Abstract
Background and Purpose:
Ischemic infarction of the corpus callosum is rare and infarction isolated to the corpus callosum alone rarer still, accounting for much <1% of ischemic stroke in most stroke registries. About half of callosal infarctions affect the splenium.
Methods:
During a 2-week period, at the height of the coronavirus disease 2019 (COVID-19) pandemic in New York City, 4 patients at Montefiore Medical Center in the Bronx were found to have ischemic lesions of the splenium of the corpus callosum, 2 with infarction isolated to the corpus callosum.
Results:
All patients tested positive for COVID-19 and 3 had prolonged periods of intubation. All had cardiovascular risk factors. Clinically, all presented with encephalopathy and had evidence of coagulopathy and raised inflammatory markers.
Conclusions:
Infarction of the splenium of the corpus callosum is exceedingly rare and a cluster of such cases suggests COVID-19 as an inciting agent, with the mechanisms to be elucidated.
Keywords: coronavirus, corpus callosum, COVID-19, inpatients, posterior cerebral artery
The coronavirus disease 2019 (COVID-19) epidemic in the spring of 2020 showed its greatest incidence in New York City, disproportionately high in areas of low socioeconomic standing. During the height of the epidemic in New York from mid-March to mid-April, Montefiore Health System cared for a daily census of ≈2000 COVID-19 infected inpatients, with well over 400 at the Montefiore Medical Center Moses Campus. The relationship of COVID-19 to stroke is complex and not yet fully elucidated. Many infected patients presented with respiratory symptoms and later developed strokes during their hospitalization; conversely, several presented with cerebrovascular events without respiratory symptoms and only later were found to be COVID-19 positive. During this time, the Neurology service was consulted for numerous patients with alterations in mental status. Most of these patients had presumed infectious or metabolic reasons for encephalopathy but several showed brain infarctions on imaging. Four patients seen during a 2-week period in late-April showed radiographic evidence of ischemic infarction of the splenium of the corpus callosum.
Callosal infarction is rare, ranging in stroke series from 2.9% to 8% of ischemic strokes1–3 with approximately half affecting the splenium. Strokes isolated to the callosum are even rarer, ranging in these series from 0.4% to 7%. Therefore, a cluster of infarction isolated to the corpus callosum involving the splenium is distinctly unusual and deserving of further evaluation.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. Four patients with splenial lesions were identified by the authors during a 2-week period on the Neurology Consult and Stroke services. Medical records were reviewed in terms of clinical presentation, cerebrovascular risk profile, and testing to define the mechanism of stroke. Clinical examination was conducted on each patient, although in some cases limited by pharmacological sedation and precautions to minimize COVID-19 exposures of the hospital staff. Brain imaging was reviewed. Publication of de-identified patient data was approved by Montefiore institutional review board; written consent was waived.
Results
Patient 1
An 84-year-old woman with hypertension and diabetes mellitus was admitted in respiratory failure, COVID-19 positive (see the Table for a summary of clinical data). She developed acute renal failure requiring hemodialysis. Neurology was consulted for depressed level of arousal and failure to wean from the ventilator after 4 days off sedation. On examination, she opened her eyes to request but otherwise was not interactive. A computed tomography scan of the head showed hypodensity of the splenium of the corpus callosum (Figure 1A and 1B). Mental status remained depressed; hospitalization was prolonged with little improvement in her mental status in the following 6 weeks.
Figure 1.

Imaging studies of patients 1 and 2.
A and B, Computed tomography (CT) scans of head in patient 1. C, CT scan of head in patient 2.
Table 1.
Summary of Clinical Data
Patient 2
A 52-year-old woman with hypertension and diabetes mellitus was admitted in respiratory failure, COVID-19 positive. She developed acute renal failure and required dialysis. Neurology was consulted for depressed level of arousal and transient downward eye deviation on day 16 on a ventilator. On examination, she opened her eyes spontaneously but otherwise was not interactive. A computed tomography scan of the head showed a hypodense lesion of the splenium of the corpus callosum (Figure 1C). Mental status improved and she was discharged to acute rehabilitation.
Patient 3
A 62-year-old woman with diabetes mellitus presented with acute confusion and hypoxia 2 weeks after development of persistent cough, COVID-19 positive. On examination, she was alert and oriented but could not follow complex requests. There were no focal motor signs. Venous duplex showed deep vein thrombosis in the right calf. Brain magnetic resonance imaging showed multiple infarctions involving the cerebral and cerebellar hemispheres bilaterally and the right side of the splenium of the corpus callosum (Figure 2A and 2B). She was treated with high flow oxygen, apixaban, hydroxychloroquine, and steroids. She improved and was discharged to acute rehabilitation.
Figure 2.
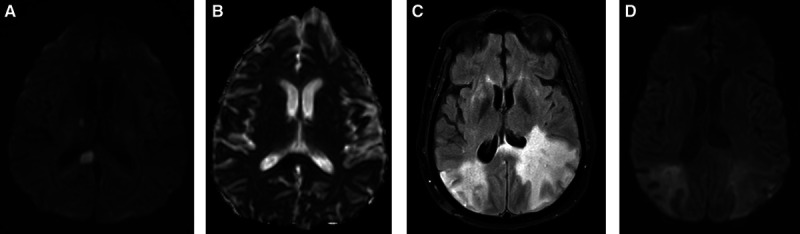
Imaging studies of patients 3 and 4.
A and B, Magnetic resonance imaging (MRI) of brain in patient 3 (diffusion weighted imaging [DWI], apparent diffusion coefficient). C and D, MRI of brain in patient 4 (fluid attenuated inversion recovery, DWI).
Patient 4
A 54-year-old woman with history of diabetes mellitus and hypothyroidism was admitted in respiratory distress, COVID-19 positive. She had prolonged intubation and developed renal failure requiring dialysis. She remained unresponsive. Magnetic resonance imaging of the brain (Figure 2C and 2D) showed edema of the hemispheres bilaterally with cortical petechiae, most pronounced in the parietal and temporal lobes, also affecting frontal lobes and splenium of the corpus callosum. She remained unresponsive and died.
Discussion
The corpus callosum is a massive commissural system interconnecting the cerebral hemispheres. It is supplied anteriorly by branches of the anterior cerebral and anterior communicating artery and posteriorly by branches of the posterior cerebral artery. The splenium is perfused by the posterior pericallosal artery, a branch of the posterior cerebral artery, forming a rich anastomosis with terminal branches of the anterior cerebral artery.4 The rarity of splenial infarction can be explained by (1) rarity of occlusion of the feeding cerebral vessels posterior cerebral artery and anterior cerebral artery, (2) the rich collateralization of the vessels supplying the callosum with blood from both anterior and posterior circulations, providing protective redundancy,5 and (3) the posterior pericallosal artery arises at almost right angles to its parent vessel which makes it resistant to occlusion by emboli.2
Callosal infarction has diverse causes including atherosclerosis of the anterior and posterior circulations, embolization, and small vessel disease.3 Zhang et al1 compared the causes of stroke isolated to the corpus callosum to that of other supratentorial infarctions in a stroke registry using the TOAST (Trial of ORG 10172 in Acute Stroke Trial) classification6 and found no statistically significant differences. However, for isolated splenial stroke, embolization was most commonly the cause.1,5,7
Other rarer causes of callosal infarction include vasospasm, venous occlusion, vasculitis, hypercoagulability, and hypoxia.2 Nonvascular causes of callosal lesions include neoplasm particularly lymphoma, multiple sclerosis and other demyelinating disorders, trauma, metabolic disorders such as Marchiafava-Bignami, carbon monoxide toxicity, viral encephalitis, and seizures.7,8 Posterior reversible encephalopathy syndrome affects the splenium in approximately 10% of cases8 although rarely in isolation. Reversible splenial lesions are seen in association with anticonvulsant medications.9
All patients had vascular risk factors that were likely exacerbated by hypoxia, renal failure, inflammation, and coagulopathy caused by COVID-19. Cases 1 and 2 showed infarction isolated to the corpus callosum. In case 3 the splenial infarction was almost certainly cardioembolic as it was associated with numerous infarctions affecting both hemispheres and both anterior and posterior circulations. Case 4 was likely a variant of posterior reversible encephalopathy syndrome due to renal failure and metabolic abnormalities causing an inflammatory as well as ischemic cerebral vasculopathy.
Clinically, all patients presented with encephalopathy, which is typical with callosal infarctions1,5,9 although metabolic derangements were certainly contributory. Callosal disconnection syndromes including Alien Hand Syndrome and tactile anomia of the left hand require larger lesions of the more anterior elements of the callosum.5 Lesions of the posterior callosum can be seen in patients with alexia without agraphia and color anomia, but the splenial lesion is neither necessary nor sufficient in causing these syndromes.10 In this case series, the patients’ mental status did not allow for detailed testing for these disconnection syndromes.
At the present time, the world-wide experience with the relationship of stroke to COVID-19 infection is beginning to be elucidated in the medical literature. Early reports11,12 indicate an increased risk of stroke in those affected with the virus that may be as much as 2.5× higher than in the uninfected.13 Paradoxically, at the same time, stroke centers in affected areas report a reduction in stroke admissions of patients with non–COVID-19, who may be avoiding acute care hospitals during the pandemic.12 The proposed role of COVID-19 as a cause of stroke14 includes the effects of hypoxia, release of inflammatory cytokines, effects of the virus on clotting, viral invasion of blood vessels causing angiitis, cardiac and hemodynamic compromise and others.
Conclusions
This small series of patients with an uncommon locus of injury provides a window into the diversity and complexity of cerebrovascular disease in the time of COVID-19.
Acknowledgments
Dr Steven Herskovitz for encouragement and assistance with editing.
Sources of Funding
None.
Disclosures
None.
Footnotes
Nonstandard Abbreviations and Acronyms
- COVID-19
- coronavirus disease 2019
- TOAST
- Trial of ORG 10172 in Acute Stroke Trial
For Sources of Funding and Disclosures, see page xxx.
References
- 1.Zhang Z, Meng X, Liu W, Liu Z. Clinical features, etiology, and 6-month prognosis of isolated corpus callosum infarction. Biomed Res Int. 2019; 2019:9458039 doi: 10.1155/2019/9458039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chrysikopoulos H, Andreou J, Roussakis A, Pappas J. Infarction of the corpus callosum: computed tomography and magnetic resonance imaging. Eur J Radiol. 1997; 25:2–8. doi: 10.1016/s0720-048x(96)01155-2 [DOI] [PubMed] [Google Scholar]
- 3.Giroud M, Dumas R. Clinical and topographical range of callosal infarction: a clinical and radiological correlation study. J Neurol Neurosurg Psychiatry. 1995; 59:238–242. doi: 10.1136/jnnp.59.3.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Türe U, Yaşargil MG, Krisht AF. The arteries of the corpus callosum: a microsurgical anatomic study. Neurosurgery. 1996; 39:1075–1084. doi: 10.1097/00006123-199612000-00001 [DOI] [PubMed] [Google Scholar]
- 5.Li S, Sun X, Bai YM, Qin HM, Wu XM, Zhang X, Jolkkonen J, Boltze J, Wang SP. Infarction of the corpus callosum: a retrospective clinical investigation. PLoS One. 2015; 10:e0120409 doi: 10.1371/journal.pone.0120409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993; 24:35–41. doi: 10.1161/01.str.24.1.35 [DOI] [PubMed] [Google Scholar]
- 7.Murthy SB, Chmayssani M, Shah S, Goldsmith CE, Kass JS. Clinical and radiologic spectrum of corpus callosum infarctions: clues to the etiology. J Clin Neurosci. 2013; 20:175–177. doi: 10.1016/j.jocn.2012.05.013 [DOI] [PubMed] [Google Scholar]
- 8.Park SE, Choi DS, Shin HS, Baek HJ, Choi HC, Kim JE, Choi HY, Park MJ. Splenial lesions of the corpus callosum: disease spectrum and MRI findings. Korean J Radiol. 2017; 18:710–721. doi: 10.3348/kjr.2017.18.4.710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unterberger I, Bauer R, Walser G, Bauer G. Corpus callosum and epilepsies. Seizure. 2016; 37:55–60. doi: 10.1016/j.seizure.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 10.Sparr SA, Miller AM. Pure Alexia without hemianopia: mapping reading specific pathways in the brain (abstract). Ann Neurol. 1993; 34:285 [Google Scholar]
- 11.Markus HS, Brainin M. COVID-19 and stroke–a global World Stroke Organization perspective. Int J Stoke. 2020; 15:361–364. doi: 10.1177/1747493020923472 [DOI] [PubMed] [Google Scholar]
- 12.Morelli N, Rota E, Terracciano C, Immovilli P, Spallazzi M, Colombi D, Zaino D, Michieletti E, Guidetti D. The baffling case of ischemic stroke disappearance for the casualty department in the COVID-19 era. Eur Neuro. 2020; 83:213–215. doi: 10.1159/000507666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aggarwal G, Lippi G, Michael Henry B. Cerebrovascular disease is associated with an increased disease severity in patients with coronavirus disease 2019 (COVID-19): a pooled analysis of published literature. Int J Stroke. 2020; 15:385–389. doi: 10.1177/1747493020921664 [DOI] [PubMed] [Google Scholar]
- 14.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, et al. ; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020; 75:2950–2973. doi: 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]


