive results, the majority of positive patients (86.8%) was from not yet peer-reviewed studies including weak data and heterogeneous techniques for sample testing. Furthermore, none of the studies available in the literature addressed the virulence of detected viral RNA in urine.
Summary
Overall, only seven studies were able to detect SARS-CoV-2 secretion in urine, all of them with a considerably low rate of positivity. However, these studies were of rather low quality considering their methodology. Despite this, as SARS-CoV-2 has been detected in urine, it is of importance to discuss safety and urinary hygiene protocols. Until further research provides valid data on viral shedding and virulence in urine, potential risk of transmission through urine cannot be ruled out. Therefore, safety and hygiene measures need to be discussed.
Keywords: COVID-19, severe respiratory coronavirus 2, urine
KEY POINTS
The presence of SARS-CoV-2 in urine is poorly investigated in the current literature (0.6% out of 5674 articles).
Overall, only 6.9% of patients in studies and case reports that analysed urine, were tested positive for SARS-CoV-2.
Remarkably, 90% of the patients with multiple urine analysis displayed a positive RT-PCR only at one single point in time.
The implementation of standardized procedures is crucial to reduce confounding factors resulting from preanalytical and analytical irregularities.
The transmission risk and virulence of SARS-CoV-2 in urine have not been assessed so far, despite this, as SARS-CoV-2 has been detected in urine, safety and urinary hygiene protocols should be discussed.
INTRODUCTION
At the end of 2019, a new virus, called novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified as the causative pathogen responsible for COVID-19. Although the transmission occurs mainly via respiratory droplets, there is a lack of evidence about the pattern, duration, timing, compartmentalization and quantity of viral shedding in different specimens. SARS-CoV-2 can be detected in different fluids such as sputum, nasal swabs, blood and faeces. The evidence regarding the presence and virulence of the virus in urine is sparse. The WHO recently proposed in its guidelines to consider urine testing for all symptomatic patients and contact persons [1].
SARS-CoV-2 targets the angiotensin-converting-enzyme 2 (ACE-2) receptor for host cell entry [2]. ACE-2 not only occurs in epithelial cells of the human airway tract but is also abundantly expressed in the kidney, predominantly in the epithelial layer of the renal ducts [3]. Hence, the kidney could constitute a site of virus replication if it can dock at that site. As the presence of an active and contagious virus in urine could be a source and route of transmission, important hygiene and safety measures for the general population and healthcare workers (HCWs) might result.
To address this lack of knowledge, we performed a systematic review of all the available (published and unpublished) literature on COVID-19 to investigate the presence of SARS-CoV-2 in human urine.
Materials and methods
On the 14th of April 2020, we searched on PubMed, medRxiv, bioRxiv and COVID-19 Open Research database to assess the incidence of SARS-CoV-2 in urine. We only included articles published in the English language without restriction with regard to the publication period. The following keywords were used in our search strategy: ‘COVID-19’ OR ‘nCoV’ OR ‘SARS-CoV-2’ OR ‘coronavirus’. Four investigators performed independently direct full-text screening of the articles based on the keywords ‘urine, urinary’. We did not exclude reviews, editorials, letters and case reports. Discrepancies were resolved by Delphi consensus. Four investigators extracted independently the information from the included articles, and one-fifth made an independent review of all the extracted data (Fig. 1 and Table 1) [4–6,7▪▪,8–25,26▪,27–37].
FIGURE 1.
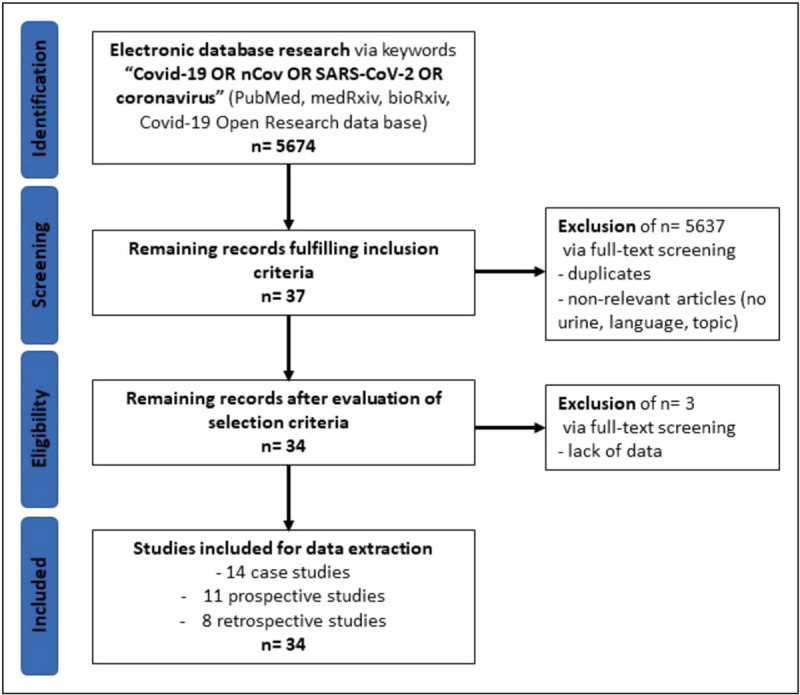
Study flow diagram. Selection of 34 studies including 2172 patients.
Table 1.
Publications analyzing SARS-CoV-2 secretion in urine
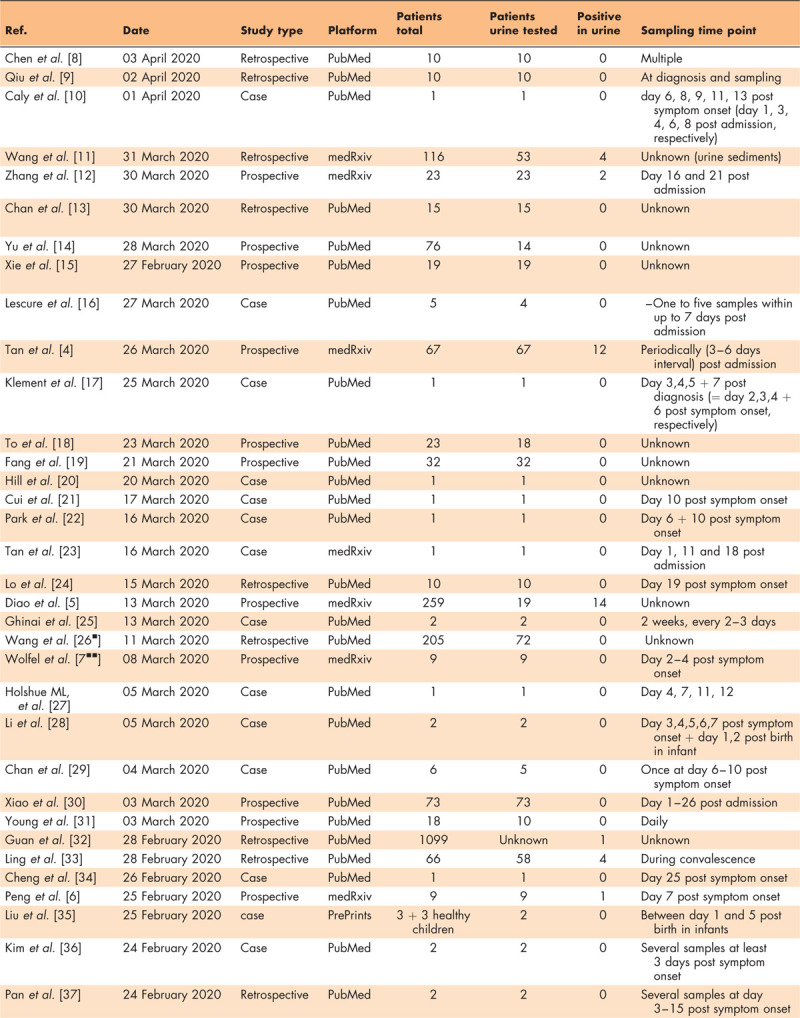
RESULTS
Overall, 5674 publications were identified in the initial search (PubMed, 4530; medRxiv and bioRxiv, 1144). Among these, after full-text screening, 5637 articles were excluded including duplicates and nonrelevant articles according to our inclusion criteria. After evaluating the selection criteria, we identified 34 articles (0.6%) reporting urinary detection of SARS-CoV-2.
We found 549 patients who had at least one urine specimen analysed. Seven publications reported at least one positive test for a total of 38 patients (6.9%). Among these positive patients, 26 (68.4%) were constituted by two studies that were not yet peer-reviewed of which one used nucleocapsid protein detection by fluorescence immunochromatography for virus detection [4,5]. The remaining studies (n = 33) performed RT-PCR to detect amplified viral RNA from urine samples. Remarkably, 90% of the patients with multiple urine analysis displayed a positive RT-PCR only at one single point in time during the study period. Twenty-seven studies (71%) testing urine in 342 patients (62.3%) did not detect any viral RNA in urine samples. Among the seven studies that reported SARS-CoV-2 secretion in urine, only one provided the viral copy number detected in RT-PCR, which was 3.22E^02 copies/ml [6]. All patients tested in the studies had COVID-19 symptoms. There was no article assessing the transmission risk and virulence of SARS-CoV-2 in urine.
DISCUSSION
Showing a high sensitivity and specificity, RT-PCR is currently considered the standard for fast and cost-efficient identification and quantification of SARS-CoV-2. Nonetheless, RT-PCR harbours the risk of false-negative and false-positive results. False-negative results are caused by low virus load and procedural errors such as inadequate sampling and processing, whereas false-positive results could result from cross-contamination occurring at some point in the entire testing procedure.
Despite WHO recommendations, the presence of SARS-CoV-2 in urine is poorly investigated in the current literature. However, urine rarely seems to contain SARS-CoV-2, even in symptomatic patients. Studies, which have been conducted so far were not designed to evaluate specifically SARS-CoV-2 in urine; therefore, most studies did not conduct multiple or systematic urine testing, which would be necessary to avoid selection bias. Potentially, the urinary secretion of the virus is highest early in the disease, when patients are not symptomatic yet and have a high virus load. Indeed, the inclusion of symptomatic patients only is a potential source of selection bias. In addition, studies failed to show consistently positive results in consecutive samples of previously positive tested patients. Urinary SARS-CoV-2 should, therefore, be measured at different disease states (e.g. asymptomatic, mild/moderate symptoms, severe symptoms and immune) and among different groups of the population (e.g. different ethnic groups, patients with renal insufficiency and so on). Moreover, validated and accurate sampling and testing strategies need to be implemented [7▪▪].
In summary, only seven studies were able to detect SARS-CoV-2 secretion in urine, all of them with a considerably low rate of positivity. However, these studies were of rather low quality considering their methodology. Despite this, as SARS-CoV-2 has been detected in urine, it is of importance to discuss safety and urinary hygiene protocols for HCW. There is a need for future studies to evaluate the virulence and risk of transmission via titre testing with virus purified from different fluid specimens such as urine.
CONCLUSION
Although the WHO recommends considering urinary testing for SARS-CoV-2 in symptomatic patients and contacts, it seems that the proportion of urine positivity is very low. Overall, we could only identify seven studies, which reported positive results, all of them with a considerably low rate of positivity. However, these studies were of rather low quality considering their methodology and none assessed the transmission risk and virulence of SARS-CoV-2 in urine. Until further research provides valid data on viral shedding and virulence in urine, a potential risk of transmission through urine cannot be ruled out. Therefore, safety and hygiene measures need to be discussed.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Footnotes
Both Stephan Bronimann and Katharina Rebhan contributed equally to this article.
REFERENCES
- 1.WHO. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases 2020 [Interim guidance]. https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117. [Accessed 05 July 2020].
- 2.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamming I, Cooper ME, Haagmans BL, et al. The emerging role of ACE2 in physiology and disease. J Pathol 2007; 212:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan W, Lu Y, Zhang J, et al. Viral kinetics and antibody responses in patients with COVID-19. medRxiv 2020. [Google Scholar]
- 5.Diao B, Wen K, Chen J, et al. Diagnosis of acute respiratory syndrome coronavirus 2 infection by detection of nucleocapsid protein. medRxiv 2020. [Google Scholar]
- 6.Peng L, Liu J, Xu W, et al. 2019 Novel Coronavirus can be detected in urine, blood, anal swabs and oropharyngeal swabs samples. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪▪.Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID. Nature 2020. [DOI] [PubMed] [Google Scholar]; The authors of this article thoroughly analysed the viral shedding patterns of SARS-CoV-2.
- 8.Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in feces of COVID-19 patients. J Med Virol 2020. [DOI] [PubMed] [Google Scholar]
- 9.Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caly L, Druce J, Roberts J, et al. Isolation and rapid sharing of the 2019 novel coronavirus (SARS-CoV-2) from the first patient diagnosed with COVID-19 in Australia. Med J Aust 2020; 212:459–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Li X, Chen H, et al. SARS-CoV-2 infection does not significantly cause acute renal injury: an analysis of 116 hospitalized patients with COVID-19 in a single hospital, Wuhan, China. medRxiv 2020. [Google Scholar]
- 12.Zhang N, Gong Y, Meng F, et al. Virus shedding patterns in nasopharyngeal and fecal specimens of COVID-19 patients. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie C, Jiang L, Huang G, et al. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int J Infect Dis 2020; 93:264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lescure FX, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis 2020; 20:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klement E, Godefroy N, Burrel S, et al. The first locally acquired novel case of 2019-nCoV infection in a healthcare worker in the Paris area. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020; 20:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang Z, Zhang Y, Hang C, et al. Comparisons of nucleic acid conversion time of SARS-CoV-2 of different samples in ICU and non-ICU patients. J Infect 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill DKJ, Russell DCD, Clifford DS, et al. The index case of SARS-CoV-2 in Scotland: a case report. J Infect 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui Y, Tian M, Huang D, et al. A 55-day-old female infant infected with COVID 19: presenting with pneumonia, liver injury, and heart damage. J Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JY, Han MS, Park KU, et al. First pediatric case of coronavirus disease 2019 in Korea. J Korean Med Sci 2020; 35:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan LV, Ngoc NM, That BTT, et al. Duration of viral detection in throat and rectum of a patient with COVID-19. medRxiv 2020. [Google Scholar]
- 24.Lo IL, Lio CF, Cheong HH, et al. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int J Biol Sci 2020; 16:1698–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghinai I, McPherson TD, Hunter JC, et al. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet 2020; 395:1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26▪.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020; 323:1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, multiple specimens were collected from more than 200 patients revealing a faecal route of viral transmission.
- 27.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Zhao R, Zheng S, et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis 2020; 26:1335–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan JF, Yip CC, To KK, et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-polymerase chain reaction assay validated in vitro and with clinical specimens. J Clin Microbiol 2020; 58:e00310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020; 158:1831–1833.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 2020; 323:1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan WJ, Zhong NS. Clinical characteristics of Covid-19 in China. Reply. N Engl J Med 2020; 382:1861–1862. [DOI] [PubMed] [Google Scholar]
- 33.Ling Y, Xu SB, Lin YX, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020; 133:1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng SC, Chang YC, Fan Chiang YL, et al. First case of Coronavirus Disease 2019 (COVID-19) pneumonia in Taiwan. J Formos Med Assoc 2020; 119:747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu WWQ, Zhang Q, Chen L, et al. Coronavirus disease 2019 (COVID-19) during pregnancy: a case series. Preprints 2020; 2020020373. [Google Scholar]
- 36.Kim JY, Ko JH, Kim Y, et al. Viral load kinetics of SARS-CoV-2 infection in first two patients in Korea. J Korean Med Sci 2020; 35:e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan Y, Zhang D, Yang P, et al. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis 2020; 20:411–412. [DOI] [PMC free article] [PubMed] [Google Scholar]


