Highlights
-
•
MSP extract increased IgG anti-Bordetella titers with the highest dosage.
-
•
Improved IgG anti-Bordetella transudation from the blood to the colostrum.
-
•
MSP extract increased total IgA titers in the milk, 7 days after farrowing.
Keywords: Sows, Piglets, Atrophic rhinitis, Immuno-stimulators, Seaweed polysaccharides, Ulvans
Abstract
The transfer of passive immunity from sows to piglets can be improved through the administration of immuno-stimulating products before farrowing. This study evaluated the immuno-stimulating effect of an algal sulfated polysaccharide extract (MSP extract) from the green algae Ulva armoricana when administrated orally to sows at the end of gestation. Four diets were tested: Control (no MSP extract), MSP1 (2 g/day of MSP extract), MSP2 (8 g/day), and MSP3 (16 g/day). The experimental diets were provided in two periods: before the last atrophic rhinitis vaccine booster, and a week before farrowing. Anti-Bordetella IgG antibodies were recorded in blood, colostrum, and milk, and total IgA were measured in colostrum and milk. Titer kinetics between the blood sampled before farrowing and colostrum displayed an increase in specific IgG for MSP3. Moreover, the MSP2 diet increased the level of total IgA in milk compared to the control group. Although the immuno-stimulating effect of MSP extract on piglet performance was not concurrent across the different supplementation levels, the present study supports the use of natural algae extract (MSP) as an immunomodulating solution in swine production.
1. Introduction
Pig respiratory diseases are a major concern for modern swine producers worldwide, accounting for nearly 54% of deaths during the nursery phase (U.S. Department of Agriculture, 2008). Bordetella bronchiseptica is one of the most important pathogens that can cause swine respiratory disease problems. It is the primary etiologic agent of atrophic rhinitis (AR), which is a contagious respiratory disease highly prevalent in swine nurseries worldwide (Magyar & Lax, 2002). A general therapeutic strategy against AR is the use of antibiotic treatments in piglets. However, the increase in antibiotic resistant bacteria has driven research on the development of alternative solutions to protect piglets from diseases such as AR. Neonatal piglets are born with an incompetent immune system, and in the first hours of life they are only protected by postnatal transfer of passive immunity through lactation (Rooke & Bland, 2002). Thus, the survival of neonatal piglets is highly reliant on the ingestion of colostrum within their first 18 to 36 h of life (Bainter, 1986), and ingestion of milk once the colostrum is no longer present. It is, therefore, important to improve the transfer of passive immunity from sows to piglets, which can be achieved through the administration of immuno-stimulating products to the sow before farrowing (Vondruskova, Slamova, Trckova, Zraly, & Pavlik, 2010).
Algal sulfated polysaccharide extracts (MSP) could provide an alternative prophylactic strategy that stimulate innate immune responses and limits infections in farm animals, and subsequently reduces the use of antibiotics (Berri et al., 2016). Seaweeds are an important source of bioactive compounds, such as MSPs, which are a complex group of macromolecules with a wide range of important biological properties (Leal et al., 2013, Xu et al., 2017, You et al., 2010). These water-soluble compounds can be found in the cell walls of seaweeds, and their structure and properties differ between the three major divisions of marine algae: Chlorophyceae, Phaeophyceae, and Rhodophyceae (green, brown, and red algae, respectively). In recent years, an increasing number of studies revealed that MSPs have a wide range of beneficial biological activities including antiviral, antioxidant, anticancer, anticoagulant, and anti-hyperlipidemic activities (Wang et al., 2014, Wijesekara et al., 2011). Moreover, several studies have demonstrated that MSP extracts from seaweeds exert immunomodulatory activities by modifying the activity of cytokines and macrophages that play a critical role in the innate immune system. This has been shown in vitro in murine macrophages (Fang et al., 2015, Jeong et al., 2015, Karnjanapratum et al., 2012, Na et al., 2010), but also in vivo in mice (Kim et al., 2011, Liu et al., 2017), and using an in vitro system of porcine intestinal epithelial (IPEC-1) cells (Berri et al., 2017). It was recently demonstrated by Guriec et al. (2018), also to be the case for chickens, both in vitro on heterophils and monocytes and in vivo when the extract was administered per os. In particular, MSP extracted from green algae have shown immunomodulating properties, both as crude algal extracts and as highly purified fractions in different models, such as in murine macrophages in vitro (Karnjanapratum et al., 2012, Tabarsa et al., 2012, Tabarsa et al., 2018), in mice and chicken in vivo (Guriec et al., 2018, Song et al., 2015), and in turbot phagocyte and peritoneal leucocytes in in vitro and in vivo trials, respectively (Castro et al., 2006). Ulvans increase the production of reactive oxygen species and nitric oxide, the secretion of pro-inflammatory cytokines, such as tumor necrosis factor (TNFα), interleukin (IL)-1, IL-6, IL-8, IL-12, interferon (IFN) (Karnjanapratum et al., 2012, Kim et al., 2011, Na et al., 2010), and the expression of the cytokine (TGF-β) involved in IgA plasmocyte activation, and of CCL20, which is a chemokine known to regulate several aspects of intestinal immunity (Berri et al., 2016, Berri et al., 2017). Seaweed extracts have therefore emerged as a rich source of bioactive natural compounds that can be used as a new generation of growth enhancers that simultaneously potentiate the immune function and improve animal health.
The current study evaluated the activity of a MSP extract from the green alga (U. armoricana) on the immune transfer between sows and piglets. Specifically, we analyzed the effects of adding different doses of MSP extract to sows feed during the final month of gestation on piglets’ performance, as well as on the levels of anti-Bordetella immunoglobulin G (IgG) antibodies in colostrum, milk, and blood, and on total immunoglobulin A (IgA) in colostrum and milk of sows.
2. Materials and Methods
U. armoricana were collected on the beach at Plestin les Grèves (Brittany, France, 48°39′28″N 3°37′47″W), and its MSP extract was produced and analyzed as previously described (Berri et al., 2016). The presence of endotoxins was assessed using the kit Etoxate (Sigma, Lyon, France), and their levels were below the detection level of the kit (0.05–0.1 endotoxin units/mL).
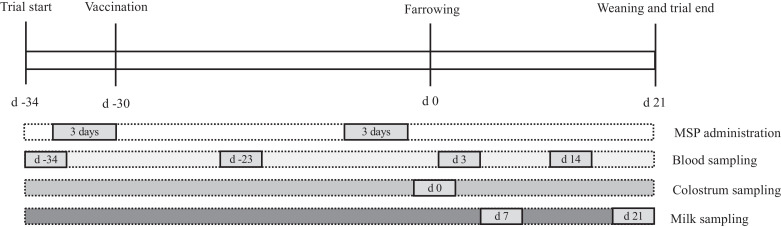
Thirty-two sows (Large white × Landrace genetics) were housed on a commercial farm with a “farrow to finish” system. The sows were born on the farm, and at 170 days old they were transferred into quarantine and given the first vaccination against AR with 2 mL of inactivated vaccine per gilt (Porcilis AR-T DF; MSD animal health, Beaucouzé, France). Two booster inoculations with the same vaccine were administrated at 200 and 330 days old. The latter date corresponded to 30 days before the anticipated farrowing date (Fig. 1). All sows received the same diet two times per day (8 a.m. and 5 p.m.) and were divided into four groups with 8 sows each: control group and three test groups with increasing MSP extract dosage (2 g/day, 8 g/day, and 16 g/day). To facilitate the distribution of MSP extract, crushed biscuit was added as excipient (40 g) to all groups. The excipient (control) or excipient-MSP extract complex (test) was administrated by top-dressing on the feed during two periods of three consecutive days: before the last vaccine booster against AR, and one week before farrowing (Fig. 1). Cross fostering during the first two days of the piglets’ life was a normal practice at the farm to improve piglet performance before weaning (Calderón Díaz, García Manzanilla, Diana, & Boyle, 2018). Adoption was performed between sows within the same experimental group, and no sample collection or measurements were performed on the adopted piglets. All sows and piglets were housed and maintained in compliance with the French Ministry of Agriculture and Finishing standards for animal protection.
Fig. 1.
Experimental timeline showing vaccination date, MSP extract supplementation periods, and sampling dates for blood, colostrum, and milk.
At farrowing, the total number of piglets and the number of live piglets within each litter were recorded (litter size), as was the weight of all live-born piglets. The first four live piglets from each litter were immediately tagged and weighed individually and these piglets were weighed again at weaning (21 days after farrowing). Blood samples were collected from the vena cava of each sow and piglets at several time points (Fig. 1). Samples were collected in 9 mL plastic tubes with the vacutainer system, and then centrifuged for 2 min at 3000× g (Labofuge 200, Thermo Fisher Scientific Inc., Waltham, MA, USA). Serum was separated and stored at −80°C for further processing. Colostrum (∼20 mL) was manually collected from each sow 2 h after the start of farrowing on the first 3 teats, and milk (∼30 mL) was manually collected from the same teats at 7 and 21 days after farrowing. Twenty I.U. (2 cc) of oxytocin (Ocytovem, CEVA animal health, Libourne, France) were injected intramuscularly on the neck of sows to facilitate milk collection. Colostrum and milk samples were frozen at −20°C immediately after collection, for posterior analyses.
Anti-Bordetella IgG was measured in the blood samples of sows and piglets and in the colostrum and milk via an anti-Bordetella ELISA test (IBL International, Hamburg, Germany) as previously described (Adogony et al., 2007). Total IgA concentration was determined in milk with an ELISA quantification kit specific for pigs, according to the manufacturer's instructions (Bethyl Laboratories Inc., Montgomery, TX, USA). These analyses were performed by LDA 37 (Laboratoire Départemental d’ Analyse de Touraine, 3 rue de l’ Aviation, 37210 Parcay-Meslay, France).
Data normality was validated by the Kolmogorov–Smirnov test, and immunoglobulin concentrations were compared between experimental groups by Student's t-tests, applying Bonferroni corrections for multiple comparisons. The variations in immunoglobulin levels between the groups were examined by comparing the slopes of the regression lines using the F test; when the lines were parallel, their origins and elevations were also compared. Data are shown as average ± standard error of the mean (SEM) and differences were considered significant at p < 0.05 in all tests. All statistical analyses were performed using GraphPad Prism 4.03 (GraphPad Software, San Diego, CA, USA).
3. Results and Discussion
Searching for new bioactive natural compounds that potentiate the immune function of farmed animals has become an important area of research for modern swine producers worldwide. MSP present in the cell walls of green algae have important immunomodulating and immunostimulant properties, which can constitute a promising prophylactic supplement to improve farm animals’ resistance against infectious diseases. The present study evaluated the immunomodulating effects of a MSP extract from the green alga U. armoricana on immunity transfer between sows and piglets, and provides evidence that supplementation with MSP can benefit maternal immunity.
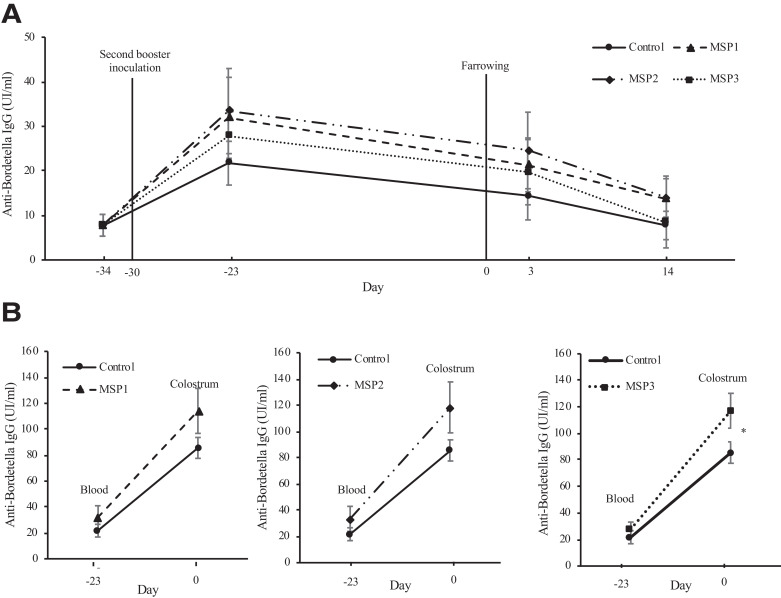
The MSP extract used in the present study had no effect on litter performance, including litter size and individual piglet weight at birth and at 21 days old (p 0.05, supplemental data). This is in agreement with previous results (Heim et al., 2015, Leonard et al., 2011), and it is likely explained by MSP extract supplementation starting too late to promote a significant effect on piglets’ early-stage performance. All experimental groups showed similar levels of IgG in sows’ serum at days 34 and 23 before farrowing (p > 0.05; Fig. 2A). After the 3rd vaccination against AR at day 30, a tendency was observed for higher IgG in MSP-supplied groups, particularly at day 23 before farrowing and day 3 after farrowing. Thereafter, IgG continued to decrease until day 14 after farrowing for the three MSP extract groups (p < 0.05). These results can be associated with the timing of blood sampling, which may not have been optimal to detect the maximum effect of MSP extract on IgG titers in the colostrum. Previous research has shown that IgG levels on blood should be measured on samples taken 3 weeks after vaccination (Kim et al., 2009). However, in the present study, 3 weeks after vaccination would be very close to the anticipated farrowing date. Although the IgG serum levels of the 14 day piglets were statistically similar among experimental treatments (Table 1), results show a relatively higher level of IgG in piglets serum, thereby supporting a positive effect of MSP extract supplementation.
Fig. 2.
Effect of algal sulfated polysaccharide (MSP extract) on the kinetics of immunoglobulin G (IgG) anti-Bordetella in the blood, colostrum, and milk of sows. (A) Levels in blood at 34 and 23 days before farrowing (−34 and −23, respectively) and at 3 and 14 days after farrowing. (B) Levels in colostrum as compared to blood level at day 23 before farrowing and in sows blood compared to colostrum. Values are given as means ± standard error of the mean (whiskers), and asterisks (*) represent significant differences (p < 0.05).
Table. 1.
Levels of immunoglobulin G (IgG) anti-Bordetella (mean ± S.E.M.) in sow colostrum and serum, and in piglet serum (at day 14 after farrowing), and immunoglobulin A (IgA) in sow colostrum observed in each experimental group.
| Ig class | Control (no MSP extract) | MSP1 (2 g/d) | MSP2 (8 g/d) | MSP3 (16 g/d) | |
|---|---|---|---|---|---|
| IgG (UI/mL) | Colostrum (day 0) | 85.7 ± 8.1 | 114.1 ± 17.3 | 118.4 ± 19.4 | 117.1 ± 13.2 |
| Gilts serum (day 14) | 7.7 ± 3.2 | 13.7 ± 5.1 | 13.9 ± 4.3 | 8.4 ± 6.5 | |
| Piglets serum (day 14) | 17.8 ± 5.7 | 23.2 ± 7.1 | 25.1 ± 6.8 | 18 ± 6.4 | |
| IgA in colostrum (day 0; mg/mL) | 12.5 ± 2.4 | 9.8 ± 1.8 | 11.2 ± 1.5 | 11.9 ± 2.8 | |
Results suggest a significant increase in the transfer rate of IgG from sows’ blood to colostrum (Fig. 2B), particularly when MSP extract was supplemented at the highest dosage (MSP3; p < 0.05). Although these findings are similar to that of Leonard, Sweeney, Bahar, Lynch, and O'Doherty (2010), it is important to note that the latter study tested a seaweed extract from Laminaria spp. and a longer supplementation period (from day 109 of gestation until weaning). The transfer of IgG from blood to colostrum is controlled by the FcRn receptor (Cervenak & Kacskovics, 2009), and an increase in the transfer rate of IgG might be attributed to either an increase in the speed of transfer by the receptor, or by an increased expression of the receptor on the mammary gland epithelial cells (Cervenak & Kacskovics, 2009). Colostrum is known to be the primary source of passive immunity in piglets, via IgG transfer from the mother to the litter. The significant increase in the transfer rate of IgG from blood to colostrum in sows supplemented with MSP3 extract was not reflected in the sera of the nursing piglets (Table 1). Nevertheless, the relative increase in IgG in colostrum of sows supplemented with MSP1 and MSP2 extracts was concurrent with the results observed for piglets (Table 1). It is also important to note that no significant differences were detected in the levels of IgG in milk among the experimental groups at any of the sampling days (data not shown). However, this could be associated with some practices previously identified, such as the timing of MSP extract supplementation and the duration of the supplementation period.
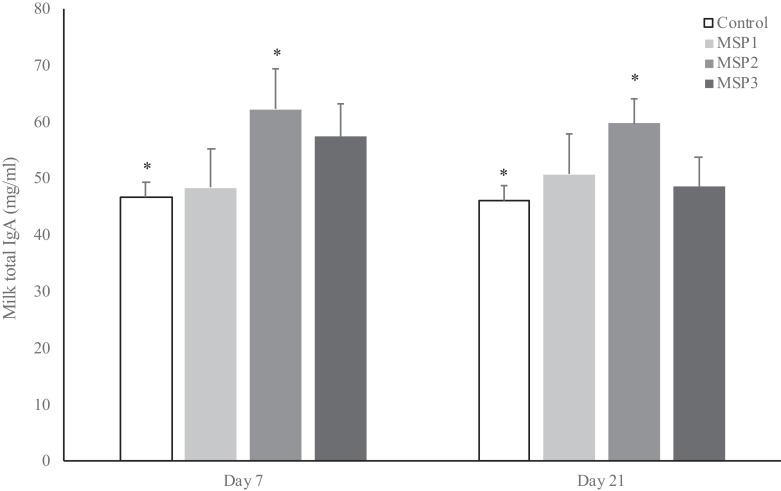
Although IgG is the major isotype in sow serum and colostrum, IgA predominates in milk (Curtis & Bourne, 1971). Neonatal protection against local pathogens is mostly conferred by milk-derived immunity until weaning—the so-called lactogenic immunity. Almost 100% of colostrum IgG and 40% of colostrum IgA are derived from sow serum, while the majority of milk IgA (>90%) results from a local synthesis in the mammary gland (Bourne & Curtis, 1973). While MSP extract supplementation had no effect on colostrum IgA concentration (Table 1), total milk IgA level during lactation was higher in the MSP2 group than in the control group (p < 0.03; Fig. 3), which is in agreement with the results of Leonard et al. (2010) and Zanello et al. (2013). The dose-dependent increase in milk IgA levels at days 7 and 21 (Fig. 3) suggests an optimal dose of 8 g MSP extract per day, and this could be associated with the optimal dose to stimulate the transfer of IgA secreting plasmocytes to the mammary gland via the entero-mammary link. Although the in vitro analysis by Berri et al. (2016) showed that MSP extract stimulates the expression of CCL20 (MIP3-α), which is a chemokine known to regulate several aspects of intestinal immunity, further studies are required to better establish the effect of MSP extracts on the immune entero-mammary link.
Fig. 3.
Effect of algal sulfated polysaccharide (MSP extract) on immunoglobulin A (IgA) in sows’ milk at days 7 and 21 after farrowing. Values are given as means (bars) ± standard error of the mean (whiskers), and asterisks (*) represent significant differences (p < 0.05).
Results from the present study show that the intake of a MSP extract has positive immunomodulating effect on sows and piglets, even though further studies are needed to show an overall immunity effect and better resistance to infections. In addition, the mechanistic drivers of such immunomodulatory effects are not clear, as evidenced by the different immunoglobulin titers at particular pathways (e.g. milk and not colostrum), and for dose-specific supplementations in some cases. The present results can contribute to the development of alternative prophylactic solutions against respiratory diseases in swine production although further studies are needed to further explain the mode of action.
Conflict of interest statement
Authors Frédérick Bussy, Matthieu Le Goff, Pi Nyvall Collén were employed by company Amadeite. All other authors declare no competing interests.
Acknowledgments
The authors wish to thank the farmer and his team, for their availability and contribution during the trial, and Miguel Leal for comments to improve the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.vas.2019.100051.
Appendix. Supplementary materials
References
- Adogony V., Respondek F., Biourge V., Rudeaux F., Delaval J., Bind J.L. Effects of dietary scFOS on immunoglobulins in colostrums and milk of bitches. Journal of Animal Physiology Animal Nutrition. 2007;91:169–174. doi: 10.1111/j.1439-0396.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- Bainter K. CRC Press; Boca Raton: 1986. Intestinal absorption of macromolecules and immune transmission from mother to young; p. 240. [Google Scholar]
- Berri M., Oliviera M., Holberta S., Dupontb J., Demaisc H., Le Goffd M. Ulvan from Ulva armoricana (Chlorophyta) activates the PI3K/Akt signalling pathway via TLR4 to induce intestinal cytokine production. Algal Research. 2017;28:39–47. [Google Scholar]
- Berri M., Slugocki C., Olivier M., Helloin E., Jacques I., Salmon H. Marine-sulfated polysaccharides extract of Ulva armoricana green algae exhibits an antimicrobial activity and stimulates cytokine expression by intestinal epithelial cells. Journal of Applied Phycology. 2016;28:2999–3008. [Google Scholar]
- Bourne F.J., Curtis J. The transfer of immunoglobulins IgG, IgA and IgM from serum to colostrum and milk in the sow. Immunology. 1973;24:157–161. [PMC free article] [PubMed] [Google Scholar]
- Calderón Díaz J.A., García Manzanilla E., Diana A., Boyle L.A. Cross-fostering implications for pig mortality, welfare and performance. Frontiers in Veterinary Science. 2018;5:123. doi: 10.3389/fvets.2018.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro R., Piazzon M.C., Zarra I., Leiro J., Noya M., Lamas J. Stimulation of turbot phagocytes by Ulva rigida C. Agardh polysaccharides. Aquaculture. 2006;254:9–20. [Google Scholar]
- Cervenak J., Kacskovics I. The neonatal Fc receptor plays a crucial role in the metabolism of IgG in livestock animals. Veterinary Immunopathology. 2009;15:171–177. doi: 10.1016/j.vetimm.2008.10.300. [DOI] [PubMed] [Google Scholar]
- Curtis J., Bourne F.J. Immunoglobulins quantitation in sow serum, colostrum and milk and the serum of young pigs. Biochemica and Biophysica Acta. 1971;236:319–332. doi: 10.1016/0005-2795(71)90181-4. [DOI] [PubMed] [Google Scholar]
- Fang Q., Wang J.F., Zha X.Q., Cui S.H., Cao L., Luo J.P. Immunomodulatory activity on macrophage of a purified polysaccharide extracted from Laminaria japonica. Carbohydrate Polymers. 2015;134:66–73. doi: 10.1016/j.carbpol.2015.07.070. [DOI] [PubMed] [Google Scholar]
- Guriec N., Bussy F., Gouin C., Mathiaud O., Quero B., Le Goff M. Ulvan activates chicken heterophils and monocytes through toll-like receptor 2 and toll-like receptor 4. Frontiers in Immunology. 2018;9:2725–2744. doi: 10.3389/fimmu.2018.02725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim G., O'Doherty J., O'Shea C., Doyle D., Egan A., Thornton K. Maternal supplementation of seaweed-derived polysaccharides improves intestinal health and immune status of suckling piglets. Journal of Nutritional Science. 2015;4:1–12. doi: 10.1017/jns.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S.C., Jeong Y.T., Lee S.M., Kim J.H. Immune-modulating activities of polysaccharides extracted from brown algae Hizikia fusiforme. Bioscience, Biotechnology, and Biochemistry. 2015;79:1362–1365. doi: 10.1080/09168451.2015.1018121. [DOI] [PubMed] [Google Scholar]
- Karnjanapratum S., Tabarsa M., MyoungLae C., You S. Characterization and immunomodulatory activities of sulfated polysaccharides from Capsosiphon fulvescens. International Journal of Biological Macromolecules. 2012;51:720–729. doi: 10.1016/j.ijbiomac.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Kim J.K., Cho M.L., Karnjanapratum S., Shin I.S., You S.G. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. International Journal of Biological Macromolecules. 2011;49:1051–1058. doi: 10.1016/j.ijbiomac.2011.08.032. [DOI] [PubMed] [Google Scholar]
- Kim T., Toan N.T., Seo J., Jung B., Lee J., Lee B. Bordetella bronchiseptica aroA mutant as a live vaccine vehicle for heterologous porcine circovirus type 2 major capsid protein expression. Veterinary Microbiology. 2009;138:318–324. doi: 10.1016/j.vetmic.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Leal M.C., Munro M.H.G., Blunt J.W., Puga J., Jesus B., Calado R. Biogeography and biodiscovery hotspots of macroalgal marine natural products. Natural Product Reports. 2013;30:1380–1390. doi: 10.1039/c3np70057g. [DOI] [PubMed] [Google Scholar]
- Leonard S.G., Sweeney T., Bahar B., Lynch B.P., O'Doherty J.V. Effect of maternal fish oil and seaweed extract supplementation on colostrum and milk composition, humoral immune response, and performance of suckled piglets. Journal of Animal Science. 2010;88:2988–2997. doi: 10.2527/jas.2009-2764. [DOI] [PubMed] [Google Scholar]
- Leonard S.G., Sweeney T., Bahar B., O'Doherty J.V. Effect of maternal seaweed extract supplementation on suckling piglet growth, humoral immunity, selected microflora and immune response after an ex vivo lipopolysaccharide challenge. Journal of Animal Science. 2011;90:505–514. doi: 10.2527/jas.2010-3243. [DOI] [PubMed] [Google Scholar]
- Liu Q.M., Xu S.S., Li L., Pan T.M., Shi C.L., Liu H. In vitro and in vivo immunomodulatory activity of sulphated polysaccharide from Porphyra haitanensis. Carbohydrate Polymers. 2017;165:189–196. doi: 10.1016/j.carbpol.2017.02.032. [DOI] [PubMed] [Google Scholar]
- Magyar T., Lax A.J. Atrophic rhinitis. In: Brogden K.A., Guthmiller J., editors. Polymicrobial diseases. ASM Press; Washington, DC: 2002. pp. 169–197. [Google Scholar]
- Na W.J., Kim S.M., Kim J.W., Park S.M., Lee S.O., Kim S.S. Purification, characterization and immunostimulating activity of water-soluble polysaccharide isolated from Capsosiphon fulvescens. International Immunopharmacology. 2010;10:364–370. doi: 10.1016/j.intimp.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Rooke J.A., Bland I.M. The acquision of passive immunity in the new-born piglet. Livestock Production Science. 2002;78:13–23. [Google Scholar]
- Song L., Chen X., Liu X., Zhang F., Hu L., Yue Y. Characterisation and comparison of the structural features, immune-modulatory and anit-avian influenza virus activities conferred by three algal sulphated polysaccharides. Marine Drugs. 2015;14:1–17. doi: 10.3390/md14010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarsa M., Han J., Kim C., You S. Molecular characteristics and immunomodulatory activities of water-soluble sulphated polysaccharides from Ulva pertusa. Journal of Medicinal Food. 2012;15:135–144. doi: 10.1089/jmf.2011.1716. [DOI] [PubMed] [Google Scholar]
- Tabarsa M., You S., Dabaghian E.H., Surayot U. Water-soluble solysaccharides from Ulva intestinalis: Molecular properties, structural elucidation and immunomodulatory activities. Journal of Food and Drug Analysis. 2018;26:599–608. doi: 10.1016/j.jfda.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Agriculture . USDA/ APHIS/VS CEAH N478.0308., USDA; Fort Collins, CO: 2008. Swine 2006, Part III: Reference of swine health and health management in the United States. [Google Scholar]
- Vondruskova H., Slamova R., Trckova M., Zraly Z., Pavlik I. Immune modulating effect of a seaweed extract from Ulva armoricana in pigs: Specific IgG and total IgA in colostrum, milk and blood. Veterinary Medicine. 2010;55:199–224. [Google Scholar]
- Wang L., Wang X., Wu H., Liu R. Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Marine Drugs. 2014;12:4984–5020. doi: 10.3390/md12094984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesekara I., Pangestuti R., Kim S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydrate Polymers. 2011;84:14–21. [Google Scholar]
- Xu S.Y., Huang X., Cheong K.L. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Marine Drugs. 2017;15:388. doi: 10.3390/md15120388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You S.G., Yang C., Lee H.Y., Lee B.Y. Molecular characteristics of partially hydrolyzed fucoidans from sporophyll of Undaria pinnatifida and their in vitro anticancer activity. Food Chemistry. 2010;119:554–559. [Google Scholar]
- Zanello G., Meurens F., Serreau D., Chevaleyre C., Melo S., Berri M. Effects of dietary yeast strains on immunoglobulin in colostrum and milk of sows. Veterinary Immunology and Immunopathology. 2013;152:20–27. doi: 10.1016/j.vetimm.2012.09.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.