Abstract
This study was conducted to estimate the prevalence and identify risk factors associated with trematode infections in cattle in and around Bahir Dar, northwest Ethiopia. Fecal samples collected from randomly selected 369 cattle were examined using simple sedimentation technique for differential trematode eggs count. The animals were found shedding eggs of three groups of trematodes, namely Fasciola spp., paramphistomes and Schistosoma spp. The overall prevalence of trematodes was 61.0%, and specific prevalence for Fasciola, paramphistomes and Schistosoma was 20.1%, 48.5% and 16.5%, respectively. A substantial overlap was observed in the occurrence of Fasciola and paramphistomes. The prevalence of all the three trematodes identified in this study was significantly (P < 0.05) associated with body condition and breed, while the prevalence of Fasciola and paramphistomes was also associated with age. The mean (± SE) fecal egg count per gram of feces (EPG) for Fasciola, paramphistomes and Schistosoma was 4.3 (± 0.55), 25.7 (± 2.11) and 3.1 (± 0.42), respectively. EPG of Fasciola was significantly correlated with EPG of paramphistomes (P < 0.001). The EPG for all the three trematodes was associated with body condition and breed of animals (P < 0.05), while EPG for paramphistomes was also affected by age of the animals (P < 0.05). The prevalence of all the three major trematodes of animal health importance with high rate of mixed infection along with poor body condition, suggests substantial economic loss incurred due to reduced productivity in cattle in the study area.
Keywords: Cattle, Fasciola, Paramphistomes, Schistosoma, Trematodes, Ethiopia
1. Introduction
Trematode infections, especially fasciolosis, are some of the most economically important helminth diseases hampering the productivity of domestic ruminants worldwide (Dargie, 1987, Mage et al., 2002, Njau et al., 1988). All the trematode species which are parasitic in livestock belong to the subclass Digenea (Hansen & Perry, 1994). The adult trematodes are commonly called ‘flukes’ and the families which include parasites of major veterinary importance are Fasciolidae, Dicrocoeliidae, Paramphistomatidae and Schistosomatidae (Andrews, 1999, Urquhart et al., 1996).
Fasciola (liver fluke), paramphistomes (rumen/stomach fluke) and Schistosoma (blood fluke) are the most important flukes recorded from different parts of the world (Dreyfuss, Alarion, Vignoles, & Rondelaud, 2006).
Fasciolosis is an economically important disease of domestic livestock, in particular cattle and sheep, and occasionally man. Fasciola hepatica and F. gigantica are the two species most commonly implicated as the etiological agents of fasciolosis (Andrews, 1999). Infection of adult cattle with liver flukes, unless in heavy infections, is usually clinically in apparent. Therefore, under normal conditions, clinical disease is only likely in young cattle (Love, 2017). However, even modest infection can result in significant reduction in milk yield and quality (Urquhart et al., 1996), reduction in weight gain (Hope-Cawdery et al., 1977, Ross, 1970) and reproductive performance (Elliott, Kelley, Rawlin, & Spithill, 2015). Infection of calves with large number of metacercariae (over 1000), on the other hand, cause clinical fasciolosis similar to the one seen in sheep characterized by weight loss, anemia and hypoproteinemia (Boray, 1969). In addition to its effect on productivity, fasciolosis is a cause of significant economic losses through liver condemnation at slaughter (Abebe et al., 2010, Abunna et al., 2010, Berhe et al., 2009, Phiri et al., 2005).
There are several genera of paramphistomes: Paramphistomum, Cotylophoron, Calicophoron, Bothriophoron, Orthocoelium and Gigantocotyle, of which Paramphistomum is the most common and widespread in ruminants (Taylor, Coop, & Wall, 2016). Paramphistomes (amphistomes) are traditionally regarded as having no clinical significance (Iglesias-Piñeiro et al., 2016). However, heavy infection with immature flukes, which attach to the lining of the upper part of small intestine, may cause severe disease which may even result in death (Lloyd et al., 2007, Rolfe et al., 1991). Moderate infections with the immature fluke may cause reduced weight gains or milk production, or ill-thrift. Most livestock, however, have only light stomach fluke infections with either adult fluke or small numbers of immature fluke that they show no signs of disease (Lloyd et al., 2007).
Schistosomosis in cattle in Africa can be caused by Schistosoma bovis, S. mattheei and S. leiperi. Schistosomosis is generally considered to be of low importance in large ruminants, and even where a high prevalence of the parasite is detected in slaughtered cattle, clinical signs of the disease are seen only rarely (Urquhart et al., 1996). However, infections sometimes may result in severe clinical signs (Hansen & Perry, 1994).
There are several reports of coproscopic and abattoir surveys indicating the widespread prevalence of liver and stomach flukes in cattle in Ethiopia (Abebe et al., 2010, Abunna et al., 2010, Ameni et al., 2001, Fromsa et al., 2011, Yeneneh et al., 2012). Prevalence of Schistosoma spp. in cattle in some parts of the country with permanent water bodies, seasonal flooding and high rainfall has also been recorded (Ameni et al., 2001, Chanie et al., 2012, Fromsa et al., 2011, Habtamu and Wolde Mariam, 2011, Habtamu et al., 2013, Yeneneh et al., 2012). Some studies demonstrated the occurrence of mixed trematode infections in cattle in Ethiopia (Ameni et al., 2001, Fromsa et al., 2011). Several studies in cattle in Ethiopia demonstrated considerable financial loss due to liver condemnation at slaughter due to Fasciola infection (Abebe et al., 2010, Abunna et al., 2010, Berhe et al., 2009).
For a rational and sustainable helminth control programme, a comprehensive knowledge of the epidemiology of parasites and their interaction with the host in a specific climate and management system is a prerequisite (Barger, 1999). Therefore this study was conducted with the objectives to estimate the prevalence and identify risk factors associated with infection of cattle with trematodes in and around Bahir Dar.
2. Materials and methods
2.1. Study area and animals
The study was conducted in Bahir Dar town and adjoining Sebatamit village (about 4 km east of Bahir Dar), northwest Ethiopia. Bahir Dar, the capital of Amhara Region, is located on the southern shore of Lake Tana, the largest lake in Ethiopia and the sources of the Abay (Blue Nile) river. The town is located approximately 578 km northwest of Addis Ababa, at a latitude and longitude of 11°36′N and 37°23′E and an elevation of 1,800 meters above sea level. The climate conforms to the Ethiopian woynadega (mid altitude area) with average annual rainfall of 1500 mm, humidity of 57.88 % and mean annual minimum and maximum temperatures of 10 and 30 °C, respectively. The main rainy season extends from late June to late September. The area has poor drainage and there is annual over flooding during the rainy seasons leaving pockets of water bodies for long period during the dry season (Aregay, Bekele, Ferede, & Hailemelekot, 2013). Both the traditional extensive and semi-intensive farming are practiced in the study area. Sebatamit is a suburban village about 4 km from Bahir Dar located on the bank of the River Abay.
The study involved cattle above 6 months of age, both sexes and local and crossbred (Friesian–Zebu) animals managed under the traditional smallholder husbandry system where cattle are often kept out-door and grazed on communal pastures all day. The animals graze in the vicinities of Lake Tana, the Blue Nile River and its tributaries (Andasa and Tikurit) (Habtamu & Wolde Mariam, 2011). The study was conducted from November 2011 to April 2012.
2.2. Study design and sampling technique
The study was a cross-sectional study involving 369 animals selected using simple random sampling method. Sample size was calculated according to Thrusfield (2005) with 40% estimated prevalence of the parasites (Habtamu and Wolde Mariam, 2011, Solomon and Wossene, 2007) and desired 95% confidence interval and 5% precision.
2.3. Fecal sample and data collection
Feces were collected directly from the rectum of the study animals with gloved hands and were placed in clean universal bottles. The locality, breed, age, sex and body condition of each study animal were recorded at the time of sample collection. Age was estimated using dentition according to Torell, Bruce, Kvasnicka, and Conley (2003) which involves noting the time of appearance and the degree of wear on the temporary and permanent teeth. Cattle estimated to be less than 4 years old were considered as young cattle while those 4 years and above as adults. Body condition was categorized into 3 broad categories (lean, medium and fat) using recommendations by Nicholson and Butterworth (1986).
2.4. Coprological examination
Coprological examination was made at Bahir Dar Regional Veterinary Laboratory; fecal samples, when not examined immediately on arrival, were stored in a refrigerator at 4 °C until examined. Simple sedimentation technique for detection and count of trematode eggs (DAFWA 2013, Hansen and Perry, 1994, Svendsen, 1997) was used with minor modifications. Briefly, 3 g of feces was put in a container and 40-50 ml tap water was added and mixed thoroughly. The suspension was filtered through a tea strainer and allowed to stand for 5 min. The supernatant was discarded carefully and the sediment was re-suspended in tap water. The sedimentation process was repeated multiple times until the fecal debris and coloring material was removed and the supernatant appear clear. After the last sedimentation the supernatant was removed very carefully and the sediment was recovered into a test tube and re-suspended in about 5 ml tap water, a drop of methylene blue was added, and allowed to stand for 5 min. for effective staining of the debris which leaves the trematode eggs un-stained, all the material was transferred into a Petri dish and examined under low power objective. Trematode egg counts were performed by moving the Petri dish in such a way that every field was examined. Yellow color of Fasciola spp. eggs was used to differentiate them from those of paramphistomes (Urquhart et al., 1996). The differential EPG was calculated by dividing the specific parasite egg count in a sample of 3 g by three.
2.5. Data management and statistical analyses
Data collected during sample collection and results of coprological examinations were entered and stored for analysis into Microsoft Excel spread sheet. The effect of age, sex, breed and body condition on infection with trematodes was analyzed using multiple logistic regression model/analysis. Univariable logistic analysis was used to assess the relationship between mixed and single trematode infections with body condition. The Goodman and Kruskal's gamma statistics was used as a measure of correlation of occurrence of the three trematodes. A Venn diagram (Dohoo, Martin, & Stryhn, 2009) was produced to illustrate the level of coinfection with trematodes. The egg count data (EPG) were log transformed [log10(EPG + 1)] and EPG difference between sex, age and breed was analyzed using two-sample t-test, while one-way analysis of variance (ANOVA) was used to test the EPG between body conditions. Pair wise comparison of means was carried using the Bonferroni adjustment. An animal positive for at least 1 egg was considered positive for the respective trematode infection. Linear regression was used to determine relationships among the EPG of the three trematodes. All statistical analyses were made using Stata 13.1 for Windows (Stata Corporation, College Station, TX). A P value of less than 0.05 was considered statistically significant.
3. Results
3.1. Prevalence
The overall prevalence of trematodes observed in this study was 61.0 % (95% CI: 55.9, 65.8). Specific prevalence was 20.1% (95% CI: 16.3, 24.5), 48.5% (95% CI: 43.4, 53.6), and 16.5% (95% CI: 13.1, 20.7) for Fasciola spp., paramphistomes and Schistosoma spp. respectively. Single infection with the respective trematodes (when an animal was infected with only one of the three trematodes) was 3.8% (95% CI: 2.3, 6.3), 28.7% (95% CI: 24.3, 33.6) and 6.0% (95% CI: 3.9, 8.9), respectively. Mixed infections with at least two parasites were recorded in 85 (23.0%, 95% CI: 19.0, 27.6) cattle (Table 1).
Table 1.
Prevalence of trematodes in cattle in and around Bahir Dar (n = 369).
| Parasite species | Number positive | Prevalence (95% CI) |
|---|---|---|
| Fasciola | 74 | 20.1% (16.3, 24.5) |
| Paramphistomes | 179 | 48.5% (43.4, 53.6) |
| Schistosoma | 61 | 16.5% (13.1, 20.7) |
| Fasciola + Paramphistomes | 45 | 12.2% (9.2, 16.0) |
| Fasciola + Schistosoma | 9 | 2.4% (1.3, 4.6) |
| Paramphistomes + Schistosoma | 27 | 7.3% (5.1, 10.5) |
| Fasciola + Paramphistomes + Schistosoma | 4 | 1.1% (0.4, 2.9) |
| Mixed | 85 | 23.0% (19.0, 27.6) |
| Trematodes | 225 | 61.0% (55.9, 65.8) |
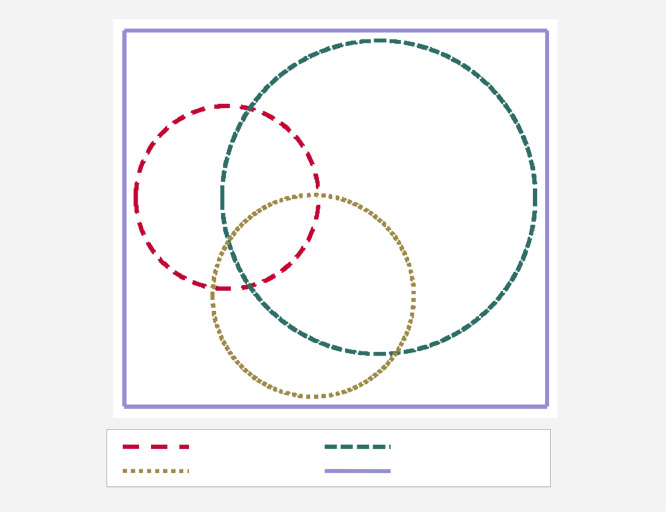
There was a substantial overlap in the infection of individual animals with Fasciola, paramphistomes and Schistosoma (Fig. 1). The overlap was significant (gamma = 0.457) between Fasciola and paramphistomes, while it was random for Schistosoma vs. Fasciola (0.104) and Schistosoma vs. paramphistomes (− 0.023).
Fig. 1.
A Venn diagram showing overlap in coprological prevalences of Fasciola, paramphistomes and Schistosoma in cattle.
Table 2 shows the results of multivariable logistic regression analysis of association between trematodes prevalence and potential predictors. Prevalences of Fasciola, paramphistomes and Schistosoma were significantly associated with body condition and breed of the study animals (P < 0.01). The prevalence of all the three trematodes was higher in animals with lean body condition than with fat body condition and in local cattle than crosses. Cattle younger than four years were associated with higher prevalence of Fasciola and paramphistomes (P < 0.05). Only Schistosoma prevalence was associated with sex, where females were more affected than their male counterparts (P < 0.05). Fasciola prevalence, however, showed a tendency to be greater in females than in males (P = 0.058).
Table 2.
Results of multivariable logistic regression analysis showing association between coprological prevalence of trematodes and risk factors in cattle in and around Bahir Dar.
| Variable | Level | N |
Fasciola |
Paramphistomes |
Schistosoma |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) positive | OR (95% CI) | P value | No. (%) positive | OR (95% CI) | P value | No. (%) positive | OR (95% CI) | P value | |||
| Sex | Male | 185 | 33 (17.8) | 1 | 85 (45.9) | 1 | 28 (15.1) | 1 | |||
| Female | 184 | 41 (22.3) | 1.7 (0.981, 3.0) | 0.058 | 94 (51.1) | 1.4 (0.9, 2.3) | 0.132 | 23 (17.9) | 2.0 (1.1, 3.7) | 0.034 | |
| BC | Fat | 94 | 3 (3.2) | 1 | 35 (37.2) | 1 | 3 (3.2) | 1 | |||
| Medium | 124 | 24 (19.4) | 7.1 (2.0, 24.5) | 0.002 | 52 (41.9) | 1.1 (0.6, 2.1) | 0.652 | 14 (11.3) | 3.4 (0.9, 12.5) | 0.061 | |
| Lean | 151 | 47 (31.1) | 13.2 (3.9, 44.5) | 0.000 | 92 (60.9) | 2.5 (1.4, 4.4) | 0.002 | 44 (29.1) | 11.7 (3.5, 39.6) | 0.000 | |
| Age | ≥4 years | 241 | 41 (17.0) | 1 | 93 (38.6) | 1 | 44 (13.3) | 1 | |||
| <4 years | 128 | 33 (25.8) | 1.956 (1.1, 3.5) | 0.022 | 86 (67.2) | 3.9 (2.4, 6.3) | 0.000 | 17 (18.3) | 0.7 (0.4, 1.4) | 0.384 | |
| Breed | Cross | 193 | 28 (14.5) | 1 | 80 (41.5) | 1 | 17 (8.8) | 1 | |||
| Local | 176 | 46 (26.1) | 2.4 (1.3, 4.3) | 0.004 | 99 (56.3) | 2.5 (1.5, 4.0) | 0.000 | 44 (25.0) | 3.6 (1.8, 6.9) | 0.000 | |
| Overall | 369 | 74 (20.1) | 179 (48.5) | 61 (16.5) | |||||||
BC, body condition
We have attempted to assess the association between mixed infections of trematodes with body condition. Body condition was significantly associated with concurrent infection with Fasciola and paramphistomes (P < 0.01) and paramphistomes and Schistosoma (P < 0.001). Highest prevalences of co-infections were observed in animals with lean body condition than animals with better body conditions. However, while co-infections involving paramphistomes were significantly associated with body condition, single infection with paramphistomes did not (P > 0.05) (Table 3).
Table 3.
Results of univariable logistic regression analysis showing association between body condition and trematode infection in cattle in and around Bahir Dar.
| Infection with | BC | No. examined | No. Positive | % | OR (95% CI) | P value |
|---|---|---|---|---|---|---|
| FasPara | Fat | 94 | 3 | 3.2 | 1 | |
| Medium | 124 | 14 | 11.3 | 3.9 (1.1, 13.9) | 0.038 | |
| Lean | 151 | 28 | 18.5 | 6.9 (2.0, 23.4) | 0.002 | |
| ParaSch | Fat | 94 | 2 | 2.1 | 1 | |
| Medium | 124 | 4 | 3.2 | 1.5 (0.3, 8.6) | 0.626 | |
| Lean | 151 | 21 | 13.9 | 7.4 (1.7, 32.5) | 0.008 | |
| Paramphistomes (single infection) | Fat | 94 | 30 | 31.9 | 1 | |
| Medium | 124 | 34 | 27.4 | 0.8 (0.5, 1.5) | 0.471 | |
| Lean | 151 | 42 | 27.8 | 0.8 (0.5, 1.4) | 0.494 |
FasPara, Fasciola and paramphistomes; ParaSch, paramphistomes and Schistosoma; BC, body condition
Interestingly, there was no animal with fat body condition score in those with single Fasciola infection. But the number of observation (n = 14) was not sufficient to make statistical comparison. The number of animals found co-infected with Fasciola and Schistosoma (n = 9) and with single Schistosoma (22) were also not sufficient to make statistical comparisons and therefore were omitted from the analysis.
3.2. Fecal egg count
The mean (± SE) count of Fasciola, paramphistomes and Schistosoma eggs was 4.3 (± 0.55), 25.7 (± 2.11) and 3.1 (± 0.42) per gram of feces (Table 4), with a range of 0 to 57, 0 to 371 and 0 to 43, respectively. Mean fecal egg count per gram of feces (EPG) for all the three trematodes identified in this study was significantly associated with body condition score of the study animals (P < 0.001). EPG was highest in cattle with lean body condition. EPG was also significantly different a mong breeds (P < 0.05); indigenous animals shed higher number of trematode eggs (for all the 3 species) compared to crossbred animals. Fecal egg count of only paramphistomes was, however, affected by age (P < 0.001) whereby EPG was higher in younger catt le of < 4 years compared to cattle ≥ 4 years. Sex did not significantly affect trematode egg counts (P > 0.05) (Tab le 4).
Table 4.
Mean (± SE) count of Fasciola, paramphistomes and Schistosoma eggs per gram of feces by risk factors in cattle in and around Bahir Dar.
| Variable | Level | N |
Fasciola |
Paramphistomes |
Schistosoma |
|||
|---|---|---|---|---|---|---|---|---|
| Mean (SE) | P value | Mean (SE) | P value | Mean (SE) | P value | |||
| Sex | Male | 185 | 4.2 (0.81) | 0.439 | 23.9 (2.86) | 0.293 | 2.8 (0.57) | 0.368 |
| Female | 184 | 4.3 (0.74) | 27.5 (3.25) | 3.5 (0.61) | ||||
| BC | Fat | 94 | 0.4 (0.29)a | 0.000 | 8.9 (1.71)a | 0.000 | 0.2 (0.13)a | 0.000 |
| Medium | 124 | 2.9 (0.68)b | 14.3 (2.17)a | 1.5 (0.45)a | ||||
| Lean | 151 | 7.7 (1.13)c | 45.6 (4.22)b | 6.2 (0.89)b | ||||
| Age | ≥4 | 241 | 4.0 (0.67) | 0.174 | 19.7 (2.21) | 0.000 | 3.6 (0.55) | 0.131 |
| <4 | 128 | 4.7 (0.94) | 37.0 (4.25) | 2.3 (0.62) | ||||
| Breed | Cross | 193 | 2.5 (0.58) | 0.001 | 24.3 (3.15) | 0.022 | 1.7 (0.45) | 0.000 |
| Local | 176 | 6.1 (0.93) | 27.3 (2.75) | 4.6 (0.71) | ||||
| Overall | 369 | 4.3 (0.55) | 25.7 (2.11) | 3.1 (0.42) | ||||
a,b,c,Means of a particular parasite EPG under a variable with different superscripts are significantly different (P < 0.05)
BC, body condition; SE, standard error of mean
A positive correlation (r2 = 0.0443, P = 0.000) was obtained between Fasciola and para mphistomes EPG. However, there were no correlations (r2 = 0.0028, P = 0.313) between Fasciola and Schistosoma EPG and between paramphistomes and Schistosoma EPG (r2 = 0.0001, P = 0.815).
4. Discussion
In this study, the highest prevalence and EPG was recorded for paramphistomes (48.5%, 25.7) followed by Fasciola (20.1%, 4.3) and Schistosoma (16.5%, 3.1). Similar pattern of occurrence, where paramphistomes top the prevalence followed by Fasciola and Schistosoma, respectively has been reported for the three trematodes from different parts of Ethiopia (Ameni et al., 2001, Yeneneh et al., 2012) and elsewhere in Africa (Nzalawahe, Kassuku, Stothard, Coles, & Eisler, 2014). Keyyu, Monrad, Kyvsgaard, and Kassuku (2005) reported consistently higher prevalence of paramphistomes than Fasciola gigantica in cattle managed under different conditions in Tanzania.
The higher prevalence and EPG of paramphistomes may partly be explained by the fact that the adult parasite is considered non pathogenic and subsequently is not targeted by anthelmintic treatment. It might also be related to the biology of the parasite and the intermediate hosts. Adult paramphistomes may survive in the host for years (Hansen & Perry, 1994) and are very prolific expelling many eggs (Dorchies, 2006) while multiplication of the parasite in infected snails is massive (Hansen & Perry, 1994). The intermediate hosts of paramphistomes are also extremely adaptable and prolific breeders (Hansen & Perry, 1994). Limitation of availability of effective drugs against paramphistomes (Dorchies, 2006) might have also contributed to the relative high prevalence of the parasite. The common anthelmintics used for routine de-worming against important nematodes and liver fluke in Ethiopia such as albendazole, ivermectin and triclabendazole have little or no effect on paramphistomes (Rolfe and Boray, 1988, Rolfe and Boray, 1993). Higher and increasing prevalence of paramphistomes compared to liver fluke has been documented in France partly due to the lack of an effective treatment against cattle paramphistomosis (Mage et al., 2002).
The prevalence of Fasciola spp. observed in our study is comparable to the 24% prevalence reported by Yeneneh et al. (2012). It was however lower compared to other recent reports from areas adjoining Lake Tana (Gebrie et al., 2015, Tsegaye et al., 2012) and reports from other parts of the country (Ameni et al., 2001, Fromsa et al., 2011, Taye et al., 2016, Telila et al., 2014). The difference in prevalence may be due to difference in the amount of rainfall and other climatic conditions over the years in the area, and differences in climato-ecological conditions among the study areas (Yilma & Mesfin, 2000). The relative low prevalence of Fasciola may also be associated with expanding veterinary services in the area. The high prevalence of Fasciola reported from parts of the country with no major permanent water bodies as compared to our study area may show the relative importance of F. hepatica in cattle fasciolosis in the country. The snail intermediate hosts of F. hepatica are amphibious (Andrews, 1999) and hence do not necessarily need aquatic environment for survival and proliferation. Several abattoir surveys conducted in different parts of the country demonstrated co-infection of cattle with F. hepatica and F. gigantica (Abebe et al., 2010, Abunna et al., 2010, Aregay et al., 2013, Berhe et al., 2009, Fromsa et al., 2011, Yilma and Mesfin, 2000).
The prevalence of paramphistomes recorded in the present study (48.5%) was comparable to an earlier report (45.8%) from an area not far from our study area (Yeneneh et al., 2012) and western Ethiopia (44.2%) (Fromsa et al., 2011) which is characterized by a humid tropical climate of heavy annual rainfall (Vanleeuwen, Tolosa, Sirak, Nemera, & Belaineh, 2014). Studies conducted near a large marshy area traversed by a river (Ameni et al., 2001) in northeastern Ethiopia (75%) and around a small lake (Kifleyohannes, Kebede, Hagos, Weldu, & Michael, 2015) in northern Ethiopia (65.3%) demonstrated higher prevalence of paramphistomes. Many other studies in Ethiopia, however, reported lower prevalence of the parasite compared to our finding (Mariam et al., 2014, Kemal and Terefe, 2013, Telila et al., 2014, Yohannes et al., 2013). The higher prevalence of paramphistomes, whose intermediate hosts are aquatic snails, observed in the present study could be explained by the fact that our study was conducted near permanent water bodies as opposed to some of the other studies which were conducted in drier areas with no major permanent water bodies.
Prevalence of Schistosoma observed in our study (16.5%) was comparable to the findings of Chanie et al. (2012) (13.7%) in an area adjacent to Lake Tana and Fromsa et al. (2011) (13.5%) from western Ethiopia. The prevalence was, however, low compared to earlier reports from Lake Tana basin (Habtamu and Wolde Mariam, 2011, Habtamu et al., 2013, Yeneneh et al., 2012) and a report from northeastern Ethiopia (28%) (Ameni et al., 2001). The variations observed among studies in trematode prevalence in general may be attributed to differences in climato-ecological conditions among study areas, difference in rainfall between study years, differences in study seasons and difference in animal management practices.
Prevalence as well as EPG of all the three trematodes considered in this study were highest in lean animals compared to animals with medium and fat body condition. Heavy infection with Fasciola in cattle, especially in young stock, may result in severe disease characterized by anemia, hypoalbuminemia (edema), ill thrift and weight loss (Boray, 1969, Love, 2017, Urquhart et al., 1996). Similarly, heavy infection with immature stomach flukes may cause decreased appetite, listlessness and weight loss (Rolfe et al., 1991).
Even moderate infections with Fasciola (Hope-Cawdery et al., 1977, Ross, 1970, Wamae et al., 1998) and immature paramphistomes (Lloyd et al., 2007) may affect weight gain. Loss of appetite, which might contribute to the poor body condition, is also one of the clinical signs of chronic fasciolosis (Love, 2017).
However, it should be noted that it is difficult to separate the effects of the different genera of trematodes on body condition as they tend to occur together. Our finding supports previous reports which associated fasciolosis (Fromsa et al., 2011, Meshesha and Tesfaye, 2017, Phiri et al., 2006), paramphistomosis (Fromsa et al., 2011, Mariam et al., 2014, Melaku and Addis, 2012) and schistosomosis (Fromsa et al., 2011, Lulie and Guadu, 2014) with poor body condition.
The significant association of paramphistomes (when an animal was considered paramphistomes positive irrespective of its status to the other two trematodes) with body condition observed in our analysis was not repeated when animals infected only with paramphistomes (single infection) were considered. It is possible that the result was influenced by those animals which were also co-infected with other trematodes (especially Fasciola) in the former, as paramphistome positive animals were more likely to be also positive to Fasciola than their paramphistome negative counterparts. It is also possible that animals found with single paramphistome infection in the study were de-wormed with anthelmintics effective against liver fluke and possibly to nematodes. The result may even suggest additive or synergistic pathogenic effect of co-infection with trematodes. High mortality rate in concurrent infection involving F. gigantica and Schistosoma bovis in dairy cows has been reported from the Sudan (A/Rahman et al., 2007). After observing a positive correlation (r2 = 0.12) between F. gigantica and paramphistomes worm count in naturally infected cattle, Yabe et al. (2008) suggested that the heterologous interaction of these two parasites may compound the economical effects of liver flukes to the livestock industry.
The prevalence of Fasciola and paramphistomes and EPG of paramphistomes were higher in young cattle, less than 4 years old, compared to their adult counterparts. Concurrent with our finding other researchers also recorded higher prevalence of Fasciola in younger cattle (Aregay et al., 2013, Meshesha and Tesfaye, 2017). However, many studies on paramphistomes in Ethiopia and elsewhere didn't find difference in prevalence among age groups (Fromsa et al., 2011, Mariam et al., 2014, Khedri et al., 2015, Kifleyohannes et al., 2015, Phiri et al., 2006). The variation might be partly attributed to differences in classification of age categories among the studies. Development of immunity due to exposure to Fasciola, which limits the lifespan of the primary infection, slows the migration of secondary infection and eventually reduces the number of flukes established (Urquhart et al., 1996), may be responsible for lower prevalence of Fasciola in older cattle. Similarly, development of a good acquired immunity against paramphistomes (Rolfe et al., 1991) has been stated.
Contrary to our expectation and some earlier reports from Ethiopia (Gebrie et al., 2015, Habtamu and Wolde Mariam, 2011), local cattle were found to be more affected with the three trematodes and shed higher number of eggs in their feces than crossbred animals. This difference is likely to be due to exposure difference rather than difference in natural resistance, as studies involving F. gigantica suggested that local cattle (Bos indicus) appear to be more resistant than B. taurus to infection with Fasciola (Bitakaramire, 1973, Castelino and Preston, 1979). It is possible that more attention is paid to valuable (crossbred) animals that their chance of grazing fluke infested areas is limited and/or they are more frequently de-wormed than local animals. In agreement with our observation some studies reported higher prevalence of trematodes in local cattle than crossbred animals (Islam et al., 2011, Meshesha and Tesfaye, 2017, Tsegaye et al., 2012).
We found a high correlation (gamma = 0.457) between Fasciola and paramphistomes occurrence and their EPG (r2 = 0.044). Similarly, Phiri et al. (2006) recorded a positive correlation (r2 = 0.043) between EPGs of F. gigantica and paramphistome species. Yabe et al. (2008) recorded even stronger correlation (r2 = 0.120) between these two trematodes using worm burdens. The correlation may be partially explained by the existence of common factors that contribute to the occurrence of these parasites.
In this study, few animals (n = 9) harbored both Fasciola and Schistosoma and neither the occurrence nor the EPG of these trematodes were correlated. Yabe et al. (2008) recorded a similar finding and explained it with the significant liver pathology both trematodes cause which may exclude establishment of the other when one infection is established.
In conclusion, the present study demonstrated that the major trematodes of animal health and welfare importance are relatively highly prevalent in the study area especially in young cattle with high rate of mixed infections. The finding suggests that there is considerable economic loss due to trematode infections through reduced production efficiency of cattle in the study area. The prevalence of Fasciola spp. and Schistosoma spp. may also show the risk these parasites could pose to public health. Introduction of measures which would help minimize exposure of cattle to the parasites, such as keeping cattle away from grazing high risk areas, and strategic use of anthelmintics effective against mixed trematode infections especially in young stock may be considered. Empirical diagnosis and treatment of cattle, especially showing poor growth or weight loss, in the study area should take trematodes into consideration.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This research work was approved by the Research and Publication Committee of the School of Veterinary Medicine of Hawassa University.
Funding
This research work was partially funded by Hawassa University, Ethiopia.
Acknowledgements
The authors gratefully appreciate the all rounded co-operations provided by the staff of Bahir Dar Regional Veterinary Laboratory especially Drs. Nuria and Almaz and Ato Getachew. We are also grateful to all the farmers who kindly co-operated with us during data collection and allowed us to use their animals for this study.
References
- Abebe R., Abunna F., Berhane M., Mekuria S., Megersa B., Regassa A. Fasciolosis: Prevalence, financial losses due to liver condemnation and evaluation of a simple sedimentation diagnostic technique in cattle slaughtered at Hawassa Municipal abattoir, southern Ethiopia. Ethiopian Veterinary Journal. 2010;14(1):39–52. [Google Scholar]
- Abunna F., Asfaw L., Megersa B., Regassa A. Bovine fasciolosis: Coprological, abattoir survey and its economic impact due to liver condemnation at Soddo municipal abattoir, Southern Ethiopia. Tropical Animal Health and Production. 2010;42:289–292. doi: 10.1007/s11250-009-9419-3. [DOI] [PubMed] [Google Scholar]
- Ameni G., Erko B., Bogale T. Preliminary study on the major bovine trematode infection around Kemissie, Northeastern Ethiopia and treatment trial with praziquantel. Bulletin of Animal Health and Production in Africa. 2001;49:62–67. [Google Scholar]
- Andrews S.J., Dalton J.P. Fasciolosis. CABI Publishing; Wallingford, UK: 1999. The life cycle of Fasciola hepatica; pp. 1–29. [Google Scholar]
- A/Rahman M.B., Mohammed Z.A., Osman A.Y., Bakhiet H.A., Mohammed-Ahmed O., Osman H.M. Concurrent infection of Schistosoma bovis and Fasciola gigantica in a dairy cattle in Khartoum State, Sudan. The Sudan Journal of Veterinary Research. 2007;22:63–70. [Google Scholar]
- Aregay F., Bekele J., Ferede Y., Hailemelekot M. Study on the prevalence of bovine fasciolosis in and around Bahir Dar, Ethiopia. Ethiopian Veterinary Journal. 2013;17(1):1–11. [Google Scholar]
- Barger I.A. The role of epidemiological knowledge and grazing management for helminth control in small ruminants. International Journal of Parasitology. 1999;29:41–47. doi: 10.1016/s0020-7519(98)00176-3. [DOI] [PubMed] [Google Scholar]
- Berhe G., Berhane K., Tadesse G. Prevalence and economic significance of fasciolosis in cattle in Mekelle Area of Ethiopia. Tropical Animal Health and Production. 2009;41(7):1503–1504. doi: 10.1007/s11250-009-9339-2. [DOI] [PubMed] [Google Scholar]
- Bitakaramire P.K. The incidence of fascioliasis in different breeds in Kenya. Bulletin of Epizootic Diseases of Africa. 1973;21:145–152. [Google Scholar]
- Boray J.C. Experimental fascioliasis in Australia. Advances in Parasitology. 1969;7:95–209. doi: 10.1016/s0065-308x(08)60435-2. [DOI] [PubMed] [Google Scholar]
- Castelino J.B., Preston J.M. The influence of breed and age on the prevalence of bovine fascioliasis in Kenya. British Veterinary Journal. 1979;135:198–203. doi: 10.1016/s0007-1935(17)32942-1. [DOI] [PubMed] [Google Scholar]
- Chanie M., Dejen B., Fentahun T. Prevalence of cattle schistosomiasis and associated risk factors in Fogera cattle, South Gondar zone, Amhara National Regional State, Ethiopia. Journal of Advanced Veterinary Research. 2012;2(3):153–156. [Google Scholar]
- DAFWA Detection of trematode eggs and Eimeria leuckarti-sedimentation method (FEST) – Faecal Samples, Department of Agriculture and Food, Western Australia. 2013. https://www.agric.wa.gov.au/sites/gateway/files/DAFWA%20approved%20fluke%20egg%20sedimentation%20test%20%28FEST%29.pdf
- Dargie J.D. The impact on production and mechanisms of pathogenesis of trematode infections in cattle and sheep. International Journal for Parasitology. 1987;17:453–463. doi: 10.1016/0020-7519(87)90121-4. [DOI] [PubMed] [Google Scholar]
- Dohoo I., Martin W., Stryhn H. 2nd ed. AVC Inc.; Charlottetown, Prince Edward Island: 2009. Veterinary Epidemiological Research. [Google Scholar]
- Dorchies Ph. Flukes: Old parasites but new emergence. Proceedings of the XXIV World Buiatrics Congress; Nice, France; Consultada; 2006. [Google Scholar]
- Dreyfuss G., Alarion N., Vignoles P., Rondelaud D. A retrospective study on the metacercarial production of Fasciola hepatica from experimentally infected Galba truncatula in central France. Parasitology Research. 2006;98(2):162–166. doi: 10.1007/s00436-005-0048-0. [DOI] [PubMed] [Google Scholar]
- Elliott T.P., Kelley J.M., Rawlin G., Spithill T.W. High prevalence of fasciolosis and evaluation of drug efficacy against Fasciola hepatica in dairy cattle in the Maffra and Bairnsdale districts of Gippsland, Victoria, Australia. Veterinary Parasitology. 2015;209(1-2):117–124. doi: 10.1016/j.vetpar.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Fromsa A., Meharenet B., Mekibib B. Major trematode infections of cattle slaughtered at Jimma municipality abattoir and the occurrence of the intermediate hosts in selected water bodies of the zone. Journal of Animal and Veterinary Advances. 2011;10(12):1592–1597. [Google Scholar]
- G. Mariam T., Mohamed A., Ibrahim N., Baye D. Prevalence of fasciolosis and paramphistomosis in dairy farm and house hold in Hawassa town. European Journal of Biological Sciences. 2014;6(2):54–58. [Google Scholar]
- Gebrie Y., Gebreyohannes M., Tesfaye A. Prevelance of bovine fasciolosis in and around Bahir Dar, north west Ethiopia. Journal of Parasitology and Vector Biology. 2015;7(4):74–79. [Google Scholar]
- Habtamu A., Wolde Mariam S. Repeated simple sedimentation technique and prevalence of bovine schistosomosis in selected sites of Bahir Dar woreda. Ethiopian Veterinary Journal. 2011;15(1):49–57. [Google Scholar]
- Habtamu A., Negash T., Sirak A., Chanie M. Pathology of natural infections of Schistosoma bovis in cattle in Ethiopia. Global Veterinaria. 2013;11(2):243–247. [Google Scholar]
- Hansen J., Perry B. International Laboratory for Research on Animal Disease (ILRAD); Nairobi, Kenya: 1994. The epidemiology, diagnosis and control of helminth parasites of ruminants, a hand book. [Google Scholar]
- Hope-Cawdery M.J., Strickland K.L., Conway A., Crowe P.J. Production effects of liver fluke in cattle. I. The effects of infection on live weight gain, food intake and food conversion efficiency in beef cattle. British Veterinary Journal. 1977;133:145–159. doi: 10.1016/s0007-1935(17)34136-2. [DOI] [PubMed] [Google Scholar]
- Iglesias-Piñeiro J., González-Warleta M., Castro-Hermida J.A., Córdoba M., González-Lanza C., Manga-González Y., Mezo M. Transmission of Calicophoron daubneyi and Fasciola hepatica in Galicia (Spain): Temporal follow-up in the intermediate and definitive hosts. Parasites and Vectors. 2016;9:610. doi: 10.1186/s13071-016-1892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.N., Begum N., Alam M.Z., Mamun M.A.A. Epidemiology of intestinal schistosomiasis in ruminants of Bangladesh. Journal of Bangladesh Agricultural University. 2011;9(2):221–228. [Google Scholar]
- Kemal J., Terefe Y. Prevalence of gastrointestinal parasitism of cattle in Gedebano Gutazer Wolene district, Ethiopia. Journal of Veterinary Medicine and Animal Health. 2013;5(12):365–370. [Google Scholar]
- Keyyu J.D., Monrad J., Kyvsgaard N.C., Kassuku A.A. Epidemiology of Fasciola gigantica and amphistomes in cattle on traditional, small-scale dairy and large-scale dairy farms in the southern highlands of Tanzania. Tropical Animal Health and Production. 2005;37(4):303–314. doi: 10.1007/s11250-005-5688-7. [DOI] [PubMed] [Google Scholar]
- Khedri J., Radfar M.H., Borji H., Mirzaei M. Prevalence and intensity of Paramphistomum spp. in cattle from South-Eastern Iran. Iranian Journal of Parasitology. 2015;10(2):268–272. [PMC free article] [PubMed] [Google Scholar]
- Kifleyohannes T., Kebede E., Hagos Y., Weldu K.,G., Michael M. Prevalence of paramphistomosis in ruminants in Ashenge, Tigray Ethiopia. Acta Parasitologica Globalis. 2015;6(2):83–86. [Google Scholar]
- Lloyd J., Boray J.C., Love S. Stomach fluke (paramphistomes) in ruminants. 2007. http://www.wormboss.com.au/files/pages/worms/flukes/stomach-fluke/Prime_Fact_452_Stomach_flukeparamphistomesin_ruminants.pdf Primefact 452, NSW DPI.
- Love, S. (2017). Liver fluke- a review. Primefact 813, NSW DPI.
- Lulie B., Guadu T. Bovine schistosomiasis: A threat in public health perspective in Bahir Dar town, northwest Ethiopia. Acta Parasitologica Globalis. 2014;5(1):1–6. [Google Scholar]
- Mage C., Bourgne H., Toullieu J.M., Rondelaud D., Dreyfuss G. Fasciola hepatica and Paramphistomum daubneyi: Changes in prevalences of natural infections in cattle and in Lymnaea truncatula from central France over the past 12 years. Veterinary Research. 2002;33(5):439–447. doi: 10.1051/vetres:2002030. [DOI] [PubMed] [Google Scholar]
- Melaku S., Addis M. Prevalence and intensity of Paramphistomum in ruminants slaughtered at Debre Zeit industrial abattoir, Ethiopia. Global Veterinaria. 2012;8(3):315–319. [Google Scholar]
- Meshesha M., Tesfaye W. Prevalence of fasciolosis in cattle slathered at Hosanna municipal abattoir, southern Ethiopia. International Journal of Advanced Research in Biological Sciences. 2017;4(2):40–46. [Google Scholar]
- Nicholson M.J., Butterworth M.H. International Livestock Center for Africa-ILCA; Addis Ababa, Ethiopia: 1986. A guide to condition scoring of Zebu Cattle; p. 29. [Google Scholar]
- Njau B.C., Kasali O.B., Scholtens R.G., Mesfin D. Review of sheep mortality in the Ethiopian highlands, 1982-1986. ILCA Bulletin, Addis Ababa, Ethiopia. 1988;31:19–22. [Google Scholar]
- Nzalawahe J., Kassuku A.A., Stothard J.R., Coles G.C., Eisler M.C. Trematode infections in cattle in Arumeru District, Tanzania are associated with irrigation. Parasites and Vectors. 2014;7:107. doi: 10.1186/1756-3305-7-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiri A.M., Phiri I.K., Sikasunge C.S., Monrad J. Prevalence of fasciolosis in Zambian cattle observed at selected abattoirs with emphasis on age, sex and origin. Journal of Veterinary Medicine, Series B. 2005;52:414–416. doi: 10.1111/j.1439-0450.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Phiri A.M., Phiri I.K., Monrad J. Prevalence of amphistomiasis and its association with Fasciola gigantica infections in Zambian cattle from communal grazing areas. Journal of Helminthology. 2006;80(1):65–68. doi: 10.1079/joh2005313. [DOI] [PubMed] [Google Scholar]
- Rolfe P.F., Boray J.C. Chemotherapy of paramphistomosis in sheep. Australian Veterinary Journal. 1988;65(5):148–150. doi: 10.1111/j.1751-0813.1988.tb14443.x. [DOI] [PubMed] [Google Scholar]
- Rolfe P.F., Boray J.C. Comparative efficacy of moxidectin, an ivermectin/clorsulon combination and closantel against immature paramphistomes in cattle. Australian Veterinary Journal. 1993;70(7):256–266. doi: 10.1111/j.1751-0813.1993.tb08047.x. [DOI] [PubMed] [Google Scholar]
- Rolfe P.F., Boray J.C., Nichols P., Collins G.H. Epidemiology of paramphistomosis in cattle. International Journal of Parasitology. 1991;21:813–819. doi: 10.1016/0020-7519(91)90150-6. [DOI] [PubMed] [Google Scholar]
- Ross J.G. The economics of Fasciola hepatica infections in cattle. British Veterinary Journal. 1970;126(4):xiii–xixv. [PubMed] [Google Scholar]
- Solomon W/M., Wossene A. Prevalence study of ruminant fasciolosis in areas adjoining upper Blue Nile basin, north western Ethiopia. Ethiopian Veterinary Journal. 2007;11:67–81. [Google Scholar]
- Svendsen E.D. 3rd ed. Whittet Books Ltd; London: 1997. The Professional Handbook of the Donkey; pp. 375–376. [Google Scholar]
- Taye M., Jagema T., Tadese A., Mulatu E., Lelisa K. Study of ruminant fasciolosis in selected districts in upper Awash River basin, south western Shoa, Ethiopia. Journal of Veterinary Science and Technology. 2016;7(5) [Google Scholar]
- Taylor M.A., Coop R.L., Wall R.L. 4th ed. Wiley Blackwell; West Sussex, UK: 2016. Veterinary parasitology; pp. 390–393. [Google Scholar]
- Telila C., Abera B., Lemma D., Eticha E. Prevalence of gastrointestinal parasitism of cattle in East Showa zone, Oromia regional state, Central Ethiopia. Journal of Veterinary Medicine and Animal Health. 2014;6(2):54–62. [Google Scholar]
- Thrusfield M. 3rd ed. Blackwell science Ltd; London: 2005. Veterinary epidemiology; pp. 228–246. [Google Scholar]
- Torell R., Bruce B., Kvasnicka B., Conley K. University of Nevada; Reno, NV: 2003. Methods of determining age of cattle. Cattle Producer's Library: CL712.http://www.unce.unr.edu/publications/files/ag/other/cl712.Pdf [Google Scholar]
- Tsegaye B., Abebaw H., Girma S. Study on coprological prevalence of bovine fasciolosis in and around Woreta, Northwestern Ethiopia. Journal of Veterinary Medicine and Animal Health. 2012;4(7):89–92. [Google Scholar]
- Urquhart G.M., Armour J., Duncan J.L., Dunn A.M., Jennings F.W. 2nd ed. Blackwell Science Ltd; Harlow, UK: 1996. Veterinary parasitology; pp. 102–120. [Google Scholar]
- Vanleeuwen J.A., Tolosa T., Sirak A., Nemera M., Belaineh Seroprevalence of Mycobacterium avium ssp paratuberculosis infection in Ethiopian dairy farms. Bulletin of Animal Health and Production in Africa. 2014;62:95–100. [Google Scholar]
- Wamae L.W., Hammond J.A., Harrison L.J.S., Onyango-Abuje J.A. Comparison of production losses caused by chronic Fasciola gigantica infection in yearling Friesian and Boran cattle. Tropical Animal Health and Production. 1998;30(1):23–30. doi: 10.1023/a:1005057225427. [DOI] [PubMed] [Google Scholar]
- Yabe J., Phiri I.K., Phiri A.M., Chembensofu M., Dorny P., Vercruysse J. Concurrent infections of Fasciola, Schistosoma and Amphistomum spp. in cattle from Kafue and Zambezi river basins of Zambia. Journal of Helminthology. 2008;82:373–376. doi: 10.1017/S0022149X08054904. [DOI] [PubMed] [Google Scholar]
- Yeneneh A., Kebede H., Fentahun T., Chanie M. Prevalence of cattle flukes infection at Andassa livestock research center in north-west of Ethiopia. Veterinary Research Forum. 2012;3(2):85–89. [PMC free article] [PubMed] [Google Scholar]
- Yilma J.M., Mesfin A. Dry season bovine fasciolosis in northwestern part of Ethiopia. Revue de Médecine Vétérinaire. 2000;151(6):493–500. [Google Scholar]
- Yohannes M., Birasa D., Damena D., Tasew S., Degefu H. Bovine trypanosomosis and gastrointestinal helminthosis in settlement villages of Bedele district, south-western Ethiopia. Ethiopian Veterinary Journal. 2013;17(1):41–45. [Google Scholar]